Introduction
Spine Anatomy
Collectively, the spine has an anterior and a posterior region (see Image. Lumbar Vertebral Anatomy). The cylindrical vertebral bodies comprise the anterior spine, separated by intervertebral (IV) disks and held in place by the anterior and posterior longitudinal ligaments. The IV disks have the gelatinous nucleus pulposus in the middle, surrounded by the cartilaginous annulus fibrosus (see Image. Intervertebral Disk). The cervical and lumbar spinal segments have the largest IV disks, owing to these regions' mobility. The anterior spine is a shock absorber of bodily movements.
The vertebral arches and processes constitute the posterior spine. Each vertebral arch has an anterior pair of cylindrical pedicles and a posterior pair of laminae (see Image. Lumbar Vertebra, Superoposterior View). Other structures emanating from the vertebral arch include 2 lateral transverse processes, 1 posterior process, and 2 superior and 2 inferior articular facets. Facet joints form from superior and inferior facet apposition. The spinal canal, which houses the spinal cord, is formed by the vertebral bodies and IV disks anteriorly and vertebral arches posteriorly. The nerve roots exit superior to their corresponding vertebral body through the intervertebral canal. The ligamentum flavum (yellow ligament) is a thick, fibrous structure passing between adjacent laminae.
The lateral recess is an anatomic space in the posterior spine bounded anteriorly by the vertebral body and disk, posteriorly by the ligamentum flavum and vertebral arch, laterally by the pedicle, and medially by the thecal sac. This region is narrow and is a potential area of nerve root compression. The posterior spine has various functions, including spinal cord and nerve root protection and muscle and ligament support.
Lumbar Spinal Stenosis
Lumbar spinal stenosis (LSS) is the narrowing of the lumbar vertebra in the central canal, lateral recess, or neural foraminal areas.[1] Central canal stenosis may compress the thecal sac and bilateral spinal segments and thus, in the severe form, may produce bilateral symptoms. Lateral recess and neural foraminal stenosis may compress the nerve roots and produce unilateral lumbar radiculopathy symptoms.[2]
Central stenosis arises from anterior ligamentum flavum hypertrophy compounded by posterior disk bulging. This condition is more prevalent at the L4 to L5 level than other spinal segments. Meanwhile, lateral recess stenosis results from facet arthropathy and osteophyte formation, compressing the nerve before it passes the intervertebral foramen. Foraminal stenosis is due to disk height loss, foraminal disk protrusion, or osteophyte formation. These changes impinge on the nerve root inside the intervertebral foramen. Extraforaminal stenosis is usually due to far lateral disk herniation. This condition compresses the nerve root after exiting the intervertebral foramen laterally.[3]
LSS is a significant cause of disability in older individuals and a common spinal surgery indication in patients older than 65 years.[4][5][6] Henk Verbiest first described relative and absolute spinal stenosis as lumbar canal midsagittal diameter of less than 12 mm and 10 mm, respectively. However, LSS clinical and radiologic diagnostic criteria have not been established despite this condition's global prevalence. Thus, this clinical entity has no universally accepted definition.[7][8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
LSS can be congenital or acquired. The condition's most frequent cause is degenerative spondylosis. Aging, chronic wear-and-tear, and trauma are the most significant risk factors. Intervertebral disk degeneration leads to posterior protrusion and increased loading of the posterior vertebral structures. These changes can result in posterior vertebral osteophyte formation (uncinate spurs), facet hypertrophy, synovial facet cysts, and ligamentum flavum hypertrophy, giving rise to LSS.
Degenerative spondylolisthesis is another cause of LSS. The pars interarticularis can be fractured when degenerative spine changes occur. The resulting instability can lead to forward translation of the vertebra. Significant anterior vertebral slippage on top of the next vertebral segment, most commonly at the L4 to L5 region, can narrow the spinal canal and cause stenosis.
Other acquired conditions, although rarer, may also cause LSS. Such conditions include space-occupying lesions, post-surgical fibrosis, rheumatologic conditions, and skeletal diseases like ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis.[9] Even more rarely, LSS may be secondary to congenital causes such as achondroplasia, which manifests with short pedicles and medially placed facets.
Epidemiology
The lack of a universally accepted definition for LSS makes the epidemiology of LSS difficult to determine. An ancillary Framingham research found that 19.4% of the participants aged 60 to 69 had a spinal internal diameter of less than 10 mm. Meanwhile, a Japanese-population-based study showed an increasing prevalence of symptomatic LSS across age groups, with 1.9% observed in the group aged 40 to 49, 4.8% in the group aged 50 to 59, 5.5% in the group aged 60 to 69, and 10.8% in the group aged 70 to 79.[10] By comparison, LSS was reported to affect more than 200,000 individuals in the US. The condition is also considered the most common spinal surgery indication in patients older than 65 years.[11]
Another study found that genetic factors predisposed individuals to developing LSS. The authors reported that aberrant genes promoted the pathological processes driving vertebral body and facet joint osteophyte proliferation, ligamentum flavum hypertrophy, and intervertebral disk degeneration.[12]
The radiological prevalence of moderate and severe stenosis in patients older than 40 years can be as high as 80% and 40%, respectively. The condition affects almost 11% of older individuals in the US, with radiological evidence of LSS observed in 20% of people above 60 years. Paradoxically, 80% of these individuals are asymptomatic.[13] A systematic review estimated the pooled prevalence of LSS to be 11% in the general population and 25% to 39% in the clinical setting. LSS is also a significant contributor to spinal surgery in the US, as 5.9 per 100 patients undergo a lumbar fusion within 1 year from the time of diagnosis of lumbar degeneration.[14]
Pathophysiology
Repeated spinal wear and tear and concomitant axial musculature weakening predispose to IV disk desiccation, shifting the body's axial loading posteriorly. Canal narrowing results from subsequent facet arthropathy, synovial cyst and osteophyte formation, ligamentum hypertrophy, and buckling.
The anteroposterior (AP) diameter of the bony canal of the L5 vertebra in males and L4 vertebra in females is the most significant risk factor for developing degenerative LSS. Combined characteristics such as bony canal and vertebral body dimensions contributed to this risk.[15]
Mechanical stress-induced ligamentum flavum hypertrophy is another important pathological variable.[16] Ligamentum flavum thickening and hypertrophy occur due to multispectral effects from fibrosis, chondroid-metaplasia, and amyloid deposition.[17] Changes like elastic fiber degeneration, increased collagen content, fibrosis, cicatrization, and calcification have been noted in the ligamentum flavum in LSS.[18]
Neurologic LSS symptoms are thought to originate from nerve root compression and ischemia.[19] The ischemic or venous blood stasis theory may account for axial back pain and neurological claudication. Lateral recess and foraminal stenosis resulting from disk prolapse, facet arthropathy, ligamentum hypertrophy, and synovial cyst formation lead to radicular pain. Nerve root compression may arise from direct mechanical compression or increased intrathecal pressure due to spinal canal narrowing. Nerve root inflammation is also a plausible but less likely mechanism of neurologic symptoms in LSS.[20]
As previously mentioned, certain individuals are predisposed to developing LSS. Additionally, conditions like spondylolisthesis and scoliosis can trigger aging-related degeneration, leading to osteophyte formation, disk prolapse, ligamental hypertrophy, and facet hypertrophy.
History and Physical
Classically, LSS presents with axial low back pain, radiculopathy, or neurological claudication aggravated by ambulation and lumbar extension. Neurogenic claudication is an important feature of LSS. Symptoms are typically bilateral but usually asymmetric. Low back pain, numbness, and tingling are present in most patients. Numbness and tingling in LSS typically involve the entire leg and rarely affect only a single nerve root distribution.[21] Approximately 43% of affected individuals experience weakness.
Patients may also report walking upstairs being easier than downstairs, as the back is forward-flexed when climbing stairs. The "shopping cart sign” has also been described, wherein forward flexion, as if pushing a shopping cart, relieves back pain. Nociceptive axial back pain is due to facet arthropathy. The "simian stance," characterized by walking with the trunk and knees slightly flexed, may be observed as patients experience pain relief in such a posture.
Central stenosis presents with neurological claudication. Lateral recess and foraminal stenosis present with radiculopathy. Radiating leg pain exacerbated by walking is the most sensitive clinical marker.
Mild LSS is mostly asymptomatic. Moderate LSS has been defined as up to 50% reduction in central canal or nerve root canal dimensions while being able to sit without pain for 50 minutes and walk 50 feet or more. Severe cases present with motor weakness, gait impairment, and abnormal postural sway.[22]
LSS may lead to cauda equina or conus medullaris syndrome, which may manifest with new-onset bowel or bladder dysfunction, saddle anesthesia, and sudden bilateral or increased lower extremity weakness. These conditions are medical emergencies needing prompt attention. Thus, progressive disease must be suspected in patients with a stooped forward posture and reduced lumbar extension suddenly experiencing these neurologic changes.
All patients with suspected LSS must undergo a thorough physical examination, paying particular attention to neurologic signs. Unilateral radicular pain from foraminal stenosis may be elicited with passive and active lumbar extension, producing what is known as the Kemp sign. Wasting of bilateral extensor digitorum brevis muscles is another bedside clinical marker of LSS.[23]
Some patients with LSS may be asymptomatic and have a normal neurologic examination. Other individuals with asymptomatic LSS may have neurologic deficits due to superimposed lumbar radiculopathy. The Valsalva maneuver often does not exacerbate LSS-related radicular pain as it does in IV disk prolapse. Only 10% of patients present with a positive straight leg raise test.[24] Pedal pulses should also be checked during the physical exam to rule out vascular claudication. A patient who can perform a five-repetition sit-to-stand (5R-STS) test in 10.4 seconds may be considered to have no functional impairment.[25]
Providers may ask patients to take clinical questionnaires such as the Oswestry Disability Index (ODI), Swiss spinal stenosis questionnaire, visual analog scale (VAS), pain disability index, short-form health survey (SF-36), and self-paced walking test (SPWT) to evaluate the disease's impact on the patient's function and quality of life.[26]
Evaluation
As mentioned previously, consensus regarding LSS' definition and radiological diagnostic criteria is lacking. However, neuroimaging is indicated when evaluating low-back pain in the presence of red-flag symptoms, and lumbosacral radiculopathy or spinal stenosis is suspected.
Plain x-ray provides vital information regarding the effect of axial loading on spinal biomechanics. Typical findings include osteophyte formation and reduced disk height. The lower limit of the normal lumbar spinal canal AP range on x-ray is 15 mm. Interlaminar space size on plain radiographs can help predict LSS.[27] Dynamic imaging is pivotal in ruling out instability and the need for spinal fixation and decompression.
Computed tomography (CT) cross-sectional area of spinal sacs less than 75 mm2 and 100 mm2 are indicative of absolute and relative LSS, respectively. Lateral recess stenosis is likely if the AP measurement is less than 4 mm. A height less than 15 mm typically causes foraminal stenosis and may present with gluteal pain. Axial imaging shows a trefoil or cloverleaf appearance. Sagittal measurements alone underestimate lateral stenosis and have been associated with poor operative outcomes.
CT-aided LSS-VGG16 model is a deep learning-based classification tool developed to diagnose LSS quickly. This method showed an efficacy of almost 90%.
Non-contrast magnetic resonance imaging (MRI) of the lumbosacral spine is the modality of choice for LSS. This imaging technique has better soft tissue resolution than CT and thus is more sensitive in detecting spinal nerve lesions. Still, CT myelography is an option when MRI is contraindicated, as this modality can detect stenosis.[28]
Many authors use an intraspinal canal area of less than 76 mm2 and 100 mm2 for severe and moderate stenosis, respectively. AP spinal canal diameters of less than 10 mm are also frequently used for diagnosing LSS. The "nerve root sedimentation" sign is a reliable radiological sign. Gravity-induced "sinking" of the dorsal dural sac region is observed on MRI in supine patients without LSS. Conversely, this sign is absent in patients with LSS.[29] MRI-based Schizas, Chen Jia, and Braz classification systems have high diagnostic accuracy. The Lee grading system for LSS, based on the cauda equina's shape, also helps predict the need for surgery.[30]
Table. MRI-Based Classification Systems for Diagnosing LSS
|
Schizas System
|
|
Braz Classification
|
Lee Grading System
|
Axial loading MRI is more effective in evaluating LSS.[34] Conventional MRI has been shown to overestimate lateral recess dimensions by nearly 13%. The Chen Jia classification, dural cross-sectional area, and presence of neurogenic claudication are important clinical indicators of the need for surgical intervention. Machine learning algorithms for lumbar canal segmentations have shown good results. Deep learning systems have been applied for MRI studies for evaluating canal stenosis and facet arthropathy.[35]
Electromyography and nerve conduction studies may also aid in diagnosing LSS, as these modalities can distinguish and rule out clinical mimics.[36] A study showed that gait assessment and electromyography may be used to assess the functional status of individuals with LSS.[37]
Treatment / Management
LSS management aims to reduce symptoms and improve functional status. Treatment strategies include the following:
- Pain medication
- Spinal bracing
- Physical therapy
- Transcutaneous electrical nerve stimulation
- Neuromodulation
- Epidural steroid injection (ESI)
- Interspinous spacer
- Surgical decompression
Lifestyle modifications improve overall health. Medical management is recommended for short-term symptomatic pain relief. However, concrete guidelines and high–quality evidence for recommendations are lacking.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-line agents for pain management. Opioids, muscle relaxants, gabapentin, vitamin B12, and calcitonin are also prescribed. Hemp-derived cannabidiol has also demonstrated effectiveness in improving pain scores.[38] However, evidence supporting the long-term use of these medications is limited.(B2)
Physical therapy used alone to improve pain scores and function has low evidence. However, physical therapy's role in conferring physiological stability 3 to 6 months postsurgery has moderate evidence. Standardized physical therapy regimens are currently lacking. However, core muscle stretching and strengthening have been shown to correct posture and improve symptoms.[39]
Flexion-distraction manipulation therapy also has only short-term benefits. Strong recommendations are lacking for semirigid lumbosacral bracing, transcutaneous electrical nerve stimulation, acupuncture, and spinal manipulation for managing LSS. Lumbosacral braces and corsets demonstrate short-term effectiveness in improving walking distance. Neurostimulation is recommended specifically for failed back surgery syndrome.
An ESI procedure should provide at least 3 months of significant pain relief. This modality is both diagnostic and therapeutic. Steroids are given as interlaminar or transforaminal injections. About 65% of patients undergo at least 1 epidural steroid injection therapy, but the treatment provides relief for 2 weeks to 6 months only. Additionally, a systematic review reported minimal improvement in walking abilities among cohorts with LSS treated with epidural injections.
Epidural anesthetic injections alone are not statistically different from injections with a mixture of anesthetics and corticosteroids.[40] Steroid and botulinum toxin type A injections in the bilateral facet joints are more effective in treating the symptoms of severe LSS than transformational epidural steroid injections since steroids cannot spread into the spinal canal. Caudal epidural steroid injections combined with ozone have been shown to improve the walking distance index significantly.[41] Meanwhile, medial branch block or radiofrequency ablation benefits almost 70% of patients with mild-to-moderate stenosis, specifically those with spondylosis and facet arthropathy.(A1)
Barring emergencies such as cauda equina syndrome, surgical management for LSS is usually elective. Options include open, minimally invasive, and endoscopic approaches. The dictum is to ensure adequate neural decompression without compromising spinal stability.
The most frequently performed surgical procedure is a liberal laminectomy (wide pedicle-to-pedicle decompression). Open lumbar decompressive laminectomy, the age-old standard surgical treatment, benefits almost 80% of patients with severe LSS. This procedure is usually indicated for persistent, refractory, and progressive pain despite maximizing conservative measures for 3 to 6 months, and progressive neurologic deterioration has been documented. The minimally invasive spinal treatment (MIST) guidelines recommend open decompression with or without fusion, as this modality has a favorable risk-to-benefit ratio among cohorts with progressive neurological decline.
Absolute surgical contraindications include spinal instability and coagulopathy, while relative contraindications include concurrent scoliosis, kyphosis, or spondylolisthesis grade 2 or higher. The Spine Patient Outcomes Research Trial (SPORT) demonstrated that surgery provided sustained functional and pain score improvement compared to conservative strategies among patients with LSS without spondylolisthesis.
A randomized trial has shown that patients undergoing laminectomy experienced greater symptomatic improvement than individuals treated nonsurgically. However, the procedure's benefits tend to diminish over time.[42] Laminectomy with fusion is indicated when further stability is required as in LSS cases with concurrent segmental instability, eg, degenerative or isthmic spondylolisthesis and degenerative scoliosis.(A1)
The Swedish Spinal Stenosis Study and similar investigations reported no clinical differences between decompression procedures with and without fusion. However, the Spinal Laminectomy versus Instrumented Pedicle screw (SLIP) study showed improved quality of life with low risks of reoperation following decompression with fusion. Decompression with fusion was 2.55 times more effective in improving ODI scores than decompression without fusion. However, the procedure is expensive and associated with more blood loss and increased hospital stays.
Percutaneous lumbar decompression is an outpatient procedure recommended for individuals with a hypertrophied ligamentum flavum (size 2.5 mm or greater). Minimally invasive unilateral or bilateral decompression may be accomplished using microscopically or endoscopically guided tubular retractors. The technique confers minimal tissue trauma, fewer complications, and intraoperative blood loss, though it is technically challenging and has a significant learning curve.[43]
Unilateral biportal endoscopy (UBE) laminectomy is a safe and effective surgical modality.[44][45] Microscopic unilateral laminotomy bilateral decompression (ULBD) and UBE-ULBD are both effective in treating symptomatic LSS (see Image. Imaging Studies for a Patient with Lumbar Spinal Stenosis Treated by Unilateral Biportal Endoscopy-Unilateral Laminectomy Bilateral Decompression). However, UBE-ULBD leads to shorter hospital stays and greater pain improvement than microscopic ULBD.[46][47] Additionally, a recent meta-analysis revealed that UBE and ULBD had equivalent efficacy. However, UBE demonstrated less intraoperative bleeding and shorter hospital stays.[48](A1)
Minimally invasive lumbar decompression (MILD) has the advantages of minimal intraoperative blood loss and surgical trauma to paraspinal muscles. Thus, the procedure can be performed in an outpatient setting. However, MILD's long-term benefits have yet to be demonstrated by high-quality studies. MILD also has a steep learning curve and great radiation hazard risk.
Interspinous spacers are used for reducing lumbar extension. These devices are safe, cost-effective, and approved for lumbar-level stenosis involving 1 or 2 segments. Interspinous spacers are comparable to decompression in producing symptomatic pain relief from intermittent claudication.[49] Interspinous spacers benefit almost 50% of patients. Supirion implants showed minimal complications compared to X-STOP. In contrast to laminectomy, interspinous devices have shown decreased reoperation rates. However, using these devices is not recommended in patients with osteoporosis and dynamic instability.(A1)
The following algorithmic approach has been recommended for managing LSS:
- Lifestyle modifications, physical therapy, and pain medications
- ESI, facet injections, and medial branch radiofrequency ablation
- Multidisciplinary panel review involving radiologists, interventional pain physicians, and spine surgeons for neuromodulation and repeat ESI
- Interspinous spacers
- Stepwise decompression from MILD to open laminectomy with or without fusion
LSS management should be individualized based on the patient's specific symptoms, functional limitations, and intervention response. This algorithm serves only as a general guide, and healthcare professionals should tailor their approach to each patient's unique circumstances.
Differential Diagnosis
The differential diagnosis of LSS includes the following:
- Vascular claudication: presents with bilateral symptoms, pain while standing, and positive shopping cart sign; vascular imaging and ankle-brachial index are critical in the diagnosis
- Peripheral neuropathy: characteristically has stocking-and-glove pain distribution present even at rest and can also disturb sleep
- Lumbar spondylosis: positive straight leg or Lasègue test (L4 to S1) and reverse straight leg or Ely test (L2 to L4) are diagnostic for this condition
- Lumbar plexopathies: may present with sensorimotor deficits without pain, depending on the etiology
- Hip or knee osteoarthritis: usually present with pain and tenderness in the hip or knee area; neurologic signs are often absent
- Metabolic neuropathies: examples are alcohol abuse disorder and vitamin deficiencies; present with sensorimotor deficits and systemic signs, depending on the etiology
A thorough evaluation can distinguish these conditions from LSS.
Prognosis
LSS is a significant cause of pain and disability. Involvement of other spinal segments occurs over time in approximately half of patients. However, the natural history is favorable in 33% to 50% of cases with mild to moderate LSS. The North American Spine Society reported that the illness course of symptomatic mild-to-moderate LSS is favorable in nearly 50% of cohorts.
Some studies have reported symptom progression in 15% of cases at 5 years and almost 30% at 10 years with conservative management. Symptoms improved in 70% and 30% of these cohorts, respectively, during the same periods. Generally, 20-40% of individuals with mild-to-moderate stenosis ultimately require surgery within 10 years.
Conditions correlating with high odds of surgery include cauda equina syndrome, degenerative scoliosis, spondylolisthesis, and persistent refractory symptomatology. However, severe canal stenosis does not always require surgery, as many cases are asymptomatic. Thus, overreliance on relating MR imaging quantitative parameters to clinical scores is not advisable.[50]
The preoperative VAS score is an independent predictor of recovery.[51] Lumbar decompression surgery has proven effective in improving VAS scores in patients with moderate-to-severe central canal stenosis.[52] Without surgery, the prevalence of polypharmacy is as high as 70% among these cohorts.[53] Decompression surgery significantly reduces LSS medications.[54]
Patients who undergo microsurgical decompression sustain postsurgical improvements in lumbar kyphosis and sagittal balance for 5 years. However, sagittal balance deterioration occurs in a third of these individuals after that period.[55]
Preoperative depression has a negative outcome. Old age, concurrent depression, and disposition to rehabilitation unit increase care costs.[56] Instability is high in cases with spondylolisthesis and facet cysts. In contrast, preoperative smoking cessation and weight loss are helpful adjuncts.
Selective microendoscopic laminotomy (MEL)—or MEL performed only on spinal levels manifesting symptoms—lessens reoperation risk in patients with multilevel spinal involvement.[57] Semirigid polyetheretherketone (PEEK) implants have shown better physiological spine movement, improved fusion rates, fewer complications, reduction in adjacent segment degeneration, and biomechanical compatibility.[58] Conventional instrumentation yields good results, such as low instrumentation failure rate, significant pain resolution, and higher bony fusion rates, even in patients with osteoporosis.[59] Two-stage decompression is recommended for concurrent cervical and lumbar canal stenosis. One-stage decompression is advocated for cervical and thoracic canal stenosis.[60] Leg pain improves more significantly than back pain following surgery.
Complications
LSS can produce the following complications:
- Chronic back and lower limb pain
- Limited exercise tolerance
- Decreased mobility and function
- Muscle atrophy
- Depression and anxiety over the symptoms
- Reduced quality of life
- Cauda equina or conus medullaris syndrome
Meanwhile, the complications of open and minimally invasive LSS treatment strategies include the following:
- Spinal epidural hematoma
- Dural tear
- Surgical site infection
- Iatrogenic neurovascular injuries
- Retained non-absorbable hemostatic material such as gossypibomas
- Instability
- Bony regrowth
- Failed back surgery syndrome
- Mortality has been reported in 0.5 to 2.3% of open laminectomy cases [61]
Patients must be counseled to seek immediate medical attention if they experience LSS symptoms for prompt treatment and complication prevention.
Deterrence and Patient Education
LSS is often aging-related. However, certain lifestyle measures may help reduce the risk of developing or exacerbating this condition. Thus, primary prevention of LSS involves the following measures:
- Regular exercise for overall fitness
- Practicing good body mechanics
- Taking breaks from prolonged sitting
- Wearing comfortable footwear
- Maintaining a good posture
- Using ergonomic furniture and equipment
- Avoiding smoking
- Staying hydrated
- Stretching regularly
Patients must also be counseled to have an annual health checkup and consult immediately for low-back pain, especially if accompanied by sensorimotor deficits or lower extremity pain.
For patients diagnosed with LSS, interventions need to be undertaken in a stepwise fashion. Conservative modalities must be considered before operative interventions unless clinical red flags are present. When considering surgery, minimally invasive strategies must be considered before open procedures. Patient education about physical and psychological pain management is also important.[62]
Enhancing Healthcare Team Outcomes
LSS is a condition that exhibits increasing prevalence with age. Management is not always straightforward because aging-related problems like cardiovascular disease increase the condition's complexity and reduce patients' tolerance for aggressive treatments. Thus, an interprofessional team approach is best to improve outcomes. The members of the team should include the following:
- Primary care physicians: likely the first to evaluate the patient, provide initial treatment, and make referrals to other specialists.
- Radiologists: aid the team in interpreting imaging findings, which is crucial to management.
- Spinal surgeons: determine the need for spinal surgery and perform minimally invasive or open procedures if necessary.
- Neurologists: may be involved in the evaluation and treatment of patients presenting with neurological deficits.
- Neurosurgeons: may share their expertise in evaluating and treating patients presenting with a neurosurgical emergency like cauda equina or conus medullaris syndrome.
- Pain specialists: may provide anesthetic care to patients requiring spinal surgery or manage pain regimens in outpatient settings.
- Pharmacists: fill prescriptions and counsel patients about medications' therapeutic effects and avoiding toxicity.
- Physical therapists: design individualized exercise programs that address functional limitations associated with LSS; emphasize exercises that strengthen core muscles, improve flexibility, and promote overall stability.
- Psychologists: help patients develop coping mechanisms for LSS symptoms and the condition's impact on quality of life.
- Nurses: ensure patient comfort, administer medications, coordinate care, and educate patients on treatment adherence and health maintenance.
Effective communication and collaboration among these team members are essential for providing comprehensive care, optimizing outcomes, and ensuring a patient-centered approach.[63]
Media
(Click Image to Enlarge)
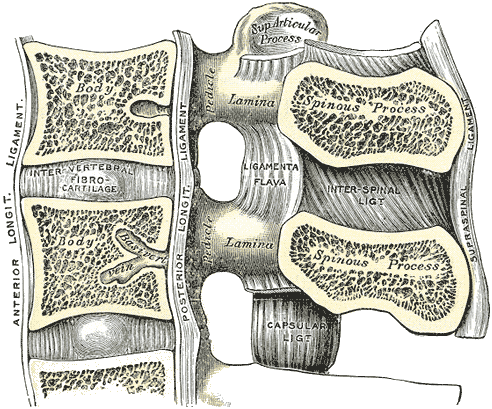
Lumbar Vertebral Anatomy. This medial sagittal section shows the relationship between the anterior longitudinal ligament, vertebral bodies, intervertebral fibrocartilage, posterior longitudinal ligament, vertebral laminae, ligamenta flava, superior articular processes, pedicles, spinous processes, interspinal ligament, capsular ligament, and supraspinal ligament.
Gray's Anatomy
(Click Image to Enlarge)
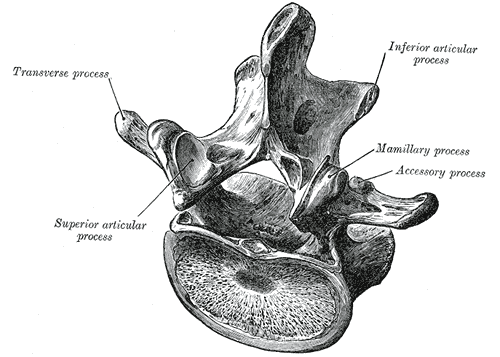
Lumbar Vertebra, Superoposterior View. This illustration shows the relationship between a typical lumbar vertebra's transverse process, superior and inferior articular processes, and mamillary and accessory processes. Shown but not labeled are the laminae, spinal canal, lateral recess, pedicles, and vertebral body.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
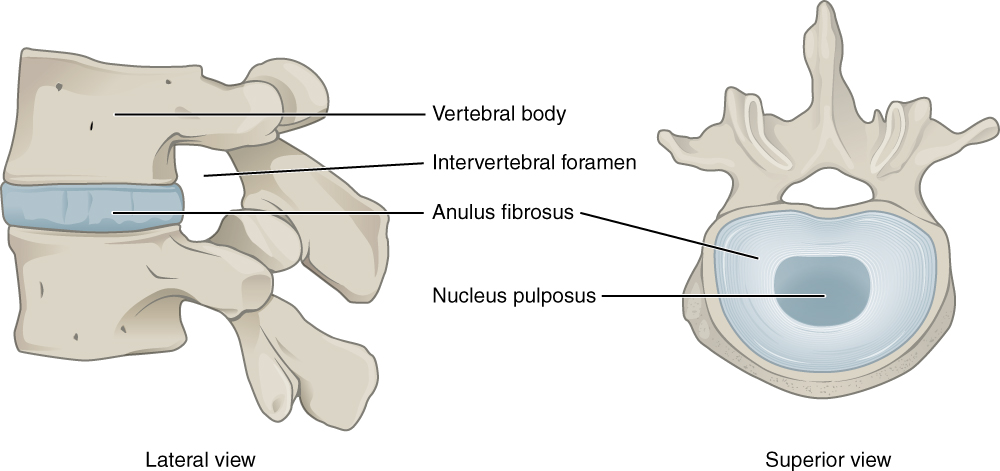
Intervertebral Disk. This lateral view shows the disc between two vertebrae. The superior view shows the annulus fibrosis at the outer layer and the nucleus pulposus in the inner layer.
Illustration from Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013. This file is licensed under the Creative Commons Attribution 3.0 Unported license.
(Click Image to Enlarge)
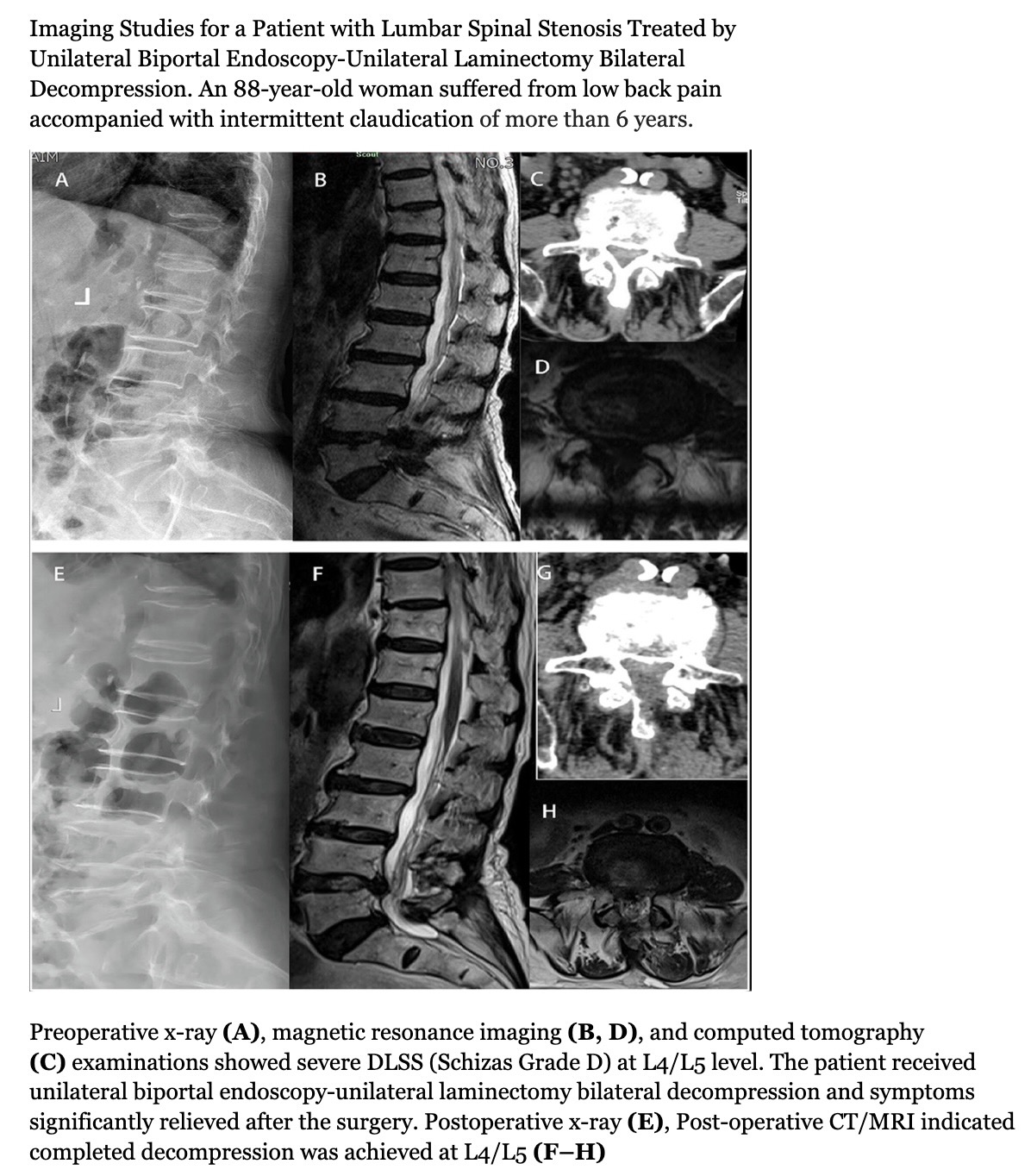
Imaging Studies for a Patient with Lumbar Spinal Stenosis Treated by Unilateral Biportal Endoscopy-Unilateral Laminectomy Bilateral Decompression.
Tan, B., Yang, Q., Fan, B., & Xiong, C. (2023). Decompression via unilateral biportal endoscopy for severe degenerative lumbar spinal stenosis: A comparative study with decompression via open discectomy. Frontiers in Neurology, 14, 1132698. https://doi.org/10.3389/fneur.2023.1132698
References
Bagley C, MacAllister M, Dosselman L, Moreno J, Aoun SG, El Ahmadieh TY. Current concepts and recent advances in understanding and managing lumbar spine stenosis. F1000Research. 2019:8():. pii: F1000 Faculty Rev-137. doi: 10.12688/f1000research.16082.1. Epub 2019 Jan 31 [PubMed PMID: 30774933]
Level 3 (low-level) evidenceCiol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. Journal of the American Geriatrics Society. 1996 Mar:44(3):285-90 [PubMed PMID: 8600197]
Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine. 2000 Feb 1:25(3):389-94 [PubMed PMID: 10703115]
Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical Versus Nonsurgical Treatment for Lumbar Spinal Stenosis. Spine. 2016 Jul 15:41(14):E857-E868. doi: 10.1097/BRS.0000000000001635. Epub [PubMed PMID: 27128388]
Lee SH, Choi HH, Chang MC. The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial. Toxins. 2022 Dec 23:15(1):. doi: 10.3390/toxins15010011. Epub 2022 Dec 23 [PubMed PMID: 36668831]
Level 1 (high-level) evidenceLaiwalla AN, Ratnaparkhi A, Zarrin D, Cook K, Li I, Wilson B, Florence TJ, Yoo B, Salehi B, Gaonkar B, Beckett J, Macyszyn L. Lumbar Spinal Canal Segmentation in Cases with Lumbar Stenosis Using Deep-U-Net Ensembles. World neurosurgery. 2023 Oct:178():e135-e140. doi: 10.1016/j.wneu.2023.07.009. Epub 2023 Jul 10 [PubMed PMID: 37437805]
Level 3 (low-level) evidenceKalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham Study. The spine journal : official journal of the North American Spine Society. 2009 Jul:9(7):545-50. doi: 10.1016/j.spinee.2009.03.005. Epub 2009 Apr 23 [PubMed PMID: 19398386]
Level 2 (mid-level) evidenceDeer TR, Grider JS, Pope JE, Lamer TJ, Wahezi SE, Hagedorn JM, Falowski S, Tolba R, Shah JM, Strand N, Escobar A, Malinowski M, Bux A, Jassal N, Hah J, Weisbein J, Tomycz ND, Jameson J, Petersen EA, Sayed D. Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST): Consensus Guidance from the American Society of Pain and Neuroscience (ASPN). Journal of pain research. 2022:15():1325-1354. doi: 10.2147/JPR.S355285. Epub 2022 May 5 [PubMed PMID: 35546905]
Level 3 (low-level) evidenceBinder DK, Schmidt MH, Weinstein PR. Lumbar spinal stenosis. Seminars in neurology. 2002 Jun:22(2):157-66 [PubMed PMID: 12524561]
Yabuki S, Fukumori N, Takegami M, Onishi Y, Otani K, Sekiguchi M, Wakita T, Kikuchi S, Fukuhara S, Konno S. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: a population-based study. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2013 Nov:18(6):893-900. doi: 10.1007/s00776-013-0455-5. Epub 2013 Aug 21 [PubMed PMID: 23963588]
Level 2 (mid-level) evidenceDiwan S, Sayed D, Deer TR, Salomons A, Liang K. An Algorithmic Approach to Treating Lumbar Spinal Stenosis: An Evidenced-Based Approach. Pain medicine (Malden, Mass.). 2019 Dec 1:20(Suppl 2):S23-S31. doi: 10.1093/pm/pnz133. Epub [PubMed PMID: 31808532]
Xu J, Si H, Zeng Y, Wu Y, Zhang S, Shen B. Transcriptome-wide association study reveals candidate causal genes for lumbar spinal stenosis. Bone & joint research. 2023 Jun 26:12(6):387-396. doi: 10.1302/2046-3758.126.BJR-2022-0160.R1. Epub 2023 Jun 26 [PubMed PMID: 37356815]
Walter KL, O'Toole JE. Lumbar Spinal Stenosis. JAMA. 2022 Jul 19:328(3):310. doi: 10.1001/jama.2022.6137. Epub [PubMed PMID: 35503646]
Buser Z, Ortega B, D'Oro A, Pannell W, Cohen JR, Wang J, Golish R, Reed M, Wang JC. Spine Degenerative Conditions and Their Treatments: National Trends in the United States of America. Global spine journal. 2018 Feb:8(1):57-67. doi: 10.1177/2192568217696688. Epub 2017 Apr 7 [PubMed PMID: 29456916]
Abbas J, Yousef M, Peled N, Hershkovitz I, Hamoud K. Predictive factors for degenerative lumbar spinal stenosis: a model obtained from a machine learning algorithm technique. BMC musculoskeletal disorders. 2023 Mar 23:24(1):218. doi: 10.1186/s12891-023-06330-z. Epub 2023 Mar 23 [PubMed PMID: 36949452]
Wang S, Qu Y, Fang X, Ding Q, Zhao H, Yu X, Xu T, Lu R, Jing S, Liu C, Wu H, Liu Y. Decorin: a potential therapeutic candidate for ligamentum flavum hypertrophy by antagonizing TGF-β1. Experimental & molecular medicine. 2023 Jul:55(7):1413-1423. doi: 10.1038/s12276-023-01023-y. Epub 2023 Jul 3 [PubMed PMID: 37394592]
Yabe Y, Hagiwara Y, Tsuchiya M, Minowa T, Takemura T, Hattori S, Yoshida S, Onoki T, Ishikawa K. Comparative proteome analysis of the ligamentum flavum of patients with lumbar spinal canal stenosis. JOR spine. 2022 Dec:5(4):e1210. doi: 10.1002/jsp2.1210. Epub 2022 Jun 9 [PubMed PMID: 36601375]
Level 2 (mid-level) evidenceJain M, Sable M, Tirpude AP, Sahu RN, Samanta SK, Das G. Histological difference in ligament flavum between degenerative lumbar canal stenosis and non-stenotic group: A prospective, comparative study. World journal of orthopedics. 2022 Sep 18:13(9):791-801. doi: 10.5312/wjo.v13.i9.791. Epub 2022 Sep 18 [PubMed PMID: 36189332]
Level 2 (mid-level) evidenceAltun S, Alkan A, Altun İ. LSS-VGG16: Diagnosis of Lumbar Spinal Stenosis With Deep Learning. Clinical spine surgery. 2023 Jun 1:36(5):E180-E190. doi: 10.1097/BSD.0000000000001418. Epub 2023 Jan 20 [PubMed PMID: 36727890]
Jinkins JR. Gd-DTPA enhanced MR of the lumbar spinal canal in patients with claudication. Journal of computer assisted tomography. 1993 Jul-Aug:17(4):555-62 [PubMed PMID: 8331225]
Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O'Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Annals of internal medicine. 1985 Aug:103(2):271-5 [PubMed PMID: 3160275]
Ujigo S, Kamei N, Yamada K, Nakamae T, Imada H, Adachi N, Fujimoto Y. Balancing ability of patients with lumbar spinal canal stenosis. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2023 Dec:32(12):4174-4183. doi: 10.1007/s00586-023-07782-6. Epub 2023 May 22 [PubMed PMID: 37217822]
Munakomi S, Kumar BM. Wasting of Extensor Digitorum Brevis as a Decisive Preoperative Clinical Indicator of Lumbar Canal Stenosis: A Single-center Prospective Cohort Study. Annals of medical and health sciences research. 2016 Sep-Oct:6(5):296-300. doi: 10.4103/amhsr.amhsr_392_15. Epub [PubMed PMID: 28503347]
Katz JN, Dalgas M, Stucki G, Katz NP, Bayley J, Fossel AH, Chang LC, Lipson SJ. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis and rheumatism. 1995 Sep:38(9):1236-41 [PubMed PMID: 7575718]
Staartjes VE, Schröder ML. The five-repetition sit-to-stand test: evaluation of a simple and objective tool for the assessment of degenerative pathologies of the lumbar spine. Journal of neurosurgery. Spine. 2018 Oct:29(4):380-387. doi: 10.3171/2018.2.SPINE171416. Epub 2018 Jun 29 [PubMed PMID: 29957147]
Oskouie IM, Rostami M, Moosavi M, Zarei M, Jouibari MF, Ataie H, Jafarieh A, Moghadam N, Kordi R, Khadivi M, Mazloumi A. Translation, cross-cultural adaptation and validation of the Persian version of selected PROMIS measures for use in lumbar canal stenosis patients. Journal of education and health promotion. 2023:12():99. doi: 10.4103/jehp.jehp_668_22. Epub 2023 Mar 31 [PubMed PMID: 37288413]
Level 1 (high-level) evidenceWang Y, Zhang P, Yan X, Wang J, Zhu M, Teng H. The correlation between lumbar interlaminar space size on plain radiograph and spinal stenosis. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2023 May:32(5):1721-1728. doi: 10.1007/s00586-023-07646-z. Epub 2023 Mar 20 [PubMed PMID: 36941496]
Saifuddin A. The imaging of lumbar spinal stenosis. Clinical radiology. 2000 Aug:55(8):581-94 [PubMed PMID: 10964728]
Pant YR, Paudel S, Lakhey RB, Pokharel RK. Nerve Root Sedimentation Sign among Lumbar Canal Stenosis Patients Visiting the Department of Orthopaedics in a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA; journal of the Nepal Medical Association. 2022 Dec 1:60(256):1030-1032. doi: 10.31729/jnma.7540. Epub 2022 Dec 1 [PubMed PMID: 36705116]
Level 2 (mid-level) evidenceAhn DY, Park HJ, Yi JW, Kim JN. To Assess Whether Lee's Grading System for Central Lumbar Spinal Stenosis Can Be Used as a Decision-Making Tool for Surgical Treatment. Taehan Yongsang Uihakhoe chi. 2022 Jan:83(1):102-111. doi: 10.3348/jksr.2021.0017. Epub 2021 Nov 4 [PubMed PMID: 36237366]
Ko YJ, Lee E, Lee JW, Park CY, Cho J, Kang Y, Ahn JM. Clinical validity of two different grading systems for lumbar central canal stenosis: Schizas and Lee classification systems. PloS one. 2020:15(5):e0233633. doi: 10.1371/journal.pone.0233633. Epub 2020 May 27 [PubMed PMID: 32459814]
Qian G, Wang Y, Huang J, Wang D, Miao C. Value of nerve root sedimentation sign in diagnosis and surgical indication of lumbar spinal stenosis. BMC musculoskeletal disorders. 2023 Apr 28:24(1):336. doi: 10.1186/s12891-023-06459-x. Epub 2023 Apr 28 [PubMed PMID: 37118727]
Level 2 (mid-level) evidenceLee GY, Lee JW, Choi HS, Oh KJ, Kang HS. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal radiology. 2011 Aug:40(8):1033-9. doi: 10.1007/s00256-011-1102-x. Epub 2011 Feb 1 [PubMed PMID: 21286714]
Fang X, Li J, Wang L, Liu L, Lv W, Tang Z, Gao D. Diagnostic value of a new axial loading MRI device in patients with suspected lumbar spinal stenosis. European radiology. 2023 May:33(5):3200-3210. doi: 10.1007/s00330-023-09447-w. Epub 2023 Feb 23 [PubMed PMID: 36814030]
Bharadwaj UU, Christine M, Li S, Chou D, Pedoia V, Link TM, Chin CT, Majumdar S. Deep learning for automated, interpretable classification of lumbar spinal stenosis and facet arthropathy from axial MRI. European radiology. 2023 May:33(5):3435-3443. doi: 10.1007/s00330-023-09483-6. Epub 2023 Mar 15 [PubMed PMID: 36920520]
Haig AJ, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, Chiodo A, Miner JA, Choksi VR, Geisser ME. The sensitivity and specificity of electrodiagnostic testing for the clinical syndrome of lumbar spinal stenosis. Spine. 2005 Dec 1:30(23):2667-76 [PubMed PMID: 16319753]
Nüesch C, Mandelli F, Przybilla P, Schären S, Mündermann A, Netzer C. Kinematics and paraspinal muscle activation patterns during walking differ between patients with lumbar spinal stenosis and controls. Gait & posture. 2023 Jan:99():44-50. doi: 10.1016/j.gaitpost.2022.10.017. Epub 2022 Oct 26 [PubMed PMID: 36327537]
Bakewell BK, Sherman M, Binsfeld K, Ilyas AM, Stache SA, Sharma S, Stolzenberg D, Greis A. The Use of Cannabidiol in Patients With Low Back Pain Caused by Lumbar Spinal Stenosis: An Observational Study. Cureus. 2022 Sep:14(9):e29196. doi: 10.7759/cureus.29196. Epub 2022 Sep 15 [PubMed PMID: 36507111]
Level 2 (mid-level) evidenceMazanec DJ, Podichetty VK, Hsia A. Lumbar canal stenosis: start with nonsurgical therapy. Cleveland Clinic journal of medicine. 2002 Nov:69(11):909-17 [PubMed PMID: 12430977]
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. The Clinical journal of pain. 1998 Jun:14(2):148-51 [PubMed PMID: 9647457]
Level 1 (high-level) evidenceRayegani SM, Soltani V, Cheraghi M, Omid Zohor MR, Babaei-Ghazani A, Raeissadat SA. Efficacy of ultrasound guided caudal epidural steroid injection with or without ozone in patients with lumbosacral canal stenosis; a randomized clinical controlled trial. BMC musculoskeletal disorders. 2023 Apr 29:24(1):339. doi: 10.1186/s12891-023-06451-5. Epub 2023 Apr 29 [PubMed PMID: 37120532]
Level 1 (high-level) evidenceMalmivaara A, Slätis P, Heliövaara M, Sainio P, Kinnunen H, Kankare J, Dalin-Hirvonen N, Seitsalo S, Herno A, Kortekangas P, Niinimäki T, Rönty H, Tallroth K, Turunen V, Knekt P, Härkänen T, Hurri H, Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007 Jan 1:32(1):1-8 [PubMed PMID: 17202885]
Level 1 (high-level) evidenceSüner HI, Castaño JP, Vargas-Jimenez A, Wagner R, Mazzei AS, Velazquez W, Jorquera M, Sallabanda K, Barcia Albacar JA, Carrascosa-Granada A. Comparison of the Tubular Approach and Uniportal Interlaminar Full-Endoscopic Approach in the Treatment of Lumbar Spinal Stenosis: Our 3-Year Results. World neurosurgery. 2023 May:173():e148-e155. doi: 10.1016/j.wneu.2023.02.022. Epub 2023 Feb 10 [PubMed PMID: 36775236]
Zhang Y, Feng B, Su W, Liu D, Hu P, Lu H, Geng X. [Early-effectiveness of unilateral biportal endoscopic laminectomy in treatment of two-level lumbar spinal stenosis]. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery. 2023 Jun 15:37(6):706-712. doi: 10.7507/1002-1892.202302014. Epub [PubMed PMID: 37331947]
Hu YT, Fu H, Yang DF, Wang X, Xu WB. [Comparative study of decompression of unilateral biportal endoscopic compared to laminectomy with fusion and internal fixation in the treatment of severe lumbar spinal stenosis]. Zhonghua yi xue za zhi. 2022 Nov 8:102(41):3281-3287. doi: 10.3760/cma.j.cn112137-20220720-01583. Epub [PubMed PMID: 36319180]
Level 2 (mid-level) evidenceLin GX, Yao ZK, Xin C, Kim JS, Chen CM, Hu BS. A meta-analysis of clinical effects of microscopic unilateral laminectomy bilateral decompression (ULBD) versus biportal endoscopic ULBD for lumbar canal stenosis. Frontiers in surgery. 2022:9():1002100. doi: 10.3389/fsurg.2022.1002100. Epub 2022 Sep 23 [PubMed PMID: 36211279]
Level 1 (high-level) evidencePatel K, Harikar MM, Venkataram T, Chavda V, Montemurro N, Assefi M, Hussain N, Yamamoto V, Kateb B, Lewandrowski KU, Umana GE. Is Minimally Invasive Spinal Surgery Superior to Endoscopic Spine Surgery in Postoperative Radiologic Outcomes of Lumbar Spine Degenerative Disease? A Systematic Review. Journal of neurological surgery. Part A, Central European neurosurgery. 2023 Aug 2:():. doi: 10.1055/a-2029-2694. Epub 2023 Aug 2 [PubMed PMID: 36746397]
Level 1 (high-level) evidenceJunjie L, Jiheng Y, Jun L, Haixiong L, Haifeng Y. Comparison of Unilateral Biportal Endoscopy Decompression and Microscopic Decompression Effectiveness in Lumbar Spinal Stenosis Treatment: A Systematic Review and Meta-analysis. Asian spine journal. 2023 Apr:17(2):418-430. doi: 10.31616/asj.2021.0527. Epub 2023 Feb 6 [PubMed PMID: 36740930]
Level 1 (high-level) evidenceMachado GC, Ferreira PH, Yoo RI, Harris IA, Pinheiro MB, Koes BW, van Tulder MW, Rzewuska M, Maher CG, Ferreira ML. Surgical options for lumbar spinal stenosis. The Cochrane database of systematic reviews. 2016 Nov 1:11(11):CD012421 [PubMed PMID: 27801521]
Level 1 (high-level) evidenceGupta S, Bansal T, Kashyap A, Sural S. Correlation between clinical scoring systems and quantitative MRI parameters in degenerative lumbar spinal stenosis. Journal of clinical orthopaedics and trauma. 2022 Dec:35():102050. doi: 10.1016/j.jcot.2022.102050. Epub 2022 Oct 20 [PubMed PMID: 36317084]
Inose H, Kato T, Matsukura Y, Hirai T, Yoshii T, Kawabata S, Takahashi K, Okawa A. Factors influencing the long-term outcomes of instrumentation surgery for degenerative lumbar spondylolisthesis: a post-hoc analysis of a prospective randomized study. The spine journal : official journal of the North American Spine Society. 2023 Jun:23(6):799-804. doi: 10.1016/j.spinee.2023.02.002. Epub 2023 Feb 11 [PubMed PMID: 36774998]
Level 1 (high-level) evidenceYeung CM, Heard JC, Lee Y, Lambrechts MJ, Somers S, Singh A, Bloom E, D'Antonio ND, Trenchfield D, Labarbiera A, Mangan JJ, Canseco JA, Woods BI, Kurd MF, Kaye ID, Lee JK, Hilibrand AS, Vaccaro AR, Kepler CK, Schroeder GD. The Implication of Preoperative Central Stenosis on Patient-Reported Outcomes After Lumbar Decompression Surgery. World neurosurgery. 2023 Jun 19:():. pii: S1878-8750(23)00806-9. doi: 10.1016/j.wneu.2023.06.038. Epub 2023 Jun 19 [PubMed PMID: 37343674]
Nagai S, Inagaki R, Michikawa T, Kawabata S, Ito K, Hachiya K, Takeda H, Ikeda D, Kaneko S, Yamada S, Fujita N. Efficacy of surgical treatment on polypharmacy of elderly patients with lumbar spinal canal stenosis: retrospective exploratory research. BMC geriatrics. 2023 Mar 24:23(1):169. doi: 10.1186/s12877-023-03853-x. Epub 2023 Mar 24 [PubMed PMID: 36964497]
Level 2 (mid-level) evidenceImai T, Nagai S, Michikawa T, Inagaki R, Kawabata S, Ito K, Hachiya K, Takeda H, Ikeda D, Yamada S, Fujita N, Kaneko S. Impact of Lumbar Surgery on Pharmacological Treatment for Patients with Lumbar Spinal Canal Stenosis: A Single-Center Retrospective Study. Journal of clinical medicine. 2023 Mar 20:12(6):. doi: 10.3390/jcm12062385. Epub 2023 Mar 20 [PubMed PMID: 36983385]
Level 2 (mid-level) evidenceYokoyama K, Ikeda N, Tanaka H, Ito Y, Sugie A, Yamada M, Wanibuchi M, Kawanishi M. Long-Term Changes in Sagittal Balance After Microsurgical Decompression of Lumbar Spinal Canal Stenosis in Elderly Patients: A Follow-Up Study for 5-Years After Surgery. World neurosurgery. 2023 Aug:176():e384-e390. doi: 10.1016/j.wneu.2023.05.069. Epub 2023 May 24 [PubMed PMID: 37236312]
D'Antonio ND, Lambrechts MJ, Trenchfield D, Sherman M, Karamian BA, Fredericks DJ, Boere P, Siegel N, Tran K, Canseco JA, Kaye ID, Rihn J, Woods BI, Hilibrand AS, Kepler CK, Vaccaro AR, Schroeder GD. Patient-specific Risk Factors Increase Episode of Care Costs After Lumbar Decompression. Clinical spine surgery. 2023 Oct 1:36(8):E339-E344. doi: 10.1097/BSD.0000000000001460. Epub 2023 Mar 30 [PubMed PMID: 37012618]
Murata S, Nagata K, Iwasaki H, Hashizume H, Yukawa Y, Minamide A, Nakagawa Y, Tsutsui S, Takami M, Taiji R, Kozaki T, J Schoenfeld A, K Simpson A, Yoshida M, Yamada H. Long-Term Outcomes after Selective Microendoscopic Laminotomy for Multilevel Lumbar Spinal Stenosis with and without Remaining Radiographic Stenosis: A 10-Year Follow-Up Study. Spine surgery and related research. 2022 Sep 27:6(5):488-496. doi: 10.22603/ssrr.2021-0200. Epub 2022 Feb 10 [PubMed PMID: 36348688]
Varol E, Etli MU, Avci F, Yaltirik CK, Ramazanoglu AF, Onen MR, Naderi S. Comparison of clinical and radiological results of dynamic and rigid instrumentation in degenerative lumbar spinal stenosis. Journal of craniovertebral junction & spine. 2022 Jul-Sep:13(3):350-356. doi: 10.4103/jcvjs.jcvjs_63_22. Epub 2022 Sep 14 [PubMed PMID: 36263334]
Aghajanloo M, Abdoli A, Poorolajal J, Abdolmaleki S. Comparison of clinical outcome of lumbar spinal stenosis surgery in patients with and without osteoporosis: a prospective cohort study. Journal of orthopaedic surgery and research. 2023 Jun 21:18(1):443. doi: 10.1186/s13018-023-03935-x. Epub 2023 Jun 21 [PubMed PMID: 37344883]
Level 2 (mid-level) evidenceLu C, Qiu H, Huang X, Yang X, Liu D, Zhang S, Zhang Y. Meta-Analysis of Simultaneous versus Staged Decompression of Stenotic Regions in Patients with Tandem Spinal Stenosis. World neurosurgery. 2023 Feb:170():e441-e454. doi: 10.1016/j.wneu.2022.11.028. Epub 2022 Nov 14 [PubMed PMID: 36396060]
Level 1 (high-level) evidenceEstefan M, Munakomi S, Camino Willhuber GO. Laminectomy. StatPearls. 2024 Jan:(): [PubMed PMID: 31194414]
Shaygan M, Zamani M, Jaberi A, Eghbal K, Dehghani A. The impact of physical and psychological pain management training on pain intensity, anxiety and disability in patients undergoing lumbar surgeries. The spine journal : official journal of the North American Spine Society. 2023 May:23(5):656-664. doi: 10.1016/j.spinee.2023.01.016. Epub 2023 Feb 1 [PubMed PMID: 36736739]
Gatam AR, Gatam L, Phedy, Mahadhipta H, Ajiantoro, Aprilya D. Full Endoscopic Lumbar Stenosis Decompression: A Future Gold Standard in Managing Degenerative Lumbar Canal Stenosis. International journal of spine surgery. 2022 Sep:16(5):821-830. doi: 10.14444/8338. Epub 2022 Sep 28 [PubMed PMID: 36171020]