Introduction
Ventilation-perfusion scan also referred to as lung scintigraphy or commonly V/Q scan, is a diagnostic test utilizing radioisotopes to evaluate pulmonary ventilation and perfusion. The history of the V/Q scan dates to 1964, when its initial clinical application in the diagnosis of pulmonary embolism was reported.[1] While CT pulmonary angiography is currently considered the gold standard and is one of the most commonly used modalities for diagnosing pulmonary embolism, a V/Q scan is useful in assessing the likelihood of pulmonary embolism when intravenous contrast is contraindicated, such as in acute or chronic kidney disease and intravenous contrast allergy.[2]
Ventilation perfusion scan consists of two portions, a ventilation (V) scintigraphy and a perfusion (Q) scintigraphy. An aerosolized tracer is administered to assess lung ventilation by evaluating the distribution of the tracer to the alveoli. The assessment of lung perfusion involves administering an injectable tracer and its distribution to the pulmonary vasculature. Over time, many criteria were designed to interpret the V/Q scan. These include the McNeil criteria reported in 1984, the Biello criteria, the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) criteria reported in 1990, the PIOPED II criteria, the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISAPED) criteria in 1996, and the European Association of Nuclear Medicine (EANM) guidelines published in 2009. [3] These criteria are aimed at increasing the diagnostic accuracy of the study.
Procedures
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Procedures
Lung scintigraphy requires a chest X-ray in the posteroanterior and lateral views as a pre-requisite before the procedure. A portable posteroanterior X-ray chest is an alternative only if the patient is unable to tolerate routine X-rays. A CT scan is an alternative to a chest X-ray.
The test consists of two parts, namely ventilation scintigraphy and perfusion scintigraphy. Ventilation scintigraphy usually precedes perfusion scintigraphy and may also be excluded if not required.[4]
The COVID-19 pandemic raised concern about the transmission of COVID-19 with the ventilation portion of the scintigraphy. The American College of Radiology (ACR) released a statement in 2020 stating the lung perfusion scan can provide helpful information and that a ventilation scan is unnecessary if the lung perfusion scan is normal or interpreted as having a low probability of pulmonary embolism. ACR also recommended that if a ventilation scan is considered necessary clinically, the risk of a patient having COVID-19 should be discussed with the referring clinician, and the scan should be performed in the context of the hospital or institution's COVID-19 policies. A negative COVID-19 test prior to the ventilation scan should be considered if feasible.[3]
Ventilation Scintigraphy (V)
This component of the scan assesses air distribution in the lungs. Ventilation radiopharmaceuticals are classified as gases, aerosolized liquid, and aerosolized solid particles.
- 99mTc-diethylenetriaminepentaacetic acid (DTPA) is the most commonly used (57%) radiopharmaceutical in the form of liquid aerosol with a median aerosol diameter of 4.5 micrometers, a half-life of 6 hours, and a photopeak of 140 keV. A dose of 25 to 35 mCi (925–1295 MBq) of Tc-DTPA is administered via a nebulizer using a mouthpiece.
- 99mtc-labeled solid graphite hydrophobic particles is an aerosolized solid with a particle diameter of fewer than 2 micrometers and is currently not approved for use in the United States. by the US Food and Drug Administration.
Inert radiolabeled gases used for lung ventilation scans include 133Xe and 81mKr. 133Xe has a half-life of 5.3 days and a photopeak of 81 keV and is one of the most commonly used radioactive gas and is currently the only radiolabeled gas approved for scintigraphy use in the United States. The inhaled dose is 5 to 20 mCi (185 to 740 MBq).[5] The advantage of 133Xe is the ability to obtain single-breath, wash-in, and washout images, which makes it more sensitive to obstructive lung disease. Disadvantages include longer half-life compared to 81mKr and lower photopeak energy; hence ventilation scintigraphy is done prior to perfusion scintigraphy.
81mKr has a shorter half-life of 13 seconds and a photopeak of 190 keV, requiring continuous administration and allowing ventilation images obtained after perfusion images. Both image sets can be matched without repositioning the patient. Another disadvantage of 81mKr is the higher cost making 133Xe a more favorable option.[4]
Perfusion Scintigraphy (Q)
Perfusion scintigraphy involves a radiopharmaceutical agent 99mTc-macro aggregated albumin (99mTc-MMA) with a particle size of 10 to 100 micrometers which is injected intravenously. The usual administered dose is 40–150 MBq (1 to 4 mCi).[4] The patient lays in the supine position during the injection to allow maximum blood flow to lung apices. The radiopharmaceutical particles then embolize in the capillaries and provide a map of pulmonary blood flow.
Afterward, lung imaging is performed using either planar imaging with a high-resolution gamma camera, SPECT, or 3D imaging with SPECT/CT.[6]
Imaging
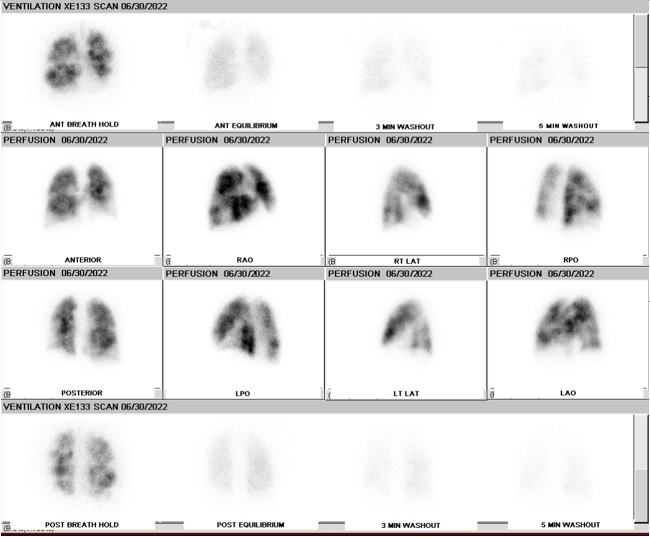
The various imaging techniques for lung imaging are planar imaging, SPECT, and SPECT/CT. Planar imaging uses a gamma camera, and images are obtained in anterior, posterior, lateral, and anterior and posterior oblique views. Planar imaging allows for two-dimensional imaging and is less sensitive compared to SPECT.[7]
SPECT imaging technique has been shown in multiple studies to have better sensitivity, specificity, and accuracy when compared to planar imaging [8]. An analysis of various pooled studies showed that the sensitivity of SPECT ranges from 80% to 100%, and its specificity ranges from 93% to 100%. [9] SPECT also has a lower indeterminate rate than planar imaging and a negative predictive value of 98%.[10][11]
SPECT/CT combines SPECT with low-dose CT, allowing for better anatomic information and visualization of an alternative etiology of patient symptoms.[12]
Indications
The commonest indication is for the diagnosis of pulmonary embolism. Other indications include pre-operative evaluation before lung surgery in lung carcinoma to estimate the loss of lung function, pre-operative evaluation and post-operative monitoring in lung transplant patients, measurement of cardiac shunts, and evaluation of new-onset pulmonary hypertension to detect chronic thromboembolic pulmonary hypertension or CTEPH.[13][4]
Normal and Critical Findings
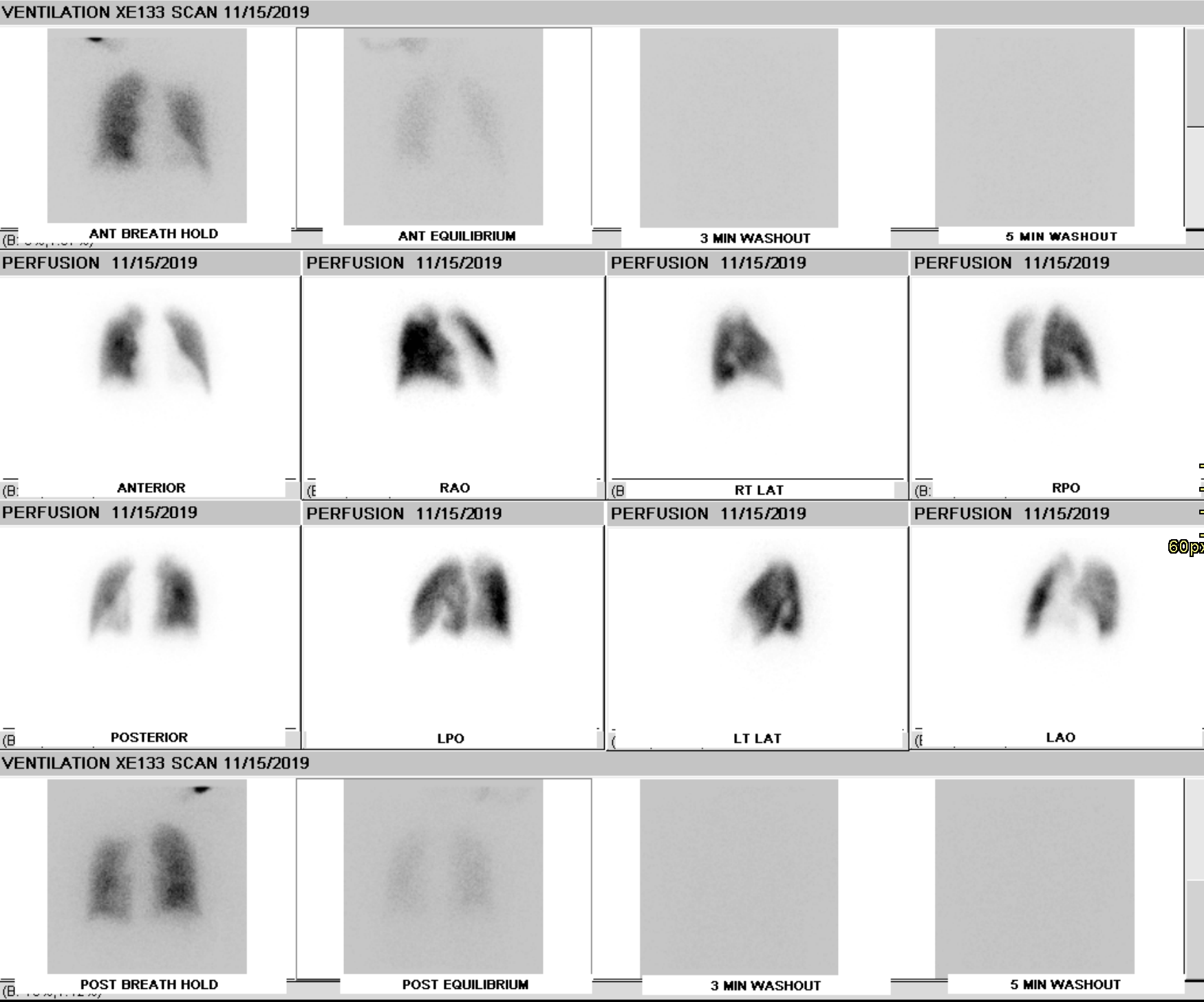
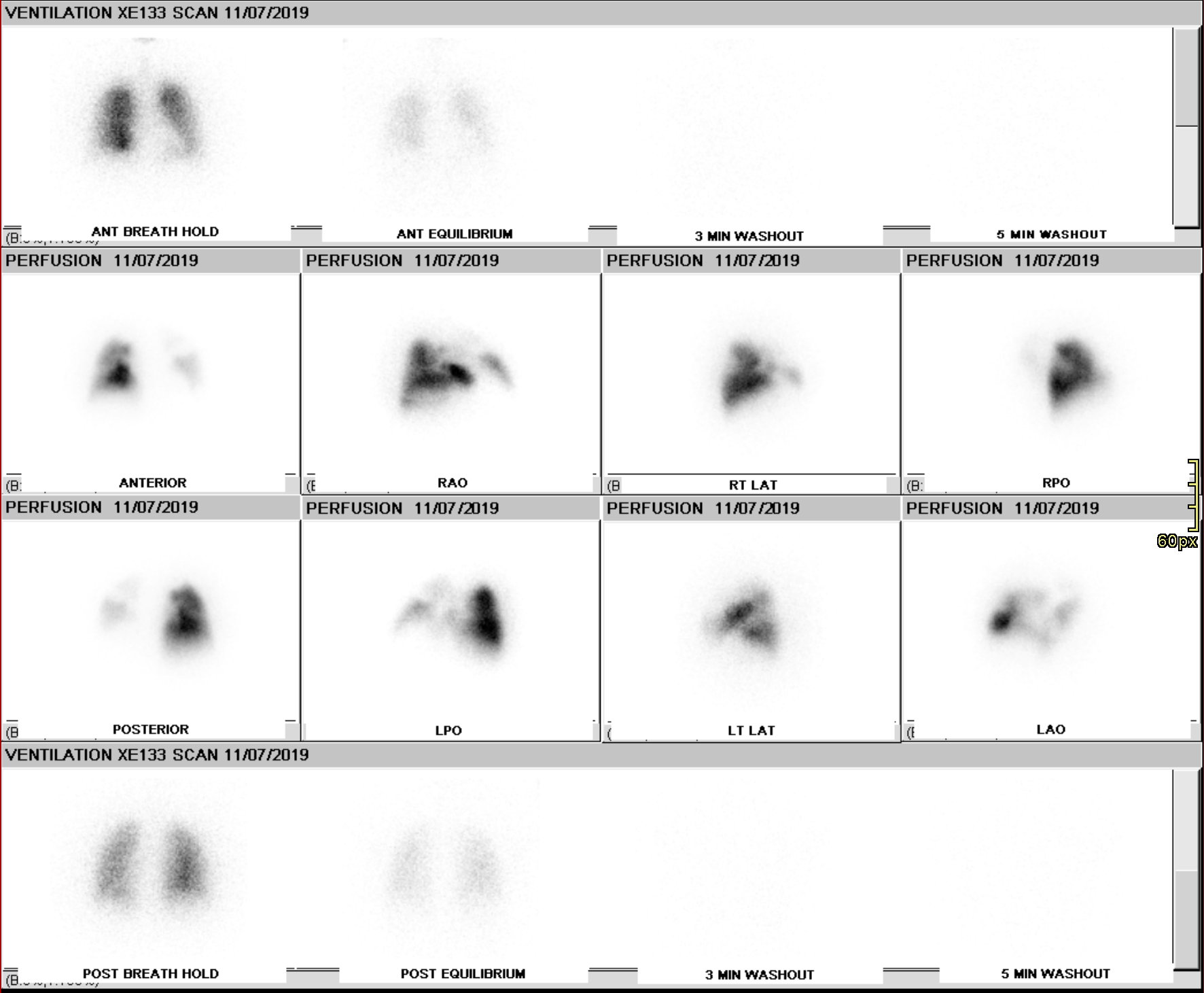
Ventilation imaging is used in conjunction with perfusion imaging to classify perfusion defects as matched (ventilation and perfusion imaging are concordant), mismatched (perfusion defect with a normal or relatively less abnormal ventilation defect), or reverse mismatched (ventilation defect with a normal or relatively less abnormal perfusion defect)
While there are multiple etiologies of ventilation-perfusion mismatch, the most common causes include acute and chronic pulmonary embolism, a tumor obstructing an artery, and radiation therapy.
The ventilation-perfusion reverse mismatch is more often attributed to emphysema, lung cysts, and lung infiltrates (pneumonia or cancer)
Diagnosis of Pulmonary Embolism
Multiple criteria are established for diagnosing PE, including but not limited to the PIOPED criteria, the PIOPED II criteria, and the modified PIOPED II criteria.[14][4] Perfusion-only modified PIOPED criteria and perfusion-only PISAPED criteria are used when a perfusion scan is performed solely without the ventilation scan.[15][16]
The modified PIOPED II and PISAPED criteria are more commonly used and will be described further.[2]
Based on modified PIOPED II criteria, the results are reported as high probability (PE present), very low probability, non-diagnostic, and normal, as summarized in the table below. Sostman et al. reported a sensitivity of 77.4% (95% CI: 69.7%, 85.0%) for a high probability (PE present) result. The specificity for the low probability or normal scan (PE absent) was 97.7% (95% CI: 96.4%, 98.9%). The percentage of a PE present or PE missing result was 73.5% (95% CI: 70.7%, 76.4%).[17]
| High probability | If two or more large mismatched segmental defects are present |
| Very low probability |
Non-segmental perfusion defect Perfusion defect smaller than X-ray chest lesion 1 to 3 small segmental defects One or less single-matched perfusion defect in the mid or upper lung Solitary large pleural effusion Stripe sign, i.e., peripheral perfusion in a perfusion defect |
| Non-diagnostic |
All other findings
|
| Normal | No perfusion defects |
Perfusion-only PISAPED criteria only utilize perfusion imaging and classify results as PE present, absent, or non-diagnostic. PISAPED criteria are classified based on the findings summarized in the table below.[4] Miniati et al. reported a sensitivity of 92% for scans reported as PE present. The specificity for PE absent results was 87%. A PE present scan had a positive predictive value of 99% with a very likely clinical presentation of PE and 92% with a possible clinical likelihood of PE. The authors concluded that a definitive diagnosis was made in 84% of the study population with abnormal scans.[16]
| PE present |
One or more large mismatched wedge-shaped perfusion defects
|
| PE absent |
Normal or near-normal perfusion Non-wedge-shaped perfusion defect Contour defect caused by the mediastinum, diaphragm, or an enlarged heart |
| Non-diagnostic | All other findings not classified as PE present or PE absent |
Pre and Post Lung Transplant
During a pre-transplant evaluation, the perfusion defects are used for a quantified perfusion analysis which detects the more dysfunctional lung.
During post-transplant monitoring, a V/Q scan can monitor complications such as diagnosing venous thromboembolism and assessing the functional impact of bronchial stenosis, which is a common complication. V/Q scan may also be used to assess air trapping that may indicate early bronchiolitis obliterans seen in rejection.
Preoperative planning for Lung Volume Reduction Surgery
Lung volume reduction surgery is performed in select patients with chronic obstructive pulmonary disease (COPD) to improve symptoms by resectioning damaged lung tissue.[18] V/Q scan can be used to evaluate patients who are candidates for surgery. Scintigraphy allows visualization of ventilation and perfusion of the lungs and can help identify the parts of the lung severely affected in heterogeneous emphysema. Newer modalities, such as quantitative CT and dual-energy CT, are now available and have the added benefit of providing structural information.[19]
Preoperative Planning for Lung Cancer
Lung scintigraphy is also used to assess the postoperative lung function reserve after partial or total pneumonectomy.[20] V/Q SPECT allows anatomic and functional information assessment and is used for radiation therapy planning for lung cancer to prevent irradiating normal lung tissues.[21] V/Q SPECT can also be helpful in the evaluation of lung injury after radiation therapy for lung cancer.[22]
Diagnosis of Chronic Thromboembolic Pulmonary Hypertension
Chronic thromboembolic pulmonary HTN (CTEPH) is a sequela of pulmonary venous thromboembolism with a reported incidence of up to 3.8% at a two-year follow-up.[23] VQ scan is a non-invasive tool for diagnosing CTEPH with a sensitivity of 96-97.4% and a specificity of 90 to 95%.[24] V/Q scan findings usually show multiple perfusion defects without matching ventilation defects.[25] SPECT imaging has increased sensitivity and specificity compared to planar imaging.[26]
Interfering Factors
Several factors may interfere with the scan and affect the examination findings. While injecting the tracer intravenously, blood drawn into the syringe may become coagulated and result in hot spots. This phenomenon was also reported after injection in an upper extremity affected by thrombophlebitis.[27] When Tc-MAA is injected through a central line or a pulmonary arterial line, it may result in inadequate mixing.[4]
If a chest X-ray shows significant abnormalities such as dense consolidation, it can result in a matching ventilation defect and perfusion defect along with an X-ray abnormality referred to as a "triple match." A triple match can result in indeterminate or non-diagnostic test results.[28]
Ventilation scintigraphy may be performed with the patient in an upright position while perfusion scintigraphy is performed with the patient in a supine position, and both are performed at different points in time which may affect the comparability of the two scans. A mismatch may also occur in decubitus or oblique positions by affecting the ventilation and perfusion distribution.[4]
Complications
A ventilation-perfusion scan is generally well tolerated. Complications or adverse effects are rare and include:
- Allergic reaction to the injected tracer
- Injection site reaction may consist of pain, erythema, and edema
Patient Safety and Education
Patients should be educated about do not need to prepare before the test. A chest X-ray will be necessary within 24 hours before the test. The test involves radiation exposure, and the total amount of exposure is relatively low. The patient will be instructed to inhale a radioactive gas in the first part and have a radioactive material injected into a vein in the second part.
Patients will need to lay still in the scanner where images are taken. The test takes from 30 minutes to about an hour to complete. The test is safely tolerated in most patients; some patients may develop redness and swelling at the injection site. Allergic reaction to the radioactive material is a rare complication that is treatable. Women should stop breastfeeding 1 to 2 days after the test and discard pumped breast milk 1 to 2 days after the test.
Clinical Significance
Using the PIOPED II criteria, the sensitivity and specificity for diagnosing PE are 85% and 93%, respectively, while using the PISAPED criteria, it is 80% and 97% and can be further improved to 97% and 91% with SPECT imaging. This is comparable to CT pulmonary angiography (CTPA), which has a sensitivity and specificity of 83% and 96%, respectively.[2] The VQ scan is currently preferred in diagnosing PE in patients who can not receive CTPA due to renal dysfunction or iodinated contrast allergy, in pregnant and very young patients, or an outpatient setting when the probability of PE is low.[2]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
QUINN JL 3rd, WHITLEY JE, HUDSPETH AS, PRICHARD RW. EARLY CLINICAL APPLICATIONS OF LUNG SCINTISCANNING. Radiology. 1964 Feb:82():315-7 [PubMed PMID: 14118400]
Moore AJE, Wachsmann J, Chamarthy MR, Panjikaran L, Tanabe Y, Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovascular diagnosis and therapy. 2018 Jun:8(3):225-243. doi: 10.21037/cdt.2017.12.01. Epub [PubMed PMID: 30057872]
Derenoncourt PR, Felder GJ, Royal HD, Bhalla S, Lang JA, Matesan MC, Itani M. Ventilation-Perfusion Scan: A Primer for Practicing Radiologists. Radiographics : a review publication of the Radiological Society of North America, Inc. 2021 Nov-Dec:41(7):2047-2070. doi: 10.1148/rg.2021210060. Epub 2021 Oct 22 [PubMed PMID: 34678101]
Parker JA, Coleman RE, Grady E, Royal HD, Siegel BA, Stabin MG, Sostman HD, Hilson AJ, Society of Nuclear Medicine. SNM practice guideline for lung scintigraphy 4.0. Journal of nuclear medicine technology. 2012 Mar:40(1):57-65. doi: 10.2967/jnmt.111.101386. Epub 2012 Jan 26 [PubMed PMID: 22282651]
Level 1 (high-level) evidenceMagnant J, Vecellio L, de Monte M, Grimbert D, Valat C, Boissinot E, Guilloteau D, Lemarié E, Diot P. Comparative analysis of different scintigraphic approaches to assess pulmonary ventilation. Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine. 2006 Summer:19(2):148-59 [PubMed PMID: 16796539]
Level 2 (mid-level) evidenceMetter D, Tulchinsky M, Freeman LM. Current Status of Ventilation-Perfusion Scintigraphy for Suspected Pulmonary Embolism. AJR. American journal of roentgenology. 2017 Mar:208(3):489-494. doi: 10.2214/AJR.16.17195. Epub 2017 Jan 17 [PubMed PMID: 28095020]
Meignan MA. Lung ventilation/perfusion SPECT: the right technique for hard times. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2002 May:43(5):648-51 [PubMed PMID: 11994529]
Level 3 (low-level) evidenceRoach PJ, Schembri GP, Bailey DL. V/Q scanning using SPECT and SPECT/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013 Sep:54(9):1588-96. doi: 10.2967/jnumed.113.124602. Epub 2013 Aug 1 [PubMed PMID: 23907760]
Stein PD, Freeman LM, Sostman HD, Goodman LR, Woodard PK, Naidich DP, Gottschalk A, Bailey DL, Matta F, Yaekoub AY, Hales CA, Hull RD, Leeper KV Jr, Tapson VF, Weg JG. SPECT in acute pulmonary embolism. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009 Dec:50(12):1999-2007. doi: 10.2967/jnumed.109.063958. Epub [PubMed PMID: 19949025]
Bajc M, Olsson B, Palmer J, Jonson B. Ventilation/Perfusion SPECT for diagnostics of pulmonary embolism in clinical practice. Journal of internal medicine. 2008 Oct:264(4):379-87. doi: 10.1111/j.1365-2796.2008.01980.x. Epub [PubMed PMID: 18823506]
Level 2 (mid-level) evidenceLeblanc M, Leveillée F, Turcotte E. Prospective evaluation of the negative predictive value of V/Q SPECT using 99mTc-Technegas. Nuclear medicine communications. 2007 Aug:28(8):667-72 [PubMed PMID: 17625390]
Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, Kjaergaard J, Kristoffersen US, Kjaer A. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009 Dec:50(12):1987-92. doi: 10.2967/jnumed.108.061606. Epub 2009 Nov 12 [PubMed PMID: 19910421]
Pinho DF, Banga A, Torres F, Mathews D. Ventilation perfusion pulmonary scintigraphy in the evaluation of pre-and post-lung transplant patients. Transplantation reviews (Orlando, Fla.). 2019 Apr:33(2):107-114. doi: 10.1016/j.trre.2018.10.003. Epub 2018 Oct 23 [PubMed PMID: 30415913]
PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990 May 23-30:263(20):2753-9 [PubMed PMID: 2332918]
Sostman HD, Miniati M, Gottschalk A, Matta F, Stein PD, Pistolesi M. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008 Nov:49(11):1741-8. doi: 10.2967/jnumed.108.052217. Epub 2008 Oct 16 [PubMed PMID: 18927339]
Miniati M, Pistolesi M, Marini C, Di Ricco G, Formichi B, Prediletto R, Allescia G, Tonelli L, Sostman HD, Giuntini C. Value of perfusion lung scan in the diagnosis of pulmonary embolism: results of the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISA-PED). American journal of respiratory and critical care medicine. 1996 Nov:154(5):1387-93 [PubMed PMID: 8912753]
Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology. 2008 Mar:246(3):941-6. doi: 10.1148/radiol.2463070270. Epub 2008 Jan 14 [PubMed PMID: 18195380]
Level 2 (mid-level) evidencevan Agteren JE, Carson KV, Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. The Cochrane database of systematic reviews. 2016 Oct 14:10(10):CD001001 [PubMed PMID: 27739074]
Level 1 (high-level) evidenceMartini K, Frauenfelder T. Emphysema and lung volume reduction: the role of radiology. Journal of thoracic disease. 2018 Aug:10(Suppl 23):S2719-S2731. doi: 10.21037/jtd.2018.05.117. Epub [PubMed PMID: 30210824]
Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT, American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007 Sep:132(3 Suppl):161S-77S [PubMed PMID: 17873167]
Level 1 (high-level) evidenceYuan ST, Frey KA, Gross MD, Hayman JA, Arenberg D, Curtis JL, Cai XW, Ramnath N, Kalemkerian GP, Ten Haken RK, Eisbruch A, Kong FM. Semiquantification and classification of local pulmonary function by V/Q single photon emission computed tomography in patients with non-small cell lung cancer: potential indication for radiotherapy planning. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011 Jan:6(1):71-8. doi: 10.1097/JTO.0b013e3181f77b40. Epub [PubMed PMID: 21119546]
Meng X, Frey K, Matuszak M, Paul S, Ten Haken R, Yu J, Kong FM. Changes in functional lung regions during the course of radiation therapy and their potential impact on lung dosimetry for non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2014 May 1:89(1):145-51. doi: 10.1016/j.ijrobp.2014.01.044. Epub [PubMed PMID: 24725697]
Level 3 (low-level) evidencePengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P, Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. The New England journal of medicine. 2004 May 27:350(22):2257-64 [PubMed PMID: 15163775]
Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, Al-Nahhas A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007 May:48(5):680-4 [PubMed PMID: 17475953]
Level 2 (mid-level) evidenceDong C, Zhou M, Liu D, Long X, Guo T, Kong X. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. PloS one. 2015:10(4):e0126985. doi: 10.1371/journal.pone.0126985. Epub 2015 Apr 29 [PubMed PMID: 25923810]
Level 1 (high-level) evidenceSoler X, Kerr KM, Marsh JJ, Renner JW, Hoh CK, Test VJ, Morris TA. Pilot study comparing SPECT perfusion scintigraphy with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Respirology (Carlton, Vic.). 2012 Jan:17(1):180-4. doi: 10.1111/j.1440-1843.2011.02061.x. Epub [PubMed PMID: 21899658]
Level 3 (low-level) evidenceLutzker LG, Perez LA. Radioactive embolization from upper-extremity thrombophlebitis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1975 Mar:16(3):241-2 [PubMed PMID: 1113175]
Waxman AD, Bajc M, Brown M, Fahey FH, Freeman LM, Haramati LB, Julien P, Le Gal G, Neilly B, Rabin J, Soudry G, Tapson V, Torbati S, Kauffman J, Ahuja S, Donohoe K. Appropriate Use Criteria for Ventilation-Perfusion Imaging in Pulmonary Embolism: Summary and Excerpts. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017 May:58(5):13N-15N [PubMed PMID: 28461589]