Introduction
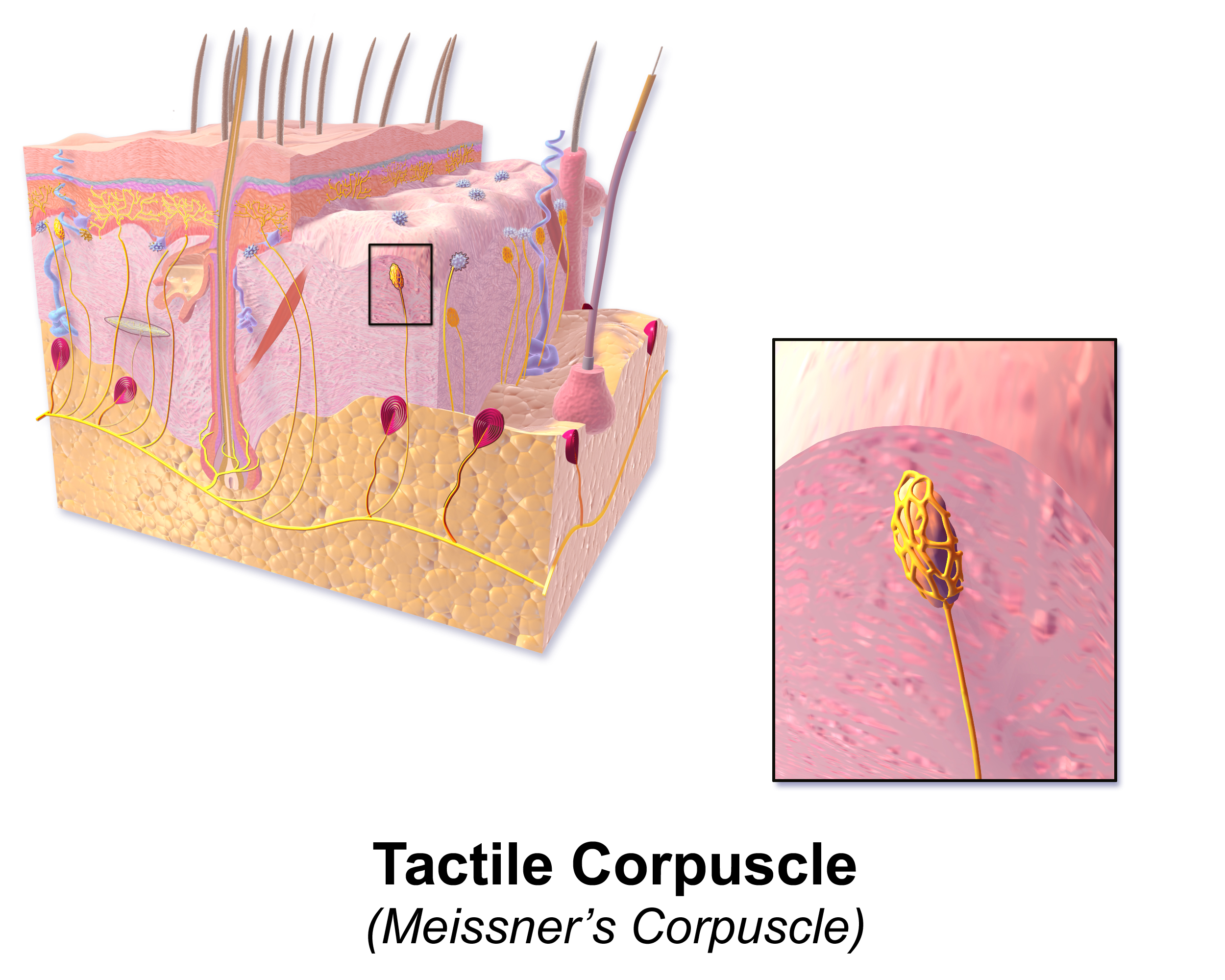
Meissner corpuscles, also known as Wagner-Meissner corpuscles or tactile corpuscles, are a subset of mechanoreceptors first described by Professor Georg Meissner and Professor Rudolf Wagner in 1852 (see Image. Tactile Corpuscle). Located in the dermal papillae of glabrous skin, these specialized encapsulated nerve endings relay delicate touch and low-frequency vibration sensations to the central nervous system (CNS). See Image. Papilla of the Hand, Treated With Acetic Acid. Magnified 350×. Meissner corpuscles play an essential role in somatosensory acuity, especially in the digital extremities and palmar skin, meriting clinical significance for peripheral and diabetic neuropathy as well as age-related degeneration of dermatological tactile sensation.
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
Meissner corpuscles are ellipsoid mechanoreceptors located superficially within the dermal papillae at a depth of approximately 150 micrometers. The corpuscles are approximately 20 to 40 micrometers in diameter and 80 to 150 micrometers in length, with their long axis oriented perpendicularly to the skin surface.[1] One corpuscle may be found within every two to four dermal papillae, with less than three corpuscles per papilla. Both the size and density of the receptors depend on the site of origin.
Each corpuscle is comprised of three primary components: elongated Schwann cells, a connective tissue capsule, and a central axon.[2][3] The flattened Schwann cells are organized in a stacked conformation in a background of an interlamellar matrix composed largely of collagen and microfilaments.[4] The capsule of Meissner corpuscles is derived from the endoneurial-perineural fibroblastic connective tissue. The deeper aspect of the corpuscle is lined by two to four layers of fibroblasts and fibrillary matrix. The apex of the capsule is incomplete. In this region, collagen fibrils from the interlamellar matrix extend into the dermis and anchor the receptor to the basal aspect of the epidermis.
Each corpuscle is supplied by a nerve ending derived from an intermediate-large amyloid-beta myelinated afferent fiber.[1][5] Innervation by additional unmyelinated C fibers has also been reported, although these fibers may simply pass through the corpuscle to reach the epidermis.[3] Typically, corpuscles are supplied by a single axon, but corpuscles with 2 to 7 accessory branches from the primary axon have been documented. The nerve fiber retains its myelin sheath as it enters the corpuscle but becomes amyelinic after a short distance.[4] The nerve fiber branches multiple times, forming bulbous expansions as it meanders tortuously throughout the lamellae. The cell body of the supplying neuron resides within a dorsal root ganglion or cranial nerve sensory ganglion. A single neuron from the sensory ganglion is capable of supplying multiple corpuscles.[6]
The development of Meissner corpuscles is dependent on brain-derived neurotrophic receptor (BDNF) signaling via tropomyosin receptor kinase B (TrkB). TrkB is an enzyme-linked transmembrane receptor encoded by the NTRK2 gene. Animal studies involving knockout of BDNF or TrkB in mice resulted in a lack of Meissner corpuscles, highlighting the importance of this signaling system in corpuscular development.[7][8][9]
Function
Meissner corpuscles consist of a cutaneous nerve ending responsible for transmitting the sensations of fine, discriminative touch and vibration.[1] Meissner corpuscles are most sensitive to low-frequency vibrations between 10 to 50 Hertz and can respond to skin indentations of less than 10 micrometers. Additionally, these corpuscles may detect the sensation of slip between an object and the skin, allowing for grip control. Meissner corpuscles have been hypothesized to function in the relay of pain sensations, as some axons may express substance P and other nociceptive peptides.[10] Further investigation into the role of these corpuscles in human nociception is warranted. The receptive field of Meissner corpuscles is 3 to 5 mm in diameter. The corpuscles respond to any stimuli within this receptive field with approximate uniformity, resulting in relatively limited spatial resolution.
The external force applied to a Meissner corpuscle is transduced by the collagen fibers connected to the lamellae. The resulting physical deformation induces bending of the nerve axon terminals to generate an action potential.[11][7] Removal of the stimulus causes normalization of the corpuscle’s shape, producing a second set of action potentials. Meissner corpuscles are considered low-threshold phasic receptors in that they adapt quickly to a stimulus. With sustained stimulation, the response of Meissner corpuscles decreases rapidly before ceasing. Such receptors are unable to convey information regarding the duration of the stimulus.
Cutaneous Meissner corpuscles are found in glabrous skin, particularly the fingertips, palms, and soles, enhancing the sensitivity of these tissues to light touch. Additional Meissner corpuscles may be found on the lips, palate, tongue, and genitalia. The density of Meissner corpuscles varies widely between studies, with a consensus on their quantity yet to be reached. One study reported corpuscular densities of 12 and 5.1 corpuscles per millimeter in digit five and the thenar eminence, respectively.[12]
Tissue Preparation
Meissner corpuscles can be identified on skin biopsy specimens under traditional light microscopy. Immediately after excision, the biopsied specimen is placed into a fixative solution of neutral buffered formalin that forms crosslinks between lysine residues to preserve tissue structure. The specimen is then placed into a small cassette before being infiltrated by paraffin. After the wax cools and hardens, the tissue-containing paraffin block is sliced into thin sections by a microtome. The sections may then be stained according to previously-described protocols for hematoxylin and eosin staining, immunohistochemistry, or immunofluorescence.
Historically, the silver impregnation technique was recommended for staining of peripheral nerve endings, including Meissner corpuscles.[13] The silver impregnation method can selectively highlight both myelinated and unmyelinated neurites and preserve the fine detail of nerves. Tissue samples are fixed in neutral or simple formalin or Bouin solution. Frozen sections are cut into thin sections and placed into the impregnating solution composed of urea, 1% silver nitrate, 95% ethyl alcohol, pyridine, 1% mercuric cyanide, and 1% picric acid in distilled water. After incubation for 5.5 hours, the specimens are submerged in a solution of 1% hydroquinone, 5% anhydrous sodium sulfate, and urea, followed by a mixture of 0.2% gold chloride and glacial acetic acid, then 5% sodium hyposulfite. After immunofluorescent staining, specimens are viewed using confocal scanning laser microscopy.
Two-photon excitation microscopy is a novel fluorescence imaging technique enabling high-resolution visualization of living tissue near the skin surface, including Meissner corpuscles.[14] Two-photon microscopy utilizes the long-lasting lipophilic fluorescent dye carbocyanine DiOC(3) and 484 nm laser for live imaging of the axonal components of the corpuscles. The persistence of the dye for several weeks allows for the ability to image the corpuscular neurites in vivo over a prolonged period to evaluate the mechanical response and structural changes of the corpuscles. A limitation of this visualization method is the inability to highlight other components of the receptors, such as the collagenous capsule or Schwann cells.
Histochemistry and Cytochemistry
Immunohistochemical staining targeting differential antigenic expression can distinguish between the neural and supportive components of Meissner corpuscles. Vega et al. reported that double immunolabelling with monoclonal antibodies against human neurofilament proteins (NFP) and S100 could reliably stain the central axon and Schwann cells, respectively.[15] Other immunohistochemical stains for the central axon include neuron-specific enolase, protein gene product 9.5, neurocalcin, and neurofilament subunits. Substance P, calcitonin gene-related peptide, and gamma-melanocyte stimulating hormone have also been utilized to stain the neuronal components of Meissner corpuscles. Lamellar cells are identifiable by using stains targeting the receptor for the vimentin and growth factor receptor TrkB.
Microscopy, Light
Under light microscopy, Meissner corpuscles appear as coiled, spring-like structures composed of stacked, disk-like lamellar cells. The orientation of the lamellae is variable, but they are typically parallel to the skin surface. The Schwann cell-derived lamellar cells have peripherally displaced nuclei and are contained within a fibroblastic capsule that is incomplete at its apex. The neurites that course through the lamellae are not visible by traditional hematoxylin and eosin staining techniques. See Image. Meissner Corpuscle (Tactile Corpuscle).
Microscopy, Electron
Much greater detail of Meissner corpuscles may be appreciated on transmission and scanning electron microscopy.[2] The lamellar cells can be visualized as peripheral nuclei and 2 to 3 micrometer-thick cytoplasmic extensions into the interior of the corpuscle. These projections are connected by desmosome-like junctions within a basal lamina and surrounded by an interlaminar substance composed of collagen and microfilaments. The internal portion of lamellar cells is smooth, although their external aspect is covered by fine 0.1 to 0.3 micrometer-thick projections, lending them a serrated appearance.[16] These projections mediate adherence to the interior portion of the collagenous capsule. Neuronal axons may be recognized on electron microscopy, as they branch and course helically throughout the lamellae. The neurites contain varicose regions rich in mitochondria and terminate in wide, bulbous endings. The apex of the corpuscle is in direct contact with the basal cells of the epidermis, with axon terminals and lamellar cells interdigitating with the stratum basale.[3]
Pathophysiology
As currently understood, Meissner corpuscles play a relatively minor role in human disease. Meissner corpuscles are often noted as a benign accessory component in some cellular nevi, schwannomas, and neurofibromas. A single case report describes the presence of Meissner-like corpuscles within a mature ovarian cystic teratoma.[17]
Occasional case reports describe benign tumors composed largely or entirely of Meissner corpuscles known as Wagner-Meissner neurilemmomas (WMNs).[18] WMNs present as slow-growing soft tissue masses involving the deep dermis and subcutaneous tissues. WMNs have not been limited to the typical distribution of Meissner corpuscles, having been reported on the cheek, lower extremity, and vulva.[19][20] These tumors are typically well-demarcated, residing within a fibrous collagen capsule, although a single case report documents an infiltrative growth pattern. On histological examination, WMNs display lamellated complexes composed of up to 20 laminar cells, resembling Meissner corpuscles. These tumors stain positively for neuron-specific enolase, vimentin, and S100 but lack the nerve fibers that supply the receptors, differentiating these abnormal structures from the functional corpuscles in the dermis.[18]
Structures morphologically identical to Meissner corpuscles are identified in abnormal locations have been termed tactile corpuscle-like bodies, Wagner-Meissner bodies, pseudo-Meissner corpuscles, and Meissner-oid corpuscles. Several cases have been published detailing the identification of proliferation of these Meissner corpuscle-like structures within the lamina propria, the gastrointestinal mucosa, including the esophagus, stomach, and colorectum.[21][22][23]
The origin of these lesions is unclear but may represent hamartomas, neural neoplasms, or a reactive process. Typically discovered incidentally during colonoscopy, these proliferations often resemble colonic polyps, leading to their biopsy and identification. Pathological examination of these lesions reveals discrete clusters of eosinophilic aggregates within the lamina propria. These structures are comprised of spindle-shaped cells, each with a single eccentric, oblong nucleus and lamellated, eosinophilic cytoplasm. Staining for S100 is positive while the histiocytic marker CD68 is negative, indicating the Schwannian or neural origin of the lesions. The differential diagnosis of these proliferations includes mucosal amyloid deposition and mucosal granulomas, although negative staining for Congo red and CD68, respectively, can be reliably used to differentiate these disorders. The presence of bodies within the gastrointestinal tract is benign.
Clinical Significance
Meissner corpuscles are an integral aspect of the human sensory system, required for discriminatory touch and grip control. The high sensitivity of these receptors also allows for the reading of Braille using the fingertips. Males and females have a similar number of corpuscles in each digit, although, given the larger average surface area of male hands and fingers, men have a lower density of receptors which may contribute to a small difference in touch receptivity.[24] The size, density, and complexity of Meissner corpuscles also decline significantly with increasing age.[25] Animal studies have revealed that with increasing age, the neurites supplying Meissner corpuscles become progressively more coarse, tortuous, and varicose with the disintegration of lamellar processes.[26] Older neurites demonstrated less parallel orientation and an increased number of axonal bifurcations per corpuscle.[27] These findings may underlie the age-related decrease in touch sensitivity.
Differences in corpuscular density have been associated with a number of neurologic disorders, including sensory neuropathy, Charcot-Marie-Tooth disease, Parkinson's disease, HIV neuropathy, and Friedreich’s ataxia.[28][29][30] Hypertrophy and hyperplasia of Meissner corpuscles have been described during the initial stages of diabetes in primate studies.[17] Following chronic hyperglycemia, hypertrophy of the corpuscles decreased; although the number of corpuscles remained greater than those of non-diabetic control animals, the receptors continued to display abnormal structure and protein expression. Additionally, the reduction of neuronal axons in the dermis, including those innervating Meissner's corpuscles, has been observed in patients with diabetes.[31] Axonal degeneration to Meissner's corpuscles and the dermis thus suggests the peripheral neuropathy experienced by people with diabetes.
Meissner corpuscles can survive for long-periods following nerve injury or denervation but can sustain alterations in protein expression. Expression of S100, a marker of lamellar cells within Meissner corpuscles, has been shown to be normal following spinal cord injury, diminished in nerve entrapment, and absent in denervated dermatomes.[32] These findings suggest that the functional integrity of axonal innervation is required for S100 protein expression by corpuscular lamellar cells.[33]
Media
(Click Image to Enlarge)
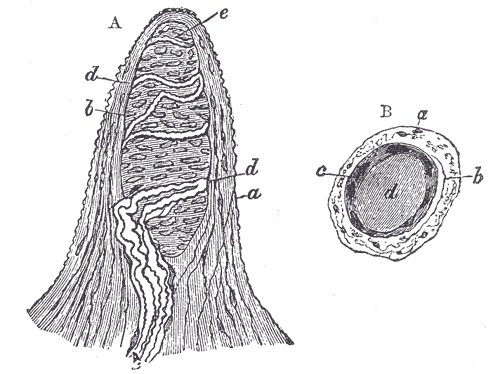
Papilla of the Hand, Treated With Acetic Acid. Magnified 350×. (A) Side view of a papilla of the hand. a. cortical layer. b. tactile corpuscle. c. small nerve of the papilla with neurolemma. d. 2 nervous fibers running with spiral coils around the tactile corpuscle. e. apparent termination of one of these fibers. B. A tactile papilla is seen from above to show its transverse section. a. cortical layer. b. nerve fiber. c. outer layer of the tactile body with nuclei. d. clear interior substance.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
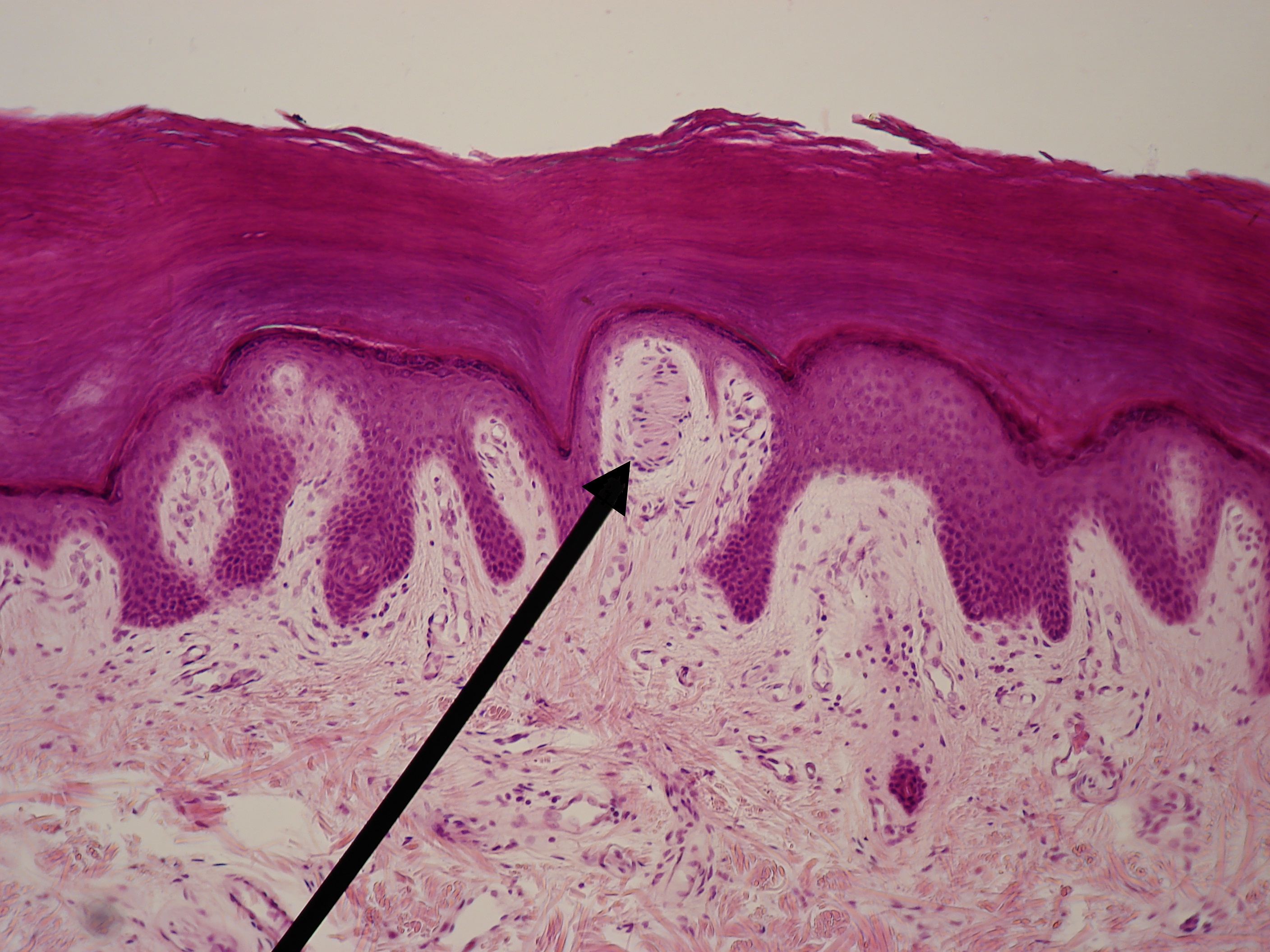
Meissner Corpuscle (Tactile Corpuscle). Image of a 100x light micrograph of Meissner's corpuscle (or tactile corpuscle) at the tip of a dermal papillus. As a type of mechanoreceptor, it is responsible for the sensitivity of light touch.
Wbensmith, Public Domain, via Wikimedia Commons
References
Vega JA, López-Muñiz A, Calavia MG, García-Suárez O, Cobo J, Otero J, Arias-Carrión O, Pérez-Piñera P, Menéndez-González M. Clinical implication of Meissner`s corpuscles. CNS & neurological disorders drug targets. 2012 Nov 1:11(7):856-68 [PubMed PMID: 23131158]
Level 3 (low-level) evidenceCAUNA N, ROSS LL. The fine structure of Meissner's touch corpuscles of human fingers. The Journal of biophysical and biochemical cytology. 1960 Oct:8(2):467-82 [PubMed PMID: 13691669]
Level 3 (low-level) evidenceIdé C. The fine structure of the digital corpuscle of the mouse toe pad, with special reference to nerve fibers. The American journal of anatomy. 1976 Nov:147(3):329-55 [PubMed PMID: 983972]
Level 3 (low-level) evidenceVega JA, García-Suárez O, Montaño JA, Pardo B, Cobo JM. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microscopy research and technique. 2009 Apr:72(4):299-309. doi: 10.1002/jemt.20651. Epub [PubMed PMID: 19012318]
Level 3 (low-level) evidenceCAUNA N. Nerve supply and nerve endings in Meissner's corpuscles. The American journal of anatomy. 1956 Sep:99(2):315-50 [PubMed PMID: 13372495]
Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science (New York, N.Y.). 2014 Nov 21:346(6212):950-4. doi: 10.1126/science.1254229. Epub [PubMed PMID: 25414303]
Level 3 (low-level) evidenceGonzález-Martínez T, Fariñas I, Del Valle ME, Feito J, Germanà G, Cobo J, Vega JA. BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neuroscience letters. 2005 Mar 22:377(1):12-5 [PubMed PMID: 15722178]
Level 3 (low-level) evidenceGonzález-Martínez T, Germanà GP, Monjil DF, Silos-Santiago I, de Carlos F, Germanà G, Cobo J, Vega JA. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain research. 2004 Mar 26:1002(1-2):120-8 [PubMed PMID: 14988041]
Level 3 (low-level) evidenceIchikawa H, Matsuo S, Silos-Santiago I, Sugimoto T. Developmental dependency of Meissner corpuscles on trkB but not trkA or trkC. Neuroreport. 2000 Feb 7:11(2):259-62 [PubMed PMID: 10674466]
Level 3 (low-level) evidenceParé M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001 Sep 15:21(18):7236-46 [PubMed PMID: 11549734]
Level 3 (low-level) evidenceHoffmann JN, Montag AG, Dominy NJ. Meissner corpuscles and somatosensory acuity: the prehensile appendages of primates and elephants. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004 Nov:281(1):1138-47 [PubMed PMID: 15470674]
Level 3 (low-level) evidenceHerrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007 Dec 4:69(23):2121-7 [PubMed PMID: 17898322]
Level 3 (low-level) evidenceCastano P, Rumio C, Morini M, Miani A Jr, Castano SM. Three-dimensional reconstruction of the Meissner corpuscle of man, after silver impregnation and immunofluorescence with PGP 9.5 antibodies using confocal scanning laser microscopy. Journal of anatomy. 1995 Apr:186 ( Pt 2)(Pt 2):261-70 [PubMed PMID: 7649825]
Pham TQ, Hoshi T, Tanaka Y, Sano A, Kawaue T, Miyata T. Two-Photon Imaging of DiO-Labelled Meissner Corpuscle in Living Mouse's Fingertip. IEEE transactions on haptics. 2016 Oct-Dec:9(4):483-491 [PubMed PMID: 27254872]
Vega JA, Llamosas MM, Huerta JJ, García-Fernández JM. Study of human cutaneous sensory corpuscles using double immunolabelling and confocal laser scanning microscopy. The Anatomical record. 1996 Dec:246(4):557-60 [PubMed PMID: 8955795]
Takahashi-Iwanaga H, Shimoda H. The three-dimensional microanatomy of Meissner corpuscles in monkey palmar skin. Journal of neurocytology. 2003 May:32(4):363-71 [PubMed PMID: 14724379]
Level 3 (low-level) evidenceFellegara G, Young RH, Kuhn E, Rosai J. Ovarian mature cystic teratoma with florid vascular proliferation and Wagner-Meissner--like corpuscles. International journal of surgical pathology. 2008 Jul:16(3):320-3. doi: 10.1177/1066896907307306. Epub [PubMed PMID: 18573789]
Level 3 (low-level) evidenceKaiserling E, Geerts ML. Tumour of Wagner-Meissner touch corpuscles. Wagner-Meissner neurilemmoma. Virchows Archiv. A, Pathological anatomy and histopathology. 1986:409(2):241-50 [PubMed PMID: 3087055]
Level 3 (low-level) evidenceWu AJ, Jarzembowski J, Morag Y, Lucas DR. Wagner-Meissner neurilemmoma of the right cheek. Annals of diagnostic pathology. 2008 Jun:12(3):204-7. doi: 10.1016/j.anndiagpath.2006.09.001. Epub 2007 Aug 20 [PubMed PMID: 18486897]
Level 3 (low-level) evidenceFerrara N, Di Marino M, Rossiello L, Baldi A. Wagner-Meissner neurilemmoma of the vulva. International journal of dermatology. 2003 Jul:42(7):550-1 [PubMed PMID: 12839606]
Level 3 (low-level) evidenceHuber AR, Agostini-Vulaj D, Drage MG, Lemmon JW. Tactile Corpuscle-Like Bodies (Wagner-Meissner Corpuscles) of the Colorectum: A Series of 5 Cases. International journal of surgical pathology. 2017 Dec:25(8):684-687. doi: 10.1177/1066896917723982. Epub 2017 Aug 7 [PubMed PMID: 28784007]
Level 3 (low-level) evidenceCeleiro-Muñoz C, Huebner TA, Robertson SA, Pittman ME, Singhi AD, Arnold CA, Bhaijee F, Voltaggio L, Montgomery EA. Tactile Corpuscle-like Bodies in Gastrointestinal-type Mucosa: A Case Series. The American journal of surgical pathology. 2015 Dec:39(12):1668-72. doi: 10.1097/PAS.0000000000000480. Epub [PubMed PMID: 26291509]
Level 2 (mid-level) evidenceWills EJ, Croker J, Brammah S. Tactile corpuscle-like bodies in colonic mucosa. Ultrastructural pathology. 2003 Mar-Apr:27(2):79-86 [PubMed PMID: 12746198]
Level 3 (low-level) evidenceDillon YK, Haynes J, Henneberg M. The relationship of the number of Meissner's corpuscles to dermatoglyphic characters and finger size. Journal of anatomy. 2001 Nov:199(Pt 5):577-84 [PubMed PMID: 11760888]
Iwasaki T, Goto N, Goto J, Ezure H, Moriyama H. The aging of human Meissner's corpuscles as evidenced by parallel sectioning. Okajimas folia anatomica Japonica. 2003 Mar:79(6):185-9 [PubMed PMID: 12776944]
Nava PB, Mathewson RC. Effect of age on the structure of Meissner corpuscles in murine digital pads. Microscopy research and technique. 1996 Jul 1:34(4):376-89 [PubMed PMID: 8807620]
Level 3 (low-level) evidenceMathewson RC, Nava PB. Effects of age on Meissner corpuscles: a study of silver-impregnated neurites in mouse digital pads. The Journal of comparative neurology. 1985 Jan 8:231(2):250-9 [PubMed PMID: 3968237]
Level 3 (low-level) evidenceNolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain : a journal of neurology. 2008 Jul:131(Pt 7):1903-11. doi: 10.1093/brain/awn102. Epub 2008 May 31 [PubMed PMID: 18515869]
Almodovar JL, Ferguson M, McDermott MP, Lewis RA, Shy ME, Herrmann DN. In vivo confocal microscopy of Meissner corpuscles as a novel sensory measure in CMT1A. Journal of the peripheral nervous system : JPNS. 2011 Sep:16(3):169-74. doi: 10.1111/j.1529-8027.2011.00342.x. Epub [PubMed PMID: 22003930]
Level 3 (low-level) evidenceAlmodovar JL, Schifitto G, McDermott MP, Ferguson M, Herrmann DN. HIV neuropathy: an in vivo confocal microscopic study. Journal of neurovirology. 2012 Dec:18(6):503-10. doi: 10.1007/s13365-012-0130-1. Epub 2012 Oct 16 [PubMed PMID: 23070817]
Level 2 (mid-level) evidenceShun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain : a journal of neurology. 2004 Jul:127(Pt 7):1593-605 [PubMed PMID: 15128619]
Level 2 (mid-level) evidenceAlbuerne M, López S, Naves FJ, Martínez-Almagro A, Represa J, Vega JA. S100alpha and S100beta proteins in human cutaneous sensory corpuscles: effects of nerve and spinal cord injury. The Anatomical record. 1998 Jul:251(3):351-9 [PubMed PMID: 9669763]
Márquez J, Pérez-Pérez M, Naves FJ, Vega JA. Effect of spinal cord and peripheral nerve injury on human cutaneous sensory corpuscles. An immunohistochemical study. Journal of the peripheral nervous system : JPNS. 1997:2(1):49-59 [PubMed PMID: 10975736]