Introduction
The lumbar spine comprises the lower end of the spinal column between the last thoracic vertebra (T12) and the first sacral vertebra (S1). The spinal cord in this region has protection from five durable and mobile vertebrae (L1-L5) that allow for the dispersion of axial forces. The spinal cord runs through the center of the vertebral column and terminates in the conus medullaris at the level of the L1-L2 vertebrae. The cauda equina, Latin for horse’s tail, is a bundle of spinal nerve roots that begin at the termination of the spinal cord and descend through the remainder of the canal. The lumbar spine is comprised of bone, cartilage, ligaments, nerves, and muscle. Each of these components plays an integral role in the form and function of the lumbar spine.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
There are three main functions of the lumbar spine. First, the lumbar spine assists in supporting the upper body. The lumbar vertebrae (L1-L5) are much larger compared to other regions of the vertebral column, allowing them to absorb axial forces delivered from the head, neck, and trunk. The lumbar vertebrae form a canal that serves to protect the spinal cord and spinal nerves. This arrangement allows for the communication of information from the central nervous system to the lower extremities and vice versa. The lumbar spine allows for diverse types of truncal motion, including flexion, extension, rotation, and side bending. From a lateral view, the lumbar spine has a concave curvature, referred to as the lumbar lordosis. This curvature is variable in degree and transfers the upper body mass over the pelvis to allow for efficient bipedal motion.[3]
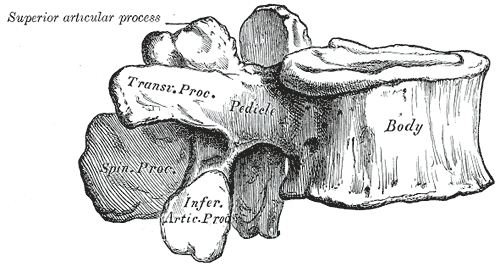
Each lumbar vertebra consists of multiple components. These include the vertebral body, and the dorsal structures termed the posterior elements. Immediately dorsal to the vertebral body lie two pedicles that attach to the laminae. The pedicles resist motion and transmit forces from the posterior elements to the vertebral body. From the junction of the two laminae, the spinous process extends posteriorly. At the junction between the pedicles and laminae, four articular processes and two transverse processes reside. The transverse processes extend laterally, serving as attachment points for ligaments and musculature. The superior and inferior articular processes create the zygapophyseal joints (the facet joints). This joint occurs between the superior articular process of a vertebra and the inferior articular process of the vertebra immediately cephalad. These joints lie in the sagittal plane and participate in flexion and extension of the lumbar spine. The pars interarticularis is the location of the lamina between the superior and inferior articular processes and is prone to the development of stress fractures (spondylolysis) in the growing spine.[1]
The lumbar disc is a fibrocartilaginous structure seated between two vertebral body endplates. It is composed of an internal gelatinous nucleus pulposus and an external fibrous annulus fibrosus. The primary function of the lumbar disc is shock absorption. Two longitudinal ligaments lie anterior and posterior to the vertebral body. The anterior longitudinal ligament resists lumbar extension, translation, and rotation. The posterior longitudinal ligament resists lumbar flexion. The segmental ligaments include the ligamentum flavum, which is perforated when performing a lumbar puncture. The remaining segmental ligaments include the supraspinous and interspinous ligaments, which lie between the spinous processes and resist lumbar flexion.[1][4]
Embryology
The development of the lumbar spine begins around the third week of gestation. The notochord initiates this process by secreting growth factors that stimulate the ectoderm to transform into the neuroectoderm. The process of neurulation produces the neural tube, which ultimately develops into the spinal cord. Errors during neurulation may result in numerous congenital anomalies ranging from mild (spina bifida) to severe (anencephaly).[5]
Also, around the third week, the paraxial mesoderm develops into pairs of somites along either side of the neural tube. Each somite differentiates into a dermomyotome and sclerotome. The sclerotome separates into cell clusters located caudally and cranially. Neurons from the neural tube penetrate these clusters to innervate individual myotomes and dermatomes. The caudally located cell clusters then fuse with the cranially located clusters of the adjacent sclerotome to create the vertebral body. Between each cell cluster, the interverbal disc develops. Simultaneously, sclerotome cells migrate around the neural tube and fuse dorsally, creating the vertebral arch.[5]
Each vertebra undergoes a process of endochondral ossification, in which the mesenchymal cells differentiate into cartilage and eventually bone. Chondrification centers develop around the sixth week, and primary ossification centers in the seventh. These processes are responsible for strengthening the eventual vertebra. Bone remodeling continues throughout the lifespan and is highly dependent on stress and mechanical loads.[5][6]
Blood Supply and Lymphatics
The spinal cord has a rich blood supply stemming from three main longitudinal arteries. A single anterior spinal artery supplies the anterior two-thirds of the cord. On the dorsal side, two posterior spinal arteries supply the posterior one-third of the cord. Several anterior and posterior radicular arteries provide collateral blood supply to the vertebral column. These radicular arteries run along with the ventral and dorsal nerve roots, supplying them with blood. The artery of Adamkiewicz is the largest radiculomedullary artery and provides vascular supply to the lumbar spinal cord. The artery has a variable origin between T8-L2, branching from a posterior intercostal or radicular artery. It typically lies left of the spinal cord and ascends the spinal canal, making a hairpin loop before joining the anterior spinal artery.[7] Specific to the lumbar spine, four pairs of lumbar arteries originate from the abdominal aorta. These paired arteries travel posteriorly along the vertebral bodies to supply each vertebra. These arteries also supply blood to the adjacent musculature, such as the transversus abdominis and internal oblique.
An extensive system of lymphatics in the lumbar region is responsible for draining lymph from the lower limb and pelvis. These lymph nodes are present along the inferior vena cava and aorta. The lumbar lymph nodes receive drainage from the common iliac nodes and deliver this lymph to the thoracic trunk.[7]
Nerves
Five pairs of mixed spinal nerves emerge from either side of the lumbar spinal cord, carrying both motor and sensory nerve fibers—the spinal nerves branch after exiting the neural foramen into ventral and dorsal rami. The dorsal rami supply motor innervation to the erector spinae musculature and sensation to the skin over the back. The ventral rami supply motor and sensory fibers to the remainder of the prevertebral musculature and lower limbs.[8]
The T12 to L4 ventral rami combine to form a network of nerves called the lumbar plexus. The lumbar plexus gives rise to the obturator (L2-L4) and femoral (L2-L4) nerves, respectively. The remaining nerves of the lumbar plexus include the iliohypogastric (T12-L1), ilioinguinal (L1), genitofemoral (L1-L2), and lateral femoral cutaneous nerve of the thigh (L2-L3)—the lumbosacral plexus form from the L4 to S4 ventral rami. The L4 and L5 roots join to form the lumbosacral trunk, which descends into the pelvis to join the sacral plexus. The lumbosacral plexus then gives rise to the sciatic nerve (L4-S3), which branches into the common peroneal and tibial nerves. The sacral plexus also includes the superior gluteal (L4-S1), inferior gluteal (L5-S2), posterior femoral cutaneous of the thigh (S1-S3), and pudendal nerve (S1-S4).[9]
Each lumbar spinal nerve exits below its corresponding vertebra—for example, the L4 nerve exits below the L4 vertebra through the L4-L5 neural foramen. Most lumbar disc herniations occur centrally and do not compress the exiting nerve root at the level of the disc. The nerve root most commonly affected exits one level below the herniated disc. For example, an L4-L5 central disc herniation will most commonly compress the L5 nerve root in the lateral recess of the spinal canal. However, in the setting of a far lateral disc herniation, the L4 nerve root is compressed, albeit less commonly. This difference is due to the more central position of the traversing spinal nerves compared to the more lateral position of the exiting spinal nerves.[8]
Each spinal nerve supplies an area of skin with afferent sensory fibers. This area of skin is referred to as a dermatome. Each lumbar spinal nerve also innervates a group of muscles with motor fibers, termed a myotome. Dermatomes and myotomes can be traced back to our embryological development. Dermatomes and myotomes are clinically relevant as they can be used to determine the lumbar spinal nerve(s) involved in the setting of pathology.
This section will focus on the sensory and major muscular innervations of the lumbar spinal nerves. L1 and L2 innervate the iliopsoas muscle and provide sensory innervation to the inguinal crease and medial thigh. L3 partially innervates the adductors, iliopsoas, and quadriceps musculature. L3 provides sensory innervation to the anterior-medial thigh. L4 contributes to the femoral and sciatic nerves, innervating the iliopsoas, adductors, quadriceps, and tibialis anterior. The L4 nerve provides sensory innervation to the anterior thigh and medial lower leg. The L3 and L4 nerves contribute to the patellar reflex arc. L5 innervates the gluteus medius, tensor fascia latae, medial hamstrings, tibialis anterior, extensor hallucis longus, extensor digitorum longus/brevis, peroneus longus, tibialis posterior, and the flexor digitorum longus. L5 provides sensory innervation to the lateral leg and dorsum of the foot. It is clinically important to note that each dermatome overlaps with adjacent dermatomes. Therefore, dense numbness is exceedingly rare in the setting of nerve root compression. Each myotome also overlaps, leading to nearly every muscle of the lower extremity receiving innervation from 2 or 3 lumbar spinal nerves.[8]
Muscles
Many muscles use the lumbar vertebrae as attachment points. These muscles allow for smooth, controlled movement in different functional planes. These muscles also serve a secondary role in stabilization, protection, and proprioception. Three major muscle groups originate or insert onto the lumbar spine and aid movement. First, the extensor group consists of the erector spinae and the multifidi. This group lies posterior to the lumbar spine. In this region, the erector spinae muscles include the longissimus thoracis and iliocostalis lumborum. The contraction of this group results in an extension moment at the lumbar spine.
The flexor group lies anterior to the lumbar spine and allows for trunk and hip flexion. The psoas major originates from the T12-L4 transverse processes and joins the iliacus in the thigh to become the iliopsoas (composite muscle). The iliopsoas plays a crucial role in hip flexion and assists with arching the lumbar spine. The abdominal musculature (internal/external oblique, rectus abdominis) plays a more important role in truncal flexion. Finally, a concerted effort involving several muscles is required to create rotation and lateral flexion (side-bending) of the lumbar spine. The quadratus lumborum, psoas major, abdominal musculature, and multifidi play an important role in creating these motions.[2]
Muscle strains in the lumbar region are typically the result of abnormal tension placed upon a tendon; this can occur from overstretching a muscle, repetitive use, or muscle tearing from excessive force. Most lumbar muscle strains will respond to conservative treatment.
Surgical Considerations
Spinal surgery for managing low back pain, lumbar radiculopathy, and lumbar spinal stenosis is a consideration after the failure of conservative treatments. Spinal surgery is the first-line treatment in more urgent/emergent conditions such as conus medullaris syndrome, cauda equina syndrome, cancer (primary/metastatic), osteomyelitis/discitis, epidural abscess, and trauma. The surgical technique and instrumentation implemented depend upon patient factors and the diagnosis requiring treatment.
Lumbar decompression is a technique used to relieve pain caused by a compressed nerve root(s). Two of the most common types of nerve root compression are lumbar central canal stenosis and lumbar radiculopathy, reviewed in the next section. Lumbar decompression comes in several forms, including microdiscectomy, foraminotomy, laminotomy, laminectomy, or a combination of these. Laminectomy (surgical removal of the laminae) is considered the standard surgical treatment for the management of lumbar spinal stenosis. If the decompression, as mentioned above, leads to iatrogenic instability, then a fusion is also performed.[10]
Lumbar fusion is a common surgery used to manage discogenic/facetogenic low back pain, radiculopathy, and spinal deformity (scoliosis/spondylolisthesis). Several surgical approaches exist within two main categories, posterior and interbody fusion. The more traditional approach is a posterior spinal fusion implementing rods and pedicular screws. Limitations of this approach include the need for thecal sac and nerve root retraction along with iatrogenic injury of the paraspinal musculature. Interbody fusion involves placing an implant such as a cage or bone graft within the intervertebral space following a discectomy (removing the intervertebral disc).
Surgical approaches for interbody fusion are multiple (posterior, transforaminal, oblique, lateral, and anterior). Anterior spinal fusion avoids the canal and nerve roots but does introduce complications such as vascular injury and incisional hernia. There is limited comparative data that one approach is superior to another. Following fusion, chronic postoperative complications include pseudoarthrosis (failure of fusion) and adjacent segment degenerative disc disease above/below the fusion level.[11]
Non-surgical treatment can include manipulation therapy and/or physical therapy. The decision regarding whether to use these methods will depend on how acute the injury is, patient compliance, and severity of symptoms.[12]
Clinical Significance
Non-specific low back pain is one of the leading causes of disease burden globally—estimates of indirect costs in the US range from 18.5 to 28.2 billion dollars. Low back pain can be triggered by physical factors (i.e., poor lifting mechanics) and psychosocial factors. Often a specific trigger is not identified. Low back pain is a symptom of several different disease processes. Triage is necessary to rule out specific disorders that may require urgent/emergent workup and treatment. ‘Red flags’ for the lumbar spine include trauma, age >70, unexplained weight loss, history of cancer, constitutional symptoms, night pain, saddle anesthesia, and impaired bowel or bladder function. Red flags are associated with vertebral fracture, malignancy, infection (osteomyelitis/discitis), and conus medullaris/cauda equina syndrome (reviewed below).[13]
The termination of the spinal cord, termed the conus medullaris, occurs at the L1-L2 vertebrae. This structure is clinically significant for procedures in this area, most notably the lumbar puncture. A lumbar puncture (spinal tap) is performed by inserting a needle between the vertebral lamina to obtain a cerebrospinal fluid sample. This procedure must occur below the level of L2, between L3-L4 or L4-L5 laminae, to avoid the spinal cord. Conus medullaris syndrome results from severe compression or injury of the conus medullaris. The most common etiologies for this condition include trauma, disc herniations, and epidural abscesses.
Signs/symptoms of conus medullaris syndrome include severe/sudden onset low back pain, symmetrical weakness of both legs, and sudden loss of bowel and bladder function. Immediate treatment is necessary to prevent permanent damage and to preserve lower extremity neurologic function. Caudal to the conus medullaris in the spinal canal is the cauda equina. The cauda equina is a collection of spinal nerves located from L1-L5 in the spinal canal. The spinal nerves that comprise the cauda equina are the L2-L5, S1-S5, and the coccygeal nerve. Compression of the cauda equina is also a surgical emergency. The most common signs and symptoms include urinary retention, saddle anesthesia (numbness of the perineum/inner thighs), as well as radicular leg pain.[14]
There are several plausible pathoanatomical disorders of the lumbar spine. The surrounding musculature, intervertebral disc, facet joints, and spinal nerves may each serve as potential pain generators. However, most low back pain is ‘non-specific and does not have a pathoanatomical explanation. Diagnostic investigations are typically not indicated in the initial management of low back pain unless a specific disease process would be managed differently due to the investigation. Immediate imaging may be indicated when red flags are present. Plain film radiography followed by MRI of the lumbar spine is the commonly performed sequence as the initial diagnostic workup. In some cases, CT scanning may still play a role.
Lumbar spondylosis (osteoarthritis) results from a degenerative cascade involving the lumbar intervertebral disc and the facet joints. The cascade progresses with age, thought to be initiated by an insult or injury. The cascade is also heavily influenced by genetic factors. The lumbar disc initially develops circumferential annular tears leading to internal disc disruption; this is followed by disc resorption, which leads to loss of disc height and eventual osteophyte (bone spur) development. Degeneration begins with a synovial reaction leading to cartilage disruption within the facet joints, which progresses to the formation of osteophytes, joint capsular laxity, and joint subluxation. The combination of these conditions can lead to segmental spinal instability, referred to as spondylolisthesis (slippage of one vertebra on another). Progressive spondylosis can lead to structural narrowing around the lumbar nerve roots, resulting in radiculopathy and/or spinal stenosis in the lumbar spine. Lumbar spondylosis is a widespread condition that is often asymptomatic, most commonly occurring at the L4-5 segment.[15]
Compression, injury, or irritation of the lumbar spinal nerve roots can occur from multiple potential sources. Most commonly, this occurs as a consequence of the degenerative cascade or an acute disc herniation. Lumbar radiculopathy (aka sciatica) describes the constellation of symptoms resulting from lumbar nerve compression. Individuals present with variable degrees of radiating pain, paresthesia (numbness/tingling), and weakness in the lower extremities. Lumbar stenosis is a condition in which the narrowing of the spinal canal occurs. This narrowing is usually secondary to degenerative spondylosis and spondylolisthesis. Classically individuals present with low back and/or lower extremity pain/paresthesias, which worsen with lumbar extension, prolonged standing, and ambulation. The pathophysiology appears to be due to mechanical compression of the lumbosacral spinal nerve roots resulting in ischemia.[16][17]
Treatment of low back pain is targeted depending on the diagnosis established. Treatment is initially conservative, incorporating a multidisciplinary approach with a combination of pharmacologic and non-pharmacologic therapies. Non-pharmacological therapies include physical therapy, manual therapy, home exercise, acupuncture, cognitive behavioral therapy, etc. Clinicians can implement interventional treatments such as epidural steroid injections to manage recalcitrant lumbar radicular pain. Pain emanating from the facet joints can be treated with medial branch radiofrequency ablation.[13]
Media
(Click Image to Enlarge)
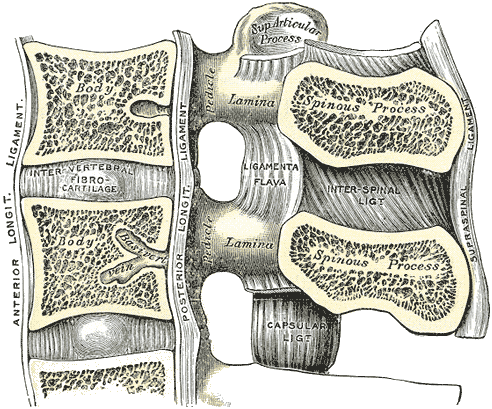
Lumbar Vertebral Anatomy. This medial sagittal section shows the relationship between the anterior longitudinal ligament, vertebral bodies, intervertebral fibrocartilage, posterior longitudinal ligament, vertebral laminae, ligamenta flava, superior articular processes, pedicles, spinous processes, interspinal ligament, capsular ligament, and supraspinal ligament.
Gray's Anatomy
(Click Image to Enlarge)
(Click Image to Enlarge)
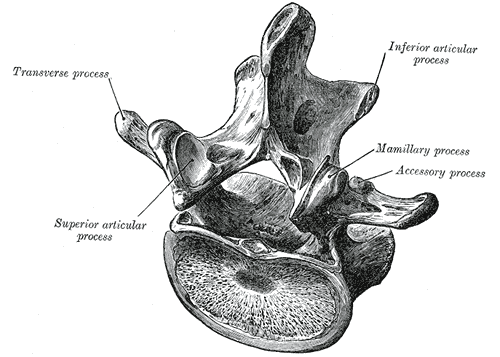
Lumbar Vertebra, Superoposterior View. This illustration shows the relationship between a typical lumbar vertebra's transverse process, superior and inferior articular processes, and mamillary and accessory processes. Shown but not labeled are the laminae, spinal canal, lateral recess, pedicles, and vertebral body.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
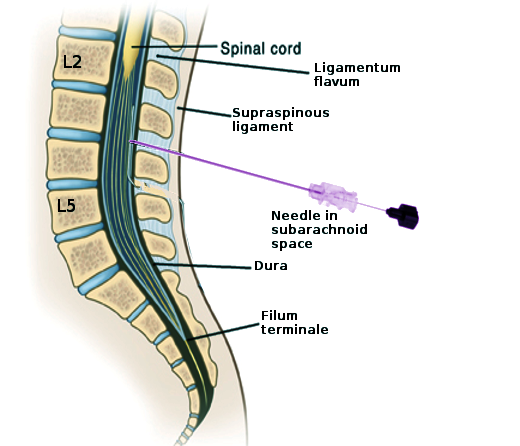
Lumbar Puncture Landmarks. This image shows the key structures encountered during a lumbar puncture for diagnostic, anesthetic, or therapeutic purposes. Labeled structures include L2, L5, the spinal cord, ligamentum flavum, supraspinous ligament, dura, and filum terminale. The needle is in the subarachnoid space at the L3-L4 level.
Contributed by S Bhimji, MD
References
Waxenbaum JA, Reddy V, Williams C, Futterman B. Anatomy, Back, Lumbar Vertebrae. StatPearls. 2025 Jan:(): [PubMed PMID: 29083618]
Gilchrist RV, Frey ME, Nadler SF. Muscular control of the lumbar spine. Pain physician. 2003 Jul:6(3):361-8 [PubMed PMID: 16880883]
Boszczyk BM, Boszczyk AA, Putz R. Comparative and functional anatomy of the mammalian lumbar spine. The Anatomical record. 2001 Oct 1:264(2):157-68 [PubMed PMID: 11590593]
Level 3 (low-level) evidenceDevereaux MW. Anatomy and examination of the spine. Neurologic clinics. 2007 May:25(2):331-51 [PubMed PMID: 17445732]
Kalamchi L, Valle C. Embryology, Vertebral Column Development. StatPearls. 2023 Jan:(): [PubMed PMID: 31751107]
O'Rahilly R, Müller F, Meyer DB. The human vertebral column at the end of the embryonic period proper. 3. The thoracicolumbar region. Journal of anatomy. 1990 Feb:168():81-93 [PubMed PMID: 2323997]
Kaiser JT, Reddy V, Lugo-Pico JG. Anatomy, Back, Spinal Cord Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 30725904]
Basit H, Reddy V, Varacallo MA. Anatomy, Back, Spinal Nerve-Muscle Innervation. StatPearls. 2025 Jan:(): [PubMed PMID: 30855906]
Singh O, Al Khalili Y. Anatomy, Back, Lumbar Plexus. StatPearls. 2023 Jan:(): [PubMed PMID: 31424721]
Williams MG, Wafai AM, Podmore MD. Functional outcomes of laminectomy and laminotomy for the surgical management lumbar spine stenosis. Journal of spine surgery (Hong Kong). 2017 Dec:3(4):580-586. doi: 10.21037/jss.2017.10.08. Epub [PubMed PMID: 29354735]
Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. Journal of spine surgery (Hong Kong). 2015 Dec:1(1):2-18. doi: 10.3978/j.issn.2414-469X.2015.10.05. Epub [PubMed PMID: 27683674]
Kuligowski T, Skrzek A, Cieślik B. Manual Therapy in Cervical and Lumbar Radiculopathy: A Systematic Review of the Literature. International journal of environmental research and public health. 2021 Jun 7:18(11):. doi: 10.3390/ijerph18116176. Epub 2021 Jun 7 [PubMed PMID: 34200510]
Level 1 (high-level) evidenceMaher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet (London, England). 2017 Feb 18:389(10070):736-747. doi: 10.1016/S0140-6736(16)30970-9. Epub 2016 Oct 11 [PubMed PMID: 27745712]
Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal cord. 2017 Oct:55(10):886-890. doi: 10.1038/sc.2017.54. Epub 2017 May 23 [PubMed PMID: 28534496]
Level 1 (high-level) evidenceKirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978 Dec:3(4):319-28 [PubMed PMID: 741238]
Alexander CE, Weisbrod LJ, Varacallo MA. Lumbosacral Radiculopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 28613587]
Wu L, Munakomi S, Cruz R. Lumbar Spinal Stenosis. StatPearls. 2025 Jan:(): [PubMed PMID: 30285388]