Introduction
The incidence of primary cutaneous melanoma has increased steadily for several decades and remains the most lethal form of cutaneous neoplasm. If diagnosed in the early stages, melanoma has high survival rates, approximating 94%.[1] According to the National Cancer Institute (NCI), from 2014 to 2018, the incidence of metastatic melanoma was estimated to be 0.9 per 100,000. Mucosal and ocular melanomas typically have worse prognoses.[2] Melanoma was once considered a very aggressive cancer that was resistant to traditional therapies such as chemotherapy, radiation, and even single-agent targeted therapies in their early stages of development. A dramatic improvement in the quality of life and overall survival of patients with metastatic melanoma has resulted after the advent of various new combinations of targeted therapies and different modalities of immunotherapies.
Melanoma is distinct from nonmelanoma skin cancers because it spreads locally, regionally, and distantly. An individual's risk of metastasis is directly related to the depth of invasion and ulceration of the primary lesion. The early stages of cancer metastasis involve invasion, angiogenesis, extravasation, dissemination, and colonization of the target organ. Patients with clinically node-negative disease and those with negative sentinel lymph node biopsy can still present with distant metastatic disease. Moreover, complete lymph node dissection has not been proven to offer a survival benefit to patients with node-positive disease.[3] There are reports of the transfer of melanoma from a donor to a recipient after an organ transplant, even when the transplant was performed years after the donor was diagnosed with melanoma.[4] Such distant seeding suggests the possibility of early subclinical micrometastasis in melanoma. According to the American Society of Clinical Oncology (ASCO), only about 4% of melanomas are present with metastasis.
Patients typically present with an asymmetrical large lesion that may also itch, bleed, ulcerate, or develop satellite lesions. Patients who present with metastatic disease or with primary sites other than the skin have signs and symptoms related to the affected organ systems. Once a suspicious skin lesion is identified, a biopsy must be performed to confirm the diagnosis of melanoma. An excisional biopsy is the preferred biopsy modality. Melanoma treatment typically involves wide local excision, Mohs micrographic surgery, digital amputations, or adjuvant therapy depending on tumor location, depth, ulceration, lymph node involvement, and metastasis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Multiple causes have been postulated in the development of malignant melanoma, including:
- Family history: A positive family history is noted in 5% to 10% of patients with melanoma. There is a 2.2-fold higher risk of developing melanoma with at least 1 affected family member.
- Personal characteristics: Blue eyes, fair or red hair, pale complexion, history of sunburns, freckled skin, history of benign or dysplastic melanocytic nevi with the number of lesions showing a stronger correlation than size, and immunocompromised state (eg, posttransplant patients, patients with hematologic malignancies) are known high-risk factors.
- Lifetime sun exposure: High UVB and UVA radiation exposure. Recent evidence has shown that the risk of melanoma is higher in people who use sunscreen. This is because sunscreen primarily blocks UVB, and therefore, people using sunscreen may be exposed to UVA more than the general public, provided those people are exposed to the sun more than the public at large. Risks of melanoma increase with decreasing or increasing latitude.[5]
- Atypical mole syndrome: Formerly termed B-K mole syndrome, dysplastic nevus syndrome, or familial atypical multiple mole (FAMM) syndrome. Over 10 years, patients with atypical mole syndrome have a 10.7% risk of melanoma versus 0.62% of controls. There is a higher risk of melanoma depending on the number of family members affected, with nearly 100% risk if ≥2 relatives have dysplastic nevi and melanoma.
- Tanning bed use
- Socioeconomic status: Lower socioeconomic status may be linked to more advanced disease at the time of detection. Several studies of newly diagnosed patients found that patients of low socioeconomic status have decreased melanoma risk perception, knowledge of the disease, and preventative practices.
Epidemiology
According to the NCI's Surveillance, Epidemiology, and End Results (SEER) program, melanoma is currently the fifth most common malignancy in both men and women. Experts estimate that in 2023, 97,610 new cases of melanoma will be diagnosed in the US, with an estimated 7,990 deaths associated with melanoma.[1]
Melanoma is caused by several factors, including environmental, genetic, and immunological.[6][7][8] In particular, melanoma research has focused on activating the immune system and understanding various cancer signal transduction pathways, with the successful development of effective immunotherapies and targeted therapies, respectively. The genes associated with melanoma predisposition are CDKN2A, CDK4, MC1R. The genetic disorder xeroderma pigmentosum (XP) is also associated with melanoma and results in the improper repair of ultraviolet (UV)-induced DNA damage and, therefore, a high mutation rate.[9][10][11][12]
Pathophysiology
Melanoma has a relatively high risk of systemic spread. Melanoma has several well-defined clinical attributes and risk factors. Molecular studies, genetics, and next-generation sequencing have revealed that the BRAF V600E mutation plays a crucial role in oncogenesis. These studies have also demonstrated that UV-induced DNA mutations play a significant role in melanoma development and carry a high mutation burden. Immunotherapy and targeted therapies have significantly improved the survival rates and outcomes associated with metastatic melanoma. Despite this, several challenges remain in understanding the biology of therapeutic resistance and relapses.
Melanocytes are neural crest-derived cells in the basal layer of the epidermis and located in the skin, hair, uvea mucosal epithelia, and meninges. The primary function of melanocytes is to synthesize melanin within melanosomes and transfer melanin via dendritic processes to neighboring keratinocytes. Melanocytes produce 2 forms of melanin pigment, eumelanin and pheomelanin, derived from precursor tyrosinase.[13]
Many factors can promote melanoma development, including exposure to UV rays.[14][15][16] [17] People of the same ethnicity experience different rates of melanoma depending on their geographical location. Locations differ in terms of atmospheric absorption, latitude, altitude, cloud cover, levels of ozone layer depletion, and seasonality, thus impacting incident UV radiation.[18] Genetic factors also influence the pathogenesis of melanoma. The BRAF mutation was detected in patients with melanoma without chronic sun damage in 2005 by Uhara et al.[19][20] Several studies have shown that nearly 40% to 50% of cutaneous melanomas have mutations in BRAF, a serine/threonine-protein kinase associated with the RAS-RAF-MEK pathway.[21] The BRAF mutation activates the mitogen-activated protein (MAP) kinase pathway, which starts at the cell surface and signals through RAS, then RAF, followed by mitogen-activated protein kinase (MEK) and extracellular signal-regulated kinase (ERK). ERK is the final protein in this cascade, which affects the expression of genes within the nucleus and leads to cell proliferation. The most common mutation is the V600E, although a different mutation called V600K has also been found in some cases.
Certain types of melanoma are associated with cumulative solar damage (CSD). However, in some cases, the etiology is not always clear.[22] The 2018 World Health Organization (WHO) Classification of Melanoma categorizes melanomas into the following categories:[22]
Melanomas Typically Associated with Cumulative Solar Damage
- Pathway I. Superficial spreading melanoma/low-CSD melanoma
- Pathway II. Lentigo maligna melanoma/high-CSD melano a
- Pathway III. Desmoplastic melanoma
Melanomas Not Consistently Associated with Cumulative Solar Damage
- Pathway IV. Spitz melanomas
- Pathway V. Acral melanoma
- Pathway VI. Mucosal melanomas
- Pathway VII. Melanomas arising in congenital nevi
- Pathway VIII. Melanomas arising in blue nevi
- Pathway IX. Uveal melanoma
Nodular Melanomas
Nodular melanoma may occur in any or most of the pathways. Four major variants of primary cutaneous melanoma are:
- Superficial spreading melanoma
- Most common type of melanoma
- Exhibition of hallmark melanoma features: asymmetry, irregular borders, color, and increased diameter [23]
- Prolonged radial growth phase, characterized by intraepidermal expansion
- No dermal invasion
- Nodular melanoma
- Located in chronically sun-exposed areas, the head and neck.
- Histologically, a vertical growth phase only and an absent radial growth phase
- Rapid growth and usually presents at an advanced Breslow depth
- 15% to 20% of primary melanomas and responsible for 40% of melanoma deaths
- Lentigo maligna melanoma
- Elderly patients with chronically sun-damaged skin on the face
- Initiation from an in situ lentigo maligna precursor that presents as a slowly enlarging and evolving brown-to-black macule with irregular borders
- Acral lentiginous melanoma
Histopathology
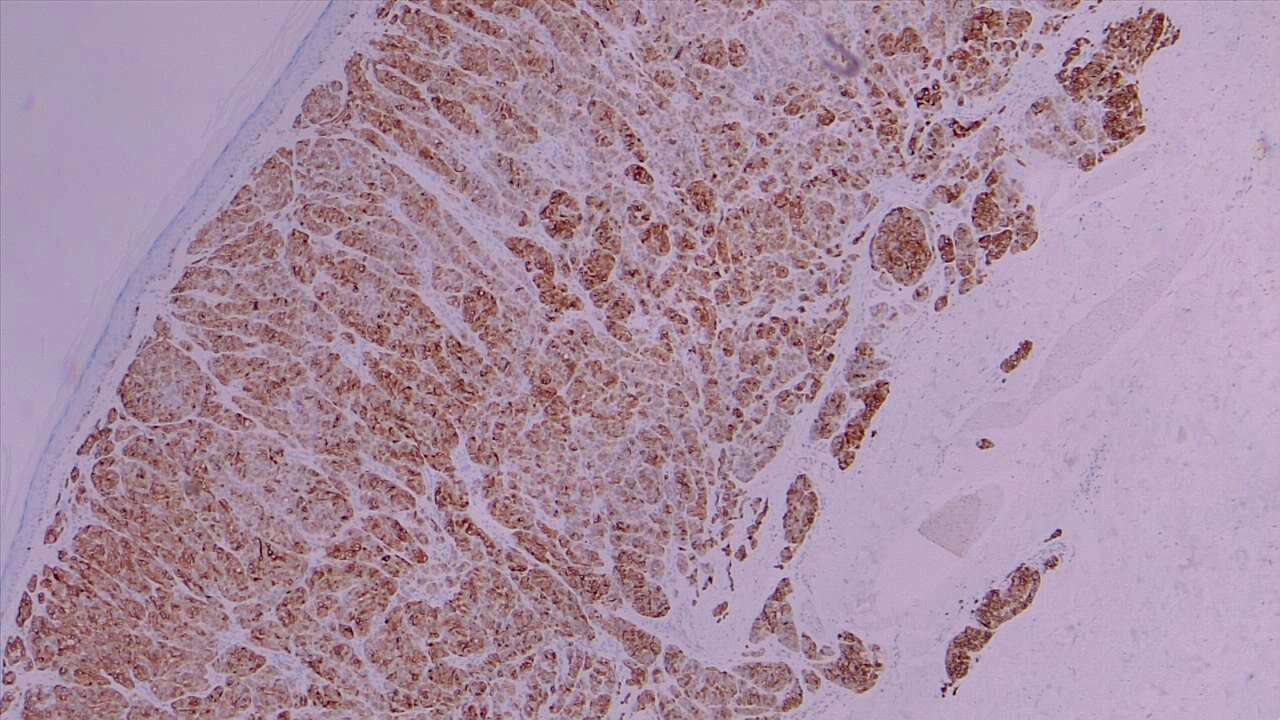
The primary types of malignant melanoma seen on histologic examination include superficial spreading, nodular, lentigo, and acral lentiginous melanomas (see Image. Malignant Melanoma).
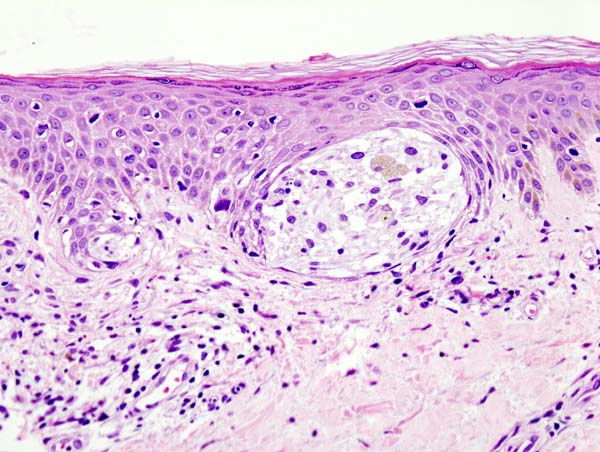
Superficial Spreading Melanomas
Epidermal findings commonly seen in superficial spreading melanomas include unrestricted melanocytes, an increased number of singular melanocytes rather than melanocyte nests, abnormally distributed and nonconjoined melanocytes, and melanocytes identified above the basal layer (ie, Pagetoid spread).[24] See Image. Superficial Melanoma.
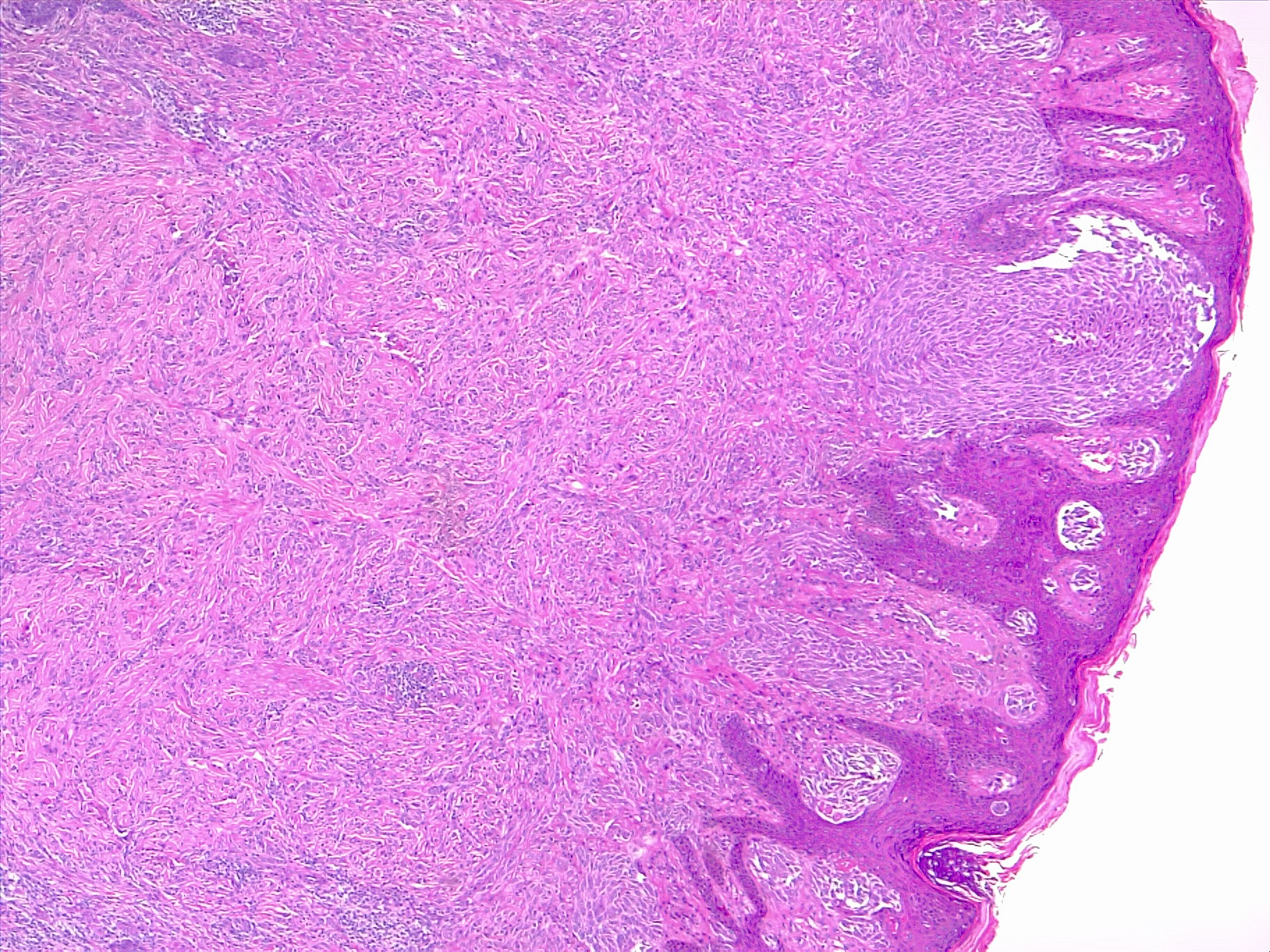
Nodular Melanomas
Nodular melanomas have similar findings to superficial spreading melanomas, except that nodular melanomas are sharply circumscribed. Instead, the epidermal component mirrors the dermal component and does not extend beyond the dermal borders. However, these neoplasms have a poor prognosis because they have a higher rate of growth and metastasis (see Image. Nodular Melanoma).[24]
Lentigo Maligna Melanomas
Characteristic histologic findings of these lesions include epidermal atrophy secondary to severe sun damage and melanocytes running together along the dermal-epidermal junction with epithelial extension. Small, hyperchromatic cells with dense nuclear chromatin are typical. However, cell nucleoli are usually not seen. Multinucleated melanoma cells are also common. Pagetoid spread is usually only seen with advanced disease.[24]
Acral Lentiginous Melanomas
This type of melanoma is rare, usually found in nailbeds, and therefore has a delayed diagnosis. Histologic findings associated with acral lentiginous melanoma include melanocytes in nests and as isolated cells along the dermal junction. Widespread pagetoid spread upwards is typical. Pagetoid spread can also be observed in benign forms of this tumor but usually has minimal extension. Melanocytes are often hyperchromatic, and nucleoli are challenging to visualize.[24]
History and Physical
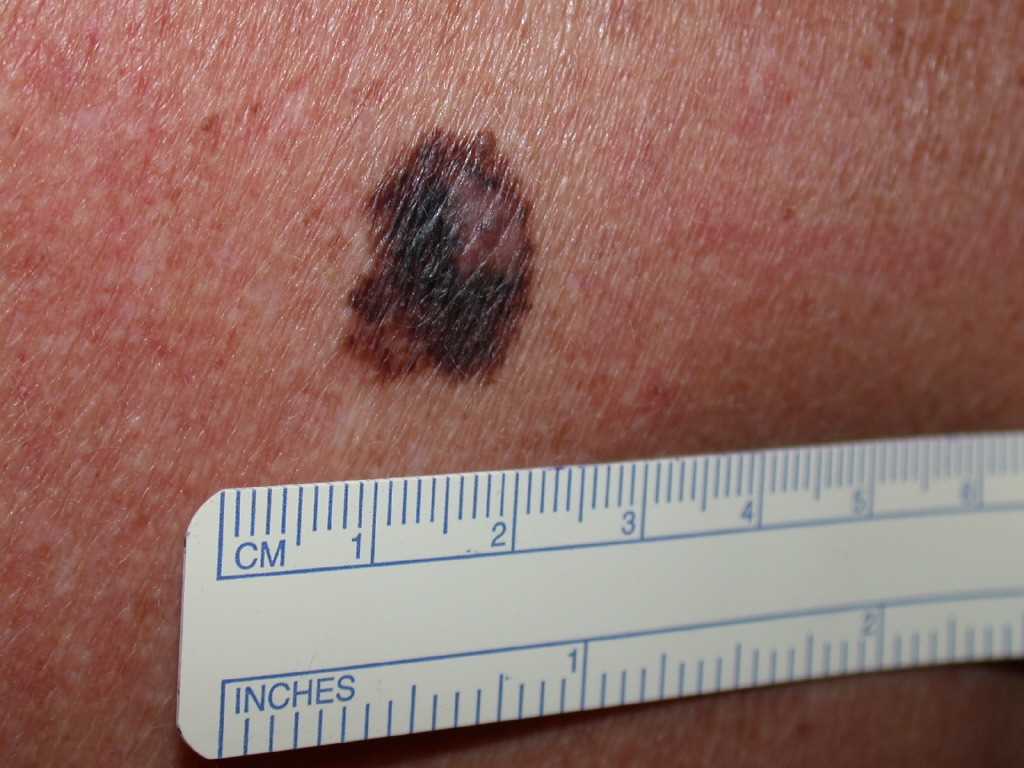
The characteristic signs of early melanoma are recognized with the following well-known ABCDE mnemonic:
- “A” stands for Asymmetry
- “B” stands for Border: irregular, ragged, notched, or blurred edges
- “C” stands for Color: nonuniform or variegated
- “D” stands for Diameter: larger than 6 millimeters
- “E” stands for Evolving: changes in size, shape, or color [25]
See Image. Melanoma. Dermoscopy can also be an important tool in distinguishing benign or malignant lesions.[26][27] Once diagnosed, melanoma is staged using American Joint Committee on Cancer (AJCC) guidelines, which guide treatment and determine prognosis.[28] Also, melanomas may itch, bleed, ulcerate, or develop satellite lesions. Patients who present with metastatic disease or with primary sites other than the skin have signs and symptoms related to the affected organ systems. Examining all lymph node groups as part of the evaluation is also important.
Evaluation
A whole-body skin examination is the most essential and fundamental evaluation needed in diagnosing melanoma, and it should be performed in the presence of optimal lighting. Skin examination is often complemented with a dermatoscopy of suspicious lesions. Using this evaluation, the assessment of "ABCDE" is completed. During this examination, attention must be paid to examining nail units. This is important for properly diagnosing abnormal melanomas, which follow a slightly different mnemonic ABCDEF, where the "F" stands for family or personal history of melanoma.
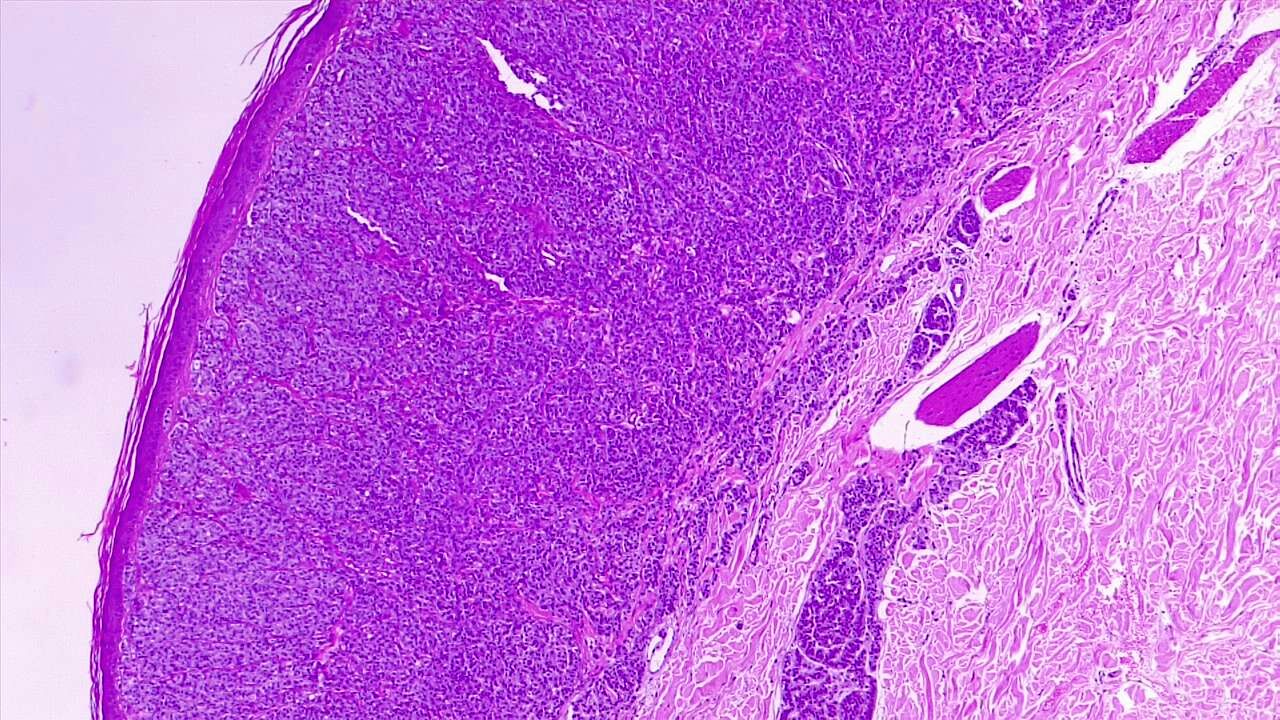
Once a suspicious skin lesion is identified, a biopsy must be performed to confirm the diagnosis of melanoma. An excisional biopsy is the preferred modality of biopsy. However, an incisional biopsy might be acceptable in certain situations, especially for subungual lesions, lesions on palms or soles, or large lesions.[29][30][31] Obtaining a complete or excisional biopsy is preferred since the tumor staging of melanoma is based on the depth of invasion. (see Image. Dermal Invasion by Melanoma). These results will further confirm the staging and necessitate additional investigations, including sentinel lymph node evaluation or the need for staging workup with imaging studies.
The following laboratory studies are indicated:
- Complete blood count
- Complete chemistry panel including alkaline phosphatase, hepatic transaminases, total protein, and albumin
- Lactate dehydrogenase
The following imaging modalities may be considered:
- Chest radiography
- Magnetic resonance imaging (MRI) of the brain
- Ultrasonography, possibly the best imaging study for diagnosing lymph node involvement
- computed tomography (CT) of the chest, abdomen, or pelvis
- Positron emission tomography (PET), PET-CT may be the best imaging study for identifying any sites of metastasis
Treatment / Management
Melanomas diagnosed in earlier stages are treated primarily with surgical intervention involving excision of the tumor using a wide local excision technique if excisional biopsy results in positive or close margins. In addition, sentinel lymph node biopsy and wide local excision for any tumor T1b or higher are completed. In cases where primary closure is impossible due to large-sized lesions, skin grafting or tissue transfer techniques are needed as part of the reconstruction.[32][33][34][35] Typically, wide local excision is performed up to the muscle fascia. The following are the optimal margins for wide excision currently derived from various large clinical trials and can be summarized below:[36][37][38][39][40][41][42](A1)
- Melanoma in situ (Tis): 0.5 to 1 cm
- T1 (≤1 mm): 1 cm
- T2 (≥1 to 2 mm): 1 to 2 cm
- T3 or T4 (>2 mm): 2 cm
For melanoma in situ, Mohs micrographic surgery may be an option, aside from wide local excision. For melanomas arising in unusual sites, like subungual melanomas, digital amputations are recommended. However, for melanoma in situ in subungual areas, there could be an option for digit-preserving surgeries. Various high-risk features could be noted on the final pathology specimen after wide excision and sentinel lymph node biopsy, including tumor depth, ulceration, and lymph node involvement that would determine the need for adjuvant therapies.[43] (B2)
Sentinel lymph node biopsy is recommended in patients with melanomas where there is an increased risk of regional node metastasis. This is determined primarily by the depth of melanoma or Breslow's depth and is recommended in melanomas with T1b or higher staging.[44][45] If lymph node involvement is noted on sentinel lymph node evaluation, the current preference, as per national oncology societies, is observation rather than completion of lymph node dissection. This is based on the results from a phase III Multicenter Selective Lymphadenectomy Trial II (MSLT-II), which demonstrated no difference in melanoma-specific survival between these 2 groups.[3] However, there was an improvement in the lymphadenectomy group compared to the observation group regarding 3-year disease-free survival or recurrence in regional lymph nodes, at the expense of higher incidences of lymphedema. (A1)
Adjuvant Therapies
- Stage IB (T2a) or stage II (≥T2b) (A1)
- Stage IIIA (sentinel node-positive) with low-risk disease (B2)
- Stage III (clinically node-positive)
- If the patient has resectable nodal disease, then after completing wide local excision and therapeutic lymph node dissection, the patient should be offered nivolumab or pembrolizumab or dabrafenib/trametinib if BRAF mutated. In addition, the patient could be considered for radiation therapy to the lymph node basin depending on the location, size, and number of lymph nodes involved.[53][54]
- If the patient has borderline resectable disease, then patients could have the option of receiving neoadjuvant therapies, raising either single-agent pembrolizumab[55] or the combination of ipilimumab and nivolumab.[56]
(A1)
In current clinical practice, interferon alfa is rarely used in adjuvant settings.
Differential Diagnosis
The differential diagnoses of malignant melanoma include:
- Atypical fibroxanthoma
- Pigmented basal cell carcinoma
- Blue nevus
- Epithelioid tumor
- Halo nevus
- Histiocytoid hemangioma
- Mycosis fungoides
- Pigmented spindle cell tumor
- Sebaceous carcinoma
- Dermatofibroma
- Seborrheic keratosis
- Cherry hemangioma
- Lentigo
- Subungual hematoma
Staging
Cutaneous melanoma staging is based on the American Joint Committee on Cancer (AJCC) 8th edition tumor, node, and metastasis (TNM) staging system guidelines. Tumor staging depends on tumor thickness and ulceration status. Nodal involvement and extent of metastatic spread define nodal and metastasis staging, respectively.
Tumor
TX: Primary tumor thickness cannot be assessed (eg, diagnosis by curettage)
T0: No evidence of primary tumor (eg, unknown primary or completely regressed melanoma)
Tis (ie, melanoma in situ)
T1: ≤1 mm
- T1a: <0.8 mm without ulceration
- T1b: <0.8 mm with ulceration
T2: <1 to 2 mm
- T2a: <1 to 2 mm without ulceration
- T2b: <1 to 2 mm with ulceration
T3: >2 to 4 mm
- T3a: >2 to 4 mm without ulceration
- T3b: >2 to 4 mm with ulceration
T4: >4.0 mm
- T4a: >4.0 mm without ulceration
- T4b: >4.0 mm with ulceration
Node
NX: Regional nodes not assessed
N0: No regional metastases detected
N1: 1 tumor-involved node or in-transit, satellite, or microsatellite metastases with no tumor-involved nodes
- N1a: 1 clinically occult (ie, detected by SLN biopsy)
- N1b: 1 clinically detected
- N1c: No regional lymph node disease but with the presence of in-transit or satellite or microsatellite metastases
N2: 2 to 3 tumor-involved nodes or in-transit, satellite, and microsatellite metastases with 1 tumor-involved node
- N2a: 2 to 3 clinically occult (ie, detected by SLN biopsy)
- N2b: 2 to 3, at least 1 clinically detected
- N2c: 1 clinically occult or clinically detected with the presence of in-transit or satellite or microsatellite metastases
N3: ≥4 tumor-involved nodes or in-transit, satellite, or microsatellite metastases with ≥2 tumor-involved nodes or any matted nodes without or with in-transit, satellite, or microsatellite metastases
- N3a: ≥4 clinically occult (ie, detected by SLN biopsy)
- N3b: ≥4, at least 1 of which was clinically detected, or the presence of any number of matted nodes
- N3c: ≥2 clinically occult or clinically detected or the presence of any matted nodes with the presence of in-transit or satellite or microsatellite metastases
Metastasis
M0: No evidence of distant metastasis
M1: Evidence of distant metastasis
- M1a(0): Distant metastasis to the skin, soft tissue including muscle, or nonregional lymph node
- M1a(1): Distant metastasis to the skin, soft tissue including muscle, or nonregional lymph node with elevated LDH
- M1b(0): Distant metastasis to the lung with or without M1a sites of disease
- M1b(1): Distant metastasis to the lung with or without M1a sites of disease with elevated LDH
- M1c(0): Distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease
- M1c(1): Distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease with elevated LDH
- M1d(0): Distant metastasis to CNS with or without M1a, M1b, or M1c sites of disease
- M1d(1): Distant metastasis to CNS with or without M1a, M1b, or M1c sites of disease with elevated LDH
Table. TNM Staging Summary
| Stage | T | N | M |
| 0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 |
| IB | T1b | N0 | M0 |
| T2a | N0 | M0 | |
| IIA | T2b | N0 | M0 |
| T3a | N0 | M0 | |
| IIB | T3b | N0 | M0 |
| T4a | N0 | M0 | |
| IIC | T4b | N0 | M0 |
| IIIA | T1a/b, T2a | N1a, N2a | M0 |
| IIIB | T0 | N1b, N1c | M0 |
| T1a/b, T2a | T1a/b, T2a | M0 | |
| T2b, T3a | N1a/b/c, N2a/b | M0 | |
| IIIC | T0 | N2b/c, N3b/c | M0 |
| T1a/b, T2a/b, T3a | N2c, N3a/b/c | M0 | |
| T3b, T4a | Any N ≥ N1 | M0 | |
| T4b | N1a/b/c, N2a/b/c | M0 | |
| IIID | T4b | N3a/b/c | M0 |
| IV | Any T, Tis | Any N | M1 |
Prognosis
Poor prognostic factors that affect survival in melanoma include:
- Tumor thickness, with a worse prognosis in thicker lesions
- Evidence of tumor in regional lymph nodes (ie, stage III disease)
- A higher number of positive lymph nodes
- Presence of distant metastasis (ie, stage IV disease)
- Anatomic site, with trunk or face lesions having a worse prognosis than extremity lesions
- Presence of ulceration
- Presence of regression on histologic examination, though this is controversial
- Male sex
The following are the reported survival rates depending on the disease stage at diagnosis:[28]
- Stage 0: 5-year overall survival rate of 99% to 100%
- Stage IA: 5-year overall survival rate of 99%
- Stage IB: 5-year overall survival rate of 97%
- Stage IIA: 5-year overall survival rate of 94%
- Stage IIB: 5-year overall survival rate of 87%
- Stage IIC: 5-year overall survival rate of 82%
- Stage IIIA: 5-year overall survival rate of 93%
- Stage IIIB: 5-year overall survival rate of 83%
- Stage IIIC: 5-year overall survival rate of 69%
- Stage IIID: 5-year overall survival rate of 32%
- Stage IV: 5-year overall survival rate of 34% to 52% [28]
Complications
Management of malignant melanoma involves a combination of surgery, radiation therapy in very few cases, adjuvant immunotherapy, or targeted therapies. These interventions, which are proven to improve survival and long-term outcomes associated with melanoma, are associated with some potential complications.
Surgery can be associated with complications, including bleeding, infection, damage to nerves, and scarring. Cosmetic outcomes from surgery might also have psychological implications, including depression and anxiety. In patients undergoing lymph node dissection, there is an increased incidence of lymphedema. In a few patients who receive radiation to the lymph node basin for extensive lymph node involvement, there could be potential radiotherapy-related complications, including skin irritation, fatigue, risk of secondary malignancies, and lymphedema.
Adjuvant therapies, including immunotherapies and targeted therapies, have various potential adverse effects that could complicate the treatment course. These include immunotherapy-related adverse events (irAEs) and targeted therapy-related drug-induced fevers, risk of secondary malignancies, fatigue, risk of infections, and immunosuppression.
All these potential complications can affect the quality of life of a patient, along with the inability to complete the treatment course, which could result in an increased risk of recurrence and poorer survival outcomes. Therefore, vigilance is essential due to the potential complications of various management strategies. Additionally, selecting the appropriate therapy and monitoring patients is critical.
Deterrence and Patient Education
Patients should be educated and counseled to practice important primary as well as secondary preventative strategies, including:
- Sun protection measures (eg, wearing protective clothing and using sunscreen with appropriate SPF strength)
- Avoiding mid-day sun exposure
- Avoiding tanning beds
- Annual dermatology evaluations
- Regular skin checks with immediate assessment of any spot changes
Enhancing Healthcare Team Outcomes
Primary care clinicians, dermatologists, surgeons, medical oncologists, and radiation oncologists frequently detect and manage melanoma, so an interprofessional/multidisciplinary team approach is needed. While many skin lesions are benign, clinicians should always consider melanoma a potentially deadly diagnosis to miss. If there is suspicion of melanoma, the patient should obtain a referral to the dermatologist for further workup, irrespective of which other healthcare clinicians first became suspicious. Surgery includes wide local excision with sentinel lymph node biopsy, followed by lymph node dissection in some cases. These surgical procedures are the definitive treatment for early-stage melanoma.
The surgical margins are the most critical consideration when performing the wide local excision. If the primary closure is not feasible, skin grafting or tissue transfers may be needed. Medical management is reserved for adjuvant therapy of patients with higher-stage melanoma. Dermatologists continue to be involved in the care of patients with melanoma indefinitely, sometimes even in the presence of metastatic disease. For localized lesions, the prognosis is with surgery. Still, advanced melanoma has a grim prognosis, but the interprofessional team approach to care will optimize the patient's prospects for a better outcome.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. 2023 Jan:73(1):17-48. doi: 10.3322/caac.21763. Epub [PubMed PMID: 36633525]
Wong VK, Lubner MG, Menias CO, Mellnick VM, Kennedy TA, Bhalla S, Pickhardt PJ. Clinical and Imaging Features of Noncutaneous Melanoma. AJR. American journal of roentgenology. 2017 May:208(5):942-959. doi: 10.2214/AJR.16.16800. Epub 2017 Mar 16 [PubMed PMID: 28301211]
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, Wouters MWJM, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, Neves RI, Trocha SD, Wright F, Byrd DR, Matter M, Hsueh E, MacKenzie-Ross A, Johnson DB, Terheyden P, Berger AC, Huston TL, Wayne JD, Smithers BM, Neuman HB, Schneebaum S, Gershenwald JE, Ariyan CE, Desai DC, Jacobs L, McMasters KM, Gesierich A, Hersey P, Bines SD, Kane JM, Barth RJ, McKinnon G, Farma JM, Schultz E, Vidal-Sicart S, Hoefer RA, Lewis JM, Scheri R, Kelley MC, Nieweg OE, Noyes RD, Hoon DSB, Wang HJ, Elashoff DA, Elashoff RM. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. The New England journal of medicine. 2017 Jun 8:376(23):2211-2222. doi: 10.1056/NEJMoa1613210. Epub [PubMed PMID: 28591523]
MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. The New England journal of medicine. 2003 Feb 6:348(6):567-8 [PubMed PMID: 12571271]
Level 3 (low-level) evidenceCrombie IK. Variation of melanoma incidence with latitude in North America and Europe. British journal of cancer. 1979 Nov:40(5):774-81 [PubMed PMID: 508580]
Level 2 (mid-level) evidenceMotofei IG. Malignant Melanoma: Autoimmunity and Supracellular Messaging as New Therapeutic Approaches. Current treatment options in oncology. 2019 May 6:20(6):45. doi: 10.1007/s11864-019-0643-4. Epub 2019 May 6 [PubMed PMID: 31056729]
Motofei IG. Melanoma and autoimmunity: spontaneous regressions as a possible model for new therapeutic approaches. Melanoma research. 2019 Jun:29(3):231-236. doi: 10.1097/CMR.0000000000000573. Epub [PubMed PMID: 30615013]
Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer. 2017 Jun 1:123(S11):2143-2153. doi: 10.1002/cncr.30444. Epub [PubMed PMID: 28543699]
Soufir N, Avril MF, Chompret A, Demenais F, Bombled J, Spatz A, Stoppa-Lyonnet D, Bénard J, Bressac-de Paillerets B. Prevalence of p16 and CDK4 germline mutations in 48 melanoma-prone families in France. The French Familial Melanoma Study Group. Human molecular genetics. 1998 Feb:7(2):209-16 [PubMed PMID: 9425228]
Puntervoll HE, Yang XR, Vetti HH, Bachmann IM, Avril MF, Benfodda M, Catricalà C, Dalle S, Duval-Modeste AB, Ghiorzo P, Grammatico P, Harland M, Hayward NK, Hu HH, Jouary T, Martin-Denavit T, Ozola A, Palmer JM, Pastorino L, Pjanova D, Soufir N, Steine SJ, Stratigos AJ, Thomas L, Tinat J, Tsao H, Veinalde R, Tucker MA, Bressac-de Paillerets B, Newton-Bishop JA, Goldstein AM, Akslen LA, Molven A. Melanoma prone families with CDK4 germline mutation: phenotypic profile and associations with MC1R variants. Journal of medical genetics. 2013 Apr:50(4):264-70. doi: 10.1136/jmedgenet-2012-101455. Epub 2013 Feb 5 [PubMed PMID: 23384855]
Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, Hayward N, Dracopoli NC. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nature genetics. 1996 Jan:12(1):97-9 [PubMed PMID: 8528263]
Kamb A, Shattuck-Eidens D, Eeles R, Liu Q, Gruis NA, Ding W, Hussey C, Tran T, Miki Y, Weaver-Feldhaus J. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nature genetics. 1994 Sep:8(1):23-6 [PubMed PMID: 7987388]
Prota G, d'Ischia M, Mascagna D. Melanogenesis as a targeting strategy against metastatic melanoma: a reassessment. Melanoma research. 1994 Dec:4(6):351-8 [PubMed PMID: 7703714]
Level 3 (low-level) evidenceRastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In vivo (Athens, Greece). 2014 Nov-Dec:28(6):1005-11 [PubMed PMID: 25398793]
Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Progress in biophysics and molecular biology. 2011 Dec:107(3):362-6. doi: 10.1016/j.pbiomolbio.2011.09.011. Epub 2011 Sep 19 [PubMed PMID: 21958910]
Vosmík F. [Malignant melanoma of the skin. Epidemiology, risk factors, clinical diagnosis]. Casopis lekaru ceskych. 1996 Jul 26:135(13):405-8 [PubMed PMID: 8925536]
Lejeune FJ. Epidemiology and etiology of malignant melanoma. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1986:40(3):91-9 [PubMed PMID: 3527290]
O'Neill CH, Scoggins CR. Melanoma. Journal of surgical oncology. 2019 Oct:120(5):873-881. doi: 10.1002/jso.25604. Epub 2019 Jun 27 [PubMed PMID: 31246291]
Uhara H. Recent advances in therapeutic strategies for unresectable or metastatic melanoma and real-world data in Japan. International journal of clinical oncology. 2019 Dec:24(12):1508-1514. doi: 10.1007/s10147-018-1246-y. Epub 2018 Feb 22 [PubMed PMID: 29470725]
Level 3 (low-level) evidenceCurtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. The New England journal of medicine. 2005 Nov 17:353(20):2135-47 [PubMed PMID: 16291983]
Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, Redman B, Thomas NE, Gruber SB. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma research. 2006 Aug:16(4):267-73 [PubMed PMID: 16845322]
Level 2 (mid-level) evidenceElder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Archives of pathology & laboratory medicine. 2020 Apr:144(4):500-522. doi: 10.5858/arpa.2019-0561-RA. Epub 2020 Feb 14 [PubMed PMID: 32057276]
Friedman RJ, Rigel DS, Kopf AW, Lieblich L, Lew R, Harris MN, Roses DF, Gumport SL, Ragaz A, Waldo E, Levine J, Levenstein M, Koenig R, Bart RS, Trau H. Favorable prognosis for malignant melanomas associated with acquired melanocytic nevi. Archives of dermatology. 1983 Jun:119(6):455-62 [PubMed PMID: 6859885]
Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Feb:19 Suppl 2():S34-40 [PubMed PMID: 16446714]
Abbasi NR, Shaw HM, Rigel DS, Friedman RJ, McCarthy WH, Osman I, Kopf AW, Polsky D. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004 Dec 8:292(22):2771-6 [PubMed PMID: 15585738]
Daniel Jensen J, Elewski BE. The ABCDEF Rule: Combining the "ABCDE Rule" and the "Ugly Duckling Sign" in an Effort to Improve Patient Self-Screening Examinations. The Journal of clinical and aesthetic dermatology. 2015 Feb:8(2):15 [PubMed PMID: 25741397]
Holmes GA, Vassantachart JM, Limone BA, Zumwalt M, Hirokane J, Jacob SE. Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination. Federal practitioner : for the health care professionals of the VA, DoD, and PHS. 2018 May:35(Suppl 4):S39-S45 [PubMed PMID: 30766399]
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, Haydu LE, Eggermont AMM, Flaherty KT, Balch CM, Thompson JF, for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017 Nov:67(6):472-492. doi: 10.3322/caac.21409. Epub 2017 Oct 13 [PubMed PMID: 29028110]
Hayek SA, Munoz A, Dove JT, Hunsinger M, Arora T, Wild J, Shabahang M, Blansfield J. Hospital-Based Study of Compliance with NCCN Guidelines and Predictive Factors of Sentinel Lymph Node Biopsy in the Setting of Thin Melanoma Using the National Cancer Database. The American surgeon. 2018 May 1:84(5):672-679 [PubMed PMID: 29966567]
Janz TA, Neskey DM, Nguyen SA, Lentsch EJ. Is imaging of the brain necessary at diagnosis for cutaneous head and neck melanomas? American journal of otolaryngology. 2018 Sep-Oct:39(5):631-635. doi: 10.1016/j.amjoto.2018.06.007. Epub 2018 Jun 8 [PubMed PMID: 29929862]
Barker CA, Salama AK. New NCCN Guidelines for Uveal Melanoma and Treatment of Recurrent or Progressive Distant Metastatic Melanoma. Journal of the National Comprehensive Cancer Network : JNCCN. 2018 May:16(5S):646-650. doi: 10.6004/jnccn.2018.0042. Epub [PubMed PMID: 29784747]
Reserva J, Janeczek M, Joyce C, Goslawski A, Hong H, Yuan FN, Balasubramanian N, Winterfield L, Swan J, Tung R. A Retrospective Analysis of Surveillance Adherence of Patients after Treatment of Primary Cutaneous Melanoma. The Journal of clinical and aesthetic dermatology. 2017 Dec:10(12):44-48 [PubMed PMID: 29399266]
Level 2 (mid-level) evidenceBlakely AM, Comissiong DS, Vezeridis MP, Miner TJ. Suboptimal Compliance With National Comprehensive Cancer Network Melanoma Guidelines: Who Is at Risk? American journal of clinical oncology. 2018 Aug:41(8):754-759. doi: 10.1097/COC.0000000000000362. Epub [PubMed PMID: 28121641]
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE 3rd, Daniels GA, DiMaio D, Fields RC, Fleming MD, Gastman B, Gonzalez R, Guild V, Johnson D, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ott P, Gupta AP, Ross MI, Salama AK, Skitzki J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. NCCN Guidelines Insights: Melanoma, Version 3.2016. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 Aug:14(8):945-58 [PubMed PMID: 27496110]
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE 3rd, Daniels GA, DiMaio D, Ernstoff M, Fields RC, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS Jr, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ross MI, Salama AK, Skitzki J, Sosman J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. Melanoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 Apr:14(4):450-73 [PubMed PMID: 27059193]
Level 1 (high-level) evidenceCascinelli N. Margin of resection in the management of primary melanoma. Seminars in surgical oncology. 1998 Jun:14(4):272-5 [PubMed PMID: 9588719]
Cohn-Cedermark G, Rutqvist LE, Andersson R, Breivald M, Ingvar C, Johansson H, Jönsson PE, Krysander L, Lindholm C, Ringborg U. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8-2.0 mm. Cancer. 2000 Oct 1:89(7):1495-501 [PubMed PMID: 11013363]
Level 1 (high-level) evidenceKhayat D, Rixe O, Martin G, Soubrane C, Banzet M, Bazex JA, Lauret P, Vérola O, Auclerc G, Harper P, Banzet P, French Group of Research on Malignant Melanoma. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer. 2003 Apr 15:97(8):1941-6 [PubMed PMID: 12673721]
Level 1 (high-level) evidenceKarakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, Bartolucci AA. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Annals of surgical oncology. 1996 Sep:3(5):446-52 [PubMed PMID: 8876886]
Level 1 (high-level) evidenceThomas JM, Newton-Bishop J, A'Hern R, Coombes G, Timmons M, Evans J, Cook M, Theaker J, Fallowfield M, O'Neill T, Ruka W, Bliss JM, United Kingdom Melanoma Study Group, British Association of Plastic Surgeons, Scottish Cancer Therapy Network. Excision margins in high-risk malignant melanoma. The New England journal of medicine. 2004 Feb 19:350(8):757-66 [PubMed PMID: 14973217]
Level 1 (high-level) evidenceGillgren P, Drzewiecki KT, Niin M, Gullestad HP, Hellborg H, Månsson-Brahme E, Ingvar C, Ringborg U. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: a randomised, multicentre trial. Lancet (London, England). 2011 Nov 5:378(9803):1635-42. doi: 10.1016/S0140-6736(11)61546-8. Epub 2011 Oct 23 [PubMed PMID: 22027547]
Level 1 (high-level) evidenceUtjés D, Malmstedt J, Teras J, Drzewiecki K, Gullestad HP, Ingvar C, Eriksson H, Gillgren P. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: long-term follow-up of a multicentre, randomised trial. Lancet (London, England). 2019 Aug 10:394(10197):471-477. doi: 10.1016/S0140-6736(19)31132-8. Epub 2019 Jul 4 [PubMed PMID: 31280965]
Level 1 (high-level) evidenceFinley RK 3rd, Driscoll DL, Blumenson LE, Karakousis CP. Subungual melanoma: an eighteen-year review. Surgery. 1994 Jul:116(1):96-100 [PubMed PMID: 8023276]
Level 2 (mid-level) evidenceMorton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Glass EC, Wang HJ, MSLT Group. Sentinel-node biopsy or nodal observation in melanoma. The New England journal of medicine. 2006 Sep 28:355(13):1307-17 [PubMed PMID: 17005948]
Level 1 (high-level) evidenceMorton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Puleo CA, Coventry BJ, Kashani-Sabet M, Smithers BM, Paul E, Kraybill WG, McKinnon JG, Wang HJ, Elashoff R, Faries MB, MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. The New England journal of medicine. 2014 Feb 13:370(7):599-609. doi: 10.1056/NEJMoa1310460. Epub [PubMed PMID: 24521106]
Level 1 (high-level) evidenceLuke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, de la Cruz Merino L, Khattak MA, Schadendorf D, Long GV, Ascierto PA, Mandala M, De Galitiis F, Haydon A, Dummer R, Grob JJ, Robert C, Carlino MS, Mohr P, Poklepovic A, Sondak VK, Scolyer RA, Kirkwood JM, Chen K, Diede SJ, Ahsan S, Ibrahim N, Eggermont AMM, KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet (London, England). 2022 Apr 30:399(10336):1718-1729. doi: 10.1016/S0140-6736(22)00562-1. Epub 2022 Apr 1 [PubMed PMID: 35367007]
Level 1 (high-level) evidenceLong GV, Luke JJ, Khattak MA, de la Cruz Merino L, Del Vecchio M, Rutkowski P, Spagnolo F, Mackiewicz J, Chiarion-Sileni V, Kirkwood JM, Robert C, Grob JJ, de Galitiis F, Schadendorf D, Carlino MS, Mohr P, Dummer R, Gershenwald JE, Yoon CH, Wu XL, Fukunaga-Kalabis M, Krepler C, Eggermont AMM, Ascierto PA, KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. The Lancet. Oncology. 2022 Nov:23(11):1378-1388. doi: 10.1016/S1470-2045(22)00559-9. Epub 2022 Oct 18 [PubMed PMID: 36265502]
Level 1 (high-level) evidenceGarbe C, Keim U, Suciu S, Amaral T, Eigentler TK, Gesierich A, Hauschild A, Heinzerling L, Kiecker F, Schadendorf D, Stadler R, Sunderkötter C, Tüting T, Utikal J, Wollina U, Zouboulis CC, Keilholz U, Testori A, Martus P, Leiter U, Eggermont AMM, German Central Malignant Melanoma Registry and the European Organisation for Research and Treatment of Cancer. Prognosis of Patients With Stage III Melanoma According to American Joint Committee on Cancer Version 8: A Reassessment on the Basis of 3 Independent Stage III Melanoma Cohorts. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Aug 1:38(22):2543-2551. doi: 10.1200/JCO.19.03034. Epub 2020 Jun 12 [PubMed PMID: 32530760]
Moncrieff MD, Lo SN, Scolyer RA, Heaton MJ, Nobes JP, Snelling AP, Carr MJ, Nessim C, Wade R, Peach AH, Kisyova R, Mason J, Wilson ED, Nolan G, Pritchard Jones R, Johansson I, Olofsson Bagge R, Wright LJ, Patel NG, Sondak VK, Thompson JF, Zager JS. Clinical Outcomes and Risk Stratification of Early-Stage Melanoma Micrometastases From an International Multicenter Study: Implications for the Management of American Joint Committee on Cancer IIIA Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022 Dec 1:40(34):3940-3951. doi: 10.1200/JCO.21.02488. Epub 2022 Jul 18 [PubMed PMID: 35849790]
Level 2 (mid-level) evidenceWeber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA, CheckMate 238 Collaborators. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine. 2017 Nov 9:377(19):1824-1835. doi: 10.1056/NEJMoa1709030. Epub 2017 Sep 10 [PubMed PMID: 28891423]
Dummer R, Hauschild A, Santinami M, Atkinson V, Mandalà M, Kirkwood JM, Chiarion Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, Robert C, Mortier L, Schachter J, Lesimple T, Plummer R, Dasgupta K, Gasal E, Tan M, Long GV, Schadendorf D. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. The New England journal of medicine. 2020 Sep 17:383(12):1139-1148. doi: 10.1056/NEJMoa2005493. Epub 2020 Sep 2 [PubMed PMID: 32877599]
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, Robert C. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. The New England journal of medicine. 2018 May 10:378(19):1789-1801. doi: 10.1056/NEJMoa1802357. Epub 2018 Apr 15 [PubMed PMID: 29658430]
Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S, Coventry BJ, Babington S, Duprat J, Hoekstra HJ, Thompson JF. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. The Lancet. Oncology. 2012 Jun:13(6):589-97. doi: 10.1016/S1470-2045(12)70138-9. Epub 2012 May 9 [PubMed PMID: 22575589]
Level 1 (high-level) evidenceHenderson MA, Burmeister BH, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S, Coventry BJ, Babington S, Duprat J, Hoekstra HJ, Thompson JF. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. The Lancet. Oncology. 2015 Sep:16(9):1049-1060. doi: 10.1016/S1470-2045(15)00187-4. Epub 2015 Jul 20 [PubMed PMID: 26206146]
Level 1 (high-level) evidencePatel SP, Othus M, Chen Y, Wright GP Jr, Yost KJ, Hyngstrom JR, Hu-Lieskovan S, Lao CD, Fecher LA, Truong TG, Eisenstein JL, Chandra S, Sosman JA, Kendra KL, Wu RC, Devoe CE, Deutsch GB, Hegde A, Khalil M, Mangla A, Reese AM, Ross MI, Poklepovic AS, Phan GQ, Onitilo AA, Yasar DG, Powers BC, Doolittle GC, In GK, Kokot N, Gibney GT, Atkins MB, Shaheen M, Warneke JA, Ikeguchi A, Najera JE, Chmielowski B, Crompton JG, Floyd JD, Hsueh E, Margolin KA, Chow WA, Grossmann KF, Dietrich E, Prieto VG, Lowe MC, Buchbinder EI, Kirkwood JM, Korde L, Moon J, Sharon E, Sondak VK, Ribas A. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. The New England journal of medicine. 2023 Mar 2:388(9):813-823. doi: 10.1056/NEJMoa2211437. Epub [PubMed PMID: 36856617]
Reijers ILM, Menzies AM, van Akkooi ACJ, Versluis JM, van den Heuvel NMJ, Saw RPM, Pennington TE, Kapiteijn E, van der Veldt AAM, Suijkerbuijk KPM, Hospers GAP, Rozeman EA, Klop WMC, van Houdt WJ, Sikorska K, van der Hage JA, Grünhagen DJ, Wouters MW, Witkamp AJ, Zuur CL, Lijnsvelt JM, Torres Acosta A, Grijpink-Ongering LG, Gonzalez M, Jóźwiak K, Bierman C, Shannon KF, Ch'ng S, Colebatch AJ, Spillane AJ, Haanen JBAG, Rawson RV, van de Wiel BA, van de Poll-Franse LV, Scolyer RA, Boekhout AH, Long GV, Blank CU. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nature medicine. 2022 Jun:28(6):1178-1188. doi: 10.1038/s41591-022-01851-x. Epub 2022 Jun 5 [PubMed PMID: 35661157]