Introduction
Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and responsible for opportunistic infections affecting both immunocompromised and immunocompetent hosts.[1] The incidence of the disease from NTM has been gradually increasing worldwide, becoming, in recent years, an emerging public health problem.[2][3][4] As a result, various international guidelines, including the American Thoracic Society (ATS) and the British Thoracic Society, have been used to diagnose and manage clinical pulmonary NTM.[5][6] In 2020, a guideline consensus was released as a collaboration between the ATS, the European Respiratory Society (ERS), the Infectious Diseases Society of America (IDSA), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID).[7]
As new data have led to an increased understanding of pulmonary NTM infections, guidelines continue to be updated to reflect emerging evidence. With new medical advances based on molecular microbiology and the recognition of pulmonary NTM as causing complicated infections, the diagnosis of most NTM is becoming more precise and efficient. This article will summarize and review key features of NTM pulmonary infections, including the epidemiology, clinical features, diagnostic workup, and management of NTM, focusing on the multidisciplinary team approach to improve the clinical care of individuals with NTM pulmonary infections.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Following the discovery of Mycobacterium tuberculosis by Robert Koch in 1882, nontuberculous mycobacteria (NTM) have gradually been identified and categorized.[8][9] The first of these, Mycobacterium avium, was first identified in birds and differentiated from tuberculosis by 1890.[10][11] Further NTM were gradually identified over the years; however, they were not recognized as responsible for human disease until the mid-twentieth century, upon which classifications were developed to categorize these mycobacteria.[12] Today, the number of identified NTM species has reached more than 150.[13]
M tuberculosis complex species and NTM are all categorized under the genus Mycobacterium. Mycobacteria are typically weakly gram-positive, acid-fast bacilli characterized by mycolic acid-rich cell walls [14]. They typically are of variable length and measure 0.3 to 0.5 μm. Further identification can be made phenotypically based on pigmentation changes in the presence or absence of light (eg, photochromogens, scotochromogens, or nonchromogenic) and growth characteristics, including the scotochromogenic Mycobacterium szulgai, Mycobacterium xenopi, and Mycobacterium gordonae, which can cause pulmonary disease in humans.[15][16]
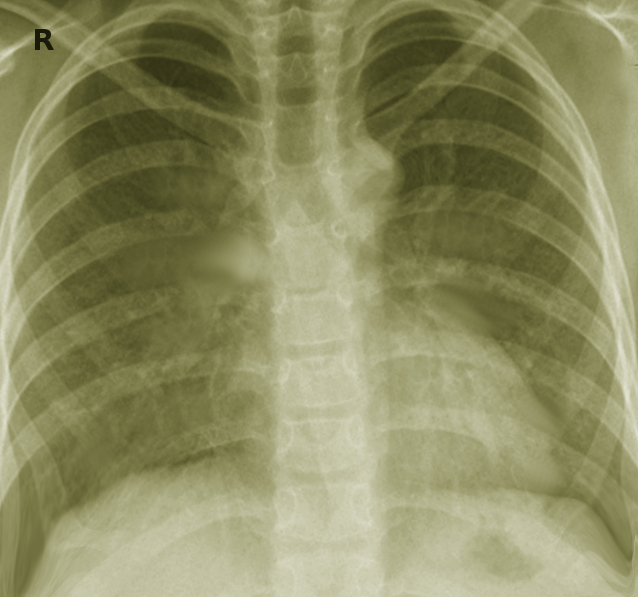
Other identification methods include growth rates, colony variation, biochemical differences, and chromatography. In particular, NTM species that grow in liquid media within 7 days are classed as rapid growers, and those that take more than 2 weeks to grow are slow growers.[17] However, older phenotypic classifications have been replaced with more advanced molecular and genomic methods for microorganism identification due to more rapid and precise results with these newer modalities.[18] As a result, further species of NTM can be further discovered and differentiated from existing species due to genomic differences. The most commonly identified pulmonary NTM species in countries such as the US, Japan, and French Guiana include Mycobacterium avium complex (MAC), M gordonae, Mycobacterium kansasii, Mycobacterium fortuitum, and Mycobacterium abscessus.[19][20][21] See Image. Mycobacterium Kansasii.
Epidemiology
The epidemiology of NTM lung infections has been challenging to determine due to multiple factors, including mandatory reporting to local public health departments not being required in many countries, differentiating between infection and disease often being difficult, unavailable diagnostic testing in all institutions making differentiation between NTM and tuberculosis infections challenging, and imprecise follow-up due to the burden of treatment and follow-up of sputum cultures to ensure clearance.[22][23][24][2][25][26]
There is evidence that individuals of Asian and Pacific Islander background may be at increased risk of NTM pulmonary disease.[27] Part of this may be attributable to a higher incidence of M tuberculosis among these populations, given a history of tuberculosis is associated with pulmonary NTM infections due to structural changes.[28][29] Advanced age, immunosuppression, and the use of corticosteroids are other known risk factors for acquiring these infections.[30]
In the 1980s, US laboratories reported an estimated prevalence of NTM infection of 1 to 2 cases per 100,000 individuals.[31] The annual prevalence increased among men and women in Florida by 3.2% and 6.5% per year, respectively; in New York, the prevalence in women increased by 4.6% per year. Conversely, there was no significant increase seen in California. The annual prevalence of NTM pulmonary diseases in US Medicare beneficiaries (ie, all persons aged ≥65) increased from 20 per 100,000 in 1997 to 47 per 100,000 in 2007.[32] Since the initial description of pulmonary NTM in the 1950s, reports of increased incidence and prevalence of pulmonary NTM infections have been documented in Slovakia, the United Kingdom, Ireland, Australia, Japan, South Korea, and the US.[33][34][35][36][37][38] In Queensland, Australia, where NTM is reportable, the incidence of clinically significant pulmonary disease rose from 2.2 per 100,000 in 1999 to 3.2 per 100,000 in 2005.[39]
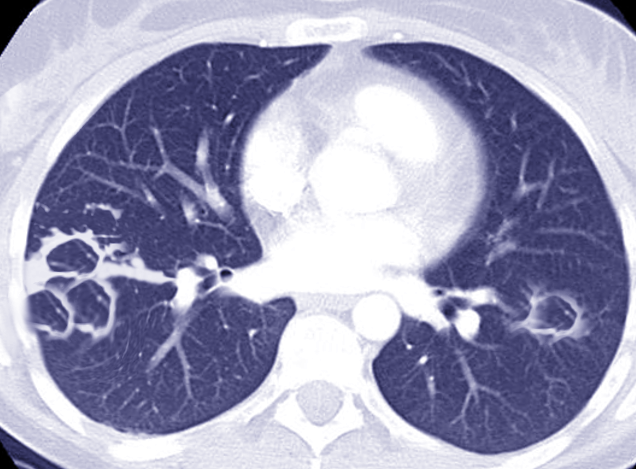
While the prevalence and incidence of NTM pulmonary disease have been increasing through the years, not only in the US but also around the world, this epidemiological variation has not been fully explained. Increased awareness among treating clinicians has been proposed as a reason for increased incidence, along with an increasingly aging and comorbid population among high-income countries.[40] Environmental associations have also been postulated, with increased population exposure to plumbing, indoor swimming pools, high rainfall events, and disruption of local soil being linked with a higher risk of NTM infections.[37] [41][42][43][44] Hot tubs themselves have been clinically linked with cases of MAC.[45] New radiological advances, especially resolution chest computed tomography (CT) scanning, improved diagnosis, and increased chest screening may also be important factors responsible for these epidemiological observations.[46][47] See Image. Chest CT of Mycobacterium Abscessus.
Pathophysiology
Nontuberculous mycobacterium may cause disease de novo in healthy human hosts; however, the pulmonary disease is commonly implicated in individuals with preexisting structural lung diseases (eg, bronchiectasis) or genetic and immune dysfunction disorders (eg, alpha-1-antitrypsin deficiency, primary ciliary dyskinesia, and granulocyte-macrophage colony-stimulating factor.)[48][49][50][51] T-cell depleting therapies have traditionally been implicated in NTM disease, altering T-cell function responses without major lymphopenia.[52] Human-immunodeficiency virus, which causes a decline in CD4+ cells, is associated with a higher incidence of NTM disease, particularly with lower CD4+ cell counts.[53][54] B-cell–depleting therapies (eg, rituximab) may also be associated with reducing the granuloma response, which contains mycobacteria.[55][56]
In those without predisposing structural lung disease, low body mass index, scoliosis, and thoracic cage abnormalities have been associated with NTM pulmonary disease.[57][58][59] Gastroesophageal reflux disease has been associated with pulmonary NTM infection, possibly as a result of the survivability of mycobacteria in low-pH environments.[60] Unlike tuberculosis, NTM pulmonary disease is not thought to be transmitted person-to-person or by exposure to droplets from an infected individual.[61] That all pulmonary NTM become acquired via inhalation of infected aerosolized droplets from the environment or water sources has been accepted. Household water and shower aerosols are known to be colonized with various NTM species, with the most frequently cited being M avium, M kansasii, M abscessus, and Mycobacterium lentiflavum.[62] Similarly, NTM has been isolated throughout the year in natural and municipal potable water in major cities globally, with species differing according to season.[63] Once the organisms enter the individual, they usually settle in the lower airways; in some cases, the bacteria incite an inflammatory reaction with an influx of lymphocytes. The resulting release of cytokines and other mediators can lead to an infectious process that presents as pneumonia. With an insufficient immune response to contain these mycobacteria, the pathogen can disseminate throughout the human host and cause extrapulmonary disease.
History and Physical
The diagnosis of pulmonary NTM infection is based on clinical, radiological, and microbiological criteria. The 2020 ATS/ERS/IDSA/ESCMID guidelines recommend clinical features, radiological opacities, exclusion of differential diagnoses, and positive microbiology from multiple sputum specimens and other respiratory laboratory studies to confirm the diagnosis of pulmonary NTM infection.[7] As such, a thorough history and physical examination are essential in detecting possible cases of pulmonary NTM infection. Compared to other bacteria, nontuberculous mycobacterium have a slow growth process, resulting in a considerable time to infect the lungs and cause symptomatic disease.[64] Sometimes, this particular factor and a low clinical suspicion index may result in either misdiagnosis or a delayed diagnosis.
Clinical History
Clinical manifestations may bear similarities with pulmonary tuberculosis infection, including respiratory symptoms such as cough, dyspnea, and increased sputum production.[65] Patients with pulmonary NTM often have fewer pulmonary cavitations and infiltrates than tuberculosis; however, constitutional symptoms (eg, fevers, night sweats, and weight loss) have been seen to occur more often in pulmonary NTM than in tuberculosis.[66] Pulmonary NTM disease is associated more with bronchiectasis than tuberculosis, and coinfection with human immunodeficiency virus (HIV) often leads to more disseminated extrapulmonary disease than in tuberculosis, which in tuberculosis patients is more associated with a miliary radiographic appearance.[67] This is mainly in the case of MAC, where a CD4 count of <50 cells/µL is at high risk for dissemination in HIV patients.[54] The duration of symptoms in pulmonary NTM disease may vary from a few days to a few weeks. Chronic pulmonary infection may occur, resulting in structural lung changes such as fibrocavitary disease.[68] This may lead to differentials such as malignancy, sarcoidosis, vasculitis, and fungal pulmonary disease being considered.[69]
Physical Examination Findings
The physical features on examination are often variable, not specific, and can mimic any infectious process of the lungs. Cutaneous lesions may be present in extrapulmonary disease and could represent NTM lymphadenitis. Disseminated MAC may present with hepatosplenomegaly and abdominal pain.[64] Furthermore, patients with NTM pulmonary infection may have preexisting structural lung disease, including findings of coarse inspiratory crackles on auscultation consistent with bronchiectasis or prolonged expiratory time on spirometry associated with chronic obstructive pulmonary disease.
Evaluation
Diagnostic Imaging Studies
As with other lung infections, radiological images are crucial for correctly diagnosing the disease. However, unlike other lung diseases, NTM have characteristic lung presentations. The major radiological patterns of NTM lung infection are fibrocavitary and nodular bronchiectatic patterns (see Image. Mycobacterium Avium-Intracellulare Pneumonia).[5] The fibrocavitary radiological pattern resembles tuberculosis lung infection. It presents with cavities with areas of increased opacity, primarily in the upper lung. On the other hand, the nodular bronchiectasis pattern characteristically shows multilobar bronchiectasis, primarily located in the middle and lower lung fields, with small nodules seen in imaging. This pattern predominantly presents in elderly female nonsmokers without previous lung conditions.[70] Finally, bronchiectasis is highly prevalent in patients with NTM lung infection. A meta-analysis demonstrated that the prevalence of NTM infection in patients with bronchiectasis was 9.3%.[71] Clinicians must be aware that NTM lung infection could present with bronchiectasis on radiological images. Disseminated disease manifestations, such as what can occur with MAC infection, can be nonspecific and reveal radiological evidence of hepatosplenomegaly and intestinal and gallbladder wall thickening.[72]
Diagnostic Laboratory Studies
- Sputum staining: The isolation of NTM in human specimens can be challenging. Since NTM are present in the environment, especially in water sources, a positive test in a sputum specimen may be a false-positive due to specimen contamination or represent upper respiratory tract colonization in patients tested for NTM.[73] Therefore, current international guidelines recommend that 3 early morning sputum specimens be collected on 3 different days to diagnose NTM lung infection accurately.[5] The methods used for acid-fast bacilli staining require the carbol fuchsin stain (eg, Ziehl-Neelsen or Kinyoun method) and the fluorochrome procedure using auramine O alone or combined with rhodamine B.[74][75] However, these staining methods cannot differentiate between tuberculosis and NTM alone, and further tests are required to identify the pathogen.
- Nuclei-acid amplification: Nuclei-acid amplification (NAA) is the most accurate test for identifying mycobacterial strains. Commercial polymerase chain reaction assays have shown a sensitivity of up to 97.7% in acid-fast bacilli smear-positive specimens, with a specificity of up to 97.7%, by targeting conserved sequences such as insertion sequence 6110 and the mpb64 gene.[76][77][78]
- Sputum culture and sensitivity: Culture remains the test of choice for NTM laboratory confirmation. Solid and liquid media are used for the culture. Solid media includes egg-based media, such as the Löwenstein-Jensen medium, or agar-based media (eg, middlebrook 7H10 and 7H11 media).[79] Solid media allows the visualization of morphology, growth rate, and species characterization. The liquid media system is more sensitive but is more prone to contamination by other microorganisms and bacteria overgrowth. Both culture methods are limited by the growth time of the organisms, the risk posed by potential contaminants, and the effect of prior empirical antimicrobials on mycobacterial growth following collection.[80][81] A timely diagnosis is important as delays between recognizing the causative organism of NTM disease, performing susceptibility testing, and initiating treatment can result in delays ranging from 1.3 to 20.8 months.[82]
Nontuberculous Mycobacterium Identification
The treatment of NTM infection is specific and different for every NTM species. Correctly identifying the species is the main factor of successful treatment. The most accurate NTM identification method is via gene sequence. Sequencing the 16S rRNA gene allows for the correct identification and discrimination between species, and whole genome sequencing has been used in public health investigations of mycobacterium outbreaks.[83][84] However, species-level identification may need several gene sequences and more complex methods. Only specialized laboratories that identify species levels using different gene sequences can perform this test. The matrix-associated laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) method has increasingly been employed for identifying NTM and is highly reproducible for this use.[85] This method identifies target bacteria by comparing mass spectral pattern molecules specific to NTM.
Drug Susceptibility
When NTM are cultured, drug susceptibility tests are always conducted to assist clinicians with choosing drug regimens due to the variable resistance patterns of different NTM organisms. However, there are difficulties with the drug susceptibility test because each species of NTM may behave differently in vitro than in vivo, and there is limited data regarding clinical outcomes based on in vitro susceptibility testing.[5][86] One exception is using macrolides in MAC, where there is a correlation between in vitro susceptibility and in vivo responses. Macrolide testing in vitro for clarithromycin is also often extrapolated to azithromycin usage in clinical practice due to safer differences in drug-drug interactions, improved dosing regimens, and similar clinical outcomes despite similarities in cross-resistance.[87] NTM species may have variable resistance to certain drugs, and thus, antimicrobial susceptibility tests should be adapted to each organism once cultured. For example, M kansasii may show resistance to rifamycins, and alternative agents (eg, tetracyclines, fluoroquinolones, and carbapenems) may need to be tested.[5] M abscessus has been shown to have inducible macrolide resistance, which can often mean prolonged therapy with intravenous amikacin, imipenem, or cefoxitin if these drugs show NTM susceptibility.[88][89]
Treatment / Management
The treatment of NTM requires a holistic and patient-centered approach based on quality-of-life and patient benefits instead of expecting mycobacterial eradication. Pulmonary NTM infection differs from a TB lung infection in that it does not require immediate initiation of treatment upon diagnosis but should be expected within a reasonable timeframe to limit disease progression.[90] The treatment of NTM lung infection includes an interprofessional approach and a discussion of risks and benefits. The therapy should remain in place for a minimum of 12 months from culture conversion.[5][90] Culture conversion is where 3 consecutive monthly sputum cultures are negative for NTM after initial positive smears.[91] Based on data in cystic fibrosis patients, sputum cultures should also be collected every 4 to 8 weeks until 12 months from culture conversion.[92] For patients unable to expectorate, meeting the definition of culture conversion may be difficult and bronchoscopy and imaging may be required to confirm conversion.[6] During the treatment phase, pulmonary NTM patients require close monitoring for drug adverse effects and medication adherence, as most patients will require a median of 5 with a range of 1 to 10 antibiotics to achieve culture conversion.[93] This is important as the cost for treatment over a lifetime for pulmonary NTM can range anywhere from $398 to $70000, based on 2009 data.(A1)
Mycobacterium avium-intracellular complex Lung Disease Treatment
MAC lung disease treatment depends on clinical presentation, including nodular or bronchiectasis disease, cavitary diseases, and advanced (ie, severe) or previously treated disease. Guideline-based treatment recommends a combination of a macrolide, ethambutol, and a rifamycin 3 times weekly for at least 12 months after culture conversion, with an option to include aminoglycosides for severe and refractory cases. Second-line agents may be required for macrolide-resistant strains.[90] Treatment 3 times per week is recommended due to its tolerability compared to daily dosing and reduced pill costs. The following treatment dosing scenarios for MAC lung disease are based on the 2020 ATS/ERS/IDSA/ESCMID guidelines.[5](B3)
Bronchiectasis or Cavitary Disease Treatment
The first regimen for the treatment of bronchiectasis disease includes clarithromycin 1000 mg 3 times per week or azithromycin 500 to 600 mg 3 times per week together with a rifamycin 600 mg 3 times per week and ethambutol 25 mg/kg 3 times per week. The primary treatment regimen for cavitary disease is clarithromycin 500 to 1000 mg daily or azithromycin 250 to 300 mg daily with ethambutol 15 mg/kg daily and rifampin 450 to 600 mg daily. Furthermore, intramuscular streptomycin or intravenous amikacin 10 mg to 15 mg/kg 3 times weekly can be an added therapy.[87]
Advanced Disease Treatment
The treatment of advanced (severe) or previously treated disease includes clarithromycin 500 to 1000 mg daily or azithromycin 250 to 300 mg daily together with rifabutin 150 to 300 mg or rifampin 450 to 600 mg once daily, ethambutol 15 mg/kg daily, and streptomycin or amikacin 10 to 15 mg/kg intramuscular or intravenous 3 times/week with a maximum dose of 500 mg for patients older than 50 years for the first 2 to 3 months. Adding moxifloxacin 400 mg orally once daily to the previous combination regimen may improve outcomes in refractory diseases or treatment failure.
Refractory Disease Treatment
Refractory pulmonary infection is a failure to achieve negative cultures for more than 6 months. The benefit of additional drugs is controversial as there is a lack of large-scale studies to guide evidence in this scenario.[94] The guideline-directed regimen of treatment is the administration of amikacin liposome inhalation suspension once daily at a dose of 590 mg/8.4 milliliters (one vial) with a specialized nebulizer system only, along with an optimized multi-drug background regimen, still typically consisting of a macrolide, rifamycin, and ethambutol.[91][90][95](A1)
Duration of Therapy
Nontuberculous mycobacterial antimicrobial therapy aims to achieve culture conversion. Those achieving 3 consecutive monthly negative sputum cultures 12 months after the last positive cultures for 6 months should continue for an additional 12 months.[91] The benefit of extended therapy in those not achieving 3 consecutive monthly negative sputum cultures by 6 months is not entirely established. However, it has been observed that patients treated for longer than 12 months achieve culture conversion within a shorter period, <12 months, associated with reduced mortality in pulmonary NTM infection.[82](A1)
Mycobacterium Kansasii Lung Disease Treatment
M kansasii lung disease remains a relatively treatable pathogen among NTM lung diseases despite being the second most common cause of pulmonary NTM in the US.[5] With the advent of rifamycin-based treatment regimens, sputum conversion remains high. Guideline-directed therapy is similar to tuberculosis treatment, with a high curative rate. The recommended primary regimens for adults are isoniazid 300 mg daily, pyridoxine 50 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg per day for 12 months of culture-negative sputum. Disseminated disease is treated in the same manner as pulmonary disease. Susceptibility testing should be used to guide treatment in rifamycin-resistant cases, which may include macrolides, sulfamethoxazole, and streptomycin. It has been demonstrated that adding an aminoglycoside such as streptomycin during the first 2 to 3 months to the 3-drug regimen has a high rate of culture conversion with a low relapse rate.[96](B2)
Mycobacterium Abscessus Complex Lung Disease Treatment
MAC lung infection treatment remains complicated because of the high treatment failure rate due to resistance against standard treatment for mycobacterial species.[97] Therefore, more clinical trials are needed to establish a successful treatment that eradicates and cures the disease. The primary regimen generally consists of a macrolide with 2 parenteral agents for an extended period in isolates without macrolide resistance. Also, the treatment is divided between the intensive phase, which uses imipenem 1000 mg intravenously (IV) every 12 hrs or cefoxitin IV 8 to 12 g daily, divided into 2 or 3 doses, azithromycin 250 to 500 mg orally once daily along with amikacin IV 15 mg/kg once daily with adjustable doses until obtaining peak serum concentration of 20 to 30 µg/mL. Generally, another oral agent, which includes clofazimine or linezolid, is used. This proposed treatment should last 2 or 3 months, depending on the severity of infection, clinical response, tolerability, and toxicity.
Finally, the continuation phase uses azithromycin 250 to 500 mg once daily, clofazimine 100 to 200 mg once daily, or linezolid 600 mg to 1200 mg once daily with inhaled liposomal amikacin 590 mg 3 times weekly for a minimum of 12 months from culture conversion.[98][99][100][101] Macrolide-resistant isolates are more difficult to treat and often require 2 to 3 parental antimicrobials in addition to 2 to 3 oral agents daily (eg, clofazimine and linezolid) in the induction phase, followed by 3 oral agents and inhaled liposomal amikacin 3 times weekly for a minimum of 12 months following culture conversion based on susceptibility testing.[90](B3)
Pharmacologic Adverse Effects
Of great importance to patient care is the pre-treatment counseling of potential medication adverse effects, reactions, and drug interactions. Clinicians and pharmacists must never forget that patients should be educated and counseled as much as possible regarding the risks and benefits of any novel treatment, particularly in pulmonary NTM disease, where antimicrobials are not without adverse effects. In the case of treatment with NTM, the most used medications are macrolides and other anti-tuberculosis medications like rifampin.
In the case of macrolides like azithromycin or erythromycin, the most common adverse effects are gastrointestinal symptoms, including nausea, vomiting, diarrhea, abdominal pain, and cholestatic hepatitis.[102] Macrolides can also be associated with QT prolongation, leading to cardiac arrhythmias.[103] Having a baseline EKG when using this medication is essential. Otoxicity and transient reversible tinnitus or deafness have occurred with over 4 g daily of erythromycin IV in renal allograft patients.[104] This effect is thought to be due to drug accumulation and has been seen with azithromycin and clarithromycin.[105](A1)
Rifampin is a potentially hepatotoxic cause of drug-induced liver injury, particularly in those with predisposing genotypes.[106][107] It requires caution in patients with preexisting liver impairment or excessive use of alcohol. If elevated bilirubin or a substantive increase in liver-associated enzymes occurs, discontinue rifampin therapy. A visual adverse effect of rifampin is red-orange discoloration of urine, feces, and saliva.[108] It can stain contact lenses permanently. In instances of rifampin overdosage of >14 grams per day, cardiopulmonary arrest may occur.[109] There are also reports of thrombocytopenia and vasculitis, thought to be secondary to the formation of autoantibodies.[110][111] Rifampin is also a potent inducer of cytochrome p450 liver enzymes and can interact with other NTM drugs, including ethambutol and clofazimine.[112]
Surgical Therapy
Nontuberculosis and mycobacterial pulmonary infections can be challenging to manage with antibiotic therapy. By targeting areas of the lung where antimicrobials have poor perfusion due to parenchymal injury, adjuvant surgical therapy may remove the most severely destroyed lung and promote treatment success. Surgical therapy is recommended in the settings of treatment-refractory disease and symptom control.[113] Surgery may also be the best choice for disease control in the case of massive hemoptysis. Some case series and reviews have reported successful treatment, with 80% to 100% sputum conversion in patients after adjuvant surgical reception[114][115]. Surgery requires a comprehensive preoperative evaluation since recent research has shown that postoperative complications have a morbidity of 7% to 25% and a mortality rate of 0% to 3%.[113](B2)
Differential Diagnosis
The clinical manifestation of NTM could mimic other lung infections, such as M tuberculosis, atypical bacterial infections, and chronic lung diseases.[66] Though NTM, including MAC, M abscessus, and M kansasii, are frequently associated with pulmonary disease, others, such as Mycobacterium ulcerans and Mycobacterium marinum, are less associated.[116] Clinicians should approach the differential diagnosis of pulmonary pathologies based on the patient's background risk factors, the disease's clinical manifestations, and any radiological characteristics. Among the differentials, infections, neoplasias, and connective tissue diseases most commonly share similarities to NTM lung infections. Klebsiella pneumoniae, Staphylococcus aureus, and Burkholderia pseudomallei are relevant entities to include in the differential.[117][118][69]
Other pertinent infectious differentials include fungal lung infections such as Aspergillus, Cryptococcus, Mucor, Histoplasma, and Coccidiodes species, particularly if cavitary lung disease is present.[69][119][120][121][122] Given their similar radiological appearances, small-cell and non-small-cell lung cancers are important to consider among neoplasias. Moreover, connective tissue disorders, including rheumatoid arthritis, lung diseases, granulomatosis with polyangiitis, or eosinophilic granulomatosis with polyangiitis, represent an essential part of the differential.[123] Overall, any entity that presents with respiratory manifestations, and either cavitary or bronchiectasis radiological lung pictures on images, is part of the differential diagnostic, and these conditions need to be ruled out by the treating clinician.
Prognosis
The prognosis of NTM lung infection is guarded, particularly if left untreated. A South Korean population study analyzed 183267 NTM infections and found the 6-, 10- and 14-year survival probabilities were 86.3%, 80.8%, and 77.1%, respectively, despite treatment.[124] Similar findings have also been found in Croatia, where a study of 436 pulmonary NTM patients observed a 5-year survival rate of 60% compared to 70% in patients without pulmonary NTM.
Patients with a fragile immune system are more prone to worse outcomes when compared with immunocompetent patients. Furthermore, pulmonary NTM patients with comorbidities have been observed to have survival expectancy following diagnosis reduced to as low as 8.6 years compared to those without comorbidities.[125] The method of diagnosis has also been shown to correlate with disease progression, with patients who have NTM pulmonary disease diagnosed through means other than sputum having a lower risk of disease progression over 5 years compared to those diagnosed by sputum culture.[126] The prognosis also depends on the type of NTM lung infection. Studies have shown that patients with MAC lung infections have a better prognosis when compared to patients with other NTM infections.[127] Conversely, the mortality rate of patients with pulmonary infections with M abscessus is greater when compared with patients with other NTM lung infections.[128] Other prognostic risk factors for mortality identified on multivariable analyses from 15-year cohort studies include older age ≥65 years, male sex, a body mass index <18.5 kg per square meter, underlying chronic cardiac and liver diseases, chronic pulmonary aspergillosis and cavitary lung disease at presentation.[129]
Complications
Pulmonary NTM infections can affect both immunocompetent and immunocompromised hosts in several ways. Affected patients can be asymptomatic, minimally symptomatic, or have serious complications such as pulmonary cavitations or constitutional disease.[66][130] In a systemic review of 206 pulmonary NTM infections, 29% had pleural disease alone, 43% developed pneumothorax, 16% empyema, and 16.5% broncho-pleural fistulas. From this cohort, 53% required pleural effusion drainage, and 26% required surgical intervention.[131] The cohort experienced a mortality rate of 24%, with 53% requiring pleural effusion drainage and 26% requiring surgical intervention. Necrotizing granulomas and chronic interstitial pneumonia and organization may occur.[132] In patients with NTM and chronic thromboembolic pulmonary disease, those with NTM have been observed to be more thrombus-obstructed than those without NTM.[133]
Among lung transplant recipients, survival is similar among NTM-infected and uninfected patients, but there is a trend toward having NTM infection and having bronchiolitis obliterans.[134] Treatment among cystic fibrosis patients may also be challenging as host physiology may mean altered drug absorption and clearance of drugs, resulting in persisting NTM disease if drug monitoring is not available.[135]
Extrapulmonary complications may also develop following initial pulmonary NTM infection, particularly in immunocompromised patients. Osteoarticular diseases such as tenosynovitis, arthritis, and vertebral osteomyelitis can occur following initial pleural infection with NTM.[136] The most frequently cited NTM associated with osteoarticular infection includes M kansasii and M marinum, though other NTM species such as MAC, M chelonae, M szulgai, and M abscessus have also been implicated.[137] In severe immunosuppression, disseminated MAC has been associated with impaired erythropoiesis and elevated liver alkaline phosphatase.[138][139] This may be linked to the clinical manifestations in disseminated MAC of hepatosplenomegaly and osteoarticular disease.[136][137]
Deterrence and Patient Education
Prevention of NTM is a rather challenging task due to its ubiquitous presence in the environment, limiting the precautions that can be put in place to limit exposure to susceptible individuals. Compounding this challenge is the difficulty of assessing which patients are susceptible to NTM infection. A notable example is that hot showers, indoor swimming pools, and plumbing may be associated with viable NTM, but not all individuals who take hot showers have NTM infection.[41][42][44] Whether educating the patient on avoiding hot showers will decrease the chances of infection is unclear. There is limited data that supports evidence-based environmental strategies to limit the transmission of NTM. Therefore, discussing general precautions with patients at risk for pulmonary NTM infection may be prudent.
Concerning management, the patient should be advised regarding the estimated duration of any proposed treatment, as this could be very lengthy. An organized and structured care plan agreed to by the patient and their family that details dosing regimens, follow-up appointments, and contact details of healthcare clinicians can optimize adherence and patient engagement. The plan may also include adverse effects and a timeframe for treatment completeness. Patients must be aware of potential complications from any disease progression. They also should be counseled on the expected time for sputum culture conversion and the anticipated duration of antimicrobial treatment for more than 12 months following culture conversion to achieve disease-free status. Building patient rapport and healthy relationships with the patient to obtain the best results is essential.
Enhancing Healthcare Team Outcomes
The diagnosis and treatment of NTM are complex and best done with an interprofessional, multidisciplinary team with a holistic approach to management. The pharmacist should educate the patient on the importance of drug adherence and counsel on potential adverse effects while checking the medication record and verifying dosing and drug interactions. An infectious disease pharmacist can also review antibiogram data with the clinician to optimize antimicrobial effectiveness, limit adverse effects, and determine antibiotic resistance. Infectious disease or respiratory nurses are essential for patient care, infection control measures while tuberculosis is being excluded, medication administration, verifying adherence, monitoring for adverse events, offering patients counsel, and reporting any concerns to the treating physicians.
Daily observed therapy may sometimes be necessary to maintain medication adherence until treatment completion.[140] The patient should be urged to cease smoking to limit symptom burden and any progression of disease. A holistic approach considers all these factors in partnership with the patient to determine the buy-in and optimal pathway to complete the long-anticipated treatment duration for pulmonary NTM.[141] Therefore, a patient-centered approach can improve clinical outcomes, patient safety, and multidisciplinary team performance.
Media
(Click Image to Enlarge)
References
Pinner M. IV. Atypical Acid Fast Organisms: II. Some Observations on Filtration Experiments. Journal of bacteriology. 1933 Jun:25(6):576-9 [PubMed PMID: 16559638]
Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Seminars in respiratory and critical care medicine. 2013 Feb:34(1):87-94. doi: 10.1055/s-0033-1333567. Epub 2013 Mar 4 [PubMed PMID: 23460008]
Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clinics in chest medicine. 2015 Mar:36(1):13-34. doi: 10.1016/j.ccm.2014.10.002. Epub 2014 Nov 6 [PubMed PMID: 25676516]
Kee SJ,Suh SP, Increasing Burden of Nontuberculous Mycobacteria in Korea. Journal of Korean medical science. 2017 Aug [PubMed PMID: 28665053]
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine. 2007 Feb 15:175(4):367-416 [PubMed PMID: 17277290]
Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017 Nov:72(Suppl 2):ii1-ii64. doi: 10.1136/thoraxjnl-2017-210927. Epub [PubMed PMID: 29054853]
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. The European respiratory journal. 2020 Jul:56(1):. doi: 10.1183/13993003.00535-2020. Epub 2020 Jul 7 [PubMed PMID: 32636299]
Level 1 (high-level) evidenceCambau E, Drancourt M. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014 Mar:20(3):196-201. doi: 10.1111/1469-0691.12555. Epub [PubMed PMID: 24450600]
RUNYON EH. Anonymous mycobacteria in pulmonary disease. The Medical clinics of North America. 1959 Jan:43(1):273-90 [PubMed PMID: 13612432]
Grange JM, Yates MD, Boughton E. The avian tubercle bacillus and its relatives. The Journal of applied bacteriology. 1990 May:68(5):411-31 [PubMed PMID: 2196253]
Wolinsky E. Nontuberculous mycobacteria and associated diseases. The American review of respiratory disease. 1979 Jan:119(1):107-59 [PubMed PMID: 369415]
Runyon EH. Micobacterium intracellulare. The American review of respiratory disease. 1967 May:95(5):861-5 [PubMed PMID: 6023519]
Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clinical microbiology reviews. 2014 Oct:27(4):727-52. doi: 10.1128/CMR.00035-14. Epub [PubMed PMID: 25278573]
Cook GM, Berney M, Gebhard S, Heinemann M, Cox RA, Danilchanka O, Niederweis M. Physiology of mycobacteria. Advances in microbial physiology. 2009:55():81-182, 318-9. doi: 10.1016/S0065-2911(09)05502-7. Epub [PubMed PMID: 19573696]
Level 3 (low-level) evidenceGangadharam PR. Microbiology of nontuberculosis mycobacteria. Seminars in respiratory infections. 1996 Dec:11(4):231-43 [PubMed PMID: 8976577]
Bittner MJ, Preheim LC. Other Slow-Growing Nontuberculous Mycobacteria. Microbiology spectrum. 2016 Nov:4(6):. doi: 10.1128/microbiolspec.TNMI7-0012-2016. Epub [PubMed PMID: 27837745]
Kim CJ, Kim NH, Song KH, Choe PG, Kim ES, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. Differentiating rapid- and slow-growing mycobacteria by difference in time to growth detection in liquid media. Diagnostic microbiology and infectious disease. 2013 Jan:75(1):73-6. doi: 10.1016/j.diagmicrobio.2012.09.019. Epub 2012 Oct 29 [PubMed PMID: 23114094]
Springer B, Stockman L, Teschner K, Roberts GD, Böttger EC. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. Journal of clinical microbiology. 1996 Feb:34(2):296-303 [PubMed PMID: 8789004]
Asaoka M, Hagiwara E, Etori S, Higa K, Ikeda S, Sekine A, Kitamura H, Baba T, Komatsu S, Ogura T. Identification and Characteristics of Co-isolation of Multiple Nontuberculous Mycobacteria. Internal medicine (Tokyo, Japan). 2021 Oct 15:60(20):3213-3219. doi: 10.2169/internalmedicine.5300-20. Epub 2021 Apr 26 [PubMed PMID: 33896860]
Jun HJ, Jeon K, Um SW, Kwon OJ, Lee NY, Koh WJ. Nontuberculous mycobacteria isolated during the treatment of pulmonary tuberculosis. Respiratory medicine. 2009 Dec:103(12):1936-40. doi: 10.1016/j.rmed.2009.05.025. Epub 2009 Jul 2 [PubMed PMID: 19576745]
Chaptal M, Andrejak C, Bonifay T, Beillard E, Guillot G, Guyomard-Rabenirina S, Demar M, Trombert-Paolantoni S, Jacomo V, Mosnier E, Veziris N, Djossou F, Epelboin L, French Guiana PNTM working group. Epidemiology of infection by pulmonary non-tuberculous mycobacteria in French Guiana 2008-2018. PLoS neglected tropical diseases. 2022 Sep:16(9):e0010693. doi: 10.1371/journal.pntd.0010693. Epub 2022 Sep 9 [PubMed PMID: 36084148]
Chindam A, Vengaldas S, Srigiri VR, Syed U, Kilaru H, Chenimilla NP, Kilaru SC, Patil E. Challenges of diagnosing and treating non-tuberculous mycobacterial pulmonary disease [NTM-PD]: A case series. Journal of clinical tuberculosis and other mycobacterial diseases. 2021 Dec:25():100271. doi: 10.1016/j.jctube.2021.100271. Epub 2021 Aug 30 [PubMed PMID: 34541338]
Level 2 (mid-level) evidencePravosud V, Mannino DM, Prieto D, Zhang Q, Choate R, Malanga E, Aksamit TR. Symptom Burden and Medication Use Among Patients with Nontuberculous Mycobacterial Lung Disease. Chronic obstructive pulmonary diseases (Miami, Fla.). 2021 Apr 27:8(2):243-254. doi: 10.15326/jcopdf.2020.0184. Epub [PubMed PMID: 33610137]
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Aug 14:71(4):e1-e36. doi: 10.1093/cid/ciaa241. Epub [PubMed PMID: 32628747]
Level 1 (high-level) evidenceSchildkraut JA, Zweijpfenning SMH, Nap M, He K, Dacheva E, Overbeek J, Tostmann A, Wertheim HFL, Hoefsloot W, van Ingen J. The epidemiology of nontuberculous mycobacterial pulmonary disease in the Netherlands. ERJ open research. 2021 Jul:7(3):. pii: 00207-2021. doi: 10.1183/23120541.00207-2021. Epub 2021 Jul 12 [PubMed PMID: 34262970]
Thomson R, Donnan E, Konstantinos A. Notification of Nontuberculous Mycobacteria: An Australian Perspective. Annals of the American Thoracic Society. 2017 Mar:14(3):318-323. doi: 10.1513/AnnalsATS.201612-994OI. Epub [PubMed PMID: 28118021]
Level 3 (low-level) evidenceAdjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, Honda S, Prevots DR. Epidemiology of Nontuberculous Mycobacterial Lung Disease and Tuberculosis, Hawaii, USA. Emerging infectious diseases. 2017 Mar:23(3):439-447. doi: 10.3201/eid2303.161827. Epub [PubMed PMID: 28221128]
Deutsch-Feldman M, Springer YP, Felix D, Tsang CA, Brostrom R, Haddad M. Tuberculosis Among Native Hawaiian and Other Pacific Islander Persons: United States and U.S.-Affiliated Pacific Islands, 2010-2019. Health equity. 2022:6(1):476-484. doi: 10.1089/heq.2022.0065. Epub 2022 Jun 27 [PubMed PMID: 35801148]
Lin C, Russell C, Soll B, Chow D, Bamrah S, Brostrom R, Kim W, Scott J, Bankowski MJ. Increasing Prevalence of Nontuberculous Mycobacteria in Respiratory Specimens from US-Affiliated Pacific Island Jurisdictions(1). Emerging infectious diseases. 2018 Mar:24(3):485-491. doi: 10.3201/eid2403.171301. Epub [PubMed PMID: 29460734]
Hojo M, Iikura M, Hirano S, Sugiyama H, Kobayashi N, Kudo K. Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology (Carlton, Vic.). 2012 Jan:17(1):185-90. doi: 10.1111/j.1440-1843.2011.02076.x. Epub [PubMed PMID: 21995339]
O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. The American review of respiratory disease. 1987 May:135(5):1007-14 [PubMed PMID: 3579001]
Level 2 (mid-level) evidenceAdjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. American journal of respiratory and critical care medicine. 2012 Apr 15:185(8):881-6. doi: 10.1164/rccm.201111-2016OC. Epub 2012 Feb 3 [PubMed PMID: 22312016]
Level 2 (mid-level) evidenceShah NM, Davidson JA, Anderson LF, Lalor MK, Kim J, Thomas HL, Lipman M, Abubakar I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007-2012. BMC infectious diseases. 2016 May 6:16():195. doi: 10.1186/s12879-016-1521-3. Epub 2016 May 6 [PubMed PMID: 27154015]
Dohál M, Porvazník I, Krivošová M, Solovič I, Mokrý J. Epidemiology of non-tuberculous mycobacterial diseases in Slovakia during the years 2016-2021. Respiratory physiology & neurobiology. 2023 Aug:314():104090. doi: 10.1016/j.resp.2023.104090. Epub 2023 Jun 12 [PubMed PMID: 37315773]
Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014 Nov:18(11):1370-7. doi: 10.5588/ijtld.14.0120. Epub [PubMed PMID: 25299873]
Level 1 (high-level) evidence. Rapid increase of the incidence of lung disease due to Mycobacterium kansasii in Japan. Chest. 1983 Jun:83(6):890-2 [PubMed PMID: 6851691]
Thomson RM, Furuya-Kanamori L, Coffey C, Bell SC, Knibbs LD, Lau CL. Influence of climate variables on the rising incidence of nontuberculous mycobacterial (NTM) infections in Queensland, Australia 2001-2016. The Science of the total environment. 2020 Oct 20:740():139796. doi: 10.1016/j.scitotenv.2020.139796. Epub 2020 Jun 13 [PubMed PMID: 32563864]
Lee SJ, Ju S, You JW, Jeong YY, Lee JD, Kim HC, Choi H, Lee H, Oh YM, Ra SW. Trends in the Prevalence of Non-TB Mycobacterial Infection in Patients With Non-Cystic Fibrosis Bronchiectasis in South Korea, 2012-2016. Chest. 2021 Mar:159(3):959-962. doi: 10.1016/j.chest.2020.10.093. Epub 2020 Dec 7 [PubMed PMID: 33301746]
Thomson RM, NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerging infectious diseases. 2010 Oct:16(10):1576-83. doi: 10.3201/eid1610.091201. Epub [PubMed PMID: 20875283]
Park SC, Kang MJ, Han CH, Lee SM, Kim CJ, Lee JM, Kang YA. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC pulmonary medicine. 2019 Aug 1:19(1):140. doi: 10.1186/s12890-019-0901-z. Epub 2019 Aug 1 [PubMed PMID: 31370826]
Falkinham JO 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerging infectious diseases. 2011 Mar:17(3):419-24. doi: 10.3201/eid1703.101510. Epub [PubMed PMID: 21392432]
Shen Y, Haig SJ, Prussin AJ 2nd, LiPuma JJ, Marr LC, Raskin L. Shower water contributes viable nontuberculous mycobacteria to indoor air. PNAS nexus. 2022 Nov:1(5):pgac145. doi: 10.1093/pnasnexus/pgac145. Epub 2022 Nov 10 [PubMed PMID: 36712351]
Dirac MA, Horan KL, Doody DR, Meschke JS, Park DR, Jackson LA, Weiss NS, Winthrop KL, Cangelosi GA. Environment or host?: A case-control study of risk factors for Mycobacterium avium complex lung disease. American journal of respiratory and critical care medicine. 2012 Oct 1:186(7):684-91. doi: 10.1164/rccm.201205-0825OC. Epub 2012 Aug 2 [PubMed PMID: 22859521]
Level 2 (mid-level) evidencePrevots DR, Adjemian J, Fernandez AG, Knowles MR, Olivier KN. Environmental risks for nontuberculous mycobacteria. Individual exposures and climatic factors in the cystic fibrosis population. Annals of the American Thoracic Society. 2014 Sep:11(7):1032-8. doi: 10.1513/AnnalsATS.201404-184OC. Epub [PubMed PMID: 25068620]
Kahana LM, Kay JM, Yakrus MA, Waserman S. Mycobacterium avium complex infection in an immunocompetent young adult related to hot tub exposure. Chest. 1997 Jan:111(1):242-5 [PubMed PMID: 8996025]
Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. The European respiratory journal. 2019 Jul:54(1):. pii: 1900250. doi: 10.1183/13993003.00250-2019. Epub 2019 Jul 11 [PubMed PMID: 31221809]
Garcia B, Wilmskoetter J, Grady A, Mingora C, Dorman S, Flume P. Chest Computed Tomography Features of Nontuberculous Mycobacterial Pulmonary Disease Versus Asymptomatic Colonization: A Cross-sectional Cohort Study. Journal of thoracic imaging. 2022 May 1:37(3):140-145. doi: 10.1097/RTI.0000000000000610. Epub 2021 Jul 21 [PubMed PMID: 34292274]
Level 2 (mid-level) evidenceFowler SJ, French J, Screaton NJ, Foweraker J, Condliffe A, Haworth CS, Exley AR, Bilton D. Nontuberculous mycobacteria in bronchiectasis: Prevalence and patient characteristics. The European respiratory journal. 2006 Dec:28(6):1204-10 [PubMed PMID: 16807259]
Bai X, Bai A, Honda JR, Eichstaedt C, Musheyev A, Feng Z, Huitt G, Harbeck R, Kosmider B, Sandhaus RA, Chan ED. Alpha-1-Antitrypsin Enhances Primary Human Macrophage Immunity Against Non-tuberculous Mycobacteria. Frontiers in immunology. 2019:10():1417. doi: 10.3389/fimmu.2019.01417. Epub 2019 Jun 26 [PubMed PMID: 31293581]
Wijers CD, Chmiel JF, Gaston BM. Bacterial infections in patients with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chronic respiratory disease. 2017 Nov:14(4):392-406. doi: 10.1177/1479972317694621. Epub 2017 Mar 6 [PubMed PMID: 29081265]
Benmerzoug S, Marinho FV, Rose S, Mackowiak C, Gosset D, Sedda D, Poisson E, Uyttenhove C, Van Snick J, Jacobs M, Garcia I, Ryffel B, Quesniaux VFJ. GM-CSF targeted immunomodulation affects host response to M. tuberculosis infection. Scientific reports. 2018 Jun 5:8(1):8652. doi: 10.1038/s41598-018-26984-3. Epub 2018 Jun 5 [PubMed PMID: 29872095]
Lombardi A, Villa S, Castelli V, Bandera A, Gori A. T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives. Microorganisms. 2021 Nov 28:9(12):. doi: 10.3390/microorganisms9122460. Epub 2021 Nov 28 [PubMed PMID: 34946062]
Level 3 (low-level) evidenceMcCarthy KD, Cain KP, Winthrop KL, Udomsantisuk N, Lan NT, Sar B, Kimerling ME, Kanara N, Lynen L, Monkongdee P, Tasaneeyapan T, Varma JK. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. American journal of respiratory and critical care medicine. 2012 May 1:185(9):981-8. doi: 10.1164/rccm.201107-1327OC. Epub 2012 Feb 16 [PubMed PMID: 22345581]
Horsburgh CR Jr. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. The New England journal of medicine. 1991 May 9:324(19):1332-8 [PubMed PMID: 2017230]
Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. Journal of immunology (Baltimore, Md. : 1950). 2007 Jun 1:178(11):7222-34 [PubMed PMID: 17513771]
Lutt JR, Pisculli ML, Weinblatt ME, Deodhar A, Winthrop KL. Severe nontuberculous mycobacterial infection in 2 patients receiving rituximab for refractory myositis. The Journal of rheumatology. 2008 Aug:35(8):1683-5 [PubMed PMID: 18671331]
Okumura M, Iwai K, Ogata H, Ueyama M, Kubota M, Aoki M, Kokuto H, Tadokoro E, Uchiyama T, Saotome M, Yoshiyama T, Yoshimori K, Yoshida N, Azuma A, Kudoh S. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Internal medicine (Tokyo, Japan). 2008:47(16):1465-72 [PubMed PMID: 18703856]
Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gender medicine. 2010 Feb:7(1):5-18. doi: 10.1016/j.genm.2010.01.005. Epub [PubMed PMID: 20189150]
Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. The American review of respiratory disease. 1991 Oct:144(4):914-6 [PubMed PMID: 1928970]
Dawrs SN, Kautz M, Chan ED, Honda JR. Mycobacterium abscessus and Gastroesophageal Reflux: An In Vitro Study. American journal of respiratory and critical care medicine. 2020 Aug 1:202(3):466-469. doi: 10.1164/rccm.202001-0011LE. Epub [PubMed PMID: 32298605]
Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. Journal of Korean medical science. 2005 Dec:20(6):913-25 [PubMed PMID: 16361797]
Level 3 (low-level) evidenceThomson R, Tolson C, Carter R, Coulter C, Huygens F, Hargreaves M. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. Journal of clinical microbiology. 2013 Sep:51(9):3006-11. doi: 10.1128/JCM.00899-13. Epub 2013 Jul 10 [PubMed PMID: 23843489]
Thomson RM, Carter R, Tolson C, Coulter C, Huygens F, Hargreaves M. Factors associated with the isolation of Nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC microbiology. 2013 Apr 22:13():89. doi: 10.1186/1471-2180-13-89. Epub 2013 Apr 22 [PubMed PMID: 23601969]
Pennington KM, Vu A, Challener D, Rivera CG, Shweta FNU, Zeuli JD, Temesgen Z. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. Journal of clinical tuberculosis and other mycobacterial diseases. 2021 Aug:24():100244. doi: 10.1016/j.jctube.2021.100244. Epub 2021 May 8 [PubMed PMID: 34036184]
Pathak K, Hart S, Lande L. Nontuberculous Mycobacteria Lung Disease (NTM-LD): Current Recommendations on Diagnosis, Treatment, and Patient Management. International journal of general medicine. 2022:15():7619-7629. doi: 10.2147/IJGM.S272690. Epub 2022 Oct 1 [PubMed PMID: 36213301]
Kendall BA, Varley CD, Choi D, Cassidy PM, Hedberg K, Ware MA, Winthrop KL. Distinguishing tuberculosis from nontuberculous mycobacteria lung disease, Oregon, USA. Emerging infectious diseases. 2011 Mar:17(3):506-9. doi: 10.3201/eid1703.101164. Epub [PubMed PMID: 21392445]
Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. Journal of biomedical science. 2020 Jun 17:27(1):74. doi: 10.1186/s12929-020-00667-6. Epub 2020 Jun 17 [PubMed PMID: 32552732]
Level 2 (mid-level) evidenceMusaddaq B, Cleverley JR. Diagnosis of non-tuberculous mycobacterial pulmonary disease (NTM-PD): modern challenges. The British journal of radiology. 2020 Feb 1:93(1106):20190768. doi: 10.1259/bjr.20190768. Epub 2019 Dec 11 [PubMed PMID: 31794241]
Parkar AP, Kandiah P. Differential Diagnosis of Cavitary Lung Lesions. Journal of the Belgian Society of Radiology. 2016 Nov 19:100(1):100. doi: 10.5334/jbr-btr.1202. Epub 2016 Nov 19 [PubMed PMID: 30151493]
Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, Strand MJ, Bai X, Ramamoorthy P, Rothman MS, Nagabhushanam V, McDermott M, Levin AR, Frazer-Abel A, Giclas PC, Korner J, Iseman MD, Shapiro L, Chan ED. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. American journal of respiratory and critical care medicine. 2013 Jan 15:187(2):197-205. doi: 10.1164/rccm.201206-1035OC. Epub 2012 Nov 9 [PubMed PMID: 23144328]
Chu H, Zhao L, Xiao H, Zhang Z, Zhang J, Gui T, Gong S, Xu L, Sun X. Prevalence of nontuberculous mycobacteria in patients with bronchiectasis: a meta-analysis. Archives of medical science : AMS. 2014 Aug 29:10(4):661-8. doi: 10.5114/aoms.2014.44857. Epub [PubMed PMID: 25276148]
Level 1 (high-level) evidencePursner M, Haller JO, Berdon WE. Imaging features of Mycobacterium avium-intracellulare complex (MAC) in children with AIDS. Pediatric radiology. 2000 Jun:30(6):426-9 [PubMed PMID: 10876832]
Soetaert K, Subissi L, Ceyssens PJ, Vanfleteren B, Chantrenne M, Asikainen T, Duysburgh E, Mathys V. Strong increase of true and false positive mycobacterial cultures sent to the National Reference Centre in Belgium, 2007 to 2016. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2019 Mar:24(11):. doi: 10.2807/1560-7917.ES.2019.24.11.1800205. Epub [PubMed PMID: 30892180]
Kim N, Yi J, Chang CL. Recovery Rates of Non-Tuberculous Mycobacteria from Clinical Specimens Are Increasing in Korean Tertiary-Care Hospitals. Journal of Korean medical science. 2017 Aug:32(8):1263-1267. doi: 10.3346/jkms.2017.32.8.1263. Epub [PubMed PMID: 28665061]
Somoskövi A, Hotaling JE, Fitzgerald M, O'Donnell D, Parsons LM, Salfinger M. Lessons from a proficiency testing event for acid-fast microscopy. Chest. 2001 Jul:120(1):250-7 [PubMed PMID: 11451846]
Sawatpanich A, Petsong S, Tumwasorn S, Rotcheewaphan S. Diagnostic performance of the Anyplex MTB/NTM real-time PCR in detection of Mycobacterium tuberculosis complex and nontuberculous mycobacteria from pulmonary and extrapulmonary specimens. Heliyon. 2022 Dec:8(12):e11935. doi: 10.1016/j.heliyon.2022.e11935. Epub 2022 Nov 26 [PubMed PMID: 36471833]
Alonso H, Samper S, Martín C, Otal I. Mapping IS6110 in high-copy number Mycobacterium tuberculosis strains shows specific insertion points in the Beijing genotype. BMC genomics. 2013 Jun 25:14():422. doi: 10.1186/1471-2164-14-422. Epub 2013 Jun 25 [PubMed PMID: 23800083]
Harboe M, Nagai S, Patarroyo ME, Torres ML, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infection and immunity. 1986 Apr:52(1):293-302 [PubMed PMID: 3514457]
Alexander KJ, Furlong JL, Baron JL, Rihs JD, Stephenson D, Perry JD, Stout JE. Evaluation of a new culture medium for isolation of nontuberculous mycobacteria from environmental water samples. PloS one. 2021:16(3):e0247166. doi: 10.1371/journal.pone.0247166. Epub 2021 Mar 3 [PubMed PMID: 33657154]
Ryu YJ, Koh WJ, Daley CL. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease: Clinicians' Perspectives. Tuberculosis and respiratory diseases. 2016 Apr:79(2):74-84. doi: 10.4046/trd.2016.79.2.74. Epub 2016 Mar 31 [PubMed PMID: 27066084]
Level 3 (low-level) evidenceCaverly LJ, Carmody LA, Haig SJ, Kotlarz N, Kalikin LM, Raskin L, LiPuma JJ. Culture-Independent Identification of Nontuberculous Mycobacteria in Cystic Fibrosis Respiratory Samples. PloS one. 2016:11(4):e0153876. doi: 10.1371/journal.pone.0153876. Epub 2016 Apr 19 [PubMed PMID: 27093603]
Im Y, Hwang NY, Kim K, Kim H, Kwon OJ, Jhun BW. Impact of Time Between Diagnosis and Treatment for Nontuberculous Mycobacterial Pulmonary Disease on Culture Conversion and All-Cause Mortality. Chest. 2022 May:161(5):1192-1200. doi: 10.1016/j.chest.2021.10.048. Epub 2021 Nov 16 [PubMed PMID: 34793759]
Dohál M, Porvazník I, Solovič I, Mokrý J. Whole Genome Sequencing in the Management of Non-Tuberculous Mycobacterial Infections. Microorganisms. 2021 Oct 27:9(11):. doi: 10.3390/microorganisms9112237. Epub 2021 Oct 27 [PubMed PMID: 34835363]
Donnan EJ, Marais BJ, Coulter C, Waring J, Bastian I, Williamson DA, Sherry NL, Bond K, Sintchenko V, Meumann EM, Horan K, Cooley L, Denholm JT. The use of whole genome sequencing for tuberculosis public health activities in Australia: a joint statement of the National Tuberculosis Advisory Committee and Communicable Diseases Genomics Network. Communicable diseases intelligence (2018). 2023 Feb 28:47():. doi: 10.33321/cdi.2023.47.8. Epub 2023 Feb 28 [PubMed PMID: 36850064]
Rodriguez-Temporal D, Alcaide F, Mareković I, O'Connor JA, Gorton R, van Ingen J, Van den Bossche A, Héry-Arnaud G, Beauruelle C, Orth-Höller D, Palacios-Gutiérrez JJ, Tudó G, Bou G, Ceyssens PJ, Garrigó M, González-Martin J, Greub G, Hrabak J, Ingebretsen A, Mediavilla-Gradolph MC, Oviaño M, Palop B, Pranada AB, Quiroga L, Ruiz-Serrano MJ, Rodríguez-Sánchez B. Multicentre study on the reproducibility of MALDI-TOF MS for nontuberculous mycobacteria identification. Scientific reports. 2022 Jan 24:12(1):1237. doi: 10.1038/s41598-022-05315-7. Epub 2022 Jan 24 [PubMed PMID: 35075208]
Johnson TM, Byrd TF, Drummond WK, Childs-Kean LM, Mahoney MV, Pearson JC, Rivera CG. Contemporary Pharmacotherapies for Nontuberculosis Mycobacterial Infections: A Narrative Review. Infectious diseases and therapy. 2023 Feb:12(2):343-365. doi: 10.1007/s40121-022-00750-5. Epub 2023 Jan 7 [PubMed PMID: 36609820]
Level 3 (low-level) evidenceKwon YS, Koh WJ. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease. Journal of Korean medical science. 2016 May:31(5):649-59. doi: 10.3346/jkms.2016.31.5.649. Epub 2016 Mar 22 [PubMed PMID: 27134484]
Richard M, Gutiérrez AV, Kremer L. Dissecting erm(41)-Mediated Macrolide-Inducible Resistance in Mycobacterium abscessus. Antimicrobial agents and chemotherapy. 2020 Jan 27:64(2):. doi: 10.1128/AAC.01879-19. Epub 2020 Jan 27 [PubMed PMID: 31791943]
Lyu J, Jang HJ, Song JW, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Shim TS. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respiratory medicine. 2011 May:105(5):781-7. doi: 10.1016/j.rmed.2010.12.012. Epub 2011 Jan 5 [PubMed PMID: 21211956]
Gill LI, Dominic C, Tiberi S. Atypical mycobacterial infections - management and when to treat. Current opinion in pulmonary medicine. 2021 May 1:27(3):216-223. doi: 10.1097/MCP.0000000000000764. Epub [PubMed PMID: 33560672]
Level 3 (low-level) evidenceGriffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, Addrizzo-Harris DJ, O'Donnell AE, Marras TK, Flume PA, Loebinger MR, Morgan L, Codecasa LR, Hill AT, Ruoss SJ, Yim JJ, Ringshausen FC, Field SK, Philley JV, Wallace RJ Jr, van Ingen J, Coulter C, Nezamis J, Winthrop KL, CONVERT Study Group. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. American journal of respiratory and critical care medicine. 2018 Dec 15:198(12):1559-1569. doi: 10.1164/rccm.201807-1318OC. Epub [PubMed PMID: 30216086]
Level 1 (high-level) evidenceMartiniano SL, Esther CR, Haworth CS, Kasperbauer SH, Zemanick ET, Caverly LJ. Challenging scenarios in nontuberculous mycobacterial infection in cystic fibrosis. Pediatric pulmonology. 2020 Feb:55(2):521-525. doi: 10.1002/ppul.24604. Epub 2019 Dec 10 [PubMed PMID: 31821718]
Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respiratory medicine. 2009 Oct:103(10):1448-55. doi: 10.1016/j.rmed.2009.04.026. Epub 2009 May 21 [PubMed PMID: 19467851]
Jo KW, Kim S, Lee JY, Lee SD, Kim WS, Kim DS, Shim TS. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2014 Oct:20(10):602-6. doi: 10.1016/j.jiac.2014.05.010. Epub 2014 Jun 26 [PubMed PMID: 24981714]
Zhang Y, Hill AT. Amikacin liposome inhalation suspension as a treatment for patients with refractory mycobacterium avium complex lung infection. Expert review of respiratory medicine. 2021 Jun:15(6):737-744. doi: 10.1080/17476348.2021.1875821. Epub 2021 May 26 [PubMed PMID: 34039231]
Santin M, Dorca J, Alcaide F, Gonzalez L, Casas S, Lopez M, Guerra MR. Long-term relapses after 12-month treatment for Mycobacterium kansasii lung disease. The European respiratory journal. 2009 Jan:33(1):148-52. doi: 10.1183/09031936.00024008. Epub [PubMed PMID: 19118226]
Level 2 (mid-level) evidenceLee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus Complex Infections in Humans. Emerging infectious diseases. 2015 Sep:21(9):1638-46. doi: 10.3201/2109.141634. Epub [PubMed PMID: 26295364]
Yang B, Jhun BW, Moon SM, Lee H, Park HY, Jeon K, Kim DH, Kim SY, Shin SJ, Daley CL, Koh WJ. Clofazimine-Containing Regimen for the Treatment of Mycobacterium abscessus Lung Disease. Antimicrobial agents and chemotherapy. 2017 Jun:61(6):. doi: 10.1128/AAC.02052-16. Epub 2017 May 24 [PubMed PMID: 28348153]
Winthrop KL, Ku JH, Marras TK, Griffith DE, Daley CL, Olivier KN, Aksamit TR, Varley CD, Mackey K, Prevots DR. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. The European respiratory journal. 2015 Apr:45(4):1177-9. doi: 10.1183/09031936.00169114. Epub 2015 Jan 22 [PubMed PMID: 25614169]
Inoue T, Tsunoda A, Nishimoto E, Nishida K, Komatsubara Y, Onoe R, Saji J, Mineshita M. Successful use of linezolid for refractory Mycobacterium abcessus infection: A case report. Respiratory medicine case reports. 2018:23():43-45. doi: 10.1016/j.rmcr.2017.11.007. Epub 2017 Nov 28 [PubMed PMID: 29234594]
Level 3 (low-level) evidenceSiegel SAR, Griffith DE, Philley JV, Brown-Elliott BA, Brunton AE, Sullivan PE, Fuss C, Strnad L, Wallace RJ Jr, Winthrop KL. Open-Label Trial of Amikacin Liposome Inhalation Suspension in Mycobacterium abscessus Lung Disease. Chest. 2023 Oct:164(4):846-859. doi: 10.1016/j.chest.2023.05.036. Epub 2023 Jun 17 [PubMed PMID: 37419144]
Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, Clark J, Del Mar CB. Adverse events in people taking macrolide antibiotics versus placebo for any indication. The Cochrane database of systematic reviews. 2019 Jan 18:1(1):CD011825. doi: 10.1002/14651858.CD011825.pub2. Epub 2019 Jan 18 [PubMed PMID: 30656650]
Level 1 (high-level) evidenceMosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. The New England journal of medicine. 2013 May 2:368(18):1665-8. doi: 10.1056/NEJMp1302726. Epub [PubMed PMID: 23635046]
Vasquez EM, Maddux MS, Sanchez J, Pollak R. Clinically significant hearing loss in renal allograft recipients treated with intravenous erythromycin. Archives of internal medicine. 1993 Apr 12:153(7):879-82 [PubMed PMID: 8466379]
Barbieri MA, Cicala G, Cutroneo PM, Mocciaro E, Sottosanti L, Freni F, Galletti F, Arcoraci V, Spina E. Ototoxic Adverse Drug Reactions: A Disproportionality Analysis Using the Italian Spontaneous Reporting Database. Frontiers in pharmacology. 2019:10():1161. doi: 10.3389/fphar.2019.01161. Epub 2019 Oct 8 [PubMed PMID: 31649536]
Nakajima A, Fukami T, Kobayashi Y, Watanabe A, Nakajima M, Yokoi T. Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine. Biochemical pharmacology. 2011 Dec 1:82(11):1747-56. doi: 10.1016/j.bcp.2011.08.003. Epub 2011 Aug 12 [PubMed PMID: 21856291]
Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology (Baltimore, Md.). 2003 Apr:37(4):924-30 [PubMed PMID: 12668988]
. Rifampin. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000407]
Holdiness MR. A review of the Redman syndrome and rifampicin overdosage. Medical toxicology and adverse drug experience. 1989 Nov-Dec:4(6):444-51 [PubMed PMID: 2689837]
Kim JH, Moon JI, Kim JE, Choi GS, Park HS, Ye YM, Yim H. Cutaneous leukocytoclastic vasculitis due to anti-tuberculosis medications, rifampin and pyrazinamide. Allergy, asthma & immunology research. 2010 Jan:2(1):55-8. doi: 10.4168/aair.2010.2.1.55. Epub 2009 Dec 30 [PubMed PMID: 20224679]
Mehta YS, Jijina FF, Badakere SS, Pathare AV, Mohanty D. Rifampicin-induced immune thrombocytopenia. Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1996 Dec:77(6):558-62 [PubMed PMID: 9039451]
Venkatesan K. Pharmacokinetic drug interactions with rifampicin. Clinical pharmacokinetics. 1992 Jan:22(1):47-65 [PubMed PMID: 1559307]
Tan S, Kasperbauer S. Nontuberculous Mycobacteria. Seminars in respiratory and critical care medicine. 2021 Aug:42(4):567-586. doi: 10.1055/s-0041-1730997. Epub 2021 Jul 14 [PubMed PMID: 34261181]
Yu JA, Pomerantz M, Bishop A, Weyant MJ, Mitchell JD. Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial disease. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Sep:40(3):671-5. doi: 10.1016/j.ejcts.2010.12.028. Epub 2011 Feb 15 [PubMed PMID: 21324708]
Level 2 (mid-level) evidenceShiraishi Y, Katsuragi N, Kita H, Hyogotani A, Saito MH, Shimoda K. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. The Annals of thoracic surgery. 2013 Jul:96(1):287-91. doi: 10.1016/j.athoracsur.2013.03.008. Epub 2013 Apr 22 [PubMed PMID: 23618520]
Level 2 (mid-level) evidenceBrode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus Nonpulmonary Nontuberculous Mycobacteria, Ontario, Canada. Emerging infectious diseases. 2017 Nov:23(11):1898-1901. doi: 10.3201/eid2311.170959. Epub [PubMed PMID: 29048292]
Carpenter JL. Klebsiella pulmonary infections: occurrence at one medical center and review. Reviews of infectious diseases. 1990 Jul-Aug:12(4):672-82 [PubMed PMID: 2201068]
Macfarlane J. An overview of community acquired pneumonia with lessons learned from the British Thoracic Society Study. Seminars in respiratory infections. 1994 Sep:9(3):153-65 [PubMed PMID: 7831537]
Level 3 (low-level) evidenceThambidurai L, Prabhuradhan R, Singhvi P, Ilanchezhian S, Ramachandran R, Shankar H. Cryptococcal pneumonia: the great mimicker. BJR case reports. 2017:3(2):20150358. doi: 10.1259/bjrcr.20150358. Epub 2017 Jan 5 [PubMed PMID: 30363287]
Level 3 (low-level) evidenceSobonya RE, Yanes J, Klotz SA. Cavitary pulmonary coccidioidomycosis: pathologic and clinical correlates of disease. Human pathology. 2014 Jan:45(1):153-9. doi: 10.1016/j.humpath.2013.08.014. Epub [PubMed PMID: 24321524]
Goodwin RA Jr, Owens FT, Snell JD, Hubbard WW, Buchanan RD, Terry RT, Des Prez RM. Chronic pulmonary histoplasmosis. Medicine. 1976 Nov:55(6):413-52 [PubMed PMID: 792626]
Domej W, Hermann J, Krause R, Wehrschütz M, Maier A, Flögel E. [Lung cavities, mycetomas and hemoptysis]. Wiener medizinische Wochenschrift (1946). 2007:157(19-20):466-72 [PubMed PMID: 18030549]
Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clinical microbiology reviews. 2008 Apr:21(2):305-33, table of contents. doi: 10.1128/CMR.00060-07. Epub [PubMed PMID: 18400799]
Lee H, Myung W, Lee EM, Kim H, Jhun BW. Mortality and Prognostic Factors of Nontuberculous Mycobacterial Infection in Korea: A Population-based Comparative Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 May 18:72(10):e610-e619. doi: 10.1093/cid/ciaa1381. Epub [PubMed PMID: 32926135]
Level 2 (mid-level) evidenceMourad A, Baker AW, Stout JE. Reduction in Expected Survival Associated With Nontuberculous Mycobacterial Pulmonary Disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 May 18:72(10):e552-e557. doi: 10.1093/cid/ciaa1267. Epub [PubMed PMID: 32856690]
Sin S, Han S, Lee YJ, Cho YJ, Park JS, Yoon HI, Lee CT, Lee JH. Prognosis of nontuberculous mycobacterial pulmonary disease according to the method of microbiologic diagnosis. Scientific reports. 2021 Apr 13:11(1):8036. doi: 10.1038/s41598-021-87197-9. Epub 2021 Apr 13 [PubMed PMID: 33850204]
Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in non-tuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2015 Sep:34(9):1909-18. doi: 10.1007/s10096-015-2432-8. Epub 2015 Jul 9 [PubMed PMID: 26155783]
Graybill JR, Silva J Jr, Fraser DW, Lordon R, Rogers E. Disseminated mycobacteriosis due to Mycobacterium abcessus in two recipients of renal homografts. The American review of respiratory disease. 1974 Jan:109(1):4-10 [PubMed PMID: 4588091]
Jhun BW, Moon SM, Jeon K, Kwon OJ, Yoo H, Carriere KC, Huh HJ, Lee NY, Shin SJ, Daley CL, Koh WJ. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. The European respiratory journal. 2020 Jan:55(1):. pii: 1900798. doi: 10.1183/13993003.00798-2019. Epub 2020 Jan 2 [PubMed PMID: 31619468]
Knoll BM, Kappagoda S, Gill RR, Goldberg HJ, Boyle K, Baden LR, Fuhlbrigge AL, Marty FM. Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transplant infectious disease : an official journal of the Transplantation Society. 2012 Oct:14(5):452-60. doi: 10.1111/j.1399-3062.2012.00753.x. Epub 2012 Jun 8 [PubMed PMID: 22676720]
Bachar K, Shulimzon T, Segel MJ. Nontuberculous mycobacteria infections of the pleura: A systematic review. Respiratory medicine. 2022 Dec:205():107036. doi: 10.1016/j.rmed.2022.107036. Epub 2022 Nov 1 [PubMed PMID: 36335889]
Level 1 (high-level) evidenceKhoor A, Leslie KO, Tazelaar HD, Helmers RA, Colby TV. Diffuse pulmonary disease caused by nontuberculous mycobacteria in immunocompetent people (hot tub lung). American journal of clinical pathology. 2001 May:115(5):755-62 [PubMed PMID: 11345841]
Kuroda F, Tanabe N, Igari H, Sakurai T, Sakao S, Tada Y, Kasahara Y, Tatsumi K. Nontuberculous mycobacterium diseases and chronic thromboembolic pulmonary hypertension. Internal medicine (Tokyo, Japan). 2014:53(20):2273-9 [PubMed PMID: 25318788]
Shah SK, McAnally KJ, Seoane L, Lombard GA, LaPlace SG, Lick S, Dhillon GS, Valentine VG. Analysis of pulmonary non-tuberculous mycobacterial infections after lung transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2016 Aug:18(4):585-91. doi: 10.1111/tid.12546. Epub 2016 Jul 2 [PubMed PMID: 27368989]
Olivier KN, Yankaskas JR, Knowles MR. Nontuberculous mycobacterial pulmonary disease in cystic fibrosis. Seminars in respiratory infections. 1996 Dec:11(4):272-84 [PubMed PMID: 8976581]
Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerging infectious diseases. 2009 Sep:15(9):1351-8; quiz 1544. doi: 10.3201/eid1509.081259. Epub [PubMed PMID: 19788801]
Zenone T, Boibieux A, Tigaud S, Fredenucci JF, Vincent V, Chidiac C, Peyramond D. Non-tuberculous mycobacterial tenosynovitis: a review. Scandinavian journal of infectious diseases. 1999:31(3):221-8 [PubMed PMID: 10482048]
Gascón P, Sathe SS, Rameshwar P. Impaired erythropoiesis in the acquired immunodeficiency syndrome with disseminated Mycobacterium avium complex. The American journal of medicine. 1993 Jan:94(1):41-8 [PubMed PMID: 8093587]
Havlik JA Jr, Horsburgh CR Jr, Metchock B, Williams PP, Fann SA, Thompson SE 3rd. Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. The Journal of infectious diseases. 1992 Mar:165(3):577-80 [PubMed PMID: 1347060]
Davies PD. The role of DOTS in tuberculosis treatment and control. American journal of respiratory medicine : drugs, devices, and other interventions. 2003:2(3):203-9 [PubMed PMID: 14720002]
Courtney R, Ballard E, Fauver S, Gariota M, Holland L. The partnership model: working with individuals, families, and communities toward a new vision of health. Public health nursing (Boston, Mass.). 1996 Jun:13(3):177-86 [PubMed PMID: 8677233]