Introduction
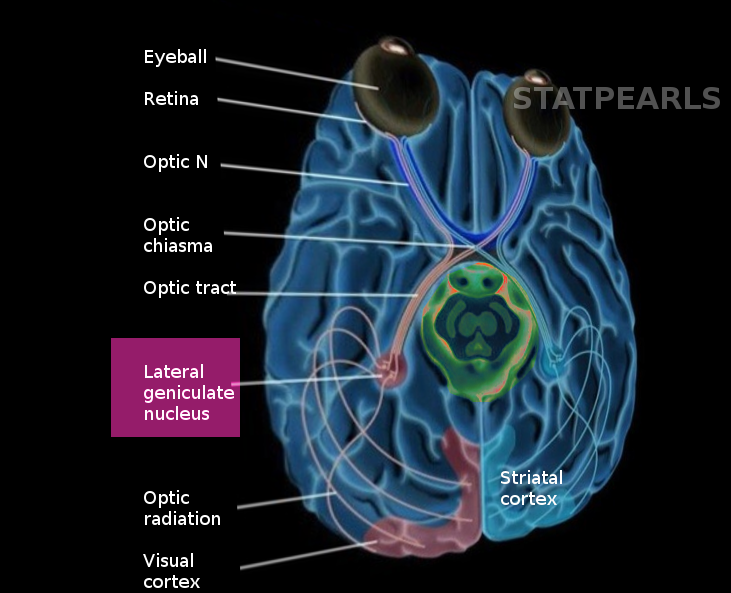
The lateral geniculate nucleus (LGN) belongs to the category of sensory projection nuclei of the thalamus and plays an essential role in normal visual processing. The lateral geniculate nucleus has broadly distributed connectivity projecting to various regions of the extrastriate cortex and receiving input from the same as well as from hindbrain and other midbrain structures. It is located in the posteroventral region of the thalamic nuclei, immediately abutting the pulvinar and posterior to the inferior choroidal point of the choroid plexus. The nucleus' name is from its lateral position relative to the medial geniculate nucleus and the sharp bend (Latin geniculum, "joint") of its laminae.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The basis of the structure of the lateral geniculate nucleus is mostly in terms of its three distinct cell types: magnocellular (M), parvocellular (P), and koniocellular (K). P and M cells are arranged in six different layers (four dorsal P layers and two ventral M layers), with retinal ganglion signals from the ipsilateral eye synapsing on layers 2, 3, and 5, and signals from the contralateral eye synapsing on layers 1, 4, and 6. M cells in the LGN receive input from the large-field, motion-sensitive Y-type retinal ganglion cells, while P cells receive input from the small-field, color-sensitive X-type retinal ganglion cells. Koniocellular cells project into regions ventral to each of the P and M laminae.
The lateral geniculate nucleus is also the point of origin for the optic radiations (Meyer's loop, central bundle, and Baum’s loop) that project via the internal capsule to the primary visual cortex (V1), primarily synapsing onto spiny stellate neurons in layers 4C-alpha and 4C-beta. Analysis of LGN-dependent fMRI activity in non-V1 extrastriate cortex suggests that the LGN also projects to regions further downstream in the visual pathway (e.g., V2-5).[1] While the LGN receives substantial input from the retinal ganglia, it receives far greater innervation from higher-order regions, such as modulatory feedback from layer 6 of V1 and the thalamic reticular nucleus.[2] It also receives varying degrees of modulatory activity from the raphe nuclei (serotonergic),[3] pedunculopontine and laterodorsal tegmental nuclei (cholinergic),[4] and locus coeruleus (noradrenergic).[5]
The extracellular matrix of the lateral geniculate nucleus is characterized by a decreased presence of the traditional aggrecan-based matrix phenotype, perineuronal nets. It instead displays a high density of axonal coats, a related structure with a more localized matrix at dendrites, suggesting a different organizational strategy possibly specialized for rapid sensory processing.[6][7]
The lateral geniculate nucleus also contains a distinct section between its dorsal and ventral regions known as the intergeniculate leaflet (IGL). The IGL projects to the suprachiasmatic nuclei of the hypothalamus via the geniculohypothalamic tract and to the pineal gland via the geniculopineal tract, implicating the LGN in the modulation of circadian/diurnal rhythms.[8][9][10]
Historically, the lateral geniculate nucleus was highlighted for its role as little more than a signal repeater, following the early conclusions of Glees and LeGros Clark, who argued that "... the geniculate cells probably serve the single function of a relay between the retinal fibers and the visual cortex."[11] However, subsequent research has suggested a more complex account of LGN function, including attentional modulation, temporal decorrelation, and binocular facilitation or suppression via monocular gain modulation.[12][13][14][15] Furthermore, some research has suggested that a subpopulation of K cells in the LGN demonstrate selective sensitivity to stimulus orientation similar to V1 cells.[16]
Embryology
Early development of the lateral geniculate nucleus characteristically demonstrates heightened retinogeniculate synaptogenesis (as early as 13 weeks of gestation) followed by the subsequent development of corticogeniculate connectivity. However, the structural development of retinogeniculate projections (without synapse formation) occurs as early as 7 weeks.[17] A critical period of increased cell metabolism and synapse development occurs at 15 to 20 weeks.[18] By the end of this period, retinogeniculate projections to the LGN have developed eye-specific segregation.[19] The development of the LGN’s laminar structure occurs at approximately 22 to 25 weeks, beginning with the ventral aspect (the magnocellular layers).[20] The later lamination of the LGN suggests that this process is a function of retinal activity. This concept receives further support from the finding in animal studies that interrupting retinogeniculate segregation severely disrupts the development of LGN's laminar organization.[21]
Blood Supply and Lymphatics
The posterior cerebral artery supplies the lateral geniculate nucleus from the lateral posterior choroidal branch and by the internal carotid artery from its anterior choroidal branch.[22]
Muscles
Evidence from multiple sources suggests that oculomotor-induced signals to the lateral geniculate nucleus are used to suppress retinal signals during saccades and facilitate the same signals immediately thereafter.[23]
Physiologic Variants
Structural variation in the lateral geniculate nucleus can occur via divergent development of or subsequent damage to upstream structures in the visual pathway. Strabismic amblyopia, the permanent reduction of visual acuity due to abnormal development in the eyes’ alignment, is associated with a significant decrease in LGN gray matter density despite normal development other major neural regions in the visual pathway.[24] Damage to the optic nerve due to primary open-angle glaucoma can similarly induce atrophy in the LGN commensurate with the degree of condition severity.[25][26] Some animal research also suggests that a lack of input from the visual cortical regions can induce cell death sharing many features of apoptosis.[27]
Surgical Considerations
Resection of intraventricular meningiomas may pose a higher risk of postoperative ischemia to the lateral geniculate nucleus due to the proximity of the choroidal arteries, the supply of which may integrate into the tumor.[28]
Clinical Significance
Lesions in the lateral geniculate nucleus, such as those caused by arteriovenous malformations, can produce contralateral homonymous hemianopias and quadrantanopias indicating specific affected laminar subregions with special vulnerability to the macular region given its disproportionate representational region.[29] Given the location of the LGN in the visual pathway and the dual, differential blood supply to the medial and lateral portions of the nucleus, it is possible to determine the specific arterial cause of such a lesion based on the presenting visual field defect.[22] Due to downstream projections to the suprachiasmatic nucleus, the insult of the LGN may also disrupt the effects of stimuli that generally modulate circadian rhythm.[30]
The lateral geniculate nucleus is among the numerous thalamic nuclei that show substantial alterations in individuals with spinocerebellar ataxia type 2 (and possibly type 3), including astrocytosis, loss of neuronal bodies, and increased levels of lipofuscin.[31][32]
For individuals diagnosed with blindsight due to a lesion of the primary visual cortex, the LGN plays an essential role in mediating visually relevant information that is below the level of conscious awareness.[1]
Media
References
Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, Leopold DA. Blindsight depends on the lateral geniculate nucleus. Nature. 2010 Jul 15:466(7304):373-7. doi: 10.1038/nature09179. Epub 2010 Jun 23 [PubMed PMID: 20574422]
Level 3 (low-level) evidenceSherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2002 Dec 29:357(1428):1695-708 [PubMed PMID: 12626004]
Level 3 (low-level) evidenceYoshida M, Sasa M, Takaori S. Serotonin-mediated inhibition from dorsal raphe nucleus of neurons in dorsal lateral geniculate and thalamic reticular nuclei. Brain research. 1984 Jan 2:290(1):95-105 [PubMed PMID: 6692141]
Level 3 (low-level) evidenceKrueger J, Disney AA. Structure and function of dual-source cholinergic modulation in early vision. The Journal of comparative neurology. 2019 Feb 15:527(3):738-750. doi: 10.1002/cne.24590. Epub 2018 Dec 20 [PubMed PMID: 30520037]
Level 2 (mid-level) evidenceHoldefer RN, Jacobs BL. Phasic stimulation of the locus coeruleus: effects on activity in the lateral geniculate nucleus. Experimental brain research. 1994:100(3):444-52 [PubMed PMID: 7813682]
Level 3 (low-level) evidenceBrückner G, Morawski M, Arendt T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience. 2008 Jan 24:151(2):489-504 [PubMed PMID: 18055126]
Lendvai D, Morawski M, Brückner G, Négyessy L, Baksa G, Glasz T, Patonay L, Matthews RT, Arendt T, Alpár A. Perisynaptic aggrecan-based extracellular matrix coats in the human lateral geniculate body devoid of perineuronal nets. Journal of neuroscience research. 2012 Feb:90(2):376-87. doi: 10.1002/jnr.22761. Epub 2011 Sep 30 [PubMed PMID: 21959900]
Moore RY, Card JP. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. The Journal of comparative neurology. 1994 Jun 15:344(3):403-30 [PubMed PMID: 8063960]
Level 3 (low-level) evidenceMuscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neuroscience. 2006 Jun 19:140(1):305-20 [PubMed PMID: 16549274]
Level 3 (low-level) evidenceCipolla-Neto J, Bartol I, Seraphim PM, Afeche SC, Scialfa JH, Peraçoli AM. The effects of lesions of the thalamic intergeniculate leaflet on the pineal metabolism. Brain research. 1995 Sep 11:691(1-2):133-41 [PubMed PMID: 8590045]
Level 3 (low-level) evidenceGlees P, le Gros Clark WE. The termination of optic fibres in the lateral geniculate body of the monkey. Journal of anatomy. 1941 Apr:75(Pt 3):295-308.3 [PubMed PMID: 17104862]
O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature neuroscience. 2002 Nov:5(11):1203-9 [PubMed PMID: 12379861]
Rathbun DL, Warland DK, Usrey WM. Spike timing and information transmission at retinogeniculate synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Oct 13:30(41):13558-66. doi: 10.1523/JNEUROSCI.0909-10.2010. Epub [PubMed PMID: 20943897]
Level 3 (low-level) evidenceDan Y, Atick JJ, Reid RC. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996 May 15:16(10):3351-62 [PubMed PMID: 8627371]
Level 3 (low-level) evidenceDougherty K, Schmid MC, Maier A. Binocular response modulation in the lateral geniculate nucleus. The Journal of comparative neurology. 2019 Feb 15:527(3):522-534. doi: 10.1002/cne.24417. Epub 2018 Mar 9 [PubMed PMID: 29473163]
Level 2 (mid-level) evidenceCheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013 Apr 17:33(16):6864-76. doi: 10.1523/JNEUROSCI.5208-12.2013. Epub [PubMed PMID: 23595745]
Level 3 (low-level) evidenceCOOPER ER. The development of the human lateral geniculate body. Brain : a journal of neurology. 1945:68(3):222-39 [PubMed PMID: 21017988]
Khan AA, Wadhwa S, Bijlani V. Development of human lateral geniculate nucleus: an electron microscopic study. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1994 Nov:12(7):661-72 [PubMed PMID: 7900548]
Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. Journal of neuropathology and experimental neurology. 2000 May:59(5):385-92 [PubMed PMID: 10888368]
Hitchcock PF, Hickey TL. Prenatal development of the human lateral geniculate nucleus. The Journal of comparative neurology. 1980 Nov 15:194(2):395-411 [PubMed PMID: 7440807]
Level 2 (mid-level) evidenceHuberman AD, Stellwagen D, Chapman B. Decoupling eye-specific segregation from lamination in the lateral geniculate nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002 Nov 1:22(21):9419-29 [PubMed PMID: 12417667]
Level 3 (low-level) evidenceLuco C, Hoppe A, Schweitzer M, Vicuña X, Fantin A. Visual field defects in vascular lesions of the lateral geniculate body. Journal of neurology, neurosurgery, and psychiatry. 1992 Jan:55(1):12-5 [PubMed PMID: 1548490]
Level 3 (low-level) evidenceWeyand TG. The multifunctional lateral geniculate nucleus. Reviews in the neurosciences. 2016 Feb:27(2):135-57. doi: 10.1515/revneuro-2015-0018. Epub [PubMed PMID: 26479339]
Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, Hess RF. Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Investigative ophthalmology & visual science. 2010 Mar:51(3):1432-8. doi: 10.1167/iovs.09-3931. Epub 2009 Oct 29 [PubMed PMID: 19875650]
Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. The British journal of ophthalmology. 2009 Jan:93(1):56-60. doi: 10.1136/bjo.2008.138172. Epub 2008 Aug 12 [PubMed PMID: 18697810]
Level 2 (mid-level) evidenceDai H, Mu KT, Qi JP, Wang CY, Zhu WZ, Xia LM, Chen ZQ, Zhang H, Ai F, Morelli JN. Assessment of lateral geniculate nucleus atrophy with 3T MR imaging and correlation with clinical stage of glaucoma. AJNR. American journal of neuroradiology. 2011 Aug:32(7):1347-53. doi: 10.3174/ajnr.A2486. Epub 2011 Jul 14 [PubMed PMID: 21757515]
Level 1 (high-level) evidenceAl-Abdulla NA, Portera-Cailliau C, Martin LJ. Occipital cortex ablation in adult rat causes retrograde neuronal death in the lateral geniculate nucleus that resembles apoptosis. Neuroscience. 1998 Sep:86(1):191-209 [PubMed PMID: 9692754]
Level 3 (low-level) evidenceSizdahkhani S, Magill ST, McDermott MW. Intraventricular Meningioma Resection with Postoperative Ischemia of the Lateral Geniculate Nucleus. World neurosurgery. 2017 Oct:106():878-883. doi: 10.1016/j.wneu.2017.07.067. Epub 2017 Jul 19 [PubMed PMID: 28735134]
Gunderson CH, Hoyt WF. Geniculate hemianopia: incongruous homonymous field defects in two patients with partial lesions of the lateral geniculate nucleus. Journal of neurology, neurosurgery, and psychiatry. 1971 Feb:34(1):1-6 [PubMed PMID: 5551688]
Johnson RF, Smale L, Moore RY, Morin LP. Lateral geniculate lesions block circadian phase-shift responses to a benzodiazepine. Proceedings of the National Academy of Sciences of the United States of America. 1988 Jul:85(14):5301-4 [PubMed PMID: 3293053]
Level 3 (low-level) evidenceRüb U, Del Turco D, Bürk K, Diaz GO, Auburger G, Mittelbronn M, Gierga K, Ghebremedhin E, Schultz C, Schöls L, Bohl J, Braak H, Deller T. Extended pathoanatomical studies point to a consistent affection of the thalamus in spinocerebellar ataxia type 2. Neuropathology and applied neurobiology. 2005 Apr:31(2):127-40 [PubMed PMID: 15771706]
Rüb U, Del Turco D, Del Tredici K, de Vos RA, Brunt ER, Reifenberger G, Seifried C, Schultz C, Auburger G, Braak H. Thalamic involvement in a spinocerebellar ataxia type 2 (SCA2) and a spinocerebellar ataxia type 3 (SCA3) patient, and its clinical relevance. Brain : a journal of neurology. 2003 Oct:126(Pt 10):2257-72 [PubMed PMID: 12847080]
Level 3 (low-level) evidence