Introduction
The lacrimal gland is a bilobed, tear-shaped gland with the primary function of secreting the aqueous portion of the tear film, thereby maintaining the ocular surface. It is primarily located in the anterior, superotemporal orbit within the lacrimal fossa of the frontal bone. The tendon of levator palpebrae superioris bisects the lacrimal gland, giving rise to 2 lobes: a smaller palpebral component found continuous with the inner eyelid, and a larger orbital component. Its histological composition is mixed serous and mucinous acini, myoepithelial cells, and small interconnecting ductules.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The lacrimal gland plays a prominent role in the formation of the tear film, a trilaminar structure that performs the following functions: 1) provides a protective barrier for the ocular surface; 2) provides a smooth optical surface at the air-cornea interface; 3) functions as a medium for removal of debris. The tear film is composed of an inner mucin layer that interfaces with the corneal surface, a middle aqueous layer, and an outer lipid layer. Serous acini are the predominant tissue found in the lacrimal gland, secreting the aqueous component, which forms most of the tear film fluid volume. The corneal surface is hydrophilic in composition, necessitating an additional component, soluble mucin, to bind to its hydrophobic surface and establish a hydrophilic character, allowing uniform adherence of the aqueous layer to the corneal surface. Mucin is secreted principally by the conjunctival goblet cells and the stratified squamous cells of the conjunctival and corneal epithelia. The contribution by the lacrimal glands of Henle and Manz is minimal.
Within the lacrimal gland, interlobular ducts connect the orbital lobe with the palpebral lobe. Tear fluid is secreted in the upper conjunctival fornix from both lobes via excretory ducts, and between 2 and 5 ducts from the palpebral lobe secrete fluid into the lower conjunctival fornix.[1]
Tear fluid originating from the lacrimal gland and other tissue drains into the nose via the nasolacrimal duct system. All tear fluid drains initially drains from the eye through the lacrimal puncti, followed by the lacrimal tubular canaliculi, nasolacrimal sac, nasolacrimal duct, the valve of Hasner, and finally into the nose.[2]
In addition to its contribution to the aqueous portion of the tear film, the lacrimal gland has adaptive immune function through myoepithelial cell secretion of IgA and IgG antibodies. It is associated with mucosa-associated lymphoid tissue (MALT) and is susceptible to autoimmune-inflicted damage commonly seen to affect MALT.
Embryology
The lacrimal gland embryologically derives from immune, epithelial, and mesenchymal cell lineages.[3] As with most tubular organs, the development of the lacrimal gland involves a series of interactions between epithelial and mesenchymal tissue that is not entirely understood. The morphogenetic protein BMP7 is expressed in the developing gland and facilitates branching into terminal ductule lobular units via signaling directed to mesenchymal tissue.[4]
Blood Supply and Lymphatics
The arterial blood supply of the lacrimal gland is the lacrimal artery, a branch of the ophthalmic artery. Venous blood returns from the lacrimal gland via the lacrimal vein, which feeds into the superior ophthalmic vein that traverses the cavernous sinus. Lymphatic draining of the orbit and its associated structures is still an area of ongoing research and debate.[5]
Nerves
The secretion of aqueous by the lacrimal gland is a response to parasympathetic and sympathetic stimulation. The gland receives parasympathetic innervation via fibers that originate in the lacrimatory nucleus of the facial nerve in the pons. Presynaptic parasympathetic fibers travel with the facial nerve to the geniculate ganglion, where they diverge, forming the greater petrosal nerve. The greater petrosal nerve passes through the foramen lacerum and is joined by the deep petrosal nerve (which contains sympathetic fibers from the superior cervical ganglion), forming the nerve of the pterygoid canal. This nerve then synapses on the pterygopalatine ganglion. Postsynaptic parasympathetic fibers leaving the pterygopalatine ganglion form the zygomatic and zygomaticotemporal nerves. These fibers travel with the lacrimal branch of the maxillary nerve to reach the lacrimal gland. Innervation of the gland by these parasympathetic fibers is either direct, indirect via communication with the lacrimal nerve, or both. In the majority of cases, the zygomaticotemporal nerve directly enters the lacrimal gland.[6]
Sympathetic innervation of the lacrimal gland originates in the superior cervical ganglion. These postsynaptic fibers travel with the internal carotid artery before joining the greater petrosal nerve, forming the nerve of the pterygoid canal. As this nerve reaches the pterygopalatine ganglion, sympathetic fibers do not synapse but continue to travel with the parasympathetic fibers that reach the lacrimal gland.
Sensory innervation of the lacrimal gland is via the lacrimal nerve, a branch of the ophthalmic nerve.
Muscles
As the aqueous fluid is secreted from serous and mucinous cells within the acini, myoepithelial cells are responsible for the expulsion of this fluid from the gland. The myoepithelial cells are organized around acini and ducts within the lacrimal gland and have a contractile response to neuronal stimulation.
Physiologic Variants
Variants of the lacrimal gland are primarily related to size and structure.[7] There also exists a variation in vascular supply. The lacrimal gland receives the bulk of its blood supply from the ophthalmic artery via the lacrimal artery branch. The ophthalmic artery is itself a branch of the internal carotid artery. However, there exists a variable arterial supply of the lacrimal gland via the infraorbital artery, a branch of the external carotid artery.
Surgical Considerations
Damage to the secretory glands of the ocular adnexa is a potential complication of periocular surgery. The lacrimal gland and its ducts can suffer injury by transconjunctival surgical intervention, such as in the recession of the lateral horn of the levator aponeurosis in correcting temporal flare. A transcutaneous approach is favored when feasible to avoid direct damage to the lacrimal secretory system.[8]
Aside from direct structural trauma incurred by surgery, fibers of the ophthalmic nerve traversing the orbicularis muscle and fascia are easily disrupted.[9] The lacrimal gland functions within a neural network in which sensory input from the ocular surface mediates its activity. Decreased ocular surface sensation via the ophthalmic nerve results in reduced stimulation of the lacrimal gland with consequent inadequate tear secretion.[10] To avoid damage to these sensory fibers, the orbicularis muscle should be preserved during surgical intervention if possible.[11]
Clinical Significance
Dry eye, often referred to as Dry Eye Syndrome (DES), is typically a multifactorial disease that results in improper tear film composition and symptoms of ocular discomfort. Risk factors of DES include aging, female gender, hormonal changes, systemic autoimmune disorders, decreased corneal sensation, drug effects, viral infections, diabetes mellitus, vitamin A deficiency, and graft-versus-host disease. The potential causes of dry eye fall under two distinct categories: evaporative and aqueous deficient. Aqueous tear deficiency (ATD) can further classify as Sjögren syndrome dry eye and Non-Sjögren dry eye. In both categories of ATD, lacrimal output diminishes, resulting in disruption of the tear film. The clinical symptoms of ATD tend to be worse at the end of the day or with prolonged use of the eyes, due to reduced blink rate. Environmental factors such as low humidity can also exacerbate symptoms. The most common symptoms associated with ATD are ocular burning, foreign body sensation, photosensitivity, and blurred vision.
Another crucial clinical consideration of the lacrimal gland is dacryoadenitis or inflammation of the lacrimal gland. The majority of cases of dacryoadenitis are idiopathic in origin, with a likely unknown autoimmune contribution. Infectious dacryoadenitis is a common feature of the Epstein-Barr virus. Autoimmune dacryoadenitis is a common feature of sarcoidosis, Sjogren, granulomatosis with polyangiitis, and IgG4-related disease. Beyond these causes, derangements of the MALT may result in lymphoproliferative tumors, seen in 7% of biopsies for orbital and lacrimal gland inflammation.[12]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
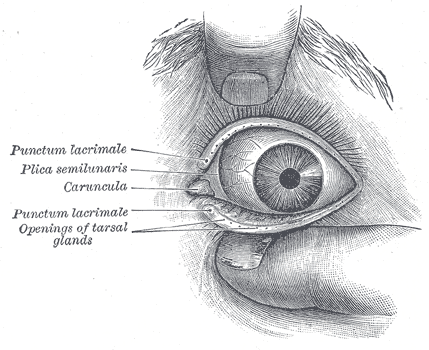
Lacrimal Glands. The front of the left eye with separated eyelids displays the medial canthus, punctum lacrimalia, plica semilunaris, caruncula, and openings of tarsal glands.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
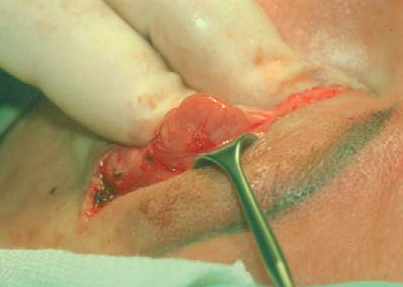
The lacrimal gland, located in the lateral aspect of the upper eyelid may be confused for a lateral fat pocket. There are two fat pockets in the pre-aponeurotic space of the upper eyelid: the medial and the central. Laterally, the lacrimal gland may prolapse forwards. It is recognized by its lobular structure and its pinkish colour. Contributed by Prof. BCK Patel MD, FRCS
References
Kang H, Takahashi Y, Ichinose A, Nakano T, Asamoto K, Ikeda H, Iwaki M, Kakizaki H. Lateral canthal anatomy: a review. Orbit (Amsterdam, Netherlands). 2012 Aug:31(4):279-85. doi: 10.3109/01676830.2012.694957. Epub 2012 Jun 12 [PubMed PMID: 22690873]
Dantas RR. Lacrimal drainage system obstruction. Seminars in ophthalmology. 2010 May:25(3):98-103. doi: 10.3109/08820538.2010.488577. Epub [PubMed PMID: 20590420]
Farmer DT, Nathan S, Finley JK, Shengyang Yu K, Emmerson E, Byrnes LE, Sneddon JB, McManus MT, Tward AD, Knox SM. Defining epithelial cell dynamics and lineage relationships in the developing lacrimal gland. Development (Cambridge, England). 2017 Jul 1:144(13):2517-2528. doi: 10.1242/dev.150789. Epub 2017 Jun 2 [PubMed PMID: 28576768]
Dean C, Ito M, Makarenkova HP, Faber SC, Lang RA. Bmp7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development (Cambridge, England). 2004 Sep:131(17):4155-65 [PubMed PMID: 15280212]
Level 3 (low-level) evidenceSherman DD, Gonnering RS, Wallow IH, Lemke BN, Doos WG, Dortzbach RK, Lyon DB, Bindley CD. Identification of orbital lymphatics: enzyme histochemical light microscopic and electron microscopic studies. Ophthalmic plastic and reconstructive surgery. 1993:9(3):153-69 [PubMed PMID: 8217957]
Level 3 (low-level) evidenceScott G, Balsiger H, Kluckman M, Fan J, Gest T. Patterns of innervation of the lacrimal gland with clinical application. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1174-7. doi: 10.1002/ca.22447. Epub 2014 Aug 4 [PubMed PMID: 25092807]
Lorber M, Vidić B. Measurements of lacrimal glands from cadavers, with descriptions of typical glands and three gross variants. Orbit (Amsterdam, Netherlands). 2009:28(2-3):137-46 [PubMed PMID: 19839898]
Takahashi Y, Watanabe A, Matsuda H, Nakamura Y, Nakano T, Asamoto K, Ikeda H, Kakizaki H. Anatomy of secretory glands in the eyelid and conjunctiva: a photographic review. Ophthalmic plastic and reconstructive surgery. 2013 May-Jun:29(3):215-9. doi: 10.1097/IOP.0b013e3182833dee. Epub [PubMed PMID: 23381567]
Scott KR, Tse DT, Kronish JW. Vertically oriented upper eyelid nerve fibers. A clinical, anatomical and immunohistochemical study. Ophthalmology. 1992 Feb:99(2):222-6 [PubMed PMID: 1553211]
Kim HH, De Paiva CS, Yen MT. Effects of upper eyelid blepharoplasty on ocular surface sensation and tear production. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2007 Oct:42(5):739-42 [PubMed PMID: 17823642]
Hamawy AH, Farkas JP, Fagien S, Rohrich RJ. Preventing and managing dry eyes after periorbital surgery: a retrospective review. Plastic and reconstructive surgery. 2009 Jan:123(1):353-359. doi: 10.1097/PRS.0b013e31819346ea. Epub [PubMed PMID: 19116572]
Level 2 (mid-level) evidenceTan LT, Davagnanam I, Isa H, Taylor SR, Rose GE, Verity DH, Pusey CD, Lightman S. Clinical and imaging features predictive of orbital granulomatosis with polyangiitis and the risk of systemic involvement. Ophthalmology. 2014 Jun:121(6):1304-9. doi: 10.1016/j.ophtha.2013.12.003. Epub 2014 Feb 20 [PubMed PMID: 24560566]
Level 2 (mid-level) evidence
