Introduction
The lungs are a pair of primary respiration organs located in the thoracic cavity on either side of the mediastinum. These organs are covered by a thin, double-layered serous membrane called the pleura.[1] The respiratory system consists of 2 components—the conducting and respiratory portions. The conducting portion transports air from the external environment to the site of respiration, whereas the respiratory portion facilitates gas exchange and blood oxygenation.
The conducting portion of the respiratory system includes the nose, nasopharynx, larynx, trachea, and a series of progressively narrowing bronchi and bronchioles, terminating at the terminal bronchiole.[2] The respiratory portion starts at the respiratory bronchiole, extends through the alveolar ducts and alveolar sacs, and culminates in the alveoli, where the primary gas exchange occurs. The branching pattern of these conducting passages looks like the branching of a tree and is hence called the tracheobronchial tree.[3]
The right lung has 3 lobes, whereas the left lung has 2 lobes. Each lobe is aerated by a secondary (lobar) bronchus. The lobes are further divided into smaller pyramidal-shaped sections called the bronchopulmonary segments. There are 10 bronchopulmonary segments in each lung, with their apex directed towards the hilum, and each segment is aerated by a tertiary (segmental) bronchus.[4]
The alveoli are the structural and functional units of the respiratory system. An adult human has approximately 300 million alveoli, providing a surface area of around 80 square meters for gas exchange.[5]
The lungs are an essential component of pulmonary circulation. Deoxygenated blood from the right ventricle is pumped through the pulmonary arteries to the alveolar-capillary beds of the lung for gaseous exchange. Oxygenated blood from the capillaries of the lungs is returned to the left atrium by the 4 pulmonary veins (see Image. The Lungs).[6]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
These are not concerns but some of the unique characteristics of the lung.
- The early prenatal development of the lungs depicts that of an exocrine tubuloalveolar gland. Initially, it begins an offshoot from the endoderm of the foregut as a lung bud. Later, it branches to form the tracheobronchial tree and canalize. This process describes how the glands develop. Therefore, the initial development of the lung is referred to as pseudo-glandular development.[7]
- The lung alveoli start developing in the prenatal stage and are very important for a live birth with the active secretion of surfactant from its type II pneumocytes.[7] Nevertheless, 95% of alveoli are formed postnatally during the first 8 years of life, the majority being in the first 3 years.
- The lymphoid tissue of the tracheobronchial system is modified to include diffuse, aggregated, and solitary lymphatic nodules specialized to be called bronchus-associated lymphoid tissue.[8][9] This tissue plays a crucial role in hypersensitivity reactions associated with respiration and serves as a key defense mechanism, preventing inhaled microorganisms from reaching the lungs.
Structure
The conduction portion of the lung begins at the trachea and extends to the terminal bronchioles. Outside the lungs, the conduction system consists of the nasal cavities, nasopharynx, larynx, and trachea. Within the lungs, the conducting portion divides into paired main bronchi. The bronchi begin as a branching pattern, splitting next into lobar (secondary) bronchial branches and then again into segmental (tertiary) bronchi. The tertiary bronchi continue to divide into small bronchioles, where the first change in histology takes place, as cartilage is no longer present in the bronchioles. The conducting portion ends at the terminal bronchioles, which open into the respiratory bronchioles, signifying the beginning of the respiratory portion of the lung.[10]
The conducting portion serves as the pathway for the movement and conditioning of air entering the lungs. Specialized cells collaborate to warm, moisturize, and remove particles that enter the airway. These cells are the respiratory epithelium and comprise the entire respiratory tree. The majority of the respiratory epithelium is composed of ciliated pseudostratified columnar epithelium. This region contains 5 distinct cell types:
- Ciliated cells
- Goblet cells
- Basal cells
- Brush cells
- Neuroendocrine cells [11]
Ciliated cells are the most abundant type of cell in the lung. These cells regulate the actions of the mucociliary escalator,[12] a primary defense mechanism of the lungs that removes debris from the respiratory system. Although the mucus provided by goblet cells traps inhaled particles, the cilia beat to move the material toward the pharynx to swallow or cough out.
Goblet cells, named for their goblet-shaped appearance, are filled with mucin granules at their apical surface, with the nucleus remaining towards the basilar layer. These cells decrease in number as the respiratory tree becomes progressively smaller and are eventually replaced by club cells, formerly known as Clara cells, when they reach the respiratory bronchioles.[13]
Basal cells connect to the basement membrane, forming the attachment layer for ciliated and goblet cells. These cells can be considered the stem cells of the respiratory epithelium, as they retain the ability to generate ciliated and goblet cells.
Brush cells, occasionally called type III pneumocyte cells, are sparsely distributed in all areas of respiratory mucosa.[11] These cells may be columnar- or flask-shaped and are identified by their short microvilli-covered apical layer, resembling a push broom or, appropriately, a brush. The function of brush cells remains uncertain, though several mechanisms have been proposed. A popular proposal suggests they have a chemoreceptor function, monitoring air quality, due to their association with unmyelinated nerve endings.[13]
The bronchial mucosa also contains a small cluster of neuroendocrine cells, also known as small granule cells or Kulchitsky cells.[14] These cells have neurosecretory-type granules and can secrete several factors, including catecholamine and polypeptide hormones, such as serotonin, calcitonin, and gastrin-releasing factors (bombesin). Like brush cells, these neuroendocrine cells make up only a small portion of mucosal epithelium, around 3%.[11]
In the bronchial submucosa, there are submucosal glands that consist of a mixture of serous and mucinous cells, similar to those found in salivary gland tissue. The secretions are emptied into ducts and then into the bronchial mucosa. Older individuals may show oncocytic metaplasia of these glands. Smooth muscle bundles are present at all levels of the airway to allow for the regulation of airflow. There are progressively fewer smooth muscle fibers progressing from the bronchi to the alveoli.
Function
Respiratory Functions of Lungs
- Respiration: The lungs are the primary organs of respiration, where the exchange of gases occurs. In the alveoli—the functional units of the lungs—oxygen is absorbed into the bloodstream. In contrast, carbon dioxide is expelled through the alveolar-capillary bed (see Image. Gaseous Exchange in the Lung).[15]
- Air conditioning: The conducting portion not only directs airflow but also functions as an efficient air conditioner. This process involves warming or cooling the inhaled air to match body temperature, humidifying it, and filtering out any foreign particles. The removal of foreign particles such as dust, bacteria, and viruses is achieved through mucous secretion, which traps the suspended particles, and the beating of the cilia, which clears the mucus from the respiratory passage.[10]
Non-Respiratory Functions of Lungs
Although the primary function of the lungs is respiration, studies indicate that they also perform several non-respiratory functions. Some of the important ones are outlined below.
- The lungs facilitate the conversion of an inactive chemical precursor into its active form, such as the conversion of angiotensin-I into angiotensin-II, which helps regulate blood pressure.[16]
- The lungs are also an essential site for degrading or inactivating important vasoactive chemical mediators such as bradykinin, serotonin, and norepinephrine.
- The bronchial mucosa also contains a small cluster of neuroendocrine cells,[16] also known as small granules or Kulchitsky cells, which can secrete several factors, including catecholamines and polypeptide hormones, such as calcitonin, serotonin, and gastrin-releasing peptide (bombesin)
- The pulmonary epithelium serves as the first line of defense for inhaled air.[17]
Tissue Preparation
Proper ethical approval is obtained before collecting the lung tissue. The lung is identified, dissected en-block, weighed, and labeled. Following this, it is perfused with 10% formalin through the trachea to the physiological peak inspiration level. Underinflation or overinflation should be prevented during this process. This approach helps in accurate assessment without any artifacts and misinterpretation of tissue structure. All lobes of the lungs are examined for any lesions. The tissue is washed well and fixed in formalin for almost 24 hours. Following tissue processing, the lung is prepared for embedding. The lung should be placed in the tissue cassette with the ventral side facing the tissue cassette. The dorsal side of the lung should be facing the open/upper side. This position guarantees the appropriate tissue section level. Post-fixation tissue trimming is crucial for obtaining high-quality sections. If the identified lesions are large or small, they can be isolated and embedded separately. Including the sections of associated lymph nodes is essential for histological evaluation, as this helps assess the extent of metastasis in lung tumors.[18]
Histochemistry and Cytochemistry
Histochemistry of the Lung
Histochemical analyses of lung tissues can be conducted using lectin histochemistry or immunohistochemistry. Both techniques can be applied to tissue slides. Normal lung cells can be classified into type I and type II pneumocytes based on histochemical analyses using lectin typing (glycotyping).[17] Alveolar macrophages in the normal lung are positive for several N-linked saccharides, including N-acetylgalactosamine, N-acetylglucosamine, terminal β-D-galactose, and sialyl groups. Some specific lectins can be used as a marker for cell types, such as Dolichos biflorus agglutinin for bronchial epithelial cells, Triticum Vulgaris (succinylated) for type I pneumocytes, and Hippeastrum hybrid or Maclura pomifera lectins for type II pneumocytes. Alveolar macrophages test positive for anti-CD68, and the alveolar lining shows positivity for cytokeratin.
Immunohistochemistry and lectin histochemistry can provide substantial insights into obstructive and restrictive pathologies and lung cancer diagnosis. Adenocarcinomas can be identified using differentiation markers such as TTF-1 and NapsinA. Both these markers are expressed in more than 85% of the cases.[19] Metastasis can be confirmed by immunohistochemistry staining to identify the primary tumor tissue of origin. Distinct expression of CK7 and CK20 profiles, in addition to the absence of markers commonly expressed in primary lung cancer, can signify metastatic cancer. With the advent of cancer treatment using tyrosine kinase inhibitors, actionable gene mutations can be screened in patients with lung cancer.[20] EGFR mutation and ALK translocation are the most effectively targeted oncogenes in non–small cell carcinomas and are now considered standard treatment procedures.[20]
Cytochemistry of the Lung
Cell analyses of the lung tissue can be performed using electron microscopy and have recently included flow cytometric assays. Assessment of nonspecific esterases, including alpha-naphthyl acetate esterase and butyrate esterase, chloroacetate esterase, acid phosphatase, intracellular glycogen (periodic acid–Schiff reaction), lipids (Sudan black B reaction), and iron (Perl's reaction), can be performed using a semiquantitative cytochemical method to identify lung diseases.[21] Extracellular matrix remodeling is fundamental to pathological changes in obstructive pulmonary diseases, and collagen alterations provide valuable insights into disease progression. The second-harmonic generation technique used to quantify collagen has shown effective analysis of lung diseases, revealing a biochemically distinct presentation of collagen organization in asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis.[22] Alveolar macrophage modulations using chloroacetate esterase and Perls' reaction (for estimation of intracellular iron) can confirm the presence of non–small cell lung cancer. The typical increase in chloroacetate esterase and iron in alveolar macrophages indicates non–small cell lung cancer.[10] Similarly, Galectin-3 can be used as a biomarker in human pulmonary fibrosis, as its levels are found to be elevated in this condition.[23]
Microscopy, Light
The conducting portion of the respiratory system extends up to the terminal bronchiole. Beyond this point, the respiratory bronchiole marks the beginning of the respiratory portion of the system. The respiratory portion of the lung includes the respiratory bronchiole, alveolar duct, alveolar sac, and, finally, alveoli, where actual respiration takes place.
In the conducting zone, the air is moistened, warmed, and filtered before it reaches the start of the respiratory region at the respiratory bronchioles. In the respiratory zone, gas exchange occurs, and blood is oxygenated for carbon dioxide. As the respiratory tree transitions from the conducting zone at the terminal bronchioles, goblet cells diminish as club cells increase, and the cartilage present in the conducting region is absent once it reaches the respiratory bronchioles.
The acinus is directly distal to the terminal bronchioles, which signals the beginning of the respiratory part. It consists of respiratory bronchioles, alveolar ducts, and alveolar sacs, forming a roughly spherical structure that resembles a cluster of grapes. Each respiratory bronchiole gives rise to several alveolar ducts and alveolar sacs, creating the distinctive appearance of a bunch of grapes. The alveolar sacs mark the termination of the respiratory tree and serve as the primary site of gaseous exchange.[24]
The alveolar epithelium is composed of type I pneumocytes, type II pneumocytes, and occasional brush cells. In addition, club cells and alveolar macrophages are present in the alveolar walls. The alveolar walls contain the pores of Kohn,[25][26] which allow communication between adjacent alveoli. These pores enable airflow between alveoli, which may be beneficial if any blockage prevents air from entering the alveoli through a direct route.
Type I pneumocytes comprise approximately 90% to 95% of the alveolar epithelium. These cells are flat, squamous epithelia that resemble plate-like structures that allow gas exchange. The fragile membrane of these cells allows for easier gas permeability between the alveoli and the blood vessels. Despite being the primary cells responsible for respiration, they cannot replicate and are highly susceptible to toxic injury.[27]
Type II pneumocytes constitute the remaining cell type in the alveoli, accounting for nearly 5% to 10%. Despite their low number, they are vital as they secrete pulmonary surfactant. The surfactant is necessary to maintain an open airway. It lowers the surface tension and prevents the alveoli from collapsing upon themselves during exhalation. Histologically, these cells have foamy cytoplasm, resulting from the surfactant stored as lamellar bodies. Type II pneumocytes are also mitotically active and can replace the easily damaged type I pneumocytes. Type II pneumocyte cells can be recognized by their rounded shapes that bulge into the alveolar space.[27]
Alveolar macrophages, also known as dust cells, may be free within the alveolar space or sometimes connected to the alveolar wall.[28] If particles reach the acinus, macrophages serve as the last line of defense and janitors of the respiratory epithelium. The black staining observed in the lungs of smokers results from macrophages cleaning and sequestering inhaled particles.
The lungs are covered by the pleural membrane, a serous membrane consisting of 2 layers—the parietal and the visceral layer. The visceral pleura of the lung is lined by a mesothelial layer with underlying connective tissue and elastic fibers. An elastin stain may be used to identify the elastic layer.
Microscopy, Electron
This section explores a more detailed study of a few essential structures of the lung, which can be better appreciated under an electron microscope.
Respiratory Bronchiole
The respiratory bronchiole is the transition region between the conducting and respiratory portions, where the exchange of gases begins. Structurally, it closely resembles the terminal bronchiole, except that its walls are interrupted by numerous sac-like alveoli for gas exchange. The respiratory bronchiole is lined by ciliated cuboidal epithelium. The cilia may be absent in more distal portions. A small number of non-ciliated club cells, formerly known as Clara cells, are also present. These club cells become dominant cells in the distal part.[24] The epithelium is devoid of goblet cells. The underlying tissue consists of smooth muscle and elastic fibers. Distally, the respiratory bronchioles divide and become narrower, and the number of alveoli increases.
Club cells have 3 functions, as follows:
- Produce a component of surfactant [29]
- Act as stem cells
- Contain enzyme systems that detoxify noxious substances
Alveolar Ducts
Respiratory bronchioles divide distally to form alveolar ducts. Alveolar ducts do not have walls of their own but are created by several openings of alveoli. These ducts terminate into clusters of alveoli known as the alveolar sac, which opens into the atrium and finally into the alveoli. Alveolar ducts are surrounded by small aggregations of smooth muscle cells, collagen, and elastic fibers. Sphincter-like smooth muscles (knob) embedded in type III collagen around respiratory bronchioles and alveolar ducts regulate air movements within alveoli.[30] Smooth muscle totally disappears at the distal end of alveolar ducts. A rich matrix of elastic and reticular fibers provides the only support for duct and alveoli
Alveoli
The alveoli are the specialized air sacs (outpouching) of 200 µm diameter. These sacs are the structural and functional units of the respiratory system and are the primary sites of oxygen and carbon dioxide exchange. There are 300 million alveoli in the lungs, providing approximately 140 m surface area for gas exchange. The alveoli are responsible for the spongy nature of the lungs. These alveoli are lined by flattened epithelial cells called pneumocytes with a single opening. The alveolar wall or septum comprises 3 tissue components—surface epithelium, supporting tissue, and an extensive network of continuous capillaries. Centrally, it has capillaries surrounded by a vibrant network of elastin, reticular, and collagen fibers with a squamous epithelial layer of 2 adjacent alveoli on either side. In certain areas, the basement membrane of the capillary endothelium comes in direct contact with the basement membrane of the surface epithelium of alveoli, with the absence of supporting tissue, thus reducing thickness (0.1-1.5 µm) for better exchange of gases. Air in the alveoli is separated from the blood in the capillary by 3 components—surface lining and cytoplasm of alveolar cells, the fused basal laminae of closely apposed alveolar, and endothelial cells and their cytoplasm. This structure forms the blood-gas barrier (air-blood barrier).[31] The capillaries are continuous, with endothelial cells that are extremely thin due to the clustering of nuclei and organelles, enhancing the efficiency of gas exchange. Occasionally, small openings called the alveolar pores (of Kohn) are present, measuring 10 to 15 µm in diameter. These pores help equalize air pressure within alveoli and allow air movement between alveoli in case of bronchiole obstruction. Elastic fibers enable alveoli to expand during inspiration and contract passively during expiration. The reticular fibers are supportive structures that prevent over-distention and damage to delicate capillaries and alveolar septa. The continuous epithelial lining of each alveolus is composed of 2 cell types—type I pneumocytes (alveolar lining cells) and type II pneumocytes.
Type I Pneumocytes (Alveolar Lining Cells)
Type I pneumocytes are simple squamous cells that are highly attenuated with a dense, small, and flattened nucleus. These cells cover most of the surface area, approximating around 95% to 97% of the total surface area. The Golgi complex, endoplasmic reticulum, and mitochondria are grouped around the nucleus, leaving a large area of cytoplasm free of organelles, thus reducing it to a fragile blood-air diffusion barrier (25 nm). The thin cytoplasm shows numerous pinocytotic vesicles. The surfactant lines the luminal surface. A basal lamina covers the adluminal side of these cells. The adjacent cells are connected by tight (occluding) junctions, preventing tissue fluid leakage into the alveolar lumen. During fetal development, the surfactant appears in the last few weeks of gestation and coincides with the appearance of lamellar bodies in the type II cells.
Type II Pneumocytes (Great Alveolar or Septal Cells)
Type II pneumocytes are cuboidal cells grouped in clusters of two to three with large, central, and plump nuclei containing dispersed chromatin and prominent nucleoli. These cells occupy about 3% to 5% of the surface area of alveoli interspersed among type I cells, with which they form occluding and desmosomal junctions. The apical surface is dome-shaped and shows numerous small microvilli associated with surfactant secretion. These cells secrete surfactant, a surface-active material that reduces surface tension, thus preventing alveolar collapse during expiration. The mitotic activity of the lining cells is 1% per day and can differentiate into type II and type I pneumocytes in response to damage to the alveolar lining epithelium. The cytoplasm has abundant rough endoplasmic reticulum, well-developed Golgi apparatus, free ribosomes, and a moderate amount of elongated mitochondria. A typical feature of these cells is the presence of lamellar bodies. These lamellar bodies are vesicles that contain phospholipids (dipalmitoyl phosphatidylcholine) and measure 1 to 2 µm in size. When discharged by exocytosis into the alveoli, these substances spread across the alveolar surface and mix with other secretory products that contain carbohydrates and proteins, some of which are secreted by Clara cells. This combination forms surfactant, a tubular lattice of lipoprotein known as tubular myelin, which helps to counteract the effects of surface tension. The reduction in surface tension leads to decreased breathing work. The surfactant is not static but is continually being turned over and removed. The removal occurs through pinocytotic vesicles of type II pneumocytes, macrophages, and type I pneumocytes.[32]
Alveolar Macrophage (Dust cells)
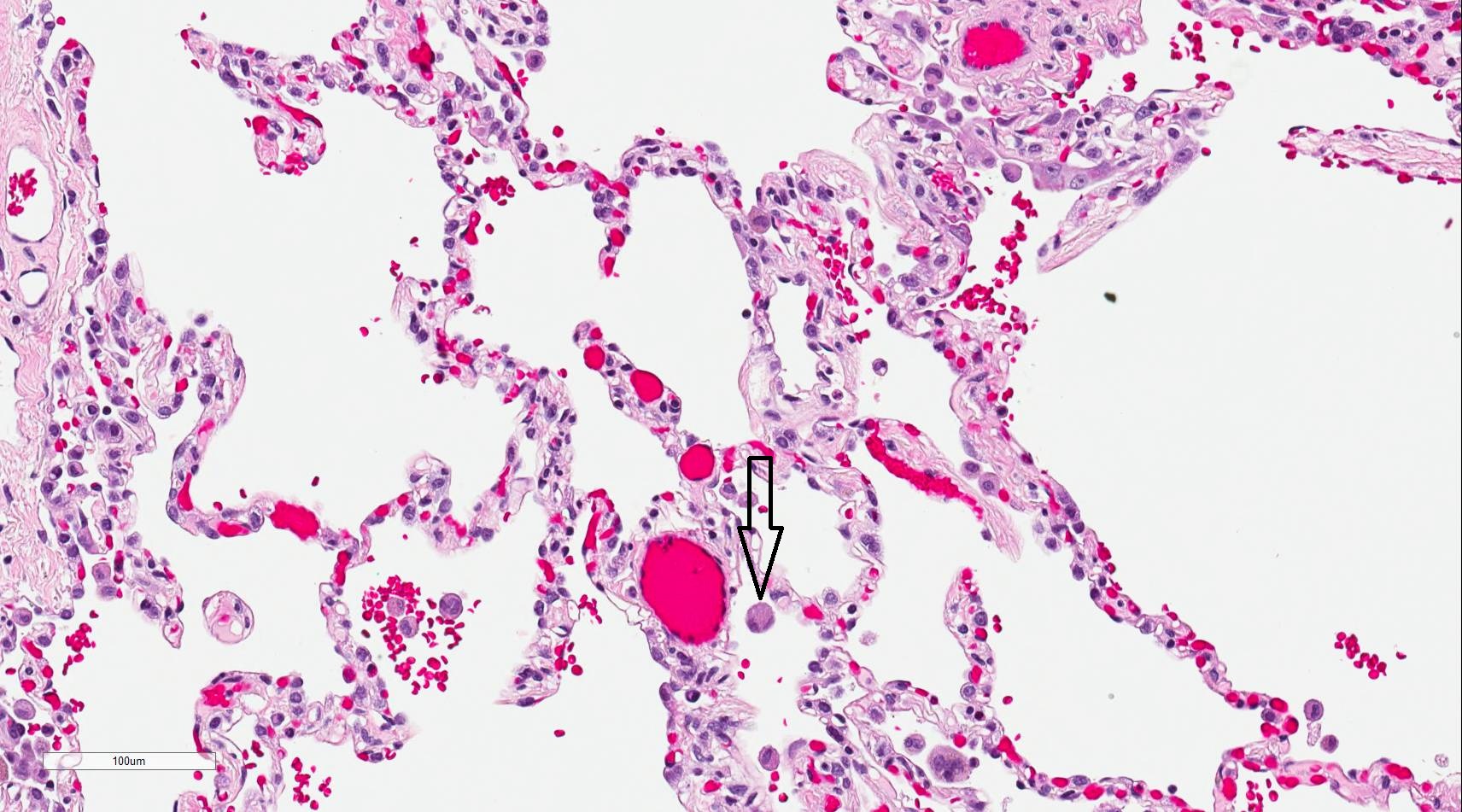
Alveolar macrophages are derived from blood monocytes and, occasionally, through the mitotic division of lung macrophages. These macrophages contain numerous secondary lysosomes and lipid droplets. Alveolar macrophages function as phagocytes and remove unwanted materials, such as inhaled particulate matter (carbon), dust, and bacteria. These macrophages are found freely within alveolar spaces and the inter-alveolar septa (spaces). Approximately 100 million macrophages migrate daily to the bronchi. The phagocytosed macrophages get trapped in mucus, are transported by ciliary action to the pharynx, and are expelled in sputum. Some alveolar macrophages also travel through lymphatic pathways to the hilar lymph nodes. Industrial lung diseases such as silicosis result from the inhalation of silica into air sacs (as tiny particles) that are phagocytosed by macrophages. Silicated macrophages stay for a long time and convert silica into silicic acid, which stimulates the proliferation of fibroblasts and collagen, leading to fibrosis of the lung and node. When inhaled extensively, a particular form of silica, such as asbestos, stimulates lung fibrosis, producing asbestosis and, occasionally, malignancy of pleura (mesothelioma). Occasionally, macrophages may phagocytose extravasated red blood cells in alveoli, especially in conditions such as pulmonary congestion and congestive heart failure. These erythrocyte phagocytosed macrophages are called heart failure cells (see Image. Alveolar Macrophage).[33]
Pathophysiology
The lung pathologies are varied, showing significant differences in their presentation due to the organ's exposure to the external environment. Lung pathologies can be broadly classified into obstructive and restrictive lung diseases.
Lung pathologies include bronchial diseases, infectious diseases, interstitial lung diseases, neoplasms, vascular diseases, and congenital abnormalities. The destruction of lung parenchymal tissue presents chronic obstructive pulmonary disease and emphysema due to chronic inflammation.[34] Morphologically, they lead to an increase in size and number of small fenestrae in alveolar walls, the breakdown of fibrovascular trabeculae, and the remodeling of acini, leading to airspace enlargement.[35]
In certain obstructive diseases, such as bronchitis, hyperplasia of goblet cells occurs, whereas in bronchiectasis, the bronchi are markedly dilated. Restrictive disorders, on the other hand, are marked by fibrous deposits that restrict lung function. Interstitial restrictive lung diseases are characterized by inflammation or scarring of the lung tissue or filling of the air spaces with exudate and debris. Extrapulmonary restrictive diseases show a thickening of alveolar septa and epithelium and are associated with an endothelial injury.
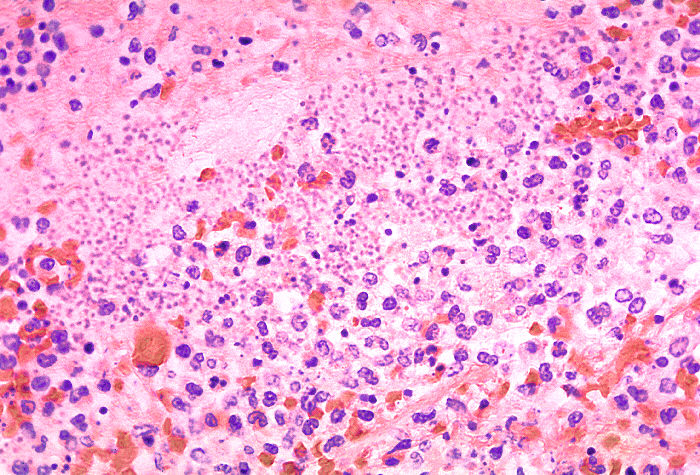
In the acute phase, restrictive lung disease demonstrates endothelial damage. In some disorders, they also show epithelial damage with fibrosis of the exudate and expansion of the interstitium through generalized fibrosis. Chronic restrictive lung diseases are marked by diffuse interstitial changes that are more prominent than morphological changes. In advanced stages, many chronic restrictive lung diseases are characterized by interstitial fibrosis, leading to a classic honeycomb-like appearance of the lung (see Image. Histological Section of Pneumonia-Affected Lung).[36]
Clinical Significance
Infant Respiratory Distress
Infant respiratory distress is the leading cause of death in premature babies.[37] Type II pneumocytes produce surfactant starting around 20 weeks of gestation, but its full secretion does not occur until nearly 30 weeks of pregnancy. Without ample surfactant, premature infants cannot overcome the collapsing surface tension in the respiratory alveoli. When a patient goes into premature labor, Clinicians administer glucocorticoids to help prevent infant respiratory distress.[38] Glucocorticoids stimulate surfactant production in the fetus and may increase its production enough to help the infant overcome any potential respiratory distress. Testing for fetal lung maturity may be performed on the amniotic fluid by several methods, including the measurement of phospholipids in amniotic fluid (phosphatidylglycerol or lecithin-sphingomyelin ratio) and lamellar body counts.
Emphysema
The primary cause of emphysema is smoking, though repeated inhalation of foreign particulate material can also contribute to its development. Emphysema, or chronic obstructive pulmonary disease, is characterized by restricted airflow and difficulty exhaling due to narrowed bronchioles and the destruction of the alveolar walls. The collapse of alveoli leads to a significant loss of surface area for gas exchange.
Healthcare professionals assume that the destruction of the alveolar wall results from excessive lysis of elastin in the interalveolar septum. The abundance of macrophages and neutrophils that migrate to the acinus due to an increase in particulate brings an equal rise of elastase and other proteases. Alpha-1 antitrypsin deficiency also leads to emphysema due to an increase in elastin. However, in this condition, the deficiency of antitrypsin typically inhibits elastin.[39]
Cystic Fibrosis
Cystic fibrosis may also cause chronic obstructive pulmonary disease. This condition is an autosomal recessive disease caused by a mutation to the CFTR gene on chromosome 7. This gene controls the Cl-channel protein involved in various cells, including goblet cells in the lungs. The defective Cl-channel affects the viscosity of the mucus in the lungs. Thickening is due to increased absorption of sodium (Na) and water from the lumen. The thickened mucus disrupts the mucociliary escalator filtration function of the lungs, resulting in obstruction. One of the supportive treatments for cystic fibrosis breaks the disulfide bonds found in mucous plugs, thinning out the sputum so it can be pushed out by the respiratory cilia.[40]
Heart Failure Cells
In heart failure, the heart's inability to pump blood efficiently results in congestion of the lungs. Increased blood pressure in the pulmonary vasculature results in erythrocytes passing into the alveolar septum. The erythrocytes are promptly engulfed by resident alveolar macrophages. As macrophages engulf red blood cells, they accumulate hemosiderin, acquiring a brown granular appearance that is visible under light microscopy with staining.[41] Hemosiderin-laden macrophages, also called siderophages, are not specific to a particular disease but may be present whenever blood cells enter the alveolus.[42]
Tumor Staging
When staging primary lung carcinoma, identifying invasion of the elastic layer of the visceral pleura is crucial. This finding increases the T stage of the tumor. As the elastic layer is difficult to visualize with routine stains, a special elastin stain may be used to demonstrate this finding.[43][44]
Media
(Click Image to Enlarge)
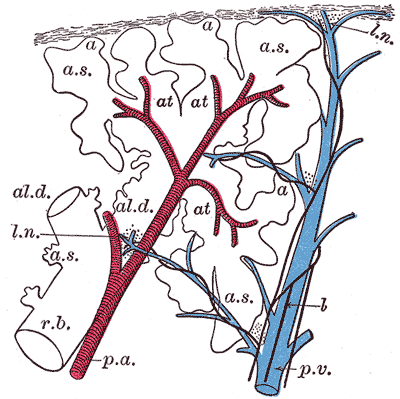
The Lungs. This image shows a schematic longitudinal section of a primary lobule of the lung.
r. b., respiratory bronchiole; al. d., alveolar duct; at., atria; a. s., alveolar sac; a, alveolus or air cell; p. a.: pulmonary artery: p. v., pulmonary vein; l., lymphatic; l. n., lymph node.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
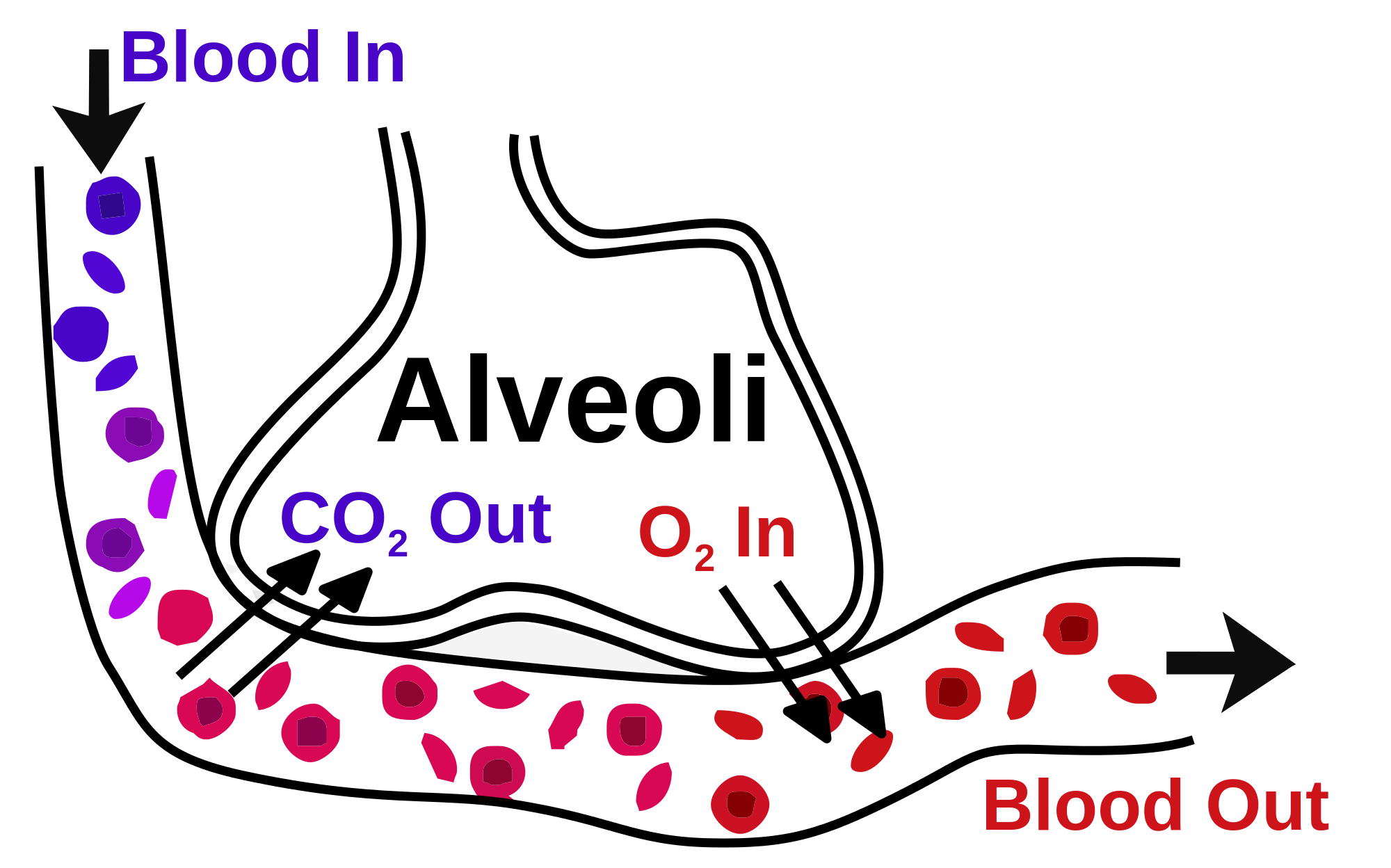
Gaseous Exchange in the Lung. The image illustrates gas exchange in the lungs, showing the movement of oxygen (O₂) into the bloodstream and carbon dioxide (CO₂) expelled through the alveolar-capillary bed.
Helix84, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Pandey K, Faridi P, Ayala R, Lee YCG, Rouse E, Krishna SSG, Dick I, Redwood A, Robinson B, Creaney J, Purcell AW. Multiple Classes of Antigen Contribute to the Antigenic Landscape of Mesothelioma. Molecular & cellular proteomics : MCP. 2025 Mar:24(3):100925. doi: 10.1016/j.mcpro.2025.100925. Epub 2025 Feb 5 [PubMed PMID: 39921204]
Warren R, Klinkhammer K, Lyu H, Knopp J, Yuan T, Yao C, Stripp B, De Langhe SP. Cell competition drives bronchiolization and pulmonary fibrosis. Nature communications. 2024 Dec 5:15(1):10624. doi: 10.1038/s41467-024-54997-2. Epub 2024 Dec 5 [PubMed PMID: 39639058]
Patwa A, Shah A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian journal of anaesthesia. 2015 Sep:59(9):533-41. doi: 10.4103/0019-5049.165849. Epub [PubMed PMID: 26556911]
Chaudhry R, Omole AE, Bordoni B. Anatomy, Thorax, Lungs. StatPearls. 2025 Jan:(): [PubMed PMID: 29262068]
Haddad M, Sharma S. Physiology, Lung. StatPearls. 2025 Jan:(): [PubMed PMID: 31424761]
Sang C, Liu Q, Lai Y, Xia S, Jiang R, Li S, Guo Q, Li Q, Gao M, Guo X, Huang L, Liu N, Jiang C, Zuo S, Liu X, Li M, Ge W, Song S, Chen L, Xie S, Zou J, Chen K, Liu X, Hu H, Wang X, Zhang J, Wang Z, Wang C, He L, Jiang C, Tang R, Zhou N, Wang Y, Long D, Du X, Jiang C, Macle L, Dong J, Ma C, PROMPT-AF investigators. Pulmonary Vein Isolation With Optimized Linear Ablation vs Pulmonary Vein Isolation Alone for Persistent AF: The PROMPT-AF Randomized Clinical Trial. JAMA. 2025 Feb 4:333(5):381-389. doi: 10.1001/jama.2024.24438. Epub [PubMed PMID: 39556379]
Level 1 (high-level) evidenceSun J, Zhao N, Zhang R, Li Y, Yu T, Nong Q, Lin L, Yang X, Luan T, Chen B, Huang Y. Metabolic landscape of human alveolar type II epithelial cells undergoing epithelial-mesenchymal transition induced directly by silica exposure. Journal of environmental sciences (China). 2025 Mar:149():676-687. doi: 10.1016/j.jes.2024.02.020. Epub 2024 Mar 18 [PubMed PMID: 39181677]
Marin ND, Dunlap MD, Kaushal D, Khader SA. Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases. Journal of immunology (Baltimore, Md. : 1950). 2019 May 1:202(9):2519-2526. doi: 10.4049/jimmunol.1801135. Epub [PubMed PMID: 31010841]
Sanchez-Guzman D, Le Guen P, Villeret B, Sola N, Le Borgne R, Guyard A, Kemmel A, Crestani B, Sallenave JM, Garcia-Verdugo I. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials. 2019 Oct:217():119308. doi: 10.1016/j.biomaterials.2019.119308. Epub 2019 Jun 26 [PubMed PMID: 31279103]
Murray JF. The structure and function of the lung. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010 Apr:14(4):391-6 [PubMed PMID: 20202294]
Chen J, Lai X, Song Y, Su X. Neuroimmune recognition and regulation in the respiratory system. European respiratory review : an official journal of the European Respiratory Society. 2024 Apr:33(172):. doi: 10.1183/16000617.0008-2024. Epub 2024 Jun 26 [PubMed PMID: 38925790]
Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue barriers. 2013 Oct 1:1(4):e24997. doi: 10.4161/tisb.24997. Epub 2013 May 30 [PubMed PMID: 24665407]
Brody AR. The brush cell. American journal of respiratory and critical care medicine. 2005 Nov 15:172(10):1349 [PubMed PMID: 16275741]
Level 3 (low-level) evidenceDrozdov I, Modlin IM, Kidd M, Goloubinov VV. From Leningrad to London: the saga of Kulchitsky and the legacy of the enterochromaffin cell. Neuroendocrinology. 2009:89(1):1-12. doi: 10.1159/000140663. Epub 2008 Jun 19 [PubMed PMID: 18562785]
Emslander HP, Heinl KW. [The respiratory tract and lung. Anatomy, physiology, pathophysiology]. Medizinische Monatsschrift fur Pharmazeuten. 1989 Nov:12(11):330-7 [PubMed PMID: 2479814]
Jin L, Wang Z, Qi X. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: Case series and a review of the literature. Medicine. 2018 Dec:97(52):e13806. doi: 10.1097/MD.0000000000013806. Epub [PubMed PMID: 30593169]
Level 2 (mid-level) evidenceBarkhordari A, Stoddart RW, McClure SF, McClure J. Lectin histochemistry of normal human lung. Journal of molecular histology. 2004 Feb:35(2):147-56 [PubMed PMID: 15328919]
Morton J, Snider TA. Guidelines for collection and processing of lungs from aged mice for histological studies. Pathobiology of aging & age related diseases. 2017:7(1):1313676. doi: 10.1080/20010001.2017.1313676. Epub 2017 Apr 21 [PubMed PMID: 28515862]
Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 Oct:24(10):1348-59. doi: 10.1038/modpathol.2011.92. Epub 2011 May 27 [PubMed PMID: 21623384]
Level 1 (high-level) evidenceLindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013 Jul:8(7):823-59. doi: 10.1097/JTO.0b013e318290868f. Epub [PubMed PMID: 23552377]
Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Current medicinal chemistry. 2010:17(7):672-97 [PubMed PMID: 20088761]
Tjin G, Xu P, Kable SH, Kable EP, Burgess JK. Quantification of collagen I in airway tissues using second harmonic generation. Journal of biomedical optics. 2014 Mar:19(3):36005. doi: 10.1117/1.JBO.19.3.036005. Epub [PubMed PMID: 24604535]
Ho JE, Gao W, Levy D, Santhanakrishnan R, Araki T, Rosas IO, Hatabu H, Latourelle JC, Nishino M, Dupuis J, Washko GR, O'Connor GT, Hunninghake GM. Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. American journal of respiratory and critical care medicine. 2016 Jul 1:194(1):77-83. doi: 10.1164/rccm.201509-1753OC. Epub [PubMed PMID: 26771117]
Brems JH, Raju S. An Issue of Caliber: The Airway Tree and Air Pollution Susceptibility. American journal of respiratory and critical care medicine. 2024 Jun 1:209(11):1294-1295. doi: 10.1164/rccm.202401-0146ED. Epub [PubMed PMID: 38394649]
Desplechain C, Foliguet B, Barrat E, Grignon G, Touati F. [The pores of Kohn in pulmonary alveoli]. Bulletin europeen de physiopathologie respiratoire. 1983 Jan-Feb:19(1):59-68 [PubMed PMID: 6850150]
Level 3 (low-level) evidenceCordingley JL. Pores of Kohn. Thorax. 1972 Jul:27(4):433-41 [PubMed PMID: 5075613]
Level 3 (low-level) evidenceBurgess CL, Huang J, Bawa PS, Alysandratos KD, Minakin K, Ayers LJ, Morley MP, Babu A, Villacorta-Martin C, Yampolskaya M, Hinds A, Thapa BR, Wang F, Matschulat A, Mehta P, Morrisey EE, Varelas X, Kotton DN. Generation of human alveolar epithelial type I cells from pluripotent stem cells. Cell stem cell. 2024 May 2:31(5):657-675.e8. doi: 10.1016/j.stem.2024.03.017. Epub 2024 Apr 19 [PubMed PMID: 38642558]
Zhang M, Lan H, Jiang M, Yang M, Chen H, Peng S, Wang X, Zhang Y, Huang X, Li L, Chen C, Hong J. NLRP3 inflammasome mediates pyroptosis of alveolar macrophages to induce radiation lung injury. Journal of hazardous materials. 2025 Feb 15:484():136740. doi: 10.1016/j.jhazmat.2024.136740. Epub 2024 Dec 1 [PubMed PMID: 39642726]
De Bernardo G, Crisci V, Centanni F, Giordano M, Perrone S, Buonocore G, Mandato C. Laryngeal Mask for Minimally-invasive Surfactant Administration: A Narrative Review. Current pediatric reviews. 2025:21(2):111-117. doi: 10.2174/0115733963328784240820062714. Epub [PubMed PMID: 39229991]
Level 3 (low-level) evidenceRen J, Zhao S, Lai J. Role and mechanism of COL3A1 in regulating the growth, metastasis, and drug sensitivity in cisplatin-resistant non-small cell lung cancer cells. Cancer biology & therapy. 2024 Dec 31:25(1):2328382. doi: 10.1080/15384047.2024.2328382. Epub 2024 Mar 26 [PubMed PMID: 38530094]
Ackermann M, Werlein C, Plucinski E, Leypold S, Kühnel MP, Verleden SE, Khalil HA, Länger F, Welte T, Mentzer SJ, Jonigk DD. The role of vasculature and angiogenesis in respiratory diseases. Angiogenesis. 2024 Aug:27(3):293-310. doi: 10.1007/s10456-024-09910-2. Epub 2024 Apr 5 [PubMed PMID: 38580869]
Vose A, Birukova A, Albright M, Schlobohm A, Garantziotis S, Tata PR, Barkauskas C, Tighe R. Hyaluronan Directs Alveolar Type II Cell Response to Acute Ozone Exposure in Mice. American journal of respiratory cell and molecular biology. 2025 Jan 6:():. doi: 10.1165/rcmb.2024-0385OC. Epub 2025 Jan 6 [PubMed PMID: 39761597]
Chen Y, Wang H, Yang X, Qi F, Qiao J, Zhang P. [A comparative study of "constant volume" animal model and "constant pressure" animal model of intra-abdominal hypertension]. Zhonghua wei zhong bing ji jiu yi xue. 2020 Apr:32(4):498-501. doi: 10.3760/cma.j.cn121430-20200227-00201. Epub [PubMed PMID: 32527361]
Level 2 (mid-level) evidenceBarnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 2016 Jul:138(1):16-27. doi: 10.1016/j.jaci.2016.05.011. Epub 2016 May 27 [PubMed PMID: 27373322]
Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, Bartolák-Suki E. Emphysema and mechanical stress-induced lung remodeling. Physiology (Bethesda, Md.). 2013 Nov:28(6):404-13. doi: 10.1152/physiol.00041.2013. Epub [PubMed PMID: 24186935]
Level 3 (low-level) evidenceMaher TM. Interstitial Lung Disease: A Review. JAMA. 2024 May 21:331(19):1655-1665. doi: 10.1001/jama.2024.3669. Epub [PubMed PMID: 38648021]
Olugbuyi O, Crosdale B, Samms-Vaughan M, Shakespeare-Pellington S, Coore-Desai C, Reece JA, Trotman H. Neonatal morbidity in the 2011 JAKIDS Jamaican birth cohort. Psychology, health & medicine. 2024 Jul:29(6):1124-1133. doi: 10.1080/13548506.2021.1975783. Epub 2021 Sep 6 [PubMed PMID: 34488500]
Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatric pulmonology. 2001 Jul:32(1):76-91 [PubMed PMID: 11416880]
Escalon JG, Girvin F. Smoking-Related Interstitial Lung Disease and Emphysema. Clinics in chest medicine. 2024 Jun:45(2):461-473. doi: 10.1016/j.ccm.2023.08.016. Epub 2023 Sep 15 [PubMed PMID: 38816100]
Tomkiewicz RP, App EM, De Sanctis GT, Coffiner M, Maes P, Rubin BK, King M. A comparison of a new mucolytic N-acetylcysteine L-lysinate with N-acetylcysteine: airway epithelial function and mucus changes in dog. Pulmonary pharmacology. 1995 Dec:8(6):259-65 [PubMed PMID: 8819180]
Level 3 (low-level) evidenceZampieri FM, Parra ER, Canzian M, Antonângelo L, Luna Filho B, de Carvalho CR, Kairalla RA, Capelozzi VL. Biopsy-proven pulmonary determinants of heart disease. Lung. 2010 Jan-Feb:188(1):63-70. doi: 10.1007/s00408-009-9193-z. Epub 2009 Oct 28 [PubMed PMID: 19862572]
Level 2 (mid-level) evidenceFranklin SD, Fierro J, Hysinger EB, Phinizy PA, Piccione J. Hemosiderin-Laden Macrophages in Bronchoalveolar Lavage Samples of Children with Bronchopulmonary Dysplasia. The Journal of pediatrics. 2023 Feb:253():79-85. doi: 10.1016/j.jpeds.2022.09.019. Epub 2022 Sep 18 [PubMed PMID: 36130636]
Detterbeck FC, Woodard GA, Bader AS, Dacic S, Grant MJ, Park HS, Tanoue LT. The Proposed Ninth Edition TNM Classification of Lung Cancer. Chest. 2024 Oct:166(4):882-895. doi: 10.1016/j.chest.2024.05.026. Epub 2024 Jun 15 [PubMed PMID: 38885896]
Li HY, Wang YY, Liu H, Liu HX, Jiang LY, Han YC, Zhou WY, Mao T, Fang WT. [The ninth edition of TNM staging for lung cancer: precise staging for precise diagnosis and treatment]. Zhonghua wai ke za zhi [Chinese journal of surgery]. 2024 Jun 1:62(6):537-542. doi: 10.3760/cma.j.cn112139-20231210-00262. Epub [PubMed PMID: 38682624]