Indications
Local anesthetics are classified into 2 primary categories: esters and amides. Esters (eg, cocaine, procaine, chloroprocaine, tetracaine) are metabolized by the enzyme pseudocholinesterase. In contrast, the liver metabolizes amides (eg, lidocaine, bupivacaine, prilocaine, and ropivacaine).[1] Formerly referred to as lignocaine, lidocaine is a tertiary amine anesthetic agent derived from xylidine and was first synthesized between 1943 and 1946 by Nils Löfgren and Bengt Lundquist (see Image. Lidocaine Molecule). This medication rapidly became used worldwide, given its superior safety profile compared to older local anesthetic agents.[2]
FDA-Approved Indications
Lidocaine is commonly used for local anesthesia and is often combined with epinephrine (which extends lidocaine's duration of action by opposing the local vasodilatory effects of lidocaine). Lidocaine can also be administered intravenously during tracheal intubation, obtunding the hypertensive response to laryngoscopy and potentially reducing the incidence of myalgia and hyperkalemia after succinylcholine is given. According to the Vaughan-Williams classification, lidocaine is a class Ib antiarrhythmic agent, and its use is indicated in the management of acute ventricular tachyarrythmias. Lidocaine is also FDA-approved as a treatment for ventricular dysrhythmias after cardiac surgery.[3]
Lidocaine may also be used as an adjuvant analgesic for patients with acute and chronic pain. According to the American Society of Regional Anesthesia and Pain Medicine (ASRA), perioperative lidocaine infusion may be a valuable adjunct in Enhanced Recovery After Surgery (ERAS) protocols for improved pain management.[4] The American College of Cardiology/American Heart Association (ACC/AHA) recommends considering lidocaine as a component of Advanced Cardiovascular Life Support (ACLS) interventions for patients experiencing cardiac arrest due to polymorphic ventricular tachycardia or ventricular fibrillation.[5] According to the American Heart Association (AHA), American College of Cardiology (ACC), and Heart Rhythm Society (HRS), amiodarone or lidocaine may be considered for patients with ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) that does not respond to defibrillation. These medications can be beneficial for patients with a witnessed cardiac arrest, as they may be administered relatively quickly.[6]
Off-Label Uses
The American Urological Society endorses intravesical lidocaine for interstitial cystitis/bladder pain syndrome.[7] Intraosseous lidocaine is administered for pain during the resuscitation of trauma patients.[8] The American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists recommend the consideration of intravenous lidocaine for adults undergoing open and laparoscopic abdominal surgeries, provided there are no contraindications. Intravenous lidocaine has been assessed as part of a multimodal analgesia approach.[9]
The Difficult Airway Society guidelines for awake tracheal intubation (ATI) suggest that topical lidocaine offers up to 40 minutes of analgesia, with variability based on concentration and administration. The return of laryngeal reflexes may be delayed. Due to lidocaine's terminal elimination half-life of up to 2 hours, patients should remain nil per os (NPO) for at least 2 hours post-application.[10]
Perioperative lidocaine infusion may be beneficial for bariatric patients, who are often more sensitive to the respiratory depressant effects of opioids. In patients undergoing bariatric surgery, lidocaine infusion has been shown to reduce 24-hour opioid consumption by 10 mg morphine equivalents compared to placebo, which is associated with improved recovery.[4]
Lidocaine is a potential treatment for chronic pain. Evidence for lidocaine's efficacy is less robust for patients with complex regional pain syndrome (CRPS) and cancer. However, lidocaine demonstrates significant efficacy as an adjunctive therapy for chronic post-surgical pain. Continued research is needed to understand better the mechanisms underlying lidocaine's effects on pain pathways.[11] Lidocaine and steroids can effectively alleviate chronic cervical radiculopathy symptoms using an ultrasound-guided selective nerve root block technique.[12] Epidural analgesia with fentanyl and lidocaine is equivalent to intrathecal fentanyl for pain relief during early labor, with similar efficacy, duration, and patient satisfaction.[13]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Similar to other local anesthetics, lidocaine acts at sodium ion channels on the internal surface of nerve cell membranes. The uncharged form of lidocaine diffuses through neural sheaths into the axoplasm before ionizing by combining with hydrogen ions. The resulting cation binds reversibly to sodium channels from the inside, locking them in the open state and preventing nerve depolarization. Lidocaine is a weak base with a dissociation constant (pKa) of 7.7. Approximately 25% of lidocaine molecules are neutrally charged at a physiological pH of 7.4 and can translocate inside the nerve cells, meaning lidocaine has a more rapid onset of action than other local anesthetics with higher pKa values.[14]
Lidocaine's efficacy is reduced at inflammation sites; this may be due to acidosis reducing the proportion of neutral lidocaine molecules, faster reductions in local lidocaine concentration due to increased blood flow, or increased production of inflammatory mediators like peroxynitrite, which act directly on sodium channels.[15] Lidocaine's function as an NMDA antagonist offers potential benefits for patients with difficult-to-control pain, such as mixed nociceptive-neuropathic pain and central sensitization.[11] In cardiac myocytes, lidocaine slows the rise of the cardiac action potential during phase 0, increasing the effective threshold potential. Increased blood levels of lidocaine can affect cardiac output, total peripheral resistance, and mean arterial pressure, likely due to its direct depressant effects on the cardiovascular system.
Pharmacokinetics
Absorption: An IV bolus of lidocaine has a very rapid onset of action. Lidocaine ointment provides local, topical anesthesia with an onset of action between 3 and 5 minutes. Absorption of lidocaine following topical application to mucous membranes varies based on the application site, concentration, duration, and dosage.
Distribution: Lidocaine is 65% protein-bound to albumin and α1-acid glycoprotein in the plasma, giving it a medium duration of action compared to other local anesthetic agents. This drug is less lipid-soluble than other agents, limiting its overall potency. Lidocaine's volume of distribution is 0.7 to 1.5 L/kg.
Metabolism: The hepatic enzymes CYP1A2 and CYP3A4 metabolize lidocaine into active and inactive molecules. The active metabolites of lidocaine are monoethylglycylxylidide (MEGX) and glycylxylidide (GX).[16]
Elimination: The half-life of lidocaine is approximately 1.5 to 2 hours and is prolonged in patients with congestive heart failure and hepatic impairment. Approximately 90% of the drug is excreted in the urine.
Administration
Available Dosage Forms and Strengths
Lidocaine is available as solutions, aqueous gels, and ointments of various strengths. Additionally, lidocaine may be combined with epinephrine or another local anesthetic (eg, prilocaine). Lidocaine is also a component of other products, including medicated plasters designed to treat chronic postherpetic neuralgia. Various administration routes require different preparations of lidocaine.
- Solutions of 0.05% to 0.1% can be injected subcutaneously in large volumes to provide tumescent local anesthesia. This results in swelling and firmness of the site, which may benefit certain surgical procedures.
- Solutions of 0.25% to 0.5% are used for intravenous regional anesthesia (Bier's block) or infiltration into subcutaneous tissue.
- Solutions of 1% to 2% are used for epidural anesthesia and regional nerve blocks and are available in intravenous preparations for antiarrhythmic use.
- Aqueous gels of 1% to 2% typically contain an antiseptic (eg, chlorhexidine) and are used to topicalize and lubricate the urethra before procedures such as Foley catheterization.
- Solutions of 4% are used for topical anesthesia of the mucous membranes of the airway, including the mouth, pharynx, and respiratory tract. These solutions are applied by gargling, spraying, or using an atomizer.
- Ointments typically contain 5% lidocaine mixed with hydrocortisone and are applied topically to other mucous membranes, such as the skin or the rectum.
- Solutions of 10% are applied topically for airway anesthesia using a metered-dose atomizer.
- An eutectic mixture of lidocaine and prilocaine (EMLA) combines the local anesthetics prilocaine and lidocaine. EMLA effectively penetrates the skin to provide local cutaneous anesthesia and is commonly used to avoid the pain associated with needle punctures.
- The aqueous preparations from 0.5% to 2% are available in plain forms or with 1 per 200000 epinephrine (dentistry sometimes uses versions with 1 per 100000 epinephrine or more) and with or without preservatives.
Adult Dosage
Doses used for infiltrative or regional anesthesia depend on the specific block. When lidocaine is used to obtund airway reflexes, the dose is 1 to 2 mg/kg, given 2 to 5 minutes before intubation. For cardiac dysrhythmias, the initial dose is 1 to 1.5 mg/kg given intravenously, optionally followed with an infusion.[5] As an adjuvant intravenous treatment for acute pain, a 2020 consensus statement suggested a loading dose of no more than 1.5mg/kg over 10 minutes, followed by an infusion of no more than 1.5mg/kg/h for no longer than 24 hours (with close monitoring for clinical response and signs of toxicity).[17] The American Society of Anesthesiologists panel noted the absence of definitive evidence regarding the optimal dosing of lidocaine. However, based on clinical experience, the ASA recommends administering an induction dose of 1.5 mg/kg, followed by an intraoperative infusion of 2 mg/kg/h for patients undergoing open and laparoscopic abdominal surgeries.[9] For awake intubation, effective topical application must be ensured. The maximum dose of lidocaine should not exceed 9 mg/kg of lean body weight. To minimize the risk of laryngospasm, clinicians should consider nebulized lidocaine and lower concentrations.[10]
Specific Patient Populations
Hepatic impairment: According to the American Association for the Study of Liver Diseases guidelines, a lidocaine patch can be used for pain relief in patients with decompensated cirrhosis; however, it should be used with caution.[18][19]
Renal impairment: Dosage adjustments are generally unnecessary for patients with renal impairment, as topical lidocaine demonstrates limited absorption.
Pregnancy considerations: Lidocaine is thought to cross the placenta by passive diffusion. According to the American College of Obstetricians and Gynecologists (ACOG), local anesthesia for the treatment of oral conditions, including lidocaine with or without epinephrine, is considered safe during pregnancy.[20] The maximum recommended dose of lidocaine is 4.5 mg/kg (300 mg) for plain formulations and 7 mg/kg (500 mg) when used with epinephrine.[1] A pregnant woman should not be denied or have necessary surgery postponed due to pregnancy, as such delays could negatively impact both the woman and the fetus. Current evidence indicates that in utero, exposure to anesthetic or sedative drugs does not appear to affect fetal brain development. Additionally, animal studies have not shown adverse effects from limited exposures lasting less than 3 hours.
Breastfeeding considerations: Lidocaine concentrations in breast milk are low following continuous intravenous infusion, epidural administration, or high-dose local anesthesia, placing breastfeeding infants at minimal risk of exposure. Therefore, lidocaine is unlikely to cause adverse effects in breastfed infants, and no special precautions are necessary. Concerns regarding lidocaine's potential interference with breastfeeding, particularly when used with other anesthetics and analgesics, are debated due to variability in study designs and methodologies. With adequate breastfeeding support, epidural lidocaine combined with opioids typically has a minimal impact on breastfeeding success, although labor pain medication may delay the onset of lactation.[21]
Pediatric patients: Neonates have an immature metabolic clearance physiology, which increases their risk of drug and metabolite accumulation. Additionally, the α1-acid glycoprotein levels in neonates and infants are lower, with AAG at birth about half that of adults. This results in a higher unbound fraction of lidocaine, an extended elimination half-life, and an increased risk of accumulation, particularly with continuous infusions.[22]
Topical lidocaine must be dosed carefully in pediatric patients to prevent overdose. The maximum dose is based on weight or age for otherwise healthy children older than 3 years. In infants and children younger than 3 years, no more than 1.2 mL of the 2% solution should be applied, with at least a 3-hour interval between doses and a maximum of 4 doses within 12 hours.
Systemic lidocaine infusions are well-established for managing postoperative acute pain in pediatric patients but are less validated for other types of pediatric pain. The NMDA antagonist properties of lidocaine offer potential benefits for difficult-to-control pain, such as mixed nociceptive-neuropathic pain and central sensitization. Dosing for these indications typically includes a 1.5 mg/kg bolus followed by a 1 mg/kg/hr infusion, with higher doses utilized in pediatric oncology settings. Additional studies are needed to understand the safety and effectiveness of lidocaine infusions in children and refine dosing recommendations for different types of pain. Further research is also required to determine the importance of monitoring plasma concentrations during continuous infusion.[23]
Older patients: Local anesthesia should be prioritized for surgical procedures in older adults. The lowest effective volume and concentration should be administered to reduce the risk of systemic toxicity. For lidocaine, the recommended concentration is 10 mg/mL, and dosing should not exceed 5 mg/kg. This approach provides effective local anesthesia for many surgical procedures while reducing the risk of postoperative complications.[24]
Adverse Effects
Most adverse reactions associated with lidocaine occur when plasma concentrations rise to toxic levels. The drug reaches the intravascular compartment most rapidly when administered into the intercostal space, followed by the caudal, epidural, brachial plexus, femoral, and subcutaneous spaces. The maximum safe dose by body weight may range from 3 mg/kg to 7 mg/kg when using formulations containing epinephrine, although various other doses have been documented. Smaller doses can still result in adverse effects and toxicity if administered intravenously. Lidocaine is thought to be more neurotoxic than other local anesthetics, especially when high concentrations are applied directly to nervous tissue. Use of highly concentrated lidocaine (2.5% to 5%) for spinal anesthesia correlates with a greater incidence of transient radicular irritation syndrome, which is a painful but self-limiting condition affecting the calves, thighs, and buttocks.[25]
Drug-Drug Interactions
- The administration of lidocaine with propranolol significantly increases lidocaine serum concentration.
- Caution is advised when administering lidocaine hydrochloride in patients with digitalis toxicity and atrioventricular block.
- Lidocaine is a substrate of CYP1A2, CYP2B6, and CYP2D6 and exerts an inhibitory effect on CYP1A2. Caution is advised when co-administering lidocaine with fluvoxamine.[26][27]
- Patients may be at increased risk of developing methemoglobinemia if co-administered local anesthetics (eg, lidocaine) and other drugs, including nitrates, nitric oxide, hydroxyurea, dapsone, sulfonamides, chloroquine, phenobarbital, and phenytoin.[28] Methemoglobinemia can also occur due to lidocaine metabolism to O-toluidine.[29] This metabolite is more likely to be present when very high doses are given, but it may also occur with lower doses when the patient is taking other medications that can precipitate methemoglobinemia or have a hemoglobinopathy or another cause of anemia.
Contraindications
Lidocaine is contraindicated for patients with a known severe adverse reaction. Anaphylactic reactions to lidocaine are possible but rare. Methemoglobinemia is possible in patients with hemoglobinopathy or another cause of anemia. Lidocaine should not be used as an antiarrhythmic if the dysrhythmia may be secondary to local anesthetic toxicity.
Box Warnings (Lidocaine Viscous)
Life-threatening events in young children: Severe adverse events, including seizures, cardiopulmonary arrest, and death, have been reported in children younger than 3 years due to improper use of lidocaine viscous 2%. This drug should not be administered for teething pain and should be used in this age group only when no safer alternatives are available.[30] Strict adherence to dosing guidelines and secure storage are essential to mitigate risks. This FDA-issued boxed warning pertains to the abovementioned circumstances and does not extend to all formulations, such as IV lidocaine.
Warning and Precautions
Previous studies have reported that lidocaine suppresses premature ventricular complexes (PVCs) and nonsustained ventricular tachycardia (NSVT), which were thought to precede ventricular fibrillation (VF) or pulseless VT (pVT). However, recent findings have linked prophylactic lidocaine administration to suppress PVCs with higher mortality following acute myocardial infarction, likely due to increased asystole and bradyarrhythmias, leading to the abandonment of routine prophylactic use. Observational studies indicate that while lidocaine reduces VF/pVT recurrence after the return of spontaneous circulation (ROSC), it does not improve survival and has no benefit for patients with non-shockable rhythms.[6]
Monitoring
Lidocaine has a narrow therapeutic index, and plasma-level monitoring may be necessary for patients with hepatic impairment who require prolonged infusions. The patient's ideal body weight should be used instead of actual body weight for dose calculations to avoid excessively high plasma concentrations (with an absolute upper limit of 120 mg/hr for lidocaine infusions).[17] Vital signs and EKG should be monitored. Clinicians should use validated tools such as the McGill Pain Questionnaire (SF-MPQ) and the Visual Analog Scale (VAS).[31][17]
Lidocaine preparations containing epinephrine cause demonstrable cardiovascular effects even if only given in small amounts, and it is prudent for essential hemodynamic monitoring to be carried out before and during the use of solutions containing vasopressors, particularly if there is any specific concern over the patient's cardiovascular status.[32]
Toxicity
Signs and Symptoms of Overdose
Signs and symptoms of mild toxicity become apparent at plasma levels greater than 5 μg/mL, beginning with slurred speech, tinnitus, circumoral paresthesia, and lightheadedness. Above 10 μg/mL, the patient may experience seizures or loss of consciousness. The myocardium and central nervous system are further depressed at 15 μg/mL, progressing to cardiac arrhythmias, respiratory arrest, and cardiac arrest above 20 μg/mL.[32] Animal studies suggest that the dose of lidocaine required to cause cardiovascular collapse is 7.1 ± 1.1 times higher than the dose needed to induce central nervous system effects.[33] This so-called "(cardiovascular collapse/CNS (CC/CNS) ratio" is significantly higher than the ratio for other local anesthetic agents; bupivacaine has a CC/CNS ratio of around 2.0. In the event of toxic dosing in the conscious patient, lidocaine may be less likely than other local anesthetics to progress rapidly from neurological effects to complete cardiovascular collapse. By contrast, neurological signs and symptoms can often be masked if the patient is under the concurrent effects of sedation or general anesthesia, meaning that cardiovascular instability or arrhythmias may be the first manifestations.[34]
Management of Overdose
Lidocaine administration should be stopped immediately if toxicity or overdose is suspected. During cardiorespiratory collapse, airway support and breathing assistance should be prioritized to prevent the development of respiratory acidosis, which may exacerbate toxicity and potentiate lidocaine's negative chronotropic and inotropic effects.[35] Vital function support, including oxygen, IV fluids, and inotropes, should be instituted if required. Intravenous lipid emulsion is indicated as rescue therapy, especially if the cardiovascular collapse is refractory.[36]
Enhancing Healthcare Team Outcomes
All interprofessional healthcare team members, including clinicians, nurses, and pharmacists, should be knowledgeable about its toxicity and management. Lidocaine may cause significant pain during initial injection due to the agent stimulating nociceptors before it exerts its effects on sodium channels; this can be counteracted by buffering the lidocaine with small volumes of sodium bicarbonate shortly before use, making the solution less acidic.[37] Injection pain can also be reduced by warming the solution to body temperature, injecting more slowly, using narrow cannulas, and injecting perpendicular to the skin.[38] Patients receiving IV infusions of lidocaine should be regarded as high-risk; a 2020 consensus statement suggested that, when used outside of the operating room or post-anesthesia care unit, patients receiving IV lidocaine infusions should be managed in a high-dependency setting/intermediate care unit (IMC) with continuous monitoring. The infusion should be delivered through a separate, dedicated cannula using a tamper-proof pump.[17]
Media
(Click Image to Enlarge)
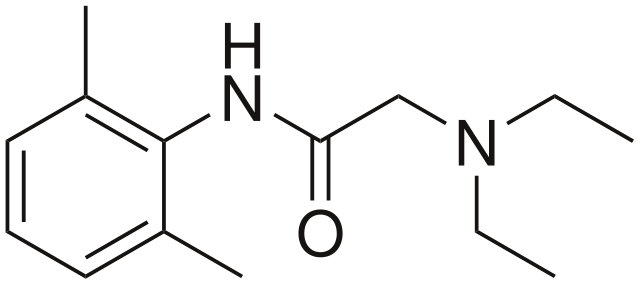
Lidocaine Molecule. The skeletal formula of the lidocaine molecule shows its aromatic ring, the amide link, and the basic amine side group.
Harbin, Public Domain, via Wikimedia Commons
References
Toledano RD, Kodali BS, Camann WR. Anesthesia drugs in the obstetric and gynecologic practice. Reviews in obstetrics & gynecology. 2009 Spring:2(2):93-100 [PubMed PMID: 19609403]
Calatayud J, González A. History of the development and evolution of local anesthesia since the coca leaf. Anesthesiology. 2003 Jun:98(6):1503-8 [PubMed PMID: 12766665]
Leeuwenburgh BP, Versteegh MI, Maas JJ, Dunning J. Should amiodarone or lidocaine be given to patients who arrest after cardiac surgery and fail to cardiovert from ventricular fibrillation? Interactive cardiovascular and thoracic surgery. 2008 Dec:7(6):1148-51. doi: 10.1510/icvts.2008.188656. Epub 2008 Sep 16 [PubMed PMID: 18796471]
Dunn LK, Durieux ME. Perioperative Use of Intravenous Lidocaine. Anesthesiology. 2017 Apr:126(4):729-737. doi: 10.1097/ALN.0000000000001527. Epub [PubMed PMID: 28114177]
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25:138(13):e272-e391. doi: 10.1161/CIR.0000000000000549. Epub [PubMed PMID: 29084731]
Level 1 (high-level) evidencePanchal AR, Berg KM, Kudenchuk PJ, Del Rios M, Hirsch KG, Link MS, Kurz MC, Chan PS, Cabañas JG, Morley PT, Hazinski MF, Donnino MW. 2018 American Heart Association Focused Update on Advanced Cardiovascular Life Support Use of Antiarrhythmic Drugs During and Immediately After Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2018 Dec 4:138(23):e740-e749. doi: 10.1161/CIR.0000000000000613. Epub [PubMed PMID: 30571262]
Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. The Journal of urology. 2022 Jul:208(1):34-42. doi: 10.1097/JU.0000000000002756. Epub 2022 May 10 [PubMed PMID: 35536143]
Qasim ZA, Joseph B. Intraosseous access in the resuscitation of patients with trauma: the good, the bad, the future. Trauma surgery & acute care open. 2024:9(Suppl 2):e001369. doi: 10.1136/tsaco-2024-001369. Epub 2024 Apr 15 [PubMed PMID: 38646033]
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The journal of pain. 2016 Feb:17(2):131-57. doi: 10.1016/j.jpain.2015.12.008. Epub [PubMed PMID: 26827847]
Level 1 (high-level) evidenceAhmad I, El-Boghdadly K, Bhagrath R, Hodzovic I, McNarry AF, Mir F, O'Sullivan EP, Patel A, Stacey M, Vaughan D. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia. 2020 Apr:75(4):509-528. doi: 10.1111/anae.14904. Epub 2019 Nov 14 [PubMed PMID: 31729018]
Onyeaka H, Adeola J, Xu R, Pappy AL, Adeola S, Smucker M, Chang A, Fraga A, Ufondu W, Osman M, Hasoon J, Orhurhu VJ. Intravenous Lidocaine for the Management of Chronic Pain: A Narrative Review of Randomized Clinical Trials. Psychopharmacology bulletin. 2024 Jul 8:54(3):73-96 [PubMed PMID: 38993659]
Level 1 (high-level) evidenceJoo HJ, Choi S, Kim BH, Kim MS, Shim GY, Chung SJ, Chon J, Yoo MC, Soh Y. Therapeutic Efficacy of Ultrasound-Guided Selective Nerve Block on Chronic Cervical Radiculopathy. Medicina (Kaunas, Lithuania). 2024 Jun 19:60(6):. doi: 10.3390/medicina60061002. Epub 2024 Jun 19 [PubMed PMID: 38929619]
Salmi L, Jernman R, Väänänen A. Is epidural analgesia non-inferior to intrathecal fentanyl as initiation for neuraxial analgesia in early non-spontaneous labour? Acta anaesthesiologica Scandinavica. 2024 May:68(5):664-674. doi: 10.1111/aas.14389. Epub 2024 Feb 16 [PubMed PMID: 38366324]
Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiology clinics of North America. 2000 Jun:18(2):217-33, v [PubMed PMID: 10935008]
Ueno T, Tsuchiya H, Mizogami M, Takakura K. Local anesthetic failure associated with inflammation: verification of the acidosis mechanism and the hypothetic participation of inflammatory peroxynitrite. Journal of inflammation research. 2008:1():41-8 [PubMed PMID: 22096346]
Kim JH, Kang DW, Choi GW, Lee SB, Lee S, Cho HY. Evaluation of Lidocaine and Metabolite Pharmacokinetics in Hyaluronic Acid Injection. Pharmaceutics. 2021 Feb 2:13(2):. doi: 10.3390/pharmaceutics13020203. Epub 2021 Feb 2 [PubMed PMID: 33540917]
Foo I, Macfarlane AJR, Srivastava D, Bhaskar A, Barker H, Knaggs R, Eipe N, Smith AF. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021 Feb:76(2):238-250. doi: 10.1111/anae.15270. Epub 2020 Nov 3 [PubMed PMID: 33141959]
Level 3 (low-level) evidenceRogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD, Kanwal F. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology (Baltimore, Md.). 2022 Sep:76(3):819-853. doi: 10.1002/hep.32378. Epub 2022 Apr 22 [PubMed PMID: 35103995]
Daraz YM, Abdelghffar OH. Lidocaine Infusion: An Antiarrhythmic With Neurologic Toxicities. Cureus. 2022 Mar:14(3):e23310. doi: 10.7759/cureus.23310. Epub 2022 Mar 19 [PubMed PMID: 35464548]
. Committee Opinion No. 569: oral health care during pregnancy and through the lifespan. Obstetrics and gynecology. 2013 Aug:122(2 Pt 1):417-422. doi: 10.1097/01.AOG.0000433007.16843.10. Epub [PubMed PMID: 23969828]
Level 3 (low-level) evidence. Lidocaine. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000289]
Heath C, Hii J, Thalayasingam P, von Ungern-Sternberg BS, Sommerfield D. Perioperative intravenous lidocaine use in children. Paediatric anaesthesia. 2023 May:33(5):336-346. doi: 10.1111/pan.14608. Epub 2022 Dec 4 [PubMed PMID: 36424875]
Hall EA, Sauer HE, Davis MS, Anghelescu DL. Lidocaine Infusions for Pain Management in Pediatrics. Paediatric drugs. 2021 Jul:23(4):349-359. doi: 10.1007/s40272-021-00454-2. Epub 2021 May 26 [PubMed PMID: 34036532]
Cuvillon P, Lefrant JY, Gricourt Y. Considerations for the Use of Local Anesthesia in the Frail Elderly: Current Perspectives. Local and regional anesthesia. 2022:15():71-75. doi: 10.2147/LRA.S325877. Epub 2022 Aug 10 [PubMed PMID: 35982729]
Level 3 (low-level) evidenceZaric D, Christiansen C, Pace NL, Punjasawadwong Y. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. The Cochrane database of systematic reviews. 2003:(2):CD003006 [PubMed PMID: 12804450]
Level 1 (high-level) evidenceKonieczny KM, Dorian P. Clinically Important Drug-Drug Interactions Between Antiarrhythmic Drugs and Anticoagulants. The Journal of innovations in cardiac rhythm management. 2019 Mar:10(3):3552-3559. doi: 10.19102/icrm.2019.100304. Epub 2019 Mar 15 [PubMed PMID: 32494414]
Isohanni MH, Neuvonen PJ, Olkkola KT. Effect of fluvoxamine and erythromycin on the pharmacokinetics of oral lidocaine. Basic & clinical pharmacology & toxicology. 2006 Aug:99(2):168-72 [PubMed PMID: 16918719]
Iolascon A, Bianchi P, Andolfo I, Russo R, Barcellini W, Fermo E, Toldi G, Ghirardello S, Rees D, Van Wijk R, Kattamis A, Gallagher PG, Roy N, Taher A, Mohty R, Kulozik A, De Franceschi L, Gambale A, De Montalembert M, Forni GL, Harteveld CL, Prchal J, SWG of red cell and iron of EHA and EuroBloodNet. Recommendations for diagnosis and treatment of methemoglobinemia. American journal of hematology. 2021 Dec 1:96(12):1666-1678. doi: 10.1002/ajh.26340. Epub 2021 Sep 23 [PubMed PMID: 34467556]
Barash M, Reich KA, Rademaker D. Lidocaine-induced methemoglobinemia: a clinical reminder. The Journal of the American Osteopathic Association. 2015 Feb:115(2):94-8. doi: 10.7556/jaoa.2015.020. Epub [PubMed PMID: 25637615]
Meyers RS, Thackray J, Matson KL, McPherson C, Lubsch L, Hellinga RC, Hoff DS. Key Potentially Inappropriate Drugs in Pediatrics: The KIDs List. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2020:25(3):175-191. doi: 10.5863/1551-6776-25.3.175. Epub [PubMed PMID: 32265601]
Carroll IR, Younger JW, Mackey SC. Pain quality predicts lidocaine analgesia among patients with suspected neuropathic pain. Pain medicine (Malden, Mass.). 2010 Apr:11(4):617-21. doi: 10.1111/j.1526-4637.2010.00807.x. Epub 2010 Mar 4 [PubMed PMID: 20210867]
Level 2 (mid-level) evidenceBecker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesthesia progress. 2012 Summer:59(2):90-101; quiz 102-3. doi: 10.2344/0003-3006-59.2.90. Epub [PubMed PMID: 22822998]
Morishima HO, Pedersen H, Finster M, Hiraoka H, Tsuji A, Feldman HS, Arthur GR, Covino BG. Bupivacaine toxicity in pregnant and nonpregnant ewes. Anesthesiology. 1985 Aug:63(2):134-9 [PubMed PMID: 4025863]
Level 3 (low-level) evidenceYukioka H, Hayashi M, Fujimori M. Lidocaine intoxication during general anesthesia. Anesthesia and analgesia. 1990 Aug:71(2):207-8 [PubMed PMID: 2375528]
Level 3 (low-level) evidenceCovino BG. Recent advances in local anaesthesia. Canadian Anaesthetists' Society journal. 1986 May:33(3 Pt 2):S5-8 [PubMed PMID: 3521804]
Level 3 (low-level) evidenceSekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute medicine & surgery. 2017 Apr:4(2):152-160. doi: 10.1002/ams2.265. Epub 2017 Mar 6 [PubMed PMID: 29123854]
Skarsvåg TI, Wågø KJ, Tangen LF, Lundbom JS, Hjelseng T, Ballo S, Finsen V. Does adjusting the pH of lidocaine reduce pain during injection? Journal of plastic surgery and hand surgery. 2015 Oct:49(5):265-267. doi: 10.3109/2000656X.2015.1047780. Epub 2015 May 19 [PubMed PMID: 25991379]
Finsen V. Reduced pain when injecting lidocaine. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2017 May:137(9):629-630. doi: 10.4045/tidsskr.16.0515. Epub 2017 May 2 [PubMed PMID: 28468478]