Introduction
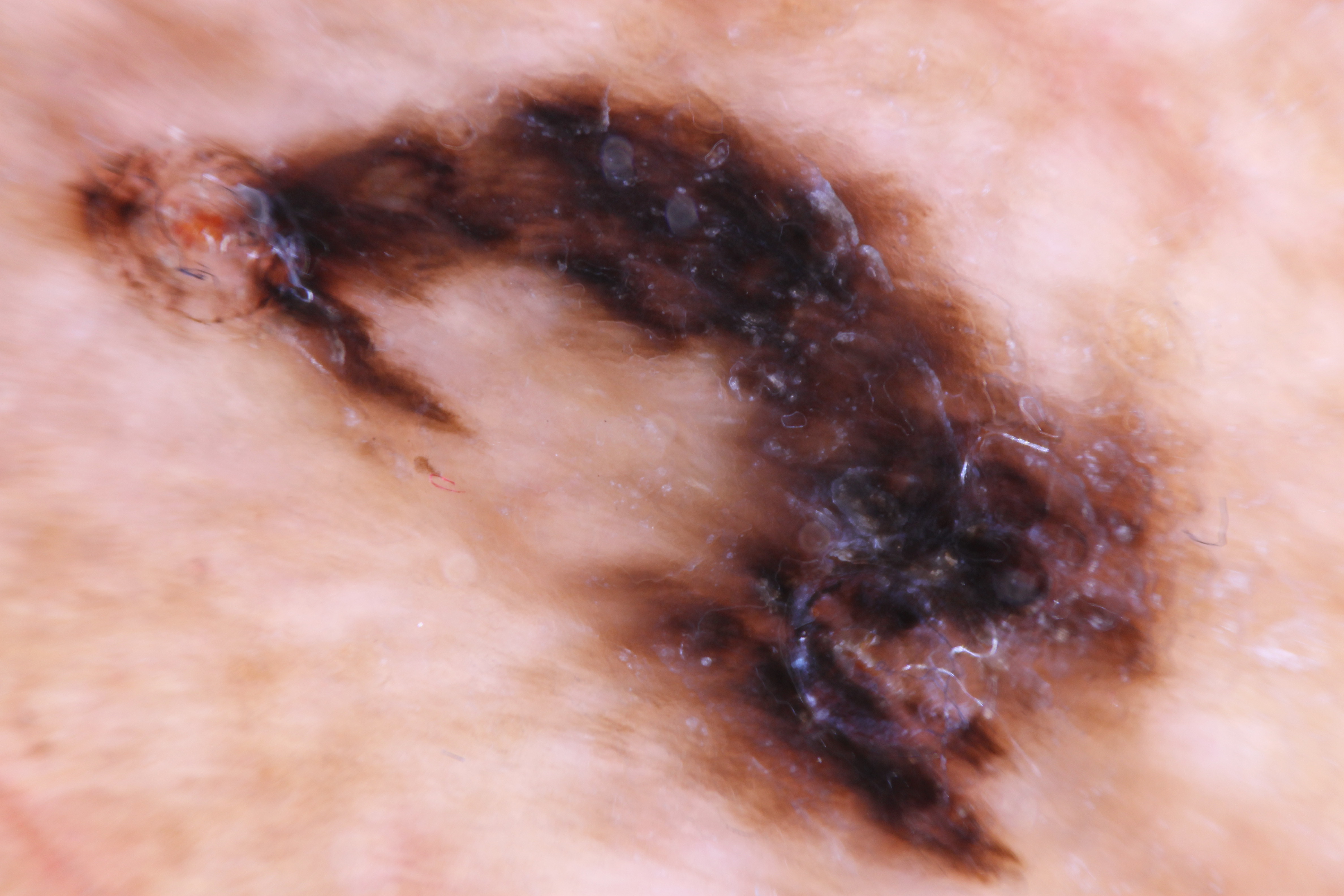
Lentigo maligna (LM) is a subtype of melanoma. It commonly presents as an irregular brown macule on chronically sun-damaged skin, particularly the head and neck, in the elderly (see Image. Asymmetric Parallel Ridge Dermoscopic Pattern Indicative of Acral Lentiginous Melanoma on the Heel of a 62-Year-Old Male).[1] It was first described by Hutchinson in 1890 and referred to as “Hutchinson’s melanotic freckle.”[2] For much of the early 20th century, LM was thought to be either benign, infectious, or precancerous due to its slow growth, with names given such as “junctional nevus,” “infective senile freckles,” and “circumscribed precancerous melanosis.”[2] It was not until the late 1970s-80s, spearheaded by research by Silvers, Ackerman, and colleagues that LM became widely recognized as malignant. Today, LM is defined as melanoma in situ (MIS) on chronically sun-damaged skin.[3][4][3][5] Therefore, by definition, LM is confined to the epidermis. The lesion is termed lentigo maligna melanoma (LMM) if it becomes invasive. See Image. Malignant Melanoma. Herein, we review the key aspects of LM/LMM and discuss this disease's unique diagnostic and treatment challenges.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The major risk factor for developing LM/LMM is ultraviolet radiation (UVR), particularly cumulative lifetime UVR exposure.[6][7][8][9] Several studies have demonstrated that LM/LMM is strongly associated with chronic UVR exposure, as opposed to nodular melanoma (NM) and superficial spreading melanoma (SSM), which are associated with intense intermittent UVR exposure.[7][8][9][10][11][12][13][14] An Australian study showed an increased risk of LM with the number of years lived in Australia, hours of sunlight, amount of actinic damage, and prior history of nonmelanoma skin cancer.[9] LM/LMM is also most likely to occur on the face, a site of chronic sun damage, whereas SSM and NM are more likely to occur on the trunk in men and legs in women, sites which are more often protected.[6] Finally, LM tends to occur in older patients compared to SSM and NM, presumably due to the increased lifetime sun exposure in older individuals.[15][16] Aside from UVR, x-ray irradiation, estrogen/progesterone, and nonpermanent hair dyes have been suggested risk factors.[2] LM is also more likely in genetic conditions predisposing to sun sensitivity, including oculocutaneous albinism, xeroderma pigmentosum, Werner syndrome, and porphyria cutanea tarda.[2] There have been no associations demonstrated with smoking or alcohol.[17]
Epidemiology
LM/LMM is the third most common subtype of melanoma (behind SSM and NM), comprising 4 to 15% of all melanomas and 10 to 26% of melanomas on the head and neck.[2] The mean age of diagnosis is 66-72 years, compared to 45- 57 years for other melanoma subtypes.[14][18][19][20] Women are more often affected than men (ratio 1.7 to 1) and are slightly older at diagnosis.[14][21] The incidence of LM/LMM has been rising over the past few decades, with data from Olmstead County, Minnesota, showing an increase from 2.2 cases/100,000 person-years between 1970 and 1989 to 13.7 cases/100,000 person-years between 2004 and 2007.[22] A similar rise in incidence was observed in the Netherlands.[23] Data from California showed a particularly rapid 52% rise in incidence among younger people ages 45 to 64 between 1990 and 2000.[18] It is unclear whether these data represent true increases in incidence or improved diagnosis.[24] The faster rise in the incidence of MIS compared to invasive melanoma suggests that detection bias plays some role at least.[25]
Pathophysiology
Melanoma has one of the highest mutational loads of all malignancies [26], and LM/LMM has an especially high mutation rate due to chronic UVR exposure.[27][28][29] UVR causes oxidative damage and produces signature C>T and CC>TT mutations, which alter genes involved in the MAPK and PI3K pathways, including BRAF, NRAS, and KIT. Secondary mutations in CCND1, CDKN2A, or p53 then lead to transformation into a malignant tumor.[30][31] LM/LMM is more likely to harbor mutations in KIT than other subtypes of melanoma, in which BRAF mutations are more common.[32][33][34] CCND1, MITF, NRAS, and p53 mutations also play a pathogenic role.[31]
Histopathology
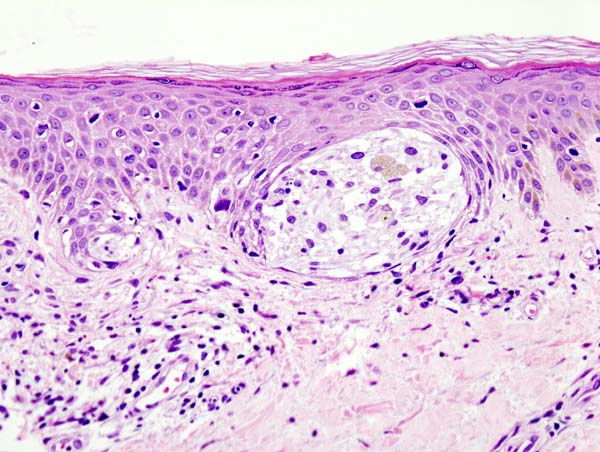
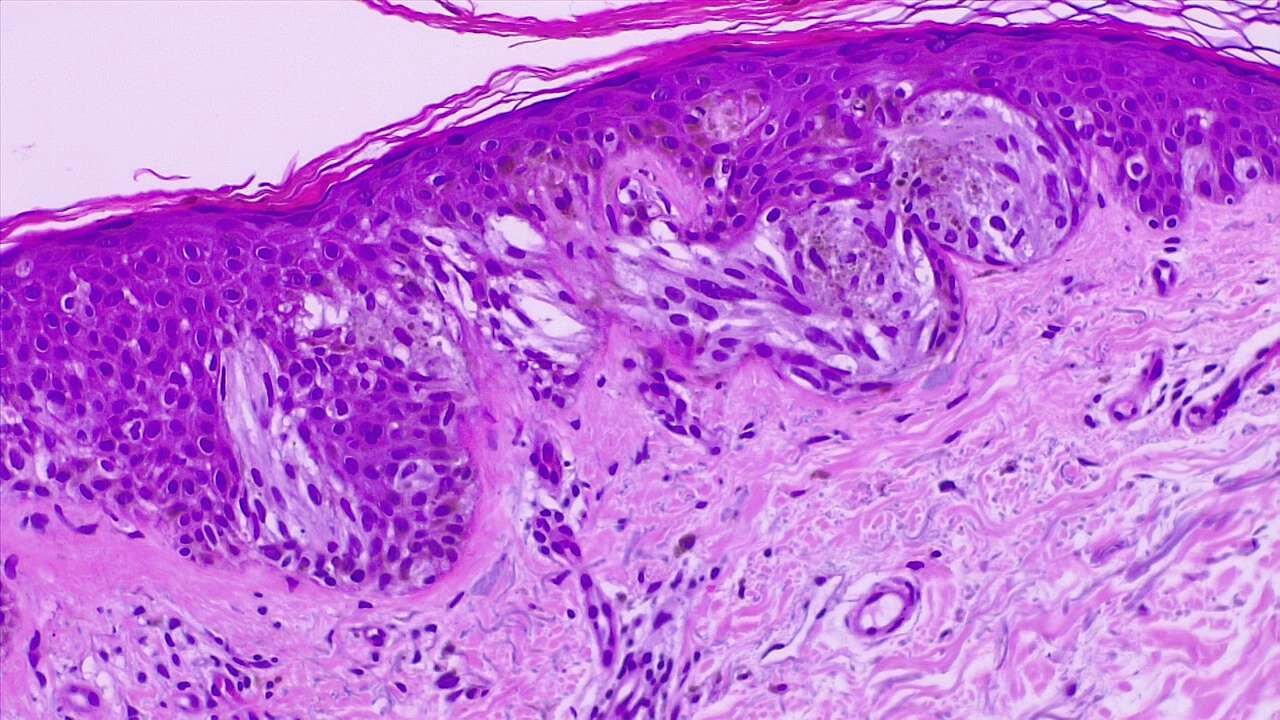
LM is characterized by a proliferation of atypical melanocytes along the dermal-epidermal junction (DEJ). Early LM may be difficult to distinguish from benign changes of chronic solar damage, which also induces melanocytic hyperplasia.[35] For this reason, a control biopsy of non-lesional but sun-damaged skin is sometimes recommended.[36] Melanocytes are typically singly arranged, although nests and multinucleated cells may be seen (see Image. Malignant Melanoma of the Skin). Pagetoid spread may occur, but less frequently than in superficial spreading melanoma. Periadnexal extension is common with the involvement of the follicular outer root sheath and eccrine duct. The tumor cells often have a conspicuous cytoplasmic retraction artifact, and nuclei are often enlarged, hyperchromatic, and angulated. Background changes of chronic sun damage are usually present, including solar elastosis, epidermal atrophy, and effacement of the rete ridges. Pigmentation is variable but may be abundant, and the papillary dermis may contain melanophages. Lymphocytes are usually seen in the superficial dermis, although prominent lymphocytic inflammation may be a sign of tumor invasion and should, therefore, prompt closer examination. As LM becomes more invasive (ie, progresses to LMM), junctional nests become larger and more spindled. Invasion is typically superficial, consisting of isolated or small aggregates of spindled cells in the papillary dermis. Desmoplasia and perineural invasion may occur, and the desmoplastic component may be mistaken for a scar. Rarely, a storiform growth pattern may be present, which may be mistaken for dermatofibrosarcoma protuberans (DFSP).[2][37][38] Immunohistochemistry with MART-1/Melan-A, HMB-45, tyrosinase, MITF, Sox10, and S100 may aid diagnosis.[2][36]
History and Physical
LM often presents as an irregular brown macule or patch on chronically sun-damaged skin in the elderly. Lesions are light-brown to black, may display color variegation, appear asymmetric, and tend to have an ill-defined border. As lesions enlarge, they may develop skip areas with a patchy, non-contiguous pattern. Lesions are usually asymptomatic, although advanced tumors may produce pain, burning, itching, or bleeding. Most cases (86%) occur on the head/neck, with a predilection for the cheek.[2][21] Extrafacial cases tend to occur in the extremities of women and back in men.[21][39] Due to its in situ nature, LM is typically smooth and non-palpable. A papular or dermal component may be felt if the lesion becomes invasive (LMM). LM exhibits slow radial growth and might be misdiagnosed for years or even decades as solar lentigo or other benign lesions (see Differential Diagnosis section).[5] The overall lifetime risk of progression from LM to LMM has been estimated to be 5% based on a retrospective epidemiologic study.[40] However, the true lifetime risk may be greater, as 10 to 20% of cases initially diagnosed as LM on biopsy are later upstaged to LMM after excision.[41][42][43][44] The timeframe of progression to LMM varies widely, from less than 10 years to more than 50 years.[45][46]
Evaluation
The clinical findings of the naked eye exam have been detailed above (see History and Physical section). Clinical diagnosis can be challenging due to overlapping features with benign lesions such as solar lentigo and pigmented actinic keratosis. For this reason, optical imaging tools such as dermoscopy and new reflectance confocal microscopy (RCM) have been developed to aid in diagnosis. Dermoscopic findings for LM/LMM reveal a stepwise progression of features based on the degree of infiltration of the follicular ostia. Early lesions exhibit peppering of pigmentation around follicular ostia, known as annular-granular structures or blue-grey dots. As the lesion grows, the dots coalesce to form short polygonal lines around and between adnexal openings. Further progression of the tumor leads to the merging and darkening of the polygonal lines into polyhedral shapes known as rhomboidal structures. Eventually, the tumor obliterates the entire follicular ostia and becomes a homogeneous dark brown to-black blotch.[47][48][49][50] RCM findings for LM/LMM are divided into major and minor criteria.[51] The two major criteria include nonedged papillae and round pagetoid cells > 20 µm. The three minor criteria include atypical cells at the DEJ, follicular localization of atypical cells, and nucleated cells within the dermal papillae. Although dermoscopy and RCM are important tools for diagnosis, skin biopsy for histopathologic examination remains the gold standard (see Histopathology section). Biopsy techniques can include excisional biopsy with narrow margins, an incisional biopsy of the most atypical appearing or thickest portion of the lesion, or broad saucerization of the entire lesion, being sure to obtain sufficient depth.[5] Finally, Wood’s lamp, dermoscopy, and RCM may help delineate surgical margins before excision.[52][53]
Treatment / Management
Surgical excision is the treatment of choice. Numerous studies have shown the traditional 0.5 cm surgical margins for MIS to be inadequate for LM, with approximately half of tumors requiring larger margins for clearance and recurrence rates between 8 to 20%.[36][54] The National Comprehensive Cancer Network (NCCN) in 2014 updated its guidelines to recommend 0.5 to 1.0 cm margins for MIS, and several single-center studies have called for larger margins up to 1.0 cm or more.[55][41][56][57][58] A recent study by Zitelli and Brodland, in which they treated over 1500 cases of LM with Mohs micrographic surgery (MMS), demonstrated that 1.2 cm margins were required to achieve a 97% clearance rate.[56] Wide local excision (WLE) among surgical modalities remains the standard of care based on expert panels.[59][60] However, a growing body of literature shows the MMS may be superior.[56][61][62][63] Several institutions that perform MMS and WLE for LM have demonstrated recurrence rates of 1.8 to 1.9% using MMS and 5.8-5.9% using WLE.[61][63] In the hands of an experienced practitioner and with the use of immunohistochemistry, recurrence rates with MMS can be as low as 0.3 to 0.5%.[56][64](A1)
For patients who wish to avoid surgery or would otherwise be poor surgical candidates, topical imiquimod 5% cream may be a viable alternative, although the data on efficacy is mixed. Clinical and histologic clearance rates for imiquimod range between 46 to 78% and 37 to 76%, respectively.[65][66] Radiation therapy may also be an acceptable non-surgical option. The method and type of radiation vary but fractionated superficial radiotherapy, or Grenz rays is most commonly delivered. Recurrence rates have been reported to be between 5 to 14%.[67][68][69] Finally, many other non-surgical modalities have been reported with varying success, including laser ablation, cryotherapy, azelaic acid, 5-fluorouracil cream, and chemical peels. Still, the data are too sparse and inconsistent to draw reliable conclusions.[2](A1)
Differential Diagnosis
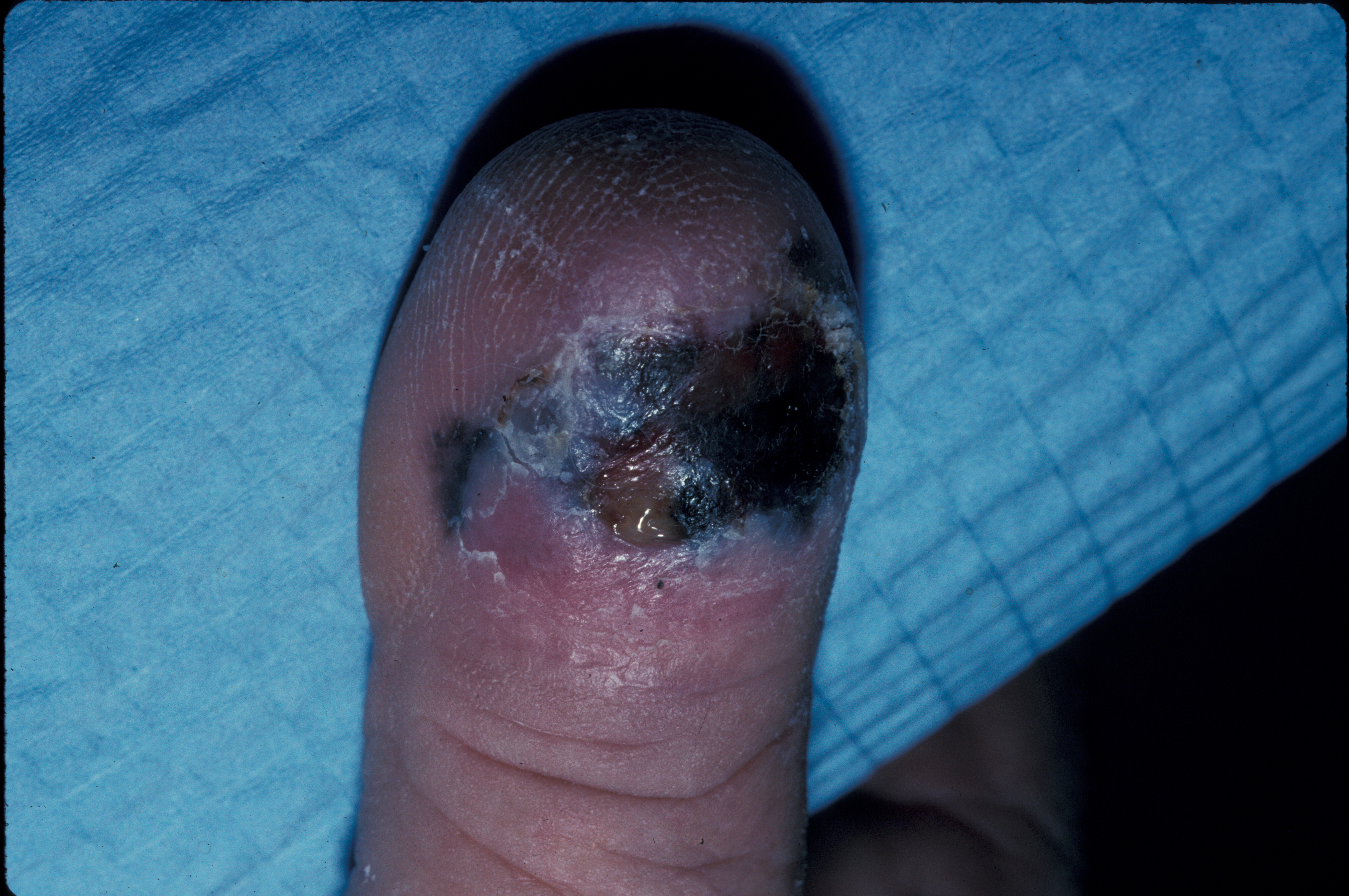
The clinical and dermoscopic differential diagnoses include solar lentigo, early/macular seborrheic keratosis, lichen planus-like keratosis (LPLK), and pigmented actinic keratosis. The histopathologic mimickers include benign melanocytic hyperplasia of sun-damaged skin, invasive lesions, desmoplastic melanoma, and rarely DFSP. See Image. Clinical Photo of an Ulcerated Acral Lentiginous Melanoma on the Dorsal Aspect of a Toe.
Staging
Staging for LM/LMM is the same as for all melanomas, using the TNM staging criteria based on the latest American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th Edition.[70] We would encourage readers to refer to that resource for an in-depth discussion. The stages are briefly summarized below:
- Stage I – Low-risk primary melanomas (T1a, T1b, T2a) without evidence of regional or distant metastases.
- Stage II – Primary melanomas at higher risk of recurrence (T2b, T3a, T3b, T4a, T4b), but without evidence of regional or distant metastases.
- Stage III – Involvement of regional lymph nodes or in-transit or satellite metastases.
- Stage IV – Distant metastases present.
Prognosis
The prognosis for LM/LMM is excellent. In a study of 270 patients with LM/LMM who were completely excised, there were zero disease-related deaths for LM and only one for LMM.[71] The 5-year and 10-year disease-specific survival was 100% and 97.1%, respectively. Thus, LM, in itself, does not reduce lifespan. However, once the tumor becomes invasive, the prognosis is the same as for all other melanomas after controlling for Breslow depth. It can be potentially quite poor if the disease becomes metastatic (5-year survival, 9-27%).[14][72] While mortality is low, morbidity for patients can be significant, given the potentially large surface area on the head/neck that may be involved and the need for extensive surgical excision and reconstruction.[73]
Complications
LMM can eventually metastasize left untreated, so early diagnosis and intervention are crucial. Surgery to remove LMM may carry cosmetic complications because it often occurs on exposed areas such as the face; modern surgical techniques can help minimize scarring.
Deterrence and Patient Education
Due to the causative nature of chronic UVR damage in inducing LM/LMM, diligent sun protection is key to prevention. The American Academy of Dermatology (AAD) periodically publishes guidelines on preventing and treating skin cancer.[59][74] Patients should wear broad-spectrum sunscreen (SPF ≥ 30) whenever outdoors, reapply every 2 hours and immediately after swimming, and use a sufficient amount (approximately 1 ounce or 1 shot-glass equivalent to cover the entire body). Wearing UPF clothing, avoiding the sun between peak hours of 10 am to 3 pm, and seeking shade provide additional sun protection. Finally, patients should be aware of the ABCDE criteria for melanoma, perform monthly skin self-examinations, and seek professional care by a dermatologist or primary care provider for any concerning lesions (see Image. Part of the ABCDs for Detection of Melanoma).
Pearls and Other Issues
LM/LMM presents diagnostic and treatment challenges due to its clinical mimicry of benign lesions, its occurrence on background sun-damaged skin, thus confounding histopathologic differentiation between true tumor and benign melanocytic activation, its occurrence on the head/neck, a cosmetically and functionally sensitive area. Thus, maintaining clinical diligence and having a high index of suspicion are key to early diagnosis, ensuring optimal treatment outcomes, and minimizing patient morbidity and mortality. Surgical excision is the treatment of choice, with MMS emerging as a surgical option that may prove superior to WLE.
Enhancing Healthcare Team Outcomes
Providing optimal care for patients with LM/LMM requires an interprofessional approach. While dermatologists detect most melanomas, primary care physicians still play an important role in early detection, as they often serve as gatekeepers in managed care plans and frequently refer patients to dermatology.[75][76] Each additional family physician per 10,000 population has been associated with a 21% increased odds of early melanoma detection.[77] Once a lesion suspicious for LM/LMM is biopsied, having an experienced dermatopathologist can be invaluable for an accurate diagnosis. Treatment of LM and early LMM may involve an interprofessional approach between the Mohs surgeon and other surgical specialists (eg, plastics, oculoplastics, ENT) for excision and reconstruction. Finally, locally advanced or metastatic tumors should be referred to surgical oncology and hematology/oncology for appropriate staging and, if indicated, systemic therapy.
Media
(Click Image to Enlarge)
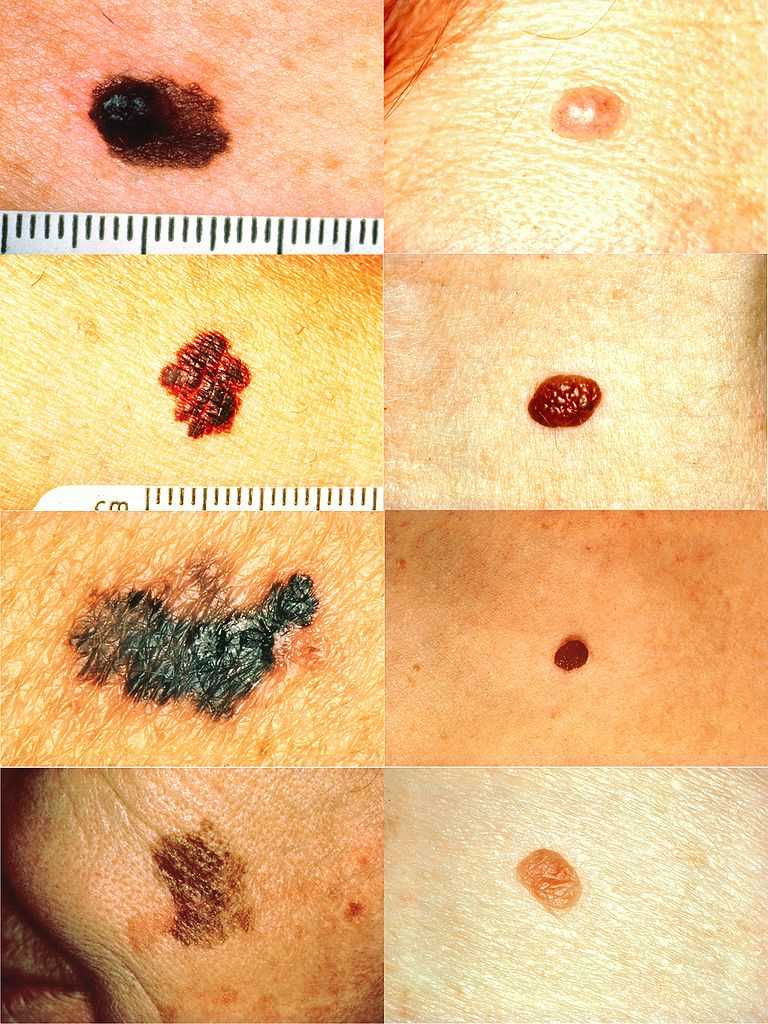
Part of the ABCDs for Detection of Melanoma. On the left side from top to bottom: melanomas showing (A) asymmetry, (B) a border that is uneven, ragged, or notched, (C) coloring of different shades of brown, black, or tan and (D) diameter that had changed in size. The normal moles on the right side do not have abnormal characteristics (no asymmetry, even border, even color, no change in diameter).
National Cancer Institute Skin Cancer Foundation
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kallini JR, Jain SK, Khachemoune A. Lentigo maligna: review of salient characteristics and management. American journal of clinical dermatology. 2013 Dec:14(6):473-80. doi: 10.1007/s40257-013-0044-6. Epub [PubMed PMID: 24019181]
Cohen LM. Lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 1995 Dec:33(6):923-36; quiz 937-40 [PubMed PMID: 7490362]
Silvers DN. Focus on melanoma: the therapeutic dilemma of lentigo maligna (Hutchinson's freckle). The Journal of dermatologic surgery. 1976 Sep:2(4):301-3 [PubMed PMID: 1026731]
Dubow BE, Ackerman AB. Ideas in pathology. Malignant melanoma in situ: the evolution of a concept. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1990 Nov:3(6):734-44 [PubMed PMID: 2263599]
DeWane ME, Kelsey A, Oliviero M, Rabinovitz H, Grant-Kels JM. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. Journal of the American Academy of Dermatology. 2019 Sep:81(3):823-833. doi: 10.1016/j.jaad.2019.03.066. Epub 2019 Mar 28 [PubMed PMID: 30930085]
Newell GR, Sider JG, Bergfelt L, Kripke ML. Incidence of cutaneous melanoma in the United States by histology with special reference to the face. Cancer research. 1988 Sep 1:48(17):5036-41 [PubMed PMID: 3409232]
Elwood JM, Gallagher RP, Worth AJ, Wood WS, Pearson JC. Etiological differences between subtypes of cutaneous malignant melanoma: Western Canada Melanoma Study. Journal of the National Cancer Institute. 1987 Jan:78(1):37-44 [PubMed PMID: 3467128]
Elwood JM, Hislop TG. Solar radiation in the etiology of cutaneous malignant melanoma in Caucasians. National Cancer Institute monograph. 1982:62():167-71 [PubMed PMID: 7167183]
Level 3 (low-level) evidenceHolman CD, Armstrong BK. Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. Journal of the National Cancer Institute. 1984 Jul:73(1):75-82 [PubMed PMID: 6588237]
Holman CD, Armstrong BK, Heenan PJ. A theory of the etiology and pathogenesis of human cutaneous malignant melanoma. Journal of the National Cancer Institute. 1983 Oct:71(4):651-6 [PubMed PMID: 6578359]
Koh HK, Kligler BE, Lew RA. Sunlight and cutaneous malignant melanoma: evidence for and against causation. Photochemistry and photobiology. 1990 Jun:51(6):765-79 [PubMed PMID: 2195564]
Schreiber MM, Moon TE, Bozzo PD. Chronic solar ultraviolet damage associated with malignant melanoma of the skin. Journal of the American Academy of Dermatology. 1984 May:10(5 Pt 1):755-9 [PubMed PMID: 6725671]
Level 2 (mid-level) evidenceArmstrong BK. Epidemiology of malignant melanoma: intermittent or total accumulated exposure to the sun? The Journal of dermatologic surgery and oncology. 1988 Aug:14(8):835-49 [PubMed PMID: 3397443]
Level 2 (mid-level) evidenceCox NH, Aitchison TC, Sirel JM, MacKie RM. Comparison between lentigo maligna melanoma and other histogenetic types of malignant melanoma of the head and neck. Scottish Melanoma Group. British journal of cancer. 1996 Apr:73(7):940-4 [PubMed PMID: 8611411]
McGovern VJ, Shaw HM, Milton GW, Farago GA. Is malignant melanoma arising in a Hutchinson's melanotic freckle a separate disease entity? Histopathology. 1980 May:4(3):235-42 [PubMed PMID: 7390408]
Holman CD, Mulroney CD, Armstrong BK. Epidemiology of pre-invasive and invasive malignant melanoma in Western Australia. International journal of cancer. 1980 Mar 15:25(3):317-23 [PubMed PMID: 7390655]
Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. IV. No association with nutritional factors, alcohol, smoking or hair dyes. International journal of cancer. 1988 Dec 15:42(6):825-8 [PubMed PMID: 3192325]
Level 2 (mid-level) evidenceSwetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990-2000. The Journal of investigative dermatology. 2005 Oct:125(4):685-91 [PubMed PMID: 16185266]
Lemish WM, Heenan PJ, Holman CD, Armstrong BK. Survival from preinvasive and invasive malignant melanoma in Western Australia. Cancer. 1983 Aug 1:52(3):580-5 [PubMed PMID: 6861096]
Gassenmaier M, Keim U, Leiter U, Eigentler TK, Röcken M, Gesierich A, Moritz RKC, Heinzerling L, Tüting T, Wollina U, Garbe C. Age as key factor for pattern, timing, and extent of distant metastasis in patients with cutaneous melanoma: A study of the German Central Malignant Melanoma Registry. Journal of the American Academy of Dermatology. 2019 May:80(5):1299-1307.e7. doi: 10.1016/j.jaad.2019.01.044. Epub 2019 Jan 29 [PubMed PMID: 30703453]
Cox NH, Aitchison TC, MacKie RM. Extrafacial lentigo maligna melanoma: analysis of 71 cases and comparison with lentigo maligna melanoma of the head and neck. The British journal of dermatology. 1998 Sep:139(3):439-43 [PubMed PMID: 9767288]
Level 2 (mid-level) evidenceMirzoyev SA, Knudson RM, Reed KB, Hou JL, Lohse CM, Frohm ML, Brewer JD, Otley CC, Roenigk RK. Incidence of lentigo maligna in Olmsted County, Minnesota, 1970 to 2007. Journal of the American Academy of Dermatology. 2014 Mar:70(3):443-8. doi: 10.1016/j.jaad.2013.11.008. Epub 2013 Dec 24 [PubMed PMID: 24373777]
Level 2 (mid-level) evidenceGreveling K, Wakkee M, Nijsten T, van den Bos RR, Hollestein LM. Epidemiology of Lentigo Maligna and Lentigo Maligna Melanoma in the Netherlands, 1989-2013. The Journal of investigative dermatology. 2016 Oct:136(10):1955-1960. doi: 10.1016/j.jid.2016.06.014. Epub 2016 Jun 24 [PubMed PMID: 27349862]
Higgins HW 2nd, Lee KC, Galan A, Leffell DJ. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. Journal of the American Academy of Dermatology. 2015 Aug:73(2):181-90, quiz 191-2. doi: 10.1016/j.jaad.2015.04.014. Epub [PubMed PMID: 26183967]
Buettner PG, Leiter U, Eigentler TK, Garbe C. Development of prognostic factors and survival in cutaneous melanoma over 25 years: An analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005 Feb 1:103(3):616-24 [PubMed PMID: 15630700]
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome Initiative, ICGC Breast Cancer Consortium, ICGC MMML-Seq Consortium, ICGC PedBrain, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013 Aug 22:500(7463):415-21. doi: 10.1038/nature12477. Epub 2013 Aug 14 [PubMed PMID: 23945592]
Mar VJ, Wong SQ, Li J, Scolyer RA, McLean C, Papenfuss AT, Tothill RW, Kakavand H, Mann GJ, Thompson JF, Behren A, Cebon JS, Wolfe R, Kelly JW, Dobrovic A, McArthur GA. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histologic and molecular signatures of UV damage. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Sep 1:19(17):4589-98. doi: 10.1158/1078-0432.CCR-13-0398. Epub 2013 Jul 5 [PubMed PMID: 23833303]
Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer causes & control : CCC. 2001 Jan:12(1):69-82 [PubMed PMID: 11227927]
Level 2 (mid-level) evidenceDavis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean? Cancer. 2018 Sep 1:124(17):3490-3499. doi: 10.1002/cncr.31345. Epub 2018 Apr 17 [PubMed PMID: 29663336]
Sanchez MI, Grichnik JM. Melanoma's high C}T mutation rate: is deamination playing a role? Experimental dermatology. 2014 Aug:23(8):551-2. doi: 10.1111/exd.12436. Epub 2014 Aug 1 [PubMed PMID: 24815223]
Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annual review of pathology. 2014:9():239-71. doi: 10.1146/annurev-pathol-012513-104658. Epub [PubMed PMID: 24460190]
Level 3 (low-level) evidenceLee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. The British journal of dermatology. 2011 Apr:164(4):776-84. doi: 10.1111/j.1365-2133.2010.10185.x. Epub 2011 Mar 21 [PubMed PMID: 21166657]
Level 1 (high-level) evidenceLang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. The Journal of investigative dermatology. 2005 Sep:125(3):575-9 [PubMed PMID: 16117801]
Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Sep 10:24(26):4340-6 [PubMed PMID: 16908931]
Weyers W, Bonczkowitz M, Weyers I, Bittinger A, Schill WB. Melanoma in situ versus melanocytic hyperplasia in sun-damaged skin. Assessment of the significance of histopathologic criteria for differential diagnosis. The American Journal of dermatopathology. 1996 Dec:18(6):560-6 [PubMed PMID: 8989926]
McGuire LK, Disa JJ, Lee EH, Busam KJ, Nehal KS. Melanoma of the lentigo maligna subtype: diagnostic challenges and current treatment paradigms. Plastic and reconstructive surgery. 2012 Feb:129(2):288e-299e. doi: 10.1097/PRS.0b013e31823aeb72. Epub [PubMed PMID: 22286443]
Green A, Little JH, Weedon D. The diagnosis of Hutchinson's melanotic freckle (lentigo maligna) in Queensland. Pathology. 1983 Jan:15(1):33-5 [PubMed PMID: 6856341]
Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Feb:19 Suppl 2():S34-40 [PubMed PMID: 16446714]
Martínez-Leboráns L, Garcías-Ladaria J, Oliver-Martínez V, Alegre de Miquel V. Extrafacial Lentigo Maligna: A Report on 14 Cases and a Review of the Literature. Actas dermo-sifiliograficas. 2016 Oct:107(8):e57-63. doi: 10.1016/j.ad.2015.10.018. Epub 2016 May 11 [PubMed PMID: 27180003]
Level 3 (low-level) evidenceWeinstock MA, Sober AJ. The risk of progression of lentigo maligna to lentigo maligna melanoma. The British journal of dermatology. 1987 Mar:116(3):303-10 [PubMed PMID: 3567069]
Bosbous MW, Dzwierzynski WW, Neuburg M. Staged excision of lentigo maligna and lentigo maligna melanoma: a 10-year experience. Plastic and reconstructive surgery. 2009 Dec:124(6):1947-1955. doi: 10.1097/PRS.0b013e3181bcf002. Epub [PubMed PMID: 19952650]
Level 2 (mid-level) evidenceHazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: A retrospective analysis of 117 cases. Journal of the American Academy of Dermatology. 2008 Jan:58(1):142-8 [PubMed PMID: 18029055]
Level 2 (mid-level) evidencePenneys NS. Microinvasive lentigo maligna melanoma. Journal of the American Academy of Dermatology. 1987 Oct:17(4):675-80 [PubMed PMID: 3312317]
Level 2 (mid-level) evidenceSomach SC, Taira JW, Pitha JV, Everett MA. Pigmented lesions in actinically damaged skin. Histopathologic comparison of biopsy and excisional specimens. Archives of dermatology. 1996 Nov:132(11):1297-302 [PubMed PMID: 8915306]
Jackson R, Williamson GS, Beattie WG. Lentigo maligna and malignant melanoma. Canadian Medical Association journal. 1966 Oct 22:95(17):846-51 [PubMed PMID: 5922502]
Clark WH Jr, Mihm MC Jr. Lentigo maligna and lentigo-maligna melanoma. The American journal of pathology. 1969 Apr:55(1):39-67 [PubMed PMID: 5776171]
Cognetta AB Jr, Stolz W, Katz B, Tullos J, Gossain S. Dermatoscopy of lentigo maligna. Dermatologic clinics. 2001 Apr:19(2):307-18 [PubMed PMID: 11556239]
Bollea-Garlatti LA, Galimberti GN, Galimberti RL. Lentigo Maligna: Keys to Dermoscopic Diagnosis. Actas dermo-sifiliograficas. 2016 Jul-Aug:107(6):489-97. doi: 10.1016/j.ad.2016.01.001. Epub 2016 Feb 11 [PubMed PMID: 26875792]
Annessi G, Bono R, Abeni D. Correlation between digital epiluminescence microscopy parameters and histopathological changes in lentigo maligna and solar lentigo: A dermoscopic index for the diagnosis of lentigo maligna. Journal of the American Academy of Dermatology. 2017 Feb:76(2):234-243. doi: 10.1016/j.jaad.2016.08.032. Epub 2016 Oct 26 [PubMed PMID: 28341252]
Mataca E, Migaldi M, Cesinaro AM. Impact of Dermoscopy and Reflectance Confocal Microscopy on the Histopathologic Diagnosis of Lentigo Maligna/Lentigo Maligna Melanoma. The American Journal of dermatopathology. 2018 Dec:40(12):884-889. doi: 10.1097/DAD.0000000000001212. Epub [PubMed PMID: 29933314]
Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S, Vinceti M, Rabinovitz H, Longo C, Menzies SW. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. The Journal of investigative dermatology. 2010 Aug:130(8):2080-91. doi: 10.1038/jid.2010.84. Epub 2010 Apr 15 [PubMed PMID: 20393481]
Chen CS, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. The British journal of dermatology. 2005 Nov:153(5):1031-6 [PubMed PMID: 16225620]
Level 3 (low-level) evidenceRobinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Archives of dermatology. 2004 Sep:140(9):1095-100 [PubMed PMID: 15381550]
Level 2 (mid-level) evidenceOsborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. British journal of plastic surgery. 2002 Dec:55(8):611-5 [PubMed PMID: 12550112]
Coit DG, Thompson JA, Andtbacka R, Anker CJ, Bichakjian CK, Carson WE 3rd, Daniels GA, Daud A, Dimaio D, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS Jr, Kelley MC, Khushalani NI, Kudchadkar RR, Lange JR, Martini MC, Olszanski AJ, Ross MI, Salama A, Swetter SM, Tanabe KK, Trisal V, Urist MM, McMillian NR, Ho M, National Comprehensive Cancer Network. Melanoma, version 4.2014. Journal of the National Comprehensive Cancer Network : JNCCN. 2014 May:12(5):621-9 [PubMed PMID: 24812131]
Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. Journal of the American Academy of Dermatology. 2019 Jul:81(1):204-212. doi: 10.1016/j.jaad.2019.01.051. Epub 2019 Apr 20 [PubMed PMID: 31014825]
Jejurikar SS, Borschel GH, Johnson TM, Lowe L, Brown DL. Immediate, optimal reconstruction of facial lentigo maligna and melanoma following total peripheral margin control. Plastic and reconstructive surgery. 2007 Oct:120(5):1249-1255. doi: 10.1097/01.prs.0000279324.35616.72. Epub [PubMed PMID: 17898597]
Bosbous MW, Dzwierzynski WW, Neuburg M. Lentigo maligna: diagnosis and treatment. Clinics in plastic surgery. 2010 Jan:37(1):35-46. doi: 10.1016/j.cps.2009.08.006. Epub [PubMed PMID: 19914456]
Bichakjian CK, Halpern AC, Johnson TM, Foote Hood A, Grichnik JM, Swetter SM, Tsao H, Barbosa VH, Chuang TY, Duvic M, Ho VC, Sober AJ, Beutner KR, Bhushan R, Smith Begolka W, American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. Journal of the American Academy of Dermatology. 2011 Nov:65(5):1032-47. doi: 10.1016/j.jaad.2011.04.031. Epub 2011 Aug 25 [PubMed PMID: 21868127]
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE 3rd, Daniels GA, DiMaio D, Ernstoff M, Fields RC, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS Jr, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ross MI, Salama AK, Skitzki J, Sosman J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. Melanoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 Apr:14(4):450-73 [PubMed PMID: 27059193]
Level 1 (high-level) evidenceNosrati A, Berliner JG, Goel S, McGuire J, Morhenn V, de Souza JR, Yeniay Y, Singh R, Lee K, Nakamura M, Wu RR, Griffin A, Grimes B, Linos E, Chren MM, Grekin R, Wei ML. Outcomes of Melanoma In Situ Treated With Mohs Micrographic Surgery Compared With Wide Local Excision. JAMA dermatology. 2017 May 1:153(5):436-441. doi: 10.1001/jamadermatol.2016.6138. Epub [PubMed PMID: 28241261]
Beaulieu D, Fathi R, Srivastava D, Nijhawan RI. Current perspectives on Mohs micrographic surgery for melanoma. Clinical, cosmetic and investigational dermatology. 2018:11():309-320. doi: 10.2147/CCID.S137513. Epub 2018 Jun 20 [PubMed PMID: 29950878]
Level 3 (low-level) evidenceHou JL, Reed KB, Knudson RM, Mirzoyev SA, Lohse CM, Frohm ML, Brewer JD, Otley CC, Roenigk RK. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2015 Feb:41(2):211-8. doi: 10.1097/DSS.0000000000000248. Epub [PubMed PMID: 25590473]
Level 2 (mid-level) evidenceEtzkorn JR, Sobanko JF, Elenitsas R, Newman JG, Goldbach H, Shin TM, Miller CJ. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. Journal of the American Academy of Dermatology. 2015 May:72(5):840-50. doi: 10.1016/j.jaad.2015.01.007. Epub 2015 Mar 13 [PubMed PMID: 25774012]
Level 2 (mid-level) evidenceMarsden JR, Fox R, Boota NM, Cook M, Wheatley K, Billingham LJ, Steven NM, NCRI Skin Cancer Clinical Studies Group, the U.K. Dermatology Clinical Trials Network and the LIMIT-1 Collaborative Group. Effect of topical imiquimod as primary treatment for lentigo maligna: the LIMIT-1 study. The British journal of dermatology. 2017 May:176(5):1148-1154. doi: 10.1111/bjd.15112. Epub 2017 Apr 10 [PubMed PMID: 27714781]
Mora AN, Karia PS, Nguyen BM. A quantitative systematic review of the efficacy of imiquimod monotherapy for lentigo maligna and an analysis of factors that affect tumor clearance. Journal of the American Academy of Dermatology. 2015 Aug:73(2):205-12. doi: 10.1016/j.jaad.2015.05.022. Epub 2015 Jun 16 [PubMed PMID: 26088690]
Level 1 (high-level) evidenceFogarty GB, Hong A, Scolyer RA, Lin E, Haydu L, Guitera P, Thompson J. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. The British journal of dermatology. 2014 Jan:170(1):52-8. doi: 10.1111/bjd.12611. Epub [PubMed PMID: 24032599]
Farshad A, Burg G, Panizzon R, Dummer R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. The British journal of dermatology. 2002 Jun:146(6):1042-6 [PubMed PMID: 12072074]
Level 2 (mid-level) evidenceTsang RW, Liu FF, Wells W, Payne DG. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Archives of dermatology. 1994 Aug:130(8):1008-12 [PubMed PMID: 8053696]
Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Annals of surgical oncology. 2018 Aug:25(8):2105-2110. doi: 10.1245/s10434-018-6513-7. Epub 2018 May 30 [PubMed PMID: 29850954]
Gambichler T, Kempka J, Kampilafkos P, Bechara FG, Altmeyer P, Stücker M. Clinicopathological characteristics of 270 patients with lentigo maligna and lentigo maligna melanoma: data from a German skin cancer centre. The British journal of dermatology. 2014 Dec:171(6):1605-7. doi: 10.1111/bjd.13204. Epub 2014 Oct 22 [PubMed PMID: 24958545]
Level 3 (low-level) evidenceBalch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Dec 20:27(36):6199-206. doi: 10.1200/JCO.2009.23.4799. Epub 2009 Nov 16 [PubMed PMID: 19917835]
Walling HW, Scupham RK, Bean AK, Ceilley RI. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 2007 Oct:57(4):659-64 [PubMed PMID: 17870430]
Level 2 (mid-level) evidenceSwetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, Sober AJ, Thompson JA, Wisco OJ, Wyatt S, Hu S, Lamina T. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology. 2019 Jan:80(1):208-250. doi: 10.1016/j.jaad.2018.08.055. Epub 2018 Nov 1 [PubMed PMID: 30392755]
Kantor J, Kantor DE. Routine dermatologist-performed full-body skin examination and early melanoma detection. Archives of dermatology. 2009 Aug:145(8):873-6. doi: 10.1001/archdermatol.2009.137. Epub [PubMed PMID: 19687416]
Gerbert B, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, Bronstone A, Caspers N. Primary care physicians as gatekeepers in managed care. Primary care physicians' and dermatologists' skills at secondary prevention of skin cancer. Archives of dermatology. 1996 Sep:132(9):1030-8 [PubMed PMID: 8795541]
Roetzheim RG, Pal N, van Durme DJ, Wathington D, Ferrante JM, Gonzalez EC, Krischer JP. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. Journal of the American Academy of Dermatology. 2000 Aug:43(2 Pt 1):211-8 [PubMed PMID: 10906640]