Introduction
Cholangiocarcinoma is an aggressive malignancy of biliary epithelium that may arise anywhere in the biliary tract, from the intrahepatic biliary canaliculi to the terminus where the common bile duct enters the duodenum at the duodenal ampulla. Cholangiocarcinoma is classified by anatomical origin as intrahepatic cholangiocarcinoma (iCCA) or extrahepatic cholangiocarcinoma (eCCA); eCCA is subdivided into perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA). More than 95% of cholangiocarcinomas are adenocarcinomas.
Several clinical conditions and premalignant lesions predispose to developing cholangiocarcinoma.[1] The clinical presentation of cholangiocarcinoma will vary with the location and size of the tumor. Diagnosing cholangiocarcinoma can be difficult, particularly for extrahepatic lesions; available biopsy techniques lack diagnostic sensitivity. Surgical intervention is indicated even without a confirmatory tissue diagnosis in the appropriate clinical setting. All patients with suspected or confirmed cholangiocarcinoma should be evaluated for distant metastatic disease; almost 75% of patients have nonresectable or metastatic disease at presentation.
Carbohydrate cell-surface antigen 19-9 (CA 19-9) is a tumor marker excreted by the biliary epithelium that assists with assessing disease severity and surveillance monitoring.[2] The overall prognosis of cholangiocarcinoma is poor, given the aggressive nature of the tumor and the usually advanced stage at presentation. Surgery is the only curative treatment modality; radiation and chemotherapy serve as adjuncts. Recent investigations into the molecular mechanisms underlying cholangiocarcinoma have yielded various targeted therapies that have improved outcomes and will hopefully improve patient care in the future.[3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Cholangiocarcinoma frequently arises in the absence of genetic predisposition and without a clear etiology. However, certain risk factors that vary with ethnicity and geography predispose to cholangiocarcinoma in some patients.[4] These predisposing risk factors include but are not limited to:
Parasitic infections: Infestation with liver flukes such as Clonorchis and Opisthorchiasis is strongly associated with cholangiocarcinoma. These infestations are endemic to Southeast Asian regions; the highest incidence rates are in northeast Thailand.[5] Liver fluke infestation is due to the consumption of undercooked fish, and parasite-induced chronic biliary inflammation is the primary driver of malignant transformation.[6]
Primary sclerosing cholangitis: Primary sclerosing cholangitis is a progressive autoimmune cholestatic liver disease. Individuals with primary sclerosing cholangitis have a significantly elevated risk, perhaps as much as 400 times the risk, of developing cholangiocarcinoma compared to the general population, especially with concomitant inflammatory bowel disease.[7] For a comprehensive discussion of this disease process, please see our StatPearls' companion reference, "Primary Sclerosing Cholangitis."
Biliary tree calculi: Hepatolithiasis, cholelithiasis, and choledocholithiasis increase the risk of developing cholangiocarcinoma, especially with larger stones and a more prolonged illness duration. Hepatolithiasis is more common in Asia and may be associated with parasitic infections.[8]
Cystic biliary lesions: Patients with choledochal cysts, biliary mucinous cystic neoplasms, or intraductal papillary biliary mucinous neoplasms are at a significantly increased risk of developing cholangiocarcinoma.
- Choledochal cysts - All choledochal cysts are associated with an increased risk of cholangiocarcinoma; types I and IV confer the greatest risk. Prompt treatment of choledochal cysts is vital in reducing the risk of cholangiocarcinoma development.[9] Please see StatPearls' companion reference, "Choledochal Cysts," for a thorough discussion of these lesions.
- Biliary mucinous cystic neoplasms - Formerly identified as cystadenomas, biliary mucinous cystic neoplasms (B-MCNs) are predominately intrahepatic cystic lesions that are more common in young women. B-MCNs are frequently asymptomatic incidental findings, but large B-MCNs may cause signs or symptoms of compression or pain secondary to liver capsular distention. B-MCNs are usually septated with thickened internal walls and internal papillary projections, distinguishing them from simple liver cysts during imaging. Complete surgical resection is the preferred treatment for B-MCNs.[10]
- Intraductal papillary biliary mucinous neoplasms - These mucin-producing lesions, most commonly found in the left lobe of the liver, are analogous to pancreatic intraductal papillary mucinous neoplasms and are frequently asymptomatic. Cross-sectional imaging may reveal an associated solid component, and mucin may be distinguishable with magnetic resonance imaging. Approximately 80% of intraductal papillary biliary mucinous neoplasms harbor a malignancy, and surgical excision is recommended.[11]
Chronic liver disease: Chronic infection with hepatitis B or C, hemochromatosis, metabolic dysfunction-associated steatotic liver disease (MASLD, formerly nonalcoholic fatty liver disease or NAFLD), and cirrhosis of any etiology are associated with an increased risk of cholangiocarcinoma.[12]
Lifestyle, environmental, and metabolic factors: Type 2 diabetes, obesity, alcohol consumption, and cigarette smoking increase the risk of developing cholangiocarcinoma. Exposure to Thorotrast, a radioactive thorium dioxide contrast media widely used between 1920 and 1950, increases the risk of cholangiocarcinoma development. Exposure to asbestos and propylene dichloride (1,2-Dichoropropane) also confers an increased risk.[12]
Genetic predisposition: Patients with hereditary nonpolyposis colorectal cancer (Lynch syndrome), BAP1-related tumor predisposition syndrome, multiple biliary papillomatosis, and cystic fibrosis carry an increased risk of cholangiocarcinoma development.
Epidemiology
Cholangiocarcinomas comprise about 3% of gastrointestinal malignancies, are the second most common primary liver tumors, and account for approximately 10% to 15% of all hepatobiliary malignancies.[13] The incidence of intrahepatic cholangiocarcinoma has been rising, possibly due to improved diagnostic and classification techniques, while the incidence of extrahepatic lesions has been falling in recent years. The incidence of cholangiocarcinoma varies markedly according to the geographic area; the incidence rates are up to 50-fold higher in parts of Thailand than in the United States. The incidence of cholangiocarcinoma increases with age, is slightly more common in men, and is most commonly diagnosed between the ages of 50 and 70 years.[14][15]
Perihilar cholangiocarcinoma is the most commonly encountered subtype, accounting for approximately 50% of cases. Distal (40%) and intrahepatic cholangiocarcinoma (10%) are less common.[1]
Pathophysiology
Cholangiocarcinoma generally arises in the setting of chronic inflammation, either from precursor lesions or de novo. Mutations in various protooncogenes and tumor suppressor genes mediate carcinogenesis. While the specific molecular carcinogenic pathways have not been identified, cholangiocarcinomas typically harbor mutations RAS, BRAF, TP53, SMAD4, and more.[16][17][18][19] K-ras and TP53 mutations are most commonly seen. However, genetic mutations will vary with the underlying disease etiology, especially for parasite-induced carcinogenesis.
Tumor Location and Morphology
The distinction between intrahepatic, perihilar, and distal cholangiocarcinoma is anatomical. Intrahepatic cholangiocarcinoma arises from the biliary epithelium proximal to the segmental bile ducts. Tumors arising from the left or right hepatic ducts or their confluence are termed perihilar cholangiocarcinoma. Tumors distal to the biliary confluence are distal cholangiocarcinoma.
Cholangiocarcinoma grows in 3 distinct morphologic forms; the Liver Cancer Study Group of Japan classifies cholangiocarcinoma into mass-forming, periductal infiltrating, and intraductal-growing types (see Table 1. Morphologic Cholangiocarcinoma Classification).[20][21] See Image. Cholangiocarcinoma Tumor Location and Morphology.
Table 1. Morphologic Cholangiocarcinoma Classification
| Morphology | Gross appearance | Radiologic appearance | Tumor characteristics and prognosis |
| Mass-forming |
Firm, nonencapsulated, well-demarcated mass Satellite nodules may be present |
Often large, well-demarcated masses with irregular borders Peripheral enhancement may be present Perihilar and distal lesions can cause biliary obstruction |
Aggressive, early invasion of portal vein branches Tendency to intrahepatic metastasis |
| Periductal-infiltrating |
Growth along the bile duct Tumors may be elongated or branching Often no associated mass Stricturing of the underlying duct |
Segmental of diffuse narrowing of the bile duct Frank stricture may occur Focal duct thickening without a mass Upstream dilation of the biliary system |
Early lymphatic spread Significant spread along proximal and distal bile duct Poor prognosis |
| Intraductal-growing |
Papillary or nodular growth into the bile duct Tumors spread superficially along ductal mucosa May be multifocal |
Intraductal mass with outer biliary wall Mass may cause biliary obstruction with upstream dilatation |
Detected at the earliest stage Best prognosis |
Histopathology
Cholangiocarcinoma arises from bile duct epithelium; 90% to 95% of cholangiocarcinomas are adenocarcinomas.[22]
Intrahepatic cholangiocarcinoma arises from ducts proximal to the segmental bile ducts, which can be divided into small and large bile ducts. Tumors arising from these ducts differ in their pathologic characteristics and are termed small- and large-duct cholangiocarcinoma. Small-duct cholangiocarcinoma arises in the liver periphery and is lined by cuboidal cholangiocytes. Cuboidal cells often form small tubular glands without mucin production. Positive expression for C-reactive protein (CRP), N-cadherin, and neural cell adhesion molecule (NCAm), with negative S100P expression, is classic. These lesions are more likely to be mass-forming.[22][23]
Large-duct cholangiocarcinoma occurs closer to the liver hilum and arises from biliary glands and columnar cholangiocytes in larger ductules and segmental bile ducts. Large-duct cholangiocarcinoma may develop in the setting of biliary intraepithelial neoplastic lesions (B-MCN, intraductal papillary biliary mucinous neoplasms). In contrast to small-duct carcinoma, mucin production is common, with negative expression for CRP, N-cadherin, and NCAm and overexpression of S100P. These tumors are more likely to be infiltrating or intraductal. Perihilar and distal cholangiocarcinoma are predominantly large-duct types.[22][23][24]
History and Physical
The clinical presentation of cholangiocarcinoma will depend on the location and size of the tumor. Intrahepatic cholangiocarcinoma typically presents with nonspecific symptoms, including abdominal pain, weight loss, and fatigue. Jaundice and cholangitis may occur in the presence of biliary obstruction. Frequently, symptoms occur late in the disease course; tumors can be large at the time of diagnosis.[25]
Extrahepatic cholangiocarcinomas are symptomatic earlier in their clinical course due to biliary obstruction, leading to jaundice, pruritus, clay-colored stools, and dark-colored urine. There may be a palpable mass and ascites in advanced disease. Uncommonly, patients can present because of signs related to metastatic disease; metastatic disease may be an incidental finding of imaging studies.[26]
The physical examination of a patient with suspected or known cholangiocarcinoma should evaluate nutritional status and liver function and include a focused abdominal exam. Rarely, a palpable mass in the right upper quadrant may be identified secondary to large intrahepatic tumors or with distal cholangiocarcinoma causing a biliary obstruction (Courvoisier sign). Signs of potentially unresectable disease include ascites or features of portal hypertension.
Evaluation
The presenting symptoms dictate the diagnostic evaluation, including laboratory, imaging, and interventional procedures. Obtaining a tissue diagnosis can be difficult depending on the location of the lesion; this is especially true for perihilar lesions. Modalities for obtaining tissue include brush cytology, fine needle aspiration, or image-guided biopsy. Despite improvement in diagnostic techniques, none of these modalities are sensitive enough to rule out cholangiocarcinoma effectively. In the appropriate clinical setting, surgery is indicated even without tissue confirmation.[27]
Laboratory Studies
It is recommended that all patients undergo a comprehensive medical evaluation that includes a complete blood count (CBC), comprehensive metabolic profile (CMP) including liver function tests (LFTs), and coagulation studies to identify anemia, evidence of portal hypertension and hypersplenism, synthetic liver dysfunction, biliary obstruction, and renal dysfunction. Additionally, elevated transaminases may indicate hepatocyte injury. In tumors that cause biliary obstruction, direct hyperbilirubinemia and elevated alkaline phosphatase are common. Tumor biomarkers, including CA 19-9, carcinoembryonic antigen (CEA), and α-fetoprotein, should be measured. CA 19-9 is typically elevated in cholangiocarcinoma, but the results are unreliable in the presence of biliary obstruction.[2]
Imaging Studies
Abdominal ultrasonography: often the initial diagnostic tool in patients with suspected cholangiocarcinoma because it is easily performed, noninvasive, cost-effective, and carries no risk of radiation exposure. However, ultrasonography may not yield a conclusive diagnosis. Ultrasonography may reveal a liver mass, signs of intra- or extrahepatic biliary dilation, or gall bladder distention.
Computerized tomography (CT): can identify liver anatomy and evaluate locoregional or metastatic disease. A triple-phase CT with arterial, venous, and portal venous phases is recommended to increase diagnostic accuracy. If the diagnosis is cholangiocarcinoma, a chest CT is recommended to evaluate for metastatic disease.
The CT findings of cholangiocarcinoma will vary with the subtype:
- Intrahepatic-peripheral cholangiocarcinoma usually presents as a mass with irregular margins with or without hepatic capsule retraction and dilatation of the peripheral intrahepatic ducts. The lesion usually demonstrates thin, incomplete rim enhancement during the arterial and portal venous phases.[28]
- Intrahepatic-intraductal disease can cause dilatation of the intrahepatic bile ducts with or without an intraductal polypoid mass or a lobar atrophy-hypertrophy complex. The neoplasm can present as irregular masses with markedly low attenuation, minimal peripheral enhancement, and focal dilatation of intrahepatic ducts around the tumor.[28]
- Perihilar cholangiocarcinoma can present with dilated proximal bile ducts, affecting the liver segments proximal to the lesion. The right and left bile ducts can appear disconnected. A primary mass lesion may not be seen. Often, a biliary stricture is the only finding.[28]
- Extrahepatic cholangiocarcinomas present as wall thickening of the extrahepatic duct, a mass at the porta hepatis, or proximal biliary dilatation in the periampullary region.
Magnetic resonance imaging (MRI): offers excellent imaging of the liver and, when combined with MR cholangiopancreatography (MRCP), provides the best noninvasive imaging of the biliary tree, allowing the identification of strictures, masses, and cysts (see Image. Cholangiocarcinoma). MRI with contrast, which is usually gadolinium-based, enables the visualization of intrahepatic masses and their relationship to hepatic vasculature (see Image. Axial T2 Haste and 20s, 3-minute, and 10-minute post-contrast).[29]
Positron emission tomography (PET): may be used to assess for distant metastatic disease, but the National Comprehensive Cancer Network (NCCN) guidelines do not recommend the routine use of PET in the diagnosis and management of cholangiocarcinoma.
Interventional Procedures
Endoscopic techniques: endoscopic retrograde cholangiopancreatography (ERCP) is used for biliary decompression and tissue diagnosis. Extrahepatic cholangiocarcinoma may be amenable to ERCP-guided biopsy or cytologic brushing. Unfortunately, the sensitivity of current biopsy techniques is too low to rule out carcinoma effectively, and operative intervention is still recommended in the appropriate clinical scenario, even without a tissue diagnosis. ERCP for biliary drainage may be indicated for severe jaundice or in patients with cholangitis.[30]
Endoscopic ultrasound (EUS): visualizes the portal structures and the surrounding lymph nodes. In addition, EUS-guided biopsies have a higher sensitivity than ERCP alone but remain inadequate in ruling out cholangiocarcinoma effectively.[31]
Percutaneous transhepatic cholangiography (PTC): Percutaneous image-guided access to the biliary tree is possible, especially in patients with intrahepatic biliary dilation. PTC allows for decompression of dilated bile ducts without contaminating the biliary tree. In patients undergoing hepatectomy, draining the functional liver remnant (FLR) is essential in improving postoperative liver regeneration and reducing the risk of liver failure.[30]
Treatment / Management
The management of cholangiocarcinoma is complex and dictated by the site, extent, and relationship of the neoplasm to surrounding structures. Surgery is the only definitive cure, either with resection or liver transplantation. Chemotherapy and radiation are adjunctive treatment modalities.
Preoperative Considerations
Biliary drainage
Biliary obstruction induces liver atrophy and impairs liver regeneration. Distal cholangiocarcinoma can cause biliary obstruction with dilation of the entire biliary tree. Preoperative biliary drainage, usually via ERCP and biliary stent placement, does not improve outcomes when compared to upfront surgery in patients with resectable disease and increases the risk of postoperative complications.[32] Drainage may be beneficial in patients who undergo neoadjuvant therapy.[33]
Biliary drainage in perihilar tumors is a more widely debated topic. Perihilar cholangiocarcinoma commonly causes biliary obstruction, with dilation of the ducts proximal to the tumor. Drainage via ERCP results in colonization of the biliary tract with intestinal organisms and has a higher rate of stent misplacement and replacement in more proximal obstruction. PTC has the advantage of biliary drainage without contamination. Routine biliary decompression is not indicated. Selective biliary decompression of the FLR in patients undergoing liver resection, especially in whom the FLR is less than 40%, is indicated, as this minimizes postoperative liver failure. Drainage is also recommended in those undergoing neoadjuvant chemotherapy or portal venous embolization.[30]
Volumetric analysis
In patients with iCCA and pCCA who require liver resection, the calculation of liver volumes and the FLR is critical to operative planning. Generally, an FLR of greater than 30% in healthy patients and 40% in those with cirrhosis is required. FLR is calculated using CT-guided imaging with software that calculates liver volumes. Patients who have inadequate FLR require interventions to increase liver volumes. Portal venous embolization (PVE) is the most commonly utilized technique and involves preoperative embolization of the portal vein branches to the liver segments to be resected.[34] Embolization results in hypertrophy of the FLR of up to 40%. The rate of hypertrophy, or the kinetic growth rate, is an important marker; a rate greater than 2.66% per week predicts lower postoperative liver failure and mortality.[35] Less common techniques for inducing hypertrophy include liver partition and portal vein ligation for staged hepatectomy and liver venous deprivation.
Local Therapies
Local therapies are the recommended treatment for intrahepatic cholangiocarcinoma when the tumor is unresectable, or the patient is not fit for surgery. There are various techniques available, but their detailed discussion is beyond the scope of this activity. The techniques are not mutually exclusive and can be used in combination depending on the size and location of the tumor and available expertise.[36] Local therapies for cholangiocarcinoma are extremely important as the majority of patients die from liver failure due to intrahepatic metastases. Delaying the onset of liver failure can significantly improve patient survival and quality of life.[35] For an overview of local therapies for the treatment of cholangiocarcinoma, please see Table 2. Local Therapies for Cholangiocarcinoma.
Transarterial chemoembolization
Transarterial chemoembolization (TACE) employs chemotherapeutic agents directly instilled into the hepatic artery, followed by embolization of the artery, thereby depriving the tumor of its arterial supply. Doxorubicin and platinum agents are most commonly used. The chemotherapeutic agent may be emulsified or incorporated into beads or microspheres and deployed via angiography into the hepatic artery branches that supply the tumor. The procedure is well-tolerated in most patients and can significantly shrink tumors.[37](A1)
Transarterial radioembolization
Transarterial radioembolization (TARE) is a newer technique that installs yttrium 90 (90Y) in either resin or glass microspheres via the hepatic artery directly into the tumor, followed by vessel embolization. This results in a high dose of radiation in the immediate vicinity of the tumor and is performed similarly to TACE.[37] (A1)
Ablative techniques
Tumor ablation using radiofrequency or microwave energy is an option for local control in patients with unresectable disease or who are not fit to undergo surgical resection.[38] These techniques can be done using image guidance and are much better tolerated than surgery. Both techniques rely on the application of energy, and the electrode is placed in the center of the tumor. The effectiveness is inversely proportional to size; tumors larger than 3 cm are much less likely to be entirely ablated. These techniques should be used cautiously near larger blood vessels or bile ducts. Ablation can be repeated as often as needed.[39]
Hepatic artery infusion
Hepatic artery infusion is a technique whereby chemotherapy is instilled directly into the hepatic artery proper via a subcutaneously placed pump. The catheter is threaded into the hepatic artery via the gastroduodenal artery; this requires a surgical procedure to accomplish. Chemotherapy is then infused over the ensuing weeks and can result in significant downstaging of the tumor. When hepatic artery infusion is performed at institutions with extensive experience, the overall results are promising. Hepatic artery infusion is not yet a widely adopted treatment modality.[40][41]
Table 2. Local Therapies for Cholangiocarcinoma
| Modality | Mechanism of action | Technique | Indications |
| Transarterial chemoembolization (TACE) | Infusion of chemotherapy-embedded carrier material followed by embolization | Angiographic access to hepatic arterial branches supplying the tumor, followed by chemotherapy injection. After instillation, bland embolization of feeding hepatic arterial branches |
Unresectable disease Borderline resectable disease to downstage tumor As a bridge to transplant Local recurrence No restriction on tumor size |
| Transarterial radioembolization (TARE) | Local high-dose radiation through yttrium microspheres | Angiographic access to hepatic arterial branches supplying the tumor, followed by radiation bead injection. After instillation, bland embolization of feeding hepatic arterial branches |
Unresectable disease Borderline resectable disease to downstage tumor As a bridge to transplant Local recurrence No restriction on tumor size |
| Radiofrequency ablation | High-frequency electric current causes heat and tissue destruction | Image-guided placement of electrodes into the tumor with ablation. |
Tumors <5 cm High-risk patients Treatment of liver remnant lesions before surgery Local recurrence |
| Microwave ablation | Electromagnetic radiation via frictional heat supplies a more uniform heat than RFA | Image-guided placement of electrodes into the tumor with ablation |
Tumors <5 cm High-risk patients Treatment of liver remnant lesions before surgery Local recurrence |
| Hepatic artery infusion | Direct infusion of chemotherapy into the hepatic artery proper with high intrahepatic doses and low systemic doses | Surgical ligation of the gastroduodenal artery with the placement of a catheter into the hepatic artery proper, supplied by a subcutaneous pump with chemotherapy | Unresectable disease
Borderline resectable disease to downstage tumor As a bridge to transplant |
| Stereotactic body radiotherapy | High-dose radiation to a small field | 3- or 4-D image-guided planning with high doses in a limited number of fractions |
Adjuvant therapy Unresectable tumors Local recurrence |
Differential Diagnosis
Since the signs and symptoms of cholangiocarcinoma, including jaundice, abdominal pain, and fatigue, are very nonspecific, the differential diagnosis can be vast. Some possible differential diagnoses include:
- Choledocholithiasis
- Pancreatic cancer
- Primary sclerosing cholangitis
- Primary biliary cirrhosis
- Hepatocellular carcinoma
- Cholangitis
- Cholecystitis
Surgical Oncology
The general principles of liver surgery apply to cholangiocarcinoma. Maintaining an adequate FLR and hepatic inflow and outflow while obtaining microscopically negative margins is critical to good outcomes. Diagnostic laparoscopy should always be performed before laparotomy; as many as 30% of patients may have radiographically occult metastatic disease that would preclude resection.[42]
Intrahepatic Cholangiocarcinoma
Hepatic resection with microscopically negative margins (R0) is the mainstay of therapy for iCCA. Positive resection margins are associated with higher rates of local recurrence and worse overall survival. Hepatic resection may be anatomic, based on segmental liver anatomy with the removal of entire segments with their surrounding Glissonian sheath, or nonanatomic, with resection of the tumor with negative margins. The extent of resection is dictated by the location and size of the tumor and may include portal vein resection and reconstruction if the vein is involved. A portal lymphadenectomy should be performed routinely.[43][1]
Perihilar Cholangiocarcinoma
Operative decision-making in pCCA is complex, as identifying the extent of the tumor may be difficult. Even with high-quality imaging and endoscopic techniques, the proximal and distal extent of the tumor may not be visualized preoperatively and only be identified intraoperatively. The extrahepatic bile duct and the involved hemiliver are usually resected with a portal lymphadenectomy. Routine resection of the caudate lobe is also recommended, given its proximity to the biliary confluence and its involvement in many tumors. To ensure negative margins, frozen section margins on the bile duct margins should be performed before reconstruction. The Bismuth-Corlette classification is commonly used for perihilar tumors and guides surgical management (see Image. Bismuth Corlette Classification of Perihilar Cholangiocarcinoma).[44][45]
- Type I: Tumors distal to the confluence of the left and right hepatic ducts
- Type II: Tumors involving the hepatic duct confluence
- Type III
- IIIa: Tumors obstructing the common hepatic duct and the proximal right hepatic duct
- IIIb: Tumors obstructing the common hepatic duct and the proximal left hepatic duct
- Type IV: Tumors that are multicentric or involve the confluence and both the right or left hepatic duct [46][47][45]
Types I and II tumors are usually managed with an extrahepatic bile duct resection and resection of adjacent liver parenchyma. Some authors and high-volume centers routinely perform right hepatectomies for these lesions, especially those of the infiltrating type.[48][45][48]
Type III tumors routinely require an extrahepatic bile duct resection and a right hepatectomy in Type IIIa or left hepatectomy in Type IIIb.[45]
Type IV tumors usually require a liver transplant. The Mayo protocol for these tumors, which involves preoperative chemoradiation followed by liver transplant, has been shown to have a 5-year survival of over 50%.[49]
Distal Cholangiocarcinoma
These neoplasms are managed with pancreaticoduodenectomy. The proximal bile duct margin should be examined intraoperatively to ensure negative margins. To obtain an R0 resection, much of the extrahepatic bile duct may need to be removed. Rarely, tumors of the mid-duct may be amenable to an extrahepatic bile duct resection alone.[50][51]
Radiation Oncology
Adjuvant Therapy
Radiotherapy (RT) may be used in the adjuvant setting for cholangiocarcinoma.[52] There is typically no role for radiotherapy for patients who undergo a complete resection with microscopically negative margins. RT, usually in combination with chemotherapy, is generally utilized to reduce local recurrence in patients with a microscopically or grossly positive surgical margin. The radiation dose depends on the tumor location, the tolerance of surrounding organs, and underlying liver function. Image-guided techniques, such as three-dimensional conformal and intensity-modulated RT, improve targeting and reduce toxicity. The radiation field should include the operative bed and the regional lymph nodes, including the portal, celiac, para-aortic, superior mesenteric artery, and others. Typical doses range from 40 Gy to 45 Gy to the lymph nodes and 55 Gy to 60 Gy to the tumor site.[49] There is no definitive data to suggest a survival benefit. Patients with node-positive disease may also receive radiotherapy. External beam radiotherapy with a chemosensitizing agent such as capecitabine or gemcitabine is typically used.[53]
Unresectable Disease
RT may be used in unresectable disease to provide local control and symptom relief.[52] Both external beam RT and stereotactic body RT (SBRT) may be used. SBRT allows very high doses of radiation to target lesions with a high degree of accuracy and provides local control rates that appear to be better than conventional fractionated external beam radiotherapy. Radiotherapy is almost always administered with concurrent radiosensitizing chemotherapy. Typical doses in this setting are higher than those in adjuvant therapy.
Medical Oncology
Adjuvant Therapy
Postoperative therapy should be offered within 8 to 12 weeks and requires preinitiation baseline laboratory tests and imaging. Adjuvant treatment may be delayed in cases where there are significant surgical complications or other patient-related factors decreasing the ability to tolerate chemotherapy. In general, the efficacy of adjuvant therapy decreases as the time to initiation increases.
After resection, adjuvant therapy should be offered to patients with positive surgical margins, positive lymph nodes, or vascular involvement. The role of adjuvant chemotherapy in patients with completely resected specimens with microscopically negative margins is unclear. The benefit of adjuvant therapy is based on 2 trials, including the Biliary Tract Cancer (BILCAP) trial and a randomized trial from Japan employing single-agent adjuvant treatment. In the BILCAP trial, adjuvant capecitabine improved overall survival without chemotherapy, although this trend was not statistically significant.[54] This is currently accepted as a standard of care after resection. Two negative trials have reported no benefit to gemcitabine-based regimens, which are presently discouraged.
The lack of randomized trials plagues trial data on cholangiocarcinoma, which is a rare cancer; most trials combined gallbladder carcinoma with all biliary tract cancers. Some of the benefits of adjuvant chemotherapy may be in the population of patients with gallbladder carcinoma, which has a higher rate of distant metastatic disease.[55][56]
Neoadjuvant setting
Neoadjuvant chemotherapy is currently used in borderline resectable tumors. Some patients may have an adequate response that allows for resection. In addition, neoadjuvant chemotherapy and radiation may be used as part of the Mayo protocol in preparation for liver transplant in patients with hilar cholangiocarcinoma. For patients with upfront resectable disease, there is no currently available data to support the routine use of neoadjuvant chemotherapy.[57]
Locally advanced or metastatic setting
Chemotherapy can significantly extend survival in patients with metastatic disease, and immunotherapy has improved this benefit more recently. Based on the recent TOPAZ-1 trial, the combination of durvalumab with gemcitabine and cisplatin was superior to gemcitabine and cisplatin alone.[58] Pembrolizumab, in combination with gemcitabine and cisplatin, has also been shown to be superior to chemotherapy alone. In general, these regimens are considered the standard of care.[59]
Chemotherapy alone remains the standard of care in several settings where immunotherapy may not be available. Multiple second- and third-line regimens exist for patients with poor performance status, persistent hyperbilirubinemia, or other considerations. With the identification of an increased number of specific tumor mutations and the availability of targeted agents, several targeted agents are now available based on the tumor mutational profile of these cancers.[60]
Prognosis
The overall prognosis for cholangiocarcinoma is poor. Only one-fourth of patients have resectable disease at the time of diagnosis; intrahepatic and perihilar tumors most commonly present at a more advanced stage. The 5-year overall survival rate for intrahepatic cholangiocarcinoma approximates 8%, with a slightly higher but sobering 10% 5-year survival rate for extrahepatic cholangiocarcinoma. Surgery remains the best opportunity to achieve a cure, but surgery is often morbid and can be associated with significant complications.[1]
Complications
Complications from cholangiocarcinoma can be related to the disease itself or due to therapeutic interventions. In patients with advanced cholangiocarcinoma, intrahepatic metastases with eventual liver failure are a significant complication. In addition, biliary obstruction due to mass-like lesions can cause cholangitis, liver atrophy, cirrhosis, and considerable deconditioning. Distant metastatic disease can occur, as can local intraperitoneal spread.
Deterrence and Patient Education
Cholangiocarcinoma is a rare but highly aggressive malignancy of the biliary tract. Symptoms may be vague, especially when the neoplasm begins within the liver, and may include nonspecific symptoms like abdominal pain, weight loss, and fatigue. Extrahepatic tumors may present with jaundice or abdominal pain. Patients with any risk factors for cholangiocarcinoma, such as chronic parasitic infections, biliary cystic disease, or unexplained symptoms such as weight loss, reduced appetite, unexplained fatigue, or unusual yellowing of the skin or urine, should be evaluated by a physician. Diagnosing cholangiocarcinoma may not be straightforward and may require several different imaging techniques or interventional procedures. Approximately one-fourth of patients with cholangiocarcinoma are curable.
Enhancing Healthcare Team Outcomes
Cholangiocarcinoma patients require personalized care that caters to their individual needs. Diagnosing this condition can be challenging and often requires the input of multiple medical specialties. The management of this disease is complex and depends on various factors such as imaging results, patient comorbidities, and the findings of interventions like endoscopy or cholangiography. As a result, a multidisciplinary team must be involved in the patient's care from the time of diagnosis. Moreover, since individualized treatment is crucial for patient-centered care, each case should receive tailored therapy that addresses its unique characteristics. Although cholangiocarcinoma is often not curable, recent advancements in local therapies and the availability of more targeted therapies have improved patient survival rates and quality of life. Therefore, a collaborative, team-based approach is vital for managing this condition effectively.
Media
(Click Image to Enlarge)
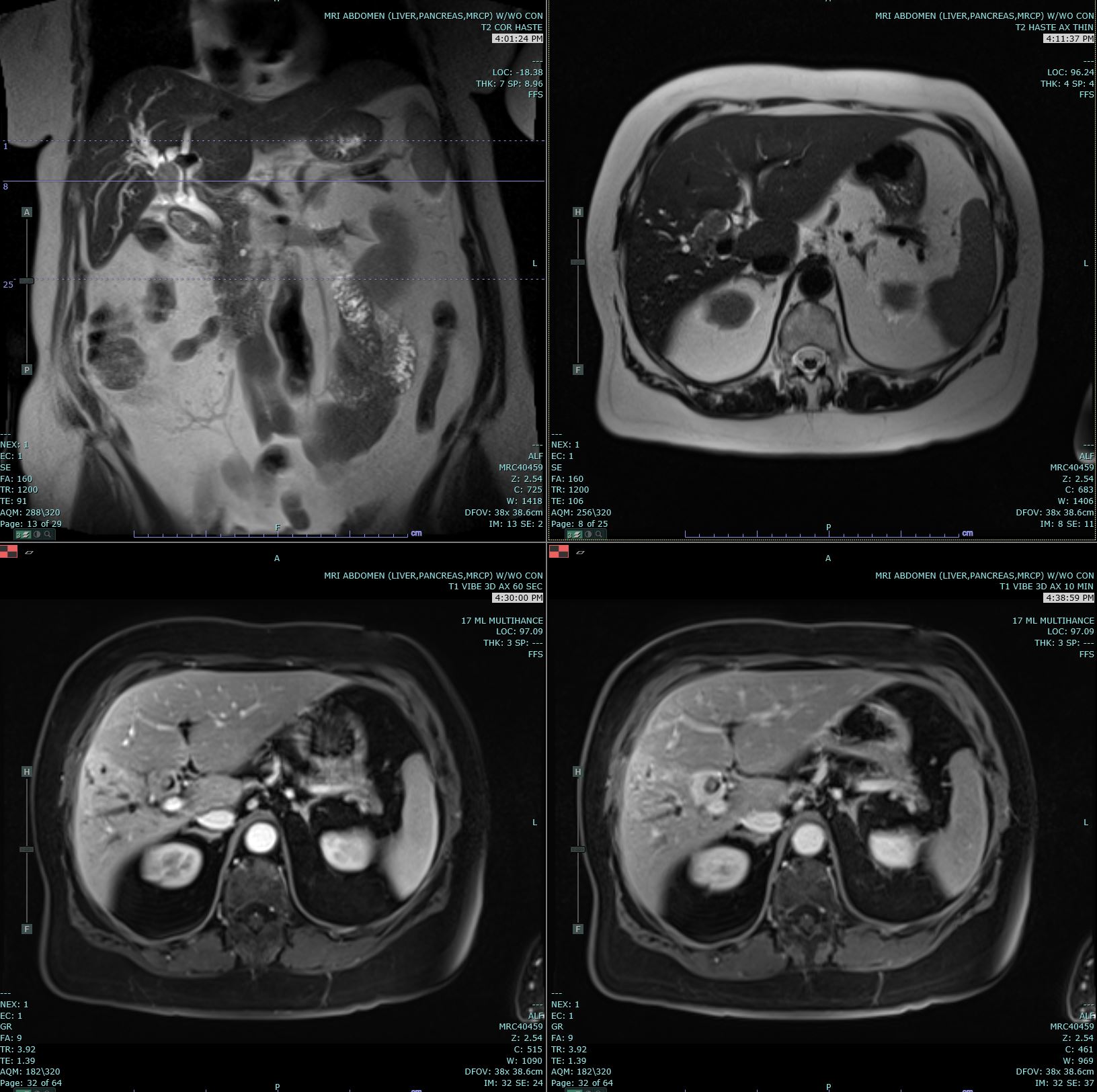
Cholangiocarcinoma. Coronal T2 Haste, Axial T2 Haste, and 60- and 10-minute post-contrast T1 Vibe images are submitted. A delayed enhancing hilar mass biopsy has proven to be cholangiocarcinoma. This mass has an increased T2 signal in comparison to the background liver parenchyma.
Contributed by M Smith, MD
(Click Image to Enlarge)
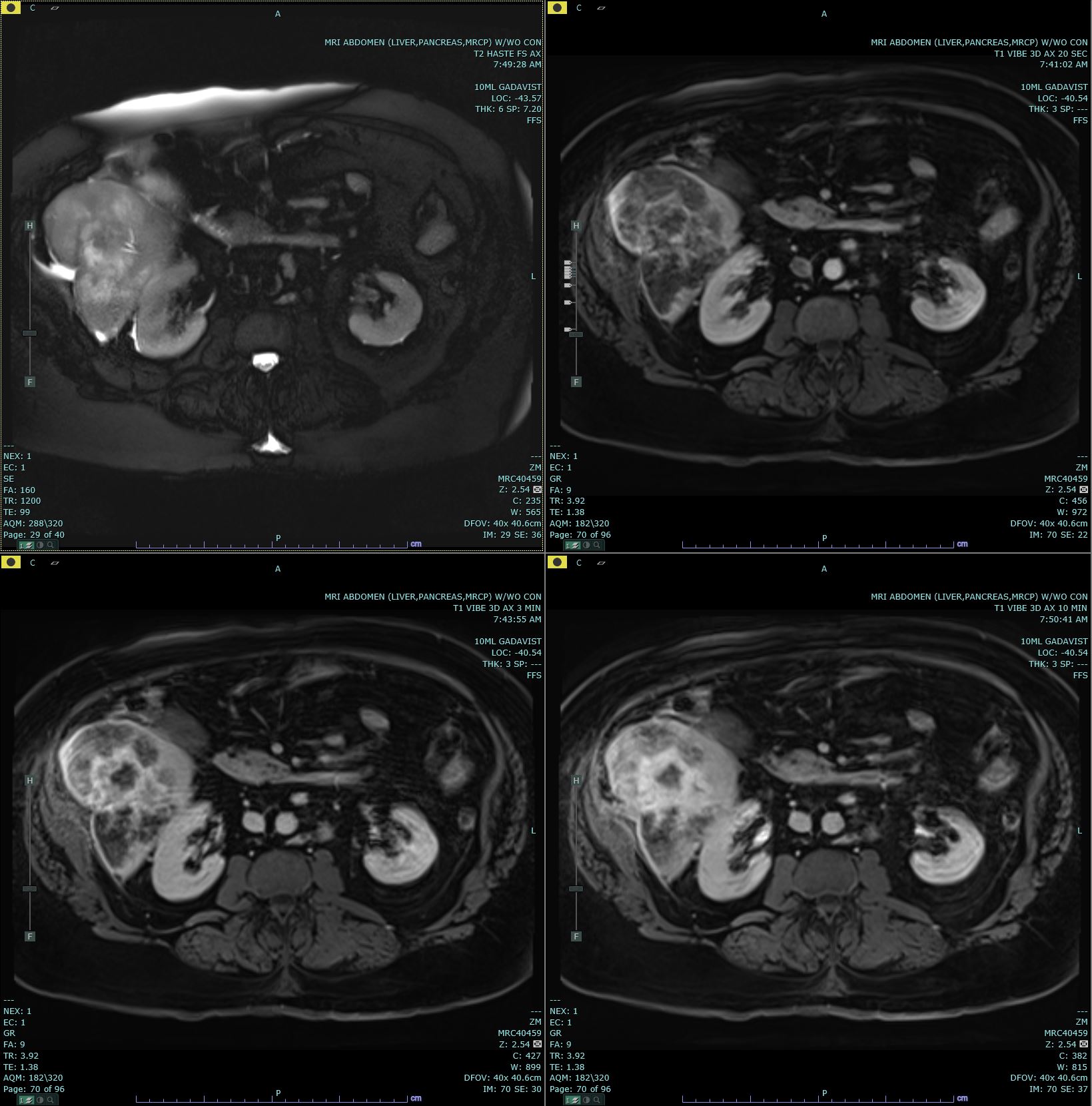
Axial T2 Haste and 20s, 3-minute, and 10-minute Post-Contrast. Axial T2 Haste and 20s, 3-minute, and 10-minute post-contrast T1 Vibe images are submitted. There is a delayed-enhancing mass in segment 7 of the right hepatic lobe, which biopsy has proven to be cholangiocarcinoma. This mass has increased T2 signal in comparison to the background liver parenchyma.
Contributed by M Smith, MD
(Click Image to Enlarge)

Bismuth Corlette Classification of Perihilar Cholangiocarcinoma
Jaax, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nature reviews. Disease primers. 2021 Sep 9:7(1):65. doi: 10.1038/s41572-021-00300-2. Epub 2021 Sep 9 [PubMed PMID: 34504109]
Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F, Cacciatore M, Guido M. Cholangiocarcinoma. Pathologica. 2021 Jun:113(3):158-169. doi: 10.32074/1591-951X-252. Epub [PubMed PMID: 34294934]
. . :(): [PubMed PMID: 34504108]
Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver international : official journal of the International Association for the Study of the Liver. 2019 May:39 Suppl 1():19-31. doi: 10.1111/liv.14095. Epub 2019 Mar 24 [PubMed PMID: 30851228]
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nature reviews. Gastroenterology & hepatology. 2016 May:13(5):261-80. doi: 10.1038/nrgastro.2016.51. Epub 2016 Apr 20 [PubMed PMID: 27095655]
Level 3 (low-level) evidenceNamsanor J, Kiatsopit N, Laha T, Andrews RH, Petney TN, Sithithaworn P. Infection Dynamics of Opisthorchis viverrini Metacercariae in Cyprinid Fishes from Two Endemic Areas in Thailand and Lao PDR. The American journal of tropical medicine and hygiene. 2020 Jan:102(1):110-116. doi: 10.4269/ajtmh.19-0432. Epub [PubMed PMID: 31701859]
Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY, EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology (Baltimore, Md.). 2013 Dec:58(6):2045-55. doi: 10.1002/hep.26565. Epub 2013 Oct 17 [PubMed PMID: 23775876]
Huang D, Joo H, Song N, Cho S, Kim W, Shin A. Association between gallstones and the risk of biliary tract cancer: a systematic review and meta-analysis. Epidemiology and health. 2021:43():e2021011. doi: 10.4178/epih.e2021011. Epub 2021 Feb 3 [PubMed PMID: 33541011]
Level 1 (high-level) evidenceHoilat GJ, John S. Choledochal Cyst. StatPearls. 2025 Jan:(): [PubMed PMID: 32491694]
Simo KA, Mckillop IH, Ahrens WA, Martinie JB, Iannitti DA, Sindram D. Invasive biliary mucinous cystic neoplasm: a review. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2012 Nov:14(11):725-40. doi: 10.1111/j.1477-2574.2012.00532.x. Epub 2012 Jul 22 [PubMed PMID: 23043661]
Krawczyk M, Ziarkiewicz-Wróblewska B, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, Grąt M, Kornasiewicz O, Skalski M, Wróblewski T. Intraductal papillary neoplasm of the bile duct - A comprehensive review. Advances in medical sciences. 2021 Mar:66(1):138-147. doi: 10.1016/j.advms.2021.01.005. Epub 2021 Feb 6 [PubMed PMID: 33556909]
Level 3 (low-level) evidenceTyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology (Baltimore, Md.). 2011 Jul:54(1):173-84. doi: 10.1002/hep.24351. Epub [PubMed PMID: 21488076]
Khan AS, Dageforde LA. Cholangiocarcinoma. The Surgical clinics of North America. 2019 Apr:99(2):315-335. doi: 10.1016/j.suc.2018.12.004. Epub 2019 Feb 10 [PubMed PMID: 30846037]
Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer control : journal of the Moffitt Cancer Center. 2017 Jul-Sep:24(3):1073274817729245. doi: 10.1177/1073274817729245. Epub [PubMed PMID: 28975830]
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014 Jun 1:74(11):2913-21. doi: 10.1158/0008-5472.CAN-14-0155. Epub [PubMed PMID: 24840647]
Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Jul 20:28(21):3531-40. doi: 10.1200/JCO.2009.27.4787. Epub 2010 Jun 14 [PubMed PMID: 20547994]
Level 3 (low-level) evidenceUson Junior PLS, Borad MJ. Precision approaches for cholangiocarcinoma: progress in clinical trials and beyond. Expert opinion on investigational drugs. 2022 Jan:31(1):125-131. doi: 10.1080/13543784.2022.2017882. Epub 2022 Jan 7 [PubMed PMID: 34904492]
Level 3 (low-level) evidenceYamada T, Nakanishi Y, Hayashi H, Tanishima S, Mori R, Fujii K, Okamura K, Tsuchikawa T, Nakamura T, Noji T, Asano T, Matsui A, Tanaka K, Watanabe Y, Kurashima Y, Ebihara Y, Murakami S, Shichinohe T, Mitsuhashi T, Hirano S. Targeted amplicon sequencing for primary tumors and matched lymph node metastases in patients with extrahepatic cholangiocarcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2022 Jul:24(7):1035-1043. doi: 10.1016/j.hpb.2021.11.008. Epub 2021 Nov 25 [PubMed PMID: 34903468]
Ahmad S, Badr B, Khan A, Rehman R, Ghias K, Muhammad JS, Khan MR. The Role of K-Ras and P53 in Biliary Tract Carcinoma. JPMA. The Journal of the Pakistan Medical Association. 2021 Oct:71(10):2378-2384. doi: 10.47391/JPMA.11-1322. Epub [PubMed PMID: 34974575]
Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR. American journal of roentgenology. 2003 Sep:181(3):819-27 [PubMed PMID: 12933488]
Chen Y, Liu X, Huang L, Chen L, Wang B. Clinicopathological, etiological and molecular characteristics of intrahepatic cholangiocarcinoma subtypes classified by mucin production and immunohistochemical features. Expert review of molecular diagnostics. 2023 May:23(5):445-456. doi: 10.1080/14737159.2023.2205588. Epub 2023 Apr 23 [PubMed PMID: 37078255]
Chung T, Park YN. Up-to-Date Pathologic Classification and Molecular Characteristics of Intrahepatic Cholangiocarcinoma. Frontiers in medicine. 2022:9():857140. doi: 10.3389/fmed.2022.857140. Epub 2022 Mar 31 [PubMed PMID: 35433771]
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nature reviews. Gastroenterology & hepatology. 2020 Sep:17(9):557-588. doi: 10.1038/s41575-020-0310-z. Epub 2020 Jun 30 [PubMed PMID: 32606456]
Guedj N. Pathology of Cholangiocarcinomas. Current oncology (Toronto, Ont.). 2022 Dec 26:30(1):370-380. doi: 10.3390/curroncol30010030. Epub 2022 Dec 26 [PubMed PMID: 36661679]
Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nature reviews. Gastroenterology & hepatology. 2011 Aug 2:8(9):512-22. doi: 10.1038/nrgastro.2011.131. Epub 2011 Aug 2 [PubMed PMID: 21808282]
Carson SW, Craven KE, Nauen D, Montemayor K, Yarchoan M, Burns WR, Merlo CA, West NE. Rapidly progressive metastatic cholangiocarcinoma in a postpartum patient with cystic fibrosis: a case report. BMC pulmonary medicine. 2020 Nov 16:20(1):298. doi: 10.1186/s12890-020-01337-x. Epub 2020 Nov 16 [PubMed PMID: 33198722]
Level 3 (low-level) evidencePelsang RE, Johlin FC. A percutaneous biopsy technique for patients with suspected biliary or pancreatic cancer without a radiographic mass. Abdominal imaging. 1997 May-Jun:22(3):307-10 [PubMed PMID: 9107656]
Engelbrecht MR, Katz SS, van Gulik TM, Laméris JS, van Delden OM. Imaging of perihilar cholangiocarcinoma. AJR. American journal of roentgenology. 2015 Apr:204(4):782-91. doi: 10.2214/AJR.14.12830. Epub [PubMed PMID: 25794067]
Shin DW, Moon SH, Kim JH. Diagnosis of Cholangiocarcinoma. Diagnostics (Basel, Switzerland). 2023 Jan 8:13(2):. doi: 10.3390/diagnostics13020233. Epub 2023 Jan 8 [PubMed PMID: 36673043]
Ellis RJ, Soares KC, Jarnagin WR. Preoperative Management of Perihilar Cholangiocarcinoma. Cancers. 2022 Apr 24:14(9):. doi: 10.3390/cancers14092119. Epub 2022 Apr 24 [PubMed PMID: 35565250]
Orzan RI, Pojoga C, Agoston R, Seicean R, Seicean A. Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023? Diagnostics (Basel, Switzerland). 2023 Mar 8:13(6):. doi: 10.3390/diagnostics13061023. Epub 2023 Mar 8 [PubMed PMID: 36980331]
Mocan T, Horhat A, Mois E, Graur F, Tefas C, Craciun R, Nenu I, Spârchez M, Sparchez Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World journal of gastrointestinal oncology. 2021 Dec 15:13(12):2050-2063. doi: 10.4251/wjgo.v13.i12.2050. Epub [PubMed PMID: 35070041]
van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. The New England journal of medicine. 2010 Jan 14:362(2):129-37. doi: 10.1056/NEJMoa0903230. Epub [PubMed PMID: 20071702]
Tran Cao HS, Vauthey JN. Portal Vein Embolization for Perihilar Cholangiocarcinoma: A Story Worth Repeating. Annals of surgical oncology. 2020 Jul:27(7):2120-2121. doi: 10.1245/s10434-020-08280-5. Epub 2020 Feb 28 [PubMed PMID: 32112210]
Narula N, Aloia TA. Portal vein embolization in extended liver resection. Langenbeck's archives of surgery. 2017 Aug:402(5):727-735. doi: 10.1007/s00423-017-1591-8. Epub 2017 May 31 [PubMed PMID: 28567528]
Owen M, Makary MS, Beal EW. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers. 2023 Apr 20:15(8):. doi: 10.3390/cancers15082384. Epub 2023 Apr 20 [PubMed PMID: 37190311]
Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, Cucchetti A, Golfieri R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma-a Systemic Review and Meta-Analysis. Cardiovascular and interventional radiology. 2021 May:44(5):728-738. doi: 10.1007/s00270-021-02800-w. Epub 2021 Mar 11 [PubMed PMID: 33709272]
Level 1 (high-level) evidenceSweeney J, Parikh N, El-Haddad G, Kis B. Ablation of Intrahepatic Cholangiocarcinoma. Seminars in interventional radiology. 2019 Oct:36(4):298-302. doi: 10.1055/s-0039-1696649. Epub 2019 Oct 31 [PubMed PMID: 31680720]
Charalampopoulos G, Iezzi R, Tsitskari M, Mazioti A, Papakonstantinou O, Kelekis A, Kelekis N, Filippiadis D. Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma. Medicina (Kaunas, Lithuania). 2023 Jun 22:59(7):. doi: 10.3390/medicina59071186. Epub 2023 Jun 22 [PubMed PMID: 37511998]
Rossi AJ, Khan TM, Luna AJ, Cercek A, Jarnagin WR, Hernandez JM. Hepatic Artery Infusion Pump (HAIP) Therapy Versus Chemotherapy in the First-Line Setting for Patients with Unresectable Intrahepatic Cholangiocarcinoma. Annals of surgical oncology. 2022 Jan:29(1):35-36. doi: 10.1245/s10434-021-10279-5. Epub 2021 Jun 11 [PubMed PMID: 34117578]
Thiels CA, D'Angelica MI. Hepatic artery infusion pumps. Journal of surgical oncology. 2020 Jul:122(1):70-77. doi: 10.1002/jso.25913. Epub 2020 Mar 25 [PubMed PMID: 32215927]
Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Annals of hepatology. 2022 Sep-Oct:27(5):100737. doi: 10.1016/j.aohep.2022.100737. Epub 2022 Jul 7 [PubMed PMID: 35809836]
Ben Khaled N, Jacob S, Rössler D, Bösch F, De Toni EN, Werner J, Ricke J, Mayerle J, Seidensticker M, Schulz C, Fabritius MP. Current State of Multidisciplinary Treatment in Cholangiocarcinoma. Digestive diseases (Basel, Switzerland). 2022:40(5):581-595. doi: 10.1159/000520346. Epub 2021 Oct 25 [PubMed PMID: 34695826]
Jena SS, Mehta NN, Nundy S. Surgical management of hilar cholangiocarcinoma: Controversies and recommendations. Annals of hepato-biliary-pancreatic surgery. 2023 Aug 31:27(3):227-240. doi: 10.14701/ahbps.23-028. Epub 2023 Jul 6 [PubMed PMID: 37408334]
Soares KC, Jarnagin WR. The Landmark Series: Hilar Cholangiocarcinoma. Annals of surgical oncology. 2021 Aug:28(8):4158-4170. doi: 10.1245/s10434-021-09871-6. Epub 2021 Apr 7 [PubMed PMID: 33829358]
Xin Y, Liu Q, Zhang J, Lu J, Song X, Zhan H, Chen X, Cao Z, Li Y, Huang Z. Hilar cholangiocarcinoma: Value of high-resolution enhanced magnetic resonance imaging for preoperative evaluation. Journal of cancer research and therapeutics. 2020:16(7):1634-1640. doi: 10.4103/jcrt.JCRT_140_20. Epub [PubMed PMID: 33565510]
Bârcu A, Kraft A, Verdea C, Croitoru A, Lupescu I, Tomescu D, Popescu I, Botea F. En-Bloc Complete Segment 1 Resection and Left Hepatectomy for Klatskin Tumor. Chirurgia (Bucharest, Romania : 1990). 2021 Oct:116(5):634-638. doi: 10.21614/chirurgia.116.5.634. Epub [PubMed PMID: 34749860]
Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver international : official journal of the International Association for the Study of the Liver. 2019 May:39 Suppl 1(Suppl Suppl 1):143-155. doi: 10.1111/liv.14089. Epub [PubMed PMID: 30843343]
Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR Jr, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Aug 20:33(24):2617-22. doi: 10.1200/JCO.2014.60.2219. Epub 2015 May 11 [PubMed PMID: 25964250]
Gorji L, Beal EW. Surgical Treatment of Distal Cholangiocarcinoma. Current oncology (Toronto, Ont.). 2022 Sep 17:29(9):6674-6687. doi: 10.3390/curroncol29090524. Epub 2022 Sep 17 [PubMed PMID: 36135093]
Zaborowski A, Heneghan HM, Fiore B, Stafford A, Gallagher T, Geoghegan J, Maguire D, Hoti E. Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Hilar Cholangiocarcinoma: The Irish Experience of the Mayo Protocol. Transplantation. 2020 Oct:104(10):2097-2104. doi: 10.1097/TP.0000000000003114. Epub [PubMed PMID: 31972704]
Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, Hallemeier CL, Koay EJ, Lasley F, Meyer J, Owen D, Pursley J, Schaub SK, Smith G, Venepalli NK, Zibari G, Cardenes H. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Practical radiation oncology. 2022 Jan-Feb:12(1):28-51. doi: 10.1016/j.prro.2021.09.004. Epub 2021 Oct 21 [PubMed PMID: 34688956]
Level 1 (high-level) evidenceIlyas SI, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nature reviews. Clinical oncology. 2018 Feb:15(2):95-111. doi: 10.1038/nrclinonc.2017.157. Epub 2017 Oct 10 [PubMed PMID: 28994423]
Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J, BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. The Lancet. Oncology. 2019 May:20(5):663-673. doi: 10.1016/S1470-2045(18)30915-X. Epub 2019 Mar 25 [PubMed PMID: 30922733]
Level 1 (high-level) evidenceRizzo A, Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert review of gastroenterology & hepatology. 2021 May:15(5):483-485. doi: 10.1080/17474124.2021.1864325. Epub 2020 Dec 18 [PubMed PMID: 33307876]
Lamarca A, Edeline J, McNamara MG, Hubner RA, Nagino M, Bridgewater J, Primrose J, Valle JW. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer treatment reviews. 2020 Mar:84():101936. doi: 10.1016/j.ctrv.2019.101936. Epub 2019 Dec 4 [PubMed PMID: 31986437]
Level 3 (low-level) evidenceRizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer treatment and research communications. 2021:27():100354. doi: 10.1016/j.ctarc.2021.100354. Epub 2021 Mar 16 [PubMed PMID: 33756174]
Ebia MI, Sankar K, Osipov A, Hendifar AE, Gong J. TOPAZ-1: a new standard of care for advanced biliary tract cancers? Immunotherapy. 2023 May:15(7):473-476. doi: 10.2217/imt-2022-0269. Epub 2023 Mar 23 [PubMed PMID: 36950948]
Almhanna K. Immune checkpoint inhibitors in combination with chemotherapy for patients with biliary tract cancer: what did we learn from TOPAZ-1 and KEYNOTE-966. Translational cancer research. 2024 Jan 31:13(1):22-24. doi: 10.21037/tcr-23-1763. Epub 2024 Jan 17 [PubMed PMID: 38410206]
Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. Journal of hepatology. 2020 Feb:72(2):353-363. doi: 10.1016/j.jhep.2019.10.009. Epub [PubMed PMID: 31954497]
