Introduction
Microbial keratitis can arise from various sources, including bacteria, viruses, fungi, and protozoa. Among these, fungal keratitis (FK) is a significant contributor. Its historical documentation dates back to 1879, and its incidence has increased over the last 30 years. It accounts for 40% to 50% of all microbial keratitis cases.[1][2]
Fungal keratitis is a serious condition that demands prompt and effective intervention. Neglecting proper treatment can result in corneal destruction and endophthalmitis, leading to profound vision loss. Early diagnosis and management are essential to prevent long-term complications, including blindness.[3][4]
Over 100 fungal species have been identified as potential culprits behind fungal keratitis.[5] The predominant fungal strain responsible for these infections may exhibit variation based on geographical regions. Approximately 40% of fungal infections are secondary to trauma.
Monomorphic fungi can be classified into yeast and filamentous fungi, both of which play a role in the occurrence of fungal keratitis. The specific type of fungal variant responsible for fungal keratitis depends on several factors, including individual susceptibility, regional temperature patterns, climate conditions, geographic location, and the degree of urbanization.[6]
Fungal keratitis is notably linked to a spectrum of personal risk factors, with trauma, immunocompromised state, ocular surface disease, and contact lens usage as the most prevalent contributors. These factors not only elevate the risk of fungal keratitis but also might predispose individuals to diverse types of fungal infections, emphasizing the multifaceted nature of this condition.[6][7]
Diagnosing fungal infections as the source of keratitis can be challenging in clinical settings, often leading to delays in confirming through culture results. This makes it crucial to strongly consider the possibility of fungal keratitis, especially when specific risk factors are present. Even after diagnosis, managing the condition is challenging because many antifungal medications struggle to penetrate the cornea effectively.[8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Various fungal species can result in fungal keratitis, with the most common culprits being Fusarium, Aspergillus, and Candida species.[5] The causative organism of fungal keratitis may differ according to several factors, including regional temperature, climate, and urbanization.
A study in India on fungal keratitis found Aspergillus species to be the most commonly isolated species, followed by Fusarium.[9] This pattern was consistently observed in several other Indian studies, with 1 study showing that Aspergillus was responsible for more than 55% of all fungal keratitis cases, highlighting its predominant role in the Indian subcontinent.[10] However, in various other parts of the world, including southern India, distinct studies have identified Fusarium as the primary cause of fungal keratitis.[11][12]
A comprehensive study conducted in the United States over 7 years found that Fusarium was responsible for 39% of fungal keratitis, while yeasts like Candida contributed to 22% of cases.[7] Meanwhile, in central China, an extensive examination of 2065 confirmed fungal keratitis cases revealed Fuasrium as the predominant species (>50%), followed by Aspergillus (>9%) and Alternaria (>7%).[13] Similar findings were echoed in a study from northern China.[1]
Contrasting these results, research conducted in the United Kingdom highlighted Candida (57%) as the prevailing causative fungus, followed by Aspergillus (17%).[6] These geographic disparities emphasize the influence of location on the specific organism responsible for fungal keratitis.
Specific patient-related risk factors also influence the prevalence of specific fungal types causing fungal keratitis. For example, Fusarium species are more commonly associated with trauma and contact lens use,[7][14] whereas Candida species tend to be linked with ocular surface disease and topical steroid use.[6] Trauma, especially involving vegetative matter, serves as a significant risk factor for filamentary fungal infections like Fusarium, though it can also lead to bacterial infections.[13][15]
Consequently, certain occupations, particularly those in agriculture, may carry an elevated risk of microbial keratitis, including fungal keratitis.[13] The likelihood of corneal fungal infections can also increase due to inadequate personal hygiene, including poor hand hygiene and overnight contact lens wear.[16]
Corneal refractive surgeries, such as laser in situ keratomileusis (LASIK), may also rarely cause fungal keratitis, carrying the potential for grave consequences if not effectively addressed.[17] Various fungal species responsible for post-LASIK keratitis have been isolated, including Candida, Fusarium, Aspergillus, and Alternaria species.[18][19][20][21] This could be due to inappropriate operative room sterilization techniques or poor postoperative hygiene.[18]
Classification of Fungal Keratitis
Filamentous septate
Nonpigmented
- Fusarium
- Aspergillus
Pigmented
- Cladosporium
- Curvilaria
- Alternaria
- Lasiodiplodia
Filamentous non-septate
- Mucor
- Rhizopus
Yeast
- Candida species
Epidemiology
Like most infectious diseases, the prevalence and underlying causes of fungal keratitis are influenced by geographic location and socioeconomic status. In the United States, regions with warmer climates, like Florida, exhibit a higher incidence of fungal keratitis than colder northern areas.[22][23] Fusarium, Candida, and Aspergillus are the most frequently isolated species causing FK in the USA [7], while in India, Aspergillus is the most common cause.[10] Fusarium is a prevalent cause of fungal keratitis in warm climates such as Brazil [24], while Candida may be more common in temperate climates.[23]
Filamentous fungi like Fusarium and Aspergillus spp are the prevailing fungal isolates in warmer climates, often arising from traumatic events. Epidemiological investigations in Brazil have revealed an incidence of 67% for Fusarium, 10.5% for Aspergillus, and 10% for Candida.
In the United States, a report highlights that fungal keratitis is more frequently encountered among older people, debilitated and immunocompromised populations, with Candida being the primary causative organism.
The incidence of fungal keratitis seems to be markedly higher in certain parts of the world. A comprehensive study over a decade in Hyderabad, India, examined 1360 patients with fungal keratitis within a single institution. Meanwhile, in central China, an investigation documented 2065 cases over 9 years.[13] In contrast, a study from Melbourne, Australia, recorded only 56 instances over 8 years.[25] Similarly, in a study from New York, documentation of fungal keratitis encompassed just 61 eyes over a 16-year timeframe.[26] These disparities could likely be attributed to variations in climate, environmental factors, occupational patterns, or individual behaviors.
Approximately 45 million residents in the United States are active users of contact lenses.[27] The utilization of contact lenses, when not adhering to proper practices, can elevate the risk of microbial keratitis. It is estimated that over 80% of contact lens users across diverse age groups in the USA engage in behaviors that heighten their susceptibility to contact lens-associated eye infections.[28] These risky behaviors include wearing contact lenses overnight and using tap water to clean them.
A significant outbreak of Fusarium keratitis across the United States and several other countries between 2005 and 2006. However, the outbreak's origins were eventually linked to a specific brand of multipurpose contact lens disinfecting solution. This incident underscores the importance of ongoing vigilance and scrutiny of these products and their constituents to decrease the potential for future contact lens-associated eye infections.[29]
Several studies have shown a higher incidence of fungal keratitis among young adult males, possibly due to more outdoor activities and a higher incidence of trauma. In an extensive study of Chinese patients with fungal keratitis, more than 60% were males, and more than 75% were aged between 30 and 60.[13]
Pathophysiology
The fungal pathogens causing keratitis are ubiquitous saprophytic microorganisms. Fungal keratitis often arises from fungi infiltrating the corneal stroma through defects in the corneal epithelium. This mechanism could explain the increased fungal keratitis risk associated with factors like trauma involving vegetative matter, soil or dust, contact lens usage, prior ocular surgeries, and foreign body presence, all compromising the protective epithelial layer.[30]
Trauma involving the vegetative matter can result in direct inoculation of fungal conidia on their surface into the corneal stroma leading to infection or harm of the overlying corneal epithelium, allowing fungal invasion.[1][14] The rapid proliferation of fungi subsequently triggers intensified inflammatory reactions and tissue necrosis.
Once within the tissue, fungi initiate replication within the stroma, exhibiting circumferential expansion with satellite lesions. Progressively they penetrate deeper into the stroma, breaching Descemet's membrane and gaining access to the anterior chamber. This progression may culminate in corneal perforation and endophthalmitis unless the body's natural defenses or appropriate treatment intervene effectively.[31]
Fungal infiltration can extend beyond the cornea, infiltrating the sclera and surrounding structures, potentially precipitating severe conditions such as scleritis and panophthalmitis. Fungal keratitis can also result in secondary sequelae of fungal endophthalmitis. In this scenario, the fungus retraces its course, spreading reverse from the posterior segment to Descemet's membrane, then permeating the stroma or infiltrating the corneoscleral trabeculae, traversing into the corneal channels.[32]
The avascular cornea possesses a barrier capacity and immune privilege, which renders it susceptible to colonization by fungi due to limited defense mechanisms, including dendritic cells, immune cells, and immunoglobulins. Fungi employ various tools to facilitate corneal invasion and colonization, including toxins (mycotoxins) and enzymes, including serine proteases and matrix metalloproteinases (proteolytic enzymes).[2] The resulting immune response, driven by immune cells like polymorphonuclear leukocytes within the cornea, can paradoxically contribute to additional tissue damage.[2]
Candida predominantly invades pre-existing epithelial defects, while filamentous fungi are commonly implicated in post-traumatic infections. The virulence of fungal infections depends on the fungal-produced substances and the host's response. Some fungi can flourish within the corneal stroma by delaying the release of chemotactic substances, evading the host's immune and inflammatory reactions. Candida albicans produces phospholipase A and lysophospholipase on blastospores surfaces, aiding tissue penetration. Fusarium is highly virulent and is known to penetrate the corneal stroma and Descemet's membrane.[33]
Histopathology
Gross granular infiltration within the corneal epithelium and the anterior stroma is a hallmark feature in fungal keratitis, accompanied by observable collagen destruction, coagulative necrosis, and stromal fungal infiltration upon microscopy. Notably, fungal keratitis typically exhibits milder purulent inflammatory cellular infiltration than bacterial keratitis.[34] The presence of low levels of neutrophil infiltration in the cornea is encouraging, as these cells play a pivotal role in corneal destruction while aiming to combat the causative organism.[2] In addition, lymphocytes and plasma cells are also often seen in FK.
Several staining methods can detect fungi in corneal tissue or scrapings. These include potassium-hydroxide, gram staining, Giemsa staining, lactophenol cotton blue, methenamine silver, and calcofluor white.[23] These stains facilitate the visualization of fungal hyphae and yeast cells, aiding in the early differentiation of the causative organism before culture results are available.[35] It may also allow the detection of a mixed fungal and bacterial infection.
Distinct histopathological patterns of hyphal growth have been observed, offering an additional tool for differentiating the responsible filamentous fungus. For instance, Fusarium hyphae tend to align parallel to the corneal stroma lamellae, while Aspergillus hyphae exhibit vertical growth.[36]
History and Physical
History
The history and clinical presentation of fungal keratitis may differ according to the causative organism, whether it is a filamentous fungus or yeast.[23] It is essential to exclude any history of trauma involving vegetative matter, foreign bodies, soil, or dust entering the eye.
A history of farming or fieldwork can indirectly indicate susceptibility to fungal keratitis. Other relevant history includes contact lens usage, prior ocular surgeries, corneal transplantation, alcohol consumption, malnourishment, presence of chronic debilitating conditions, HIV infection, hepatitis, diabetes mellitus, any form of steroid use, and systemic immunosuppressant treatment. A close differential is Pythium keratitis, wherein a history of mud and clay-related injury must be ruled out.[37]
Symptoms
Typically, the hallmark manifestations of microbial keratitis are evident, encompassing symptoms like blurred vision, pain, redness, discharge, gritty sensation, photophobia, excessive tearing, and blepharospasm. Notably, it's essential to recognize that signs often surpass symptoms in fungal keratitis, whereas the reverse holds for bacterial keratitis.[38]
Signs
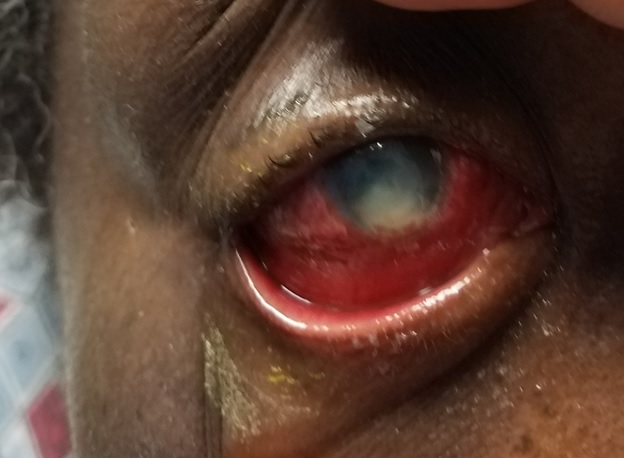
Before conducting a slit lamp examination, it is imperative to attempt a preliminary gross torch light assessment, noting prevalent clinical indicators such as lid edema, blepharospasm, matted lashes, and blepharitis (see image. Fungal Keratitis). During the slit lamp examination, a range of common clinical findings can be observed, including blepharitis, meibomian gland dysfunction, matted lashes, purulent or mucopurulent discharge, conjunctival congestion, epithelial defects, the precise location, size, depth, and extent of corneal infiltrate, distinct feathery margins, satellite lesions, stromal melt, endothelial plaque formation, thinning, descemetoceles, and perforation. Additionally, patients might display indications of inflammation within the anterior chamber, often signified by the presence of pus (hypopyon).[39]
A history of trauma involving vegetative matter is often apparent in fungal keratitis due to filamentous fungi. Clinically, patients typically present with an ulcer characterized by raised, firm slough, elongated lines of fungal hyphae extending beyond the central ulcer's border and feathery satellite stromal lesions.
Patients with fungal keratitis attributed to yeast fungi may present with a history of ocular surface disease or immunosuppression. Clinically, the presentation may resemble bacterial keratitis but with a more gradual progression. While in some cases, differentiation between fungal and bacterial causes might be possible based on clinical observations alone, this distinction is not always straightforward, and prediction of the causative fungal genus or species responsible for the condition may even be more challenging and inaccurate.[40]
Candida-induced keratitis appears as a yellow-white densely suppurative infiltrate. Keratitis due to filamentary fungus appears grey or yellow-white stromal infiltrate with indistinct fluffy margins. It is also important to note signs of scleritis and endophthalmitis in these cases, which usually develop late as a complication.[41]
Evaluation
Microscopic Examination
The laboratory assessment of fungal keratitis begins with collecting an appropriate sample for testing. These specimens undergo direct microscopic examination utilizing various stains such as 10% KOH, Gram, Giemsa, Periodic Acid- Schiff, Gomori methenamine silver stains, culture, histologic testing, and other specialized tests.[41] KOH preparation with direct microscopic examination is a highly effective and rapid diagnostic tool with over 90% sensitivity. Gram and Giemsa staining are approximately 50% sensitive. Given fungi's inclination to penetrate deep into the cornea, tissue swabbing is usually inadequate in confirming a fungal infection, necessitating the acquisition of deep corneal scrapings for a more accurate diagnosis.[42][43]
Corneal Biopsy and Polymerase Chain Reaction
Occasionally, a corneal biopsy may be needed to obtain an adequate sample. Ideally, each specimen should undergo polymerase chain reaction (PCR) and culture, especially if corneal scraping stains are negative.[42][43] Fungal cultures usually require 1 to 35 days for fungal growth to become evident. Various cultures are employed, including Sabouraud glucose neopeptone agar, blood agar, thioglycollate broth, and brain heart infusion, each serving to isolate different types of fungi.[41][43] A major disadvantage of fungal cultures is the relatively low sensitivity with false-negative results, possibly due to the small amount of material available from corneal scrapings.[42][43]
This is especially true for cases that have undergone antifungal treatment. PCR testing has been proposed as a more sensitive diagnostic method for fungal keratitis, enabling precise species identification within 4 to 8 hours. Nonetheless, it might exhibit reduced specificity, potentially leading to false-positive outcomes attributed to the amplification of non-pathogenic organisms in the sample.[23][41][43] The targets used for PCR amplification include fungal 18S rRNA and 28S rRNA sequences. While PCR can be conducted using small samples of ocular tissues or fluids like tears or aqueous humor, its application mandates specialized and costly equipment that may not always be available.
Beta-D-glucan, a component of the cell wall found in many fungal species, can be identified within the bloodstream of patients with invasive fungal infections.[44] Previous research has demonstrated that beta-d-glucan testing outpaces fungal cultures regarding speed and sensitivity when diagnosing systemic fungal infections, particularly in cases where the organisms don't thrive in blood cultures, such as Aspergillosis.[45] The presence of beta-D-glucan has the potential to be detected in tear samples from individuals affected by fungal keratitis, expediting early and prompt diagnosis.[46] Furthermore, it can also be seen in intraocular fluids of cases complicated by fungal endophthalmitis.[47]
Anterior Chamber Tap
In resistant cases, an anterior chamber tap has been advocated, mainly when an endothelial plaque is evident, as fungal infiltration is often observed to breach the endothelium.
Imaging
Non-invasive techniques, such as confocal microscopy and anterior segment optical coherence tomography (AS-OCT), can offer alternative avenues for detecting microorganisms responsible for microbial keratitis, including fungal infections, through in vivo examination techniques. Confocal microscopy can potentially reveal hyphae-like branching white lines within the infiltration area, particularly in cases associated with filamentous fungi.[48] In contrast, AS-OCT may show early localized and diffuse regions of necrotic stromal cystic spaces in cases of infection by Aspergillus species.[49] These modalities also find utility in monitoring the response to treatment as part of the follow-up process.[50]
Ophthalmic B-scan ultrasonography can be helpful in the diagnosis and follow-up of cases suspected of progressing toward endophthalmitis, especially when corneal fungal infiltration precludes examination with ophthalmoscopy.
Treatment / Management
General Measures
Hospital Admission
Hospitalization is deemed necessary for specific patient groups, including older, debilitated bedridden patients who cannot self-administer medications. Additionally, admission is warranted for cases of advanced fungal keratitis with progressive infiltration, one-eyed patients, and those originating from distant locations.[51]
Contact Lens Avoidance
It is imperative to discontinue using contact lenses in these cases promptly.[52]
Ocular Shield and Barrier
For cases at risk of or experiencing perforation, the application of a pad and bandage, along with antibiotic ointment, is essential. A transparent plastic eye shield can also be positioned between the administration of eye drops.[53]
Prophylactic Treatment and Decision to Treat
Initial, less severe corneal infiltrates may not necessitate aggressive intervention and can be managed with topical antifungals administered at a low to moderate frequency. Simultaneously, comprehensive risk management, including addressing conditions such as diabetes mellitus and refraining from contact lens use, remains crucial.
In cases where smear and culture results are pending, initiating empirical treatment is imperative to preclude the advancement of ulceration and potential complications.[54]
Scraping the Epithelium and Infiltrate
This strategy facilitates improved penetration of antifungal agents, considering their restricted ability to reach the cornea and anterior chamber. Regular removal of mucus and necrotic debris using a spatula further enhances the effectiveness of antifungal penetration.[32](B3)
Medical Treatment
Topical Antifungals
All existing antifungal agents exhibit fungistatic properties rather than being fungicidal, necessitating a prolonged treatment period until the body's defenses can completely eradicate the fungal organism. However, instances of primary treatment failure have previously been reported in 31.3% of cases.[55]
Topical agents utilized in the treatment of FK include:
- Natamycin 5%
- Amphotericin B 0.15-0.3%
- Preferred treatment of choice for yeasts
- Voriconazole 1%
- Better penetration into the eye
- Purported to be a superior alternative to natamycin
- Can be used as intrastromal or intracameral injections [58]
- Econazole 1%
- Itraconazole 1%
- Miconazole 1% (A1)
During the initial 48 hours, topical agents should be administered hourly, followed by a subsequent tapering or reduction contingent upon clinical response.
Given the predominantly fungastic nature of most antifungal agents, treatment should be continued for a minimum of 12 weeks. Candida infections warrant therapy with amphotericin B 0.15% or econazole 1%. For other cases, natamycin 5%, voriconazole 1%, clotrimazole 1% and fluconazole 2% are recommended.
Filamentous fungi infections are treated with 5% natamycin or 1% econazole, while alternative options include amphotericin B 0.15%, miconazole 1%, and voriconazole 1%.
Subconjunctival Antifungals
Subconjunctival injections of antifungal agents such as miconazole and fluconazole may be used for patients displaying suboptimal adherence to treatment or experiencing severe keratitis.[31]
Systemic Antifungals
Systemic treatment is required in severe progressive ulcers, ulcers infiltrating the limbus, scleritis, and endophthalmitis.[41] These can be found in the following forms:(B3)
- Intravenous
- Amphotericin B
- Oral
- Voriconazole 400 mg
- Itraconazole 200 mg once daily, then reduced to 100 mg once daily
- Fluconazole 200 mg twice daily
Decisions to continue therapy as based on the following biomicroscopic findings:
- A decrease in pain and size of infiltrate
- A reduction of satellite lesions
- A reduction in the density of suppuration
- Blunting of the infiltrated edges
- A decrease in inflammation in the anterior chamber
- Continued reepithelialization
Recently published clinical trials have reported equal or inferior efficacy of topical 1% voriconazole (reconstituted from injection vial) compared with topical 5% natamycin eye drops for treating fungal keratitis, especially those caused by Fusarium. These results, however, are contradictory to experimental and in vitro data. Sharma et al conducted a study involving 118 patients, 58 receiving voriconazole and 60 treated with natamycin. Notably, while the proportion of ulcers that were healing or resolving appeared similar on day 7 (natamycin 35/54, 65%; voriconazole 34/50, 68%), the final analysis unveiled a significant divergence, Specifically at the last visit, the percentage of patients exhibiting healed corneal ulcers were notably higher in the natamycin-treated group (50/56, 89.2%) compared to the voriconazole-treated group (34/51, 66.6%; p=0.005).[59] (A1)
In 2015, a systematic review of medical treatments for fungal keratitis was conducted by the Cochrane Database, concluding that natamycin may be more effective than voriconazole in treating fungal keratitis; however, most studies were underpowered with variable quality.[60] The encompassed randomized controlled trials featured a diverse array of comparisons, including topical 5% natamycin versus topical 1% voriconazole, topical 1% voriconazole vs. intrastromal voriconazole, and topical 1% natamycin versus topical 2% econazole.(A1)
Role of Antibiotics
To preempt potential bacterial co-infection, broad-spectrum antibiotics are recommended. Tetracyclines such as doxycycline 100 mg twice daily may be required due to their anti-collagenase properties.[61](B3)
Corticosteroids
Steroids are unequivocally contraindicated during active fungal keratitis due to the potential to foster rapid fungal proliferation and exacerbate vision-threatening keratitis. The functional role of steroids in fungal keratitis is post-therapeutic keratoplasty (TPK) once the eye is free of infection.
Steroids are started cautiously around 3 to 4 weeks after TPK and under close supervision. Predominantly, topical agents such as prednisolone 1% or dexamethasone 0.1% are favored due to their favorable anterior chamber penetration.
Initial dosing entails a low frequency of 3 times a day during the initial week, transitioning to a slow taper over 3 months, fostering graft survival and preventing rejection episodes. There is no role of systemic steroids in fungal keratitis.[62](B2)
Surgical Treatment
Superficial Keratectomy
A superficial keratectomy is necessary to debulk the ulcer effectively.[63](B3)
Therapeutic Keratoplasty
Patients who do not respond favorably to medical therapy may require surgical intervention, including therapeutic penetrating and lamellar keratoplasty.[13][64] Therapeutic keratoplasty can be associated with complications, including recurrence of infection, endophthalmitis, and graft rejection.[64] (B2)
Anterior Chamber Wash
In cases where the standard treatment approach proves ineffective and is met with non-responsiveness, an anterior chamber wash becomes necessary, particularly when accompanied by the presence of hypopyon and endothelial exudates.
Intracameral and Intrastromal Injection of Voriconazole
When ulcers display a slow response to topical treatment, manifest as full-thickness ulcers, exhibit a progressive course, or demonstrate resistance to conventional therapies, intracameral and intrastromal voriconazole becomes imperative for enhanced management.[65](B3)
Corneal Collagen Crosslinking
A recently available treatment modality is corneal collagen crosslinking which may sometimes be useful in medically resistant corneal ulcers or even in some early cases of fungal infection.[66][67][68] However, a recent randomized controlled clinical trial did not demonstrate any benefit from this approach in fungal keratitis and indicated an elevated incidence of complications.[69] Conjunctival flaps can also rarely be used but may increase the incidence of graft rejection following keratoplasty due to corneal vascularization.[13] (A1)
To save the eye, a tectonic or therapeutic keratoplasty is usually required in corneal perforation. Cases complicated by endophthalmitis may require intravitreal antifungal injections or even necessitate pars plana vitrectomy, which can be performed using endoscopy if extensive corneal infiltration impedes posterior segment visualization.[70][71][72] However, enucleation may eventually be the last resort in cases where the eye is rendered blind and painful, with uncontrollable inflammation.(B2)
Healing of fungal keratitis may result in central corneal scarring and opacification, which may require penetrating or lamellar keratoplasty to restore visual acuity.
Repeat Therapeutic Keratoplasty
Repeated therapeutic keratoplasty in non-resolving graft infections should be attempted to clear the infective focus.[73]
Temporary Tarsorrhaphy
After repeat keratoplasty, if there is persistent graft infection, repeat keratoplasty (third graft) along with temporary tarsorrhaphy should be performed to allow resolution of infection.[74]
Evisceration
In cases with non-resolving endophthalmitis or panophthalmitis, the option of evisceration is considered, contingent upon obtaining appropriate consent from the patient and their next of kin.[75](B2)
Differential Diagnosis
The differential diagnosis for fungal keratitis includes the following:
Prognosis
The prognosis for fungal keratitis hinges on several factors, including the causative organism, the depth and extent of the infection, the development of complications, and the promptness of treatment initiation. While certain patients may achieve microbiological cure through topical antifungal agents, others might necessitate therapeutic keratoplasty.[83]
The integrity of the Descemet membrane, positioned in the interior basement membrane near the aqueous humor, is usually impermeable to bacteria; however, fungal hyphae can breach this barrier, culminating in endophthalmitis-- a rare yet grave consequence of fungal keratitis.
Approximately 30% of fungal infections do not respond to drug therapy, potentially culminating in corneal perforation. Successful resolution of fungal keratitis usually results in central corneal scarring and opacification, which may require penetrating or lamellar keratoplasty to restore visual acuity.[84]
Complications
The complications arising from fungal keratitis encompass the following:
- Non-resolving epithelial defect
- Corneal perforation
- Corneal melting
- Corneal scarring
- Secondary glaucoma
- Iris neovascularization
- Peripheral anterior synechiae
- Posterior synechiae
- Scleritis
- Endophthalmitis
- Panophthalmitis
- Complicated cataract
- Vitritis
- Vitreous membranes
- Permanent blindness
- Phthisis bulbi
- Atrophic bulbi
Postoperative and Rehabilitation Care
Following therapeutic or tectonic keratoplasty, each patient necessitates meticulous management, consistent and timely care, and vigilant follow-up. Initially, the commencement of topical antifungal agents post-TPK is advised, typically in low doses, around 6 times daily alongside adjuvant medications. Subsequent follow-up appointments are recommended on postoperative days 1, 5, 14, 21, and 28, with later intervals of every 2 weeks or months based on the patient's clinical condition.
Topical steroids should be introduced after 3 to 4 weeks, depending on the clinical picture.[85] If the preoperative culture and button culture reports are negative and there are no signs of infection, topical steroids can be started after 3 weeks post-TPK. Conversely, if either or both reports indicate positivity, the initiation of topical steroids can be deferred to 4 weeks post-TPK.[86]
It is crucial to convey to patients the significance of diligent follow-up and consistent adherence to prescribed medication schedules. Patients should be provided with a clear understanding of the potential complications associated with keratoplasty and the prognosis specific to their case. Open communication should prepare patients for the possibility of undergoing repeat keratoplasty if warranted. It's worth noting that the prognosis tends to diminish with each subsequent keratoplasty.[87]
Consultations
Patients presenting with a corneal ulcer should be referred to a cornea and external disease specialist with the expertise to diagnose and accurately tailor appropriate management. The distinctive features of the ulcer can be readily identified by the cornea specialist through clinical evaluation.
Should the condition give rise to secondary glaucoma, referral to a glaucoma specialist is recommended for initiating antiglaucoma medications or considering procedures like trabeculectomy or glaucoma drainage devices. If the patient develops complex cataract issues, timely intervention by a cataract and IOL or cornea specialist proficient in cataract surgery becomes imperative. Expert treatment from a retina specialist is essential for instances where retinal complications emerge, such as vitritis, endophthalmitis, panophthalmitis, and retinal or choroidal detachment.
Deterrence and Patient Education
Patients should be educated about using contact lenses and the appropriate cleaning products to minimize the risk of contact lens-associated corneal infections. Proper hygienic measures should be emphasized, including frequent hand washing before handling contact lenses and avoiding overnight contact lens wear.[88]
Enhancing Healthcare Team Outcomes
Most patients with fungal keratitis are initially encountered in the emergency department or through primary care providers. To ensure a prompt referral to an ophthalmologist and avoid delays that might result in vision impairment or permanent blindness, an interprofessional approach to diagnosis and treatment is crucial. While most patients with fungal keratitis can be managed as outpatients, it's important to note that they require a sustained course of antifungal therapy spanning 12 to 16 weeks.[30]
Patient education is vital in preventing fungal keratitis. Contact lens wearers must be educated about proper hand hygiene, the correct cleaning solutions, and the importance of avoiding sleeping or swimming while wearing lenses. Regular follow-up appointments with opticians or ophthalmologists are essential for contact lens wearers. They should be informed about the symptoms of eye infections, such as pain, redness, or vision loss, and be advised to seek immediate ophthalmologic consultation if these symptoms arise.
Ophthalmology nurses are pivotal in patient education, facilitating follow-up appointments, and maintaining effective communication among the healthcare team. Pharmacists also contribute by ensuring appropriate medication dosages, identifying potential drug interactions, and reviewing proper administration methods with patients.[89]
Close follow-up throughout the treatment period is essential to monitor the condition's progression and ensure its stabilization. The eventual outcome is influenced by various factors, including the patient's overall health, immune system status, and other comorbidities. Patients with mild infections that are promptly treated tend to have favorable outcomes. However, for those with infections extending into the sclera, the prognosis becomes guarded. Available data indicates that at least 30% of patients with fungal keratitis develop corneal perforation or show inadequate response to drug therapy.[90][91]
Media
(Click Image to Enlarge)
References
Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006 Nov:113(11):1943-8 [PubMed PMID: 16935335]
Level 2 (mid-level) evidenceGopinathan U, Ramakrishna T, Willcox M, Rao CM, Balasubramanian D, Kulkarni A, Vemuganti GK, Rao GN. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Experimental eye research. 2001 Apr:72(4):433-42 [PubMed PMID: 11273671]
Level 3 (low-level) evidenceTena D, Rodríguez N, Toribio L, González-Praetorius A. Infectious Keratitis: Microbiological Review of 297 Cases. Japanese journal of infectious diseases. 2019 Mar 25:72(2):121-123. doi: 10.7883/yoken.JJID.2018.269. Epub 2018 Oct 31 [PubMed PMID: 30381686]
Level 3 (low-level) evidenceMittal R, Ahooja H, Sapra N. Corneal "Plaque" formation after anti-acanthamoeba therapy in acanthamoeba keratitis. Indian journal of ophthalmology. 2018 Nov:66(11):1623-1624. doi: 10.4103/ijo.IJO_734_18. Epub [PubMed PMID: 30355882]
Xie L, Zhai H, Zhao J, Sun S, Shi W, Dong X. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. American journal of ophthalmology. 2008 Aug:146(2):260-265. doi: 10.1016/j.ajo.2008.04.019. Epub 2008 Jun 11 [PubMed PMID: 18547535]
Level 2 (mid-level) evidenceTuft SJ, Tullo AB. Fungal keratitis in the United Kingdom 2003-2005. Eye (London, England). 2009 Jun:23(6):1308-13. doi: 10.1038/eye.2008.298. Epub 2008 Oct 3 [PubMed PMID: 18836409]
Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, Colby K, Tuli SS, Patel SR, Lee SM, Irvine J, Stulting RD, Mauger TF, Schein OD. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010 Dec:117(12):2263-7. doi: 10.1016/j.ophtha.2010.03.048. Epub 2010 Jun 29 [PubMed PMID: 20591493]
Level 2 (mid-level) evidenceHoffman JJ, Burton MJ, Leck A. Mycotic Keratitis-A Global Threat from the Filamentous Fungi. Journal of fungi (Basel, Switzerland). 2021 Apr 3:7(4):. doi: 10.3390/jof7040273. Epub 2021 Apr 3 [PubMed PMID: 33916767]
Tilak R, Singh A, Maurya OP, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: a five-year retrospective study. Journal of infection in developing countries. 2010 Mar 29:4(3):171-4 [PubMed PMID: 20351459]
Level 2 (mid-level) evidenceSaha R, Das S. Mycological profile of infectious Keratitis from Delhi. The Indian journal of medical research. 2006 Feb:123(2):159-64 [PubMed PMID: 16575115]
Level 2 (mid-level) evidenceBharathi MJ, Ramakrishnan R, Vasu S, Meenakshi, Palaniappan R. Aetiological diagnosis of microbial keratitis in South India - a study of 1618 cases. Indian journal of medical microbiology. 2002 Jan-Mar:20(1):19-24 [PubMed PMID: 17657018]
Level 3 (low-level) evidenceBharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian journal of ophthalmology. 2003 Dec:51(4):315-21 [PubMed PMID: 14750619]
Level 2 (mid-level) evidenceWang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clinical & experimental ophthalmology. 2009 Nov:37(8):763-71. doi: 10.1111/j.1442-9071.2009.02155.x. Epub [PubMed PMID: 19878220]
Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian journal of ophthalmology. 2009 Jul-Aug:57(4):273-9. doi: 10.4103/0301-4738.53051. Epub [PubMed PMID: 19574694]
Level 2 (mid-level) evidenceGalarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007 Oct:26(9):1082-6 [PubMed PMID: 17893539]
Level 2 (mid-level) evidenceLim CH, Carnt NA, Farook M, Lam J, Tan DT, Mehta JS, Stapleton F. Risk factors for contact lens-related microbial keratitis in Singapore. Eye (London, England). 2016 Mar:30(3):447-55. doi: 10.1038/eye.2015.250. Epub 2015 Dec 4 [PubMed PMID: 26634710]
Taylan Sekeroglu H, Erdem E, Yar K, Yağmur M, Ersoz TR, Uguz A. A rare devastating complication of LASIK: bilateral fungal keratitis. Journal of ophthalmology. 2010:2010():450230. doi: 10.1155/2010/450230. Epub 2010 Nov 11 [PubMed PMID: 21113441]
Level 3 (low-level) evidenceMoshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and noninfectious keratitis after laser in situ keratomileusis Occurrence, management, and visual outcomes. Journal of cataract and refractive surgery. 2007 Mar:33(3):474-83 [PubMed PMID: 17321399]
Level 2 (mid-level) evidenceChen WL, Tsai YY, Lin JM, Chiang CC. Unilateral Candida parapsilosis interface keratitis after laser in situ keratomileusis: case report and review of the literature. Cornea. 2009 Jan:28(1):105-7. doi: 10.1097/ICO.0b013e318184e69b. Epub [PubMed PMID: 19092419]
Level 3 (low-level) evidenceKocatürk T, Pineda R 2nd, Green LK, Azar DT. Post-LASIK epithelial dendritic defect associated with Alternaria. Cornea. 2007 Oct:26(9):1144-6 [PubMed PMID: 17893555]
Level 3 (low-level) evidenceSun Y, Jain A, Ta CN. Aspergillus fumigatus keratitis following laser in situ keratomileusis. Journal of cataract and refractive surgery. 2007 Oct:33(10):1806-7 [PubMed PMID: 17889780]
Level 3 (low-level) evidenceEstopinal CB, Ewald MD. Geographic Disparities in the Etiology of Bacterial and Fungal Keratitis in the United States of America. Seminars in ophthalmology. 2016:31(4):345-52. doi: 10.3109/08820538.2016.1154173. Epub 2016 Apr 21 [PubMed PMID: 27101474]
Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013 Mar:19(3):210-20. doi: 10.1111/1469-0691.12126. Epub 2013 Feb 9 [PubMed PMID: 23398543]
Godoy P, Cano J, Gené J, Guarro J, Höfling-Lima AL, Lopes Colombo A. Genotyping of 44 isolates of Fusarium solani, the main agent of fungal keratitis in Brazil. Journal of clinical microbiology. 2004 Oct:42(10):4494-7 [PubMed PMID: 15472299]
Bhartiya P, Daniell M, Constantinou M, Islam FM, Taylor HR. Fungal keratitis in Melbourne. Clinical & experimental ophthalmology. 2007 Mar:35(2):124-30 [PubMed PMID: 17362452]
Level 2 (mid-level) evidenceRitterband DC, Seedor JA, Shah MK, Koplin RS, McCormick SA. Fungal keratitis at the new york eye and ear infirmary. Cornea. 2006 Apr:25(3):264-7 [PubMed PMID: 16633023]
Level 2 (mid-level) evidenceKonne NM, Collier SA, Spangler J, Cope JR. Healthy Contact Lens Behaviors Communicated by Eye Care Providers and Recalled by Patients - United States, 2018. MMWR. Morbidity and mortality weekly report. 2019 Aug 16:68(32):693-697. doi: 10.15585/mmwr.mm6832a2. Epub 2019 Aug 16 [PubMed PMID: 31415490]
Cope JR, Collier SA, Nethercut H, Jones JM, Yates K, Yoder JS. Risk Behaviors for Contact Lens-Related Eye Infections Among Adults and Adolescents - United States, 2016. MMWR. Morbidity and mortality weekly report. 2017 Aug 18:66(32):841-845. doi: 10.15585/mmwr.mm6632a2. Epub 2017 Aug 18 [PubMed PMID: 28817556]
Epstein AB. In the aftermath of the Fusarium keratitis outbreak: What have we learned? Clinical ophthalmology (Auckland, N.Z.). 2007 Dec:1(4):355-66 [PubMed PMID: 19668512]
Acharya Y, Acharya B, Karki P. Fungal keratitis: study of increasing trend and common determinants. Nepal journal of epidemiology. 2017 Jun:7(2):685-693. doi: 10.3126/nje.v7i2.17975. Epub 2017 Jun 30 [PubMed PMID: 29181230]
Ansari Z,Miller D,Galor A, Current Thoughts in Fungal Keratitis: Diagnosis and Treatment. Current fungal infection reports. 2013 Sep 1; [PubMed PMID: 24040467]
Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clinical microbiology reviews. 2000 Oct:13(4):662-85 [PubMed PMID: 11023963]
Level 3 (low-level) evidenceMayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013 Feb 15:4(2):119-28. doi: 10.4161/viru.22913. Epub 2013 Jan 9 [PubMed PMID: 23302789]
Agrawal V, Biswas J, Madhavan HN, Mangat G, Reddy MK, Saini JS, Sharma S, Srinivasan M. Current perspectives in infectious keratitis. Indian journal of ophthalmology. 1994 Dec:42(4):171-92 [PubMed PMID: 10576995]
Level 3 (low-level) evidencePunia RS, Kundu R, Chander J, Arya SK, Handa U, Mohan H. Spectrum of fungal keratitis: clinicopathologic study of 44 cases. International journal of ophthalmology. 2014:7(1):114-7. doi: 10.3980/j.issn.2222-3959.2014.01.21. Epub 2014 Feb 18 [PubMed PMID: 24634875]
Level 3 (low-level) evidenceXie L, Zhai H, Shi W, Zhao J, Sun S, Zang X. Hyphal growth patterns and recurrence of fungal keratitis after lamellar keratoplasty. Ophthalmology. 2008 Jun:115(6):983-7 [PubMed PMID: 17919731]
Level 2 (mid-level) evidenceTabatabaee A, Mohajernezhadfard Z, Daneshgar F, Mansouri M. Keratomycosis after incidental spillage of vegetative material into the eye: Report of two cases. Oman journal of ophthalmology. 2013 May:6(2):122-6. doi: 10.4103/0974-620X.116659. Epub [PubMed PMID: 24082674]
Level 3 (low-level) evidenceUpadhyay MP, Srinivasan M, Whitcher JP. Diagnosing and managing microbial keratitis. Community eye health. 2015:28(89):3-6 [PubMed PMID: 26435583]
Martin R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian journal of ophthalmology. 2018 Feb:66(2):195-201. doi: 10.4103/ijo.IJO_649_17. Epub [PubMed PMID: 29380757]
Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. American journal of ophthalmology. 2007 Jun:143(6):940-944 [PubMed PMID: 17408586]
Level 3 (low-level) evidenceThomas PA. Current perspectives on ophthalmic mycoses. Clinical microbiology reviews. 2003 Oct:16(4):730-97 [PubMed PMID: 14557297]
Level 3 (low-level) evidenceEleinen KG, Mohalhal AA, Elmekawy HE, Abdulbaki AM, Sherif AM, El-Sherif RH, Abdul Rahman EM. Polymerase chain reaction-guided diagnosis of infective keratitis - a hospital-based study. Current eye research. 2012 Nov:37(11):1005-11. doi: 10.3109/02713683.2012.698357. Epub 2012 Jun 29 [PubMed PMID: 22746322]
Level 2 (mid-level) evidenceFerrer C, Alió JL. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. Journal of ophthalmic inflammation and infection. 2011 Feb 23:1(1):15-22. doi: 10.1007/s12348-011-0019-9. Epub 2011 Feb 23 [PubMed PMID: 21475656]
Obayashi T, Yoshida M, Tamura H, Aketagawa J, Tanaka S, Kawai T. Determination of plasma (1--}3)-beta-D-glucan: a new diagnostic aid to deep mycosis. Journal of medical and veterinary mycology : bi-monthly publication of the International Society for Human and Animal Mycology. 1992:30(4):275-80 [PubMed PMID: 1432487]
Level 3 (low-level) evidenceOdabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004 Jul 15:39(2):199-205 [PubMed PMID: 15307029]
Level 1 (high-level) evidenceKaji Y, Hiraoka T, Oshika T. Increased level of (1,3)-beta-D-glucan in tear fluid of mycotic keratitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2009 Jul:247(7):989-92. doi: 10.1007/s00417-008-1032-z. Epub 2009 Jan 17 [PubMed PMID: 19151994]
Kolomeyer AM, Murphy KM, Traband A, Frank I, Kim BJ. BETA-D-GLUCAN TESTING IN PATIENTS WITH FUNGAL ENDOPHTHALMITIS. Retina (Philadelphia, Pa.). 2018 Apr:38(4):650-659. doi: 10.1097/IAE.0000000000002049. Epub [PubMed PMID: 29370030]
Mitani A, Shiraishi A, Uno T, Miyamoto H, Hara Y, Yamaguchi M, Ohashi Y. In vivo and in vitro investigations of fungal keratitis caused by Colletotrichum gloeosporioides. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2009 Dec:25(6):563-5. doi: 10.1089/jop.2009.0069. Epub [PubMed PMID: 20028265]
Level 3 (low-level) evidenceSoliman W, Fathalla AM, El-Sebaity DM, Al-Hussaini AK. Spectral domain anterior segment optical coherence tomography in microbial keratitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013 Feb:251(2):549-53. doi: 10.1007/s00417-012-2086-5. Epub 2012 Jun 24 [PubMed PMID: 22729467]
Level 2 (mid-level) evidenceMartone G, Pichierri P, Franceschini R, Moramarco A, Ciompi L, Tosi GM, Balestrazzi A. In vivo confocal microscopy and anterior segment optical coherence tomography in a case of alternaria keratitis. Cornea. 2011 Apr:30(4):449-53. doi: 10.1097/ICO.0b013e3181dae1f3. Epub [PubMed PMID: 21099420]
Level 3 (low-level) evidenceHoffman JJ, Yadav R, Das Sanyam S, Chaudhary P, Roshan A, Singh SK, Mishra SK, Arunga S, Hu VH, Macleod D, Leck A, Burton MJ. Delay in accessing definitive care for patients with microbial keratitis in Nepal. Frontiers in medicine. 2022:9():915293. doi: 10.3389/fmed.2022.915293. Epub 2022 Jul 22 [PubMed PMID: 35935768]
Gurnani B, Kaur K. Contact Lenses. StatPearls. 2025 Jan:(): [PubMed PMID: 35593861]
Stevens S. How to apply an eye pad, shield, and bandage. Community eye health. 2010 Dec:23(74):56 [PubMed PMID: 21311669]
Tuli SS. Fungal keratitis. Clinical ophthalmology (Auckland, N.Z.). 2011:5():275-9. doi: 10.2147/OPTH.S10819. Epub 2011 Feb 27 [PubMed PMID: 21468333]
Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006 Apr:113(4):526-30 [PubMed PMID: 16581414]
Farrell S, McElnea E, Moran S, Knowles S, Murphy CC. Fungal keratitis in the Republic of Ireland. Eye (London, England). 2017 Oct:31(10):1427-1434. doi: 10.1038/eye.2017.82. Epub 2017 May 19 [PubMed PMID: 28524886]
Sun CQ, Lalitha P, Prajna NV, Karpagam R, Geetha M, O'Brien KS, Oldenburg CE, Ray KJ, McLeod SD, Acharya NR, Lietman TM, Mycotic Ulcer Treatment Trial Group. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology. 2014 Aug:121(8):1495-500.e1. doi: 10.1016/j.ophtha.2014.03.004. Epub 2014 Apr 16 [PubMed PMID: 24746358]
Level 1 (high-level) evidenceNarayana S, Krishnan T, Ramakrishnan S, Samantaray PP, Austin A, Pickel J, Porco T, Lietman T, Rose-Nussbaumer J. Mycotic Antimicrobial Localized Injection: A Randomized Clinical Trial Evaluating Intrastromal Injection of Voriconazole. Ophthalmology. 2019 Aug:126(8):1084-1089. doi: 10.1016/j.ophtha.2019.03.020. Epub 2019 Mar 20 [PubMed PMID: 30904540]
Level 1 (high-level) evidenceSharma S, Das S, Virdi A, Fernandes M, Sahu SK, Kumar Koday N, Ali MH, Garg P, Motukupally SR. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. The British journal of ophthalmology. 2015 Sep:99(9):1190-5. doi: 10.1136/bjophthalmol-2014-306485. Epub 2015 Mar 4 [PubMed PMID: 25740805]
Level 1 (high-level) evidenceFlorCruz NV, Evans JR. Medical interventions for fungal keratitis. The Cochrane database of systematic reviews. 2015 Apr 9:(4):CD004241. doi: 10.1002/14651858.CD004241.pub4. Epub 2015 Apr 9 [PubMed PMID: 25855311]
Level 1 (high-level) evidenceFair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspectives in medicinal chemistry. 2014:6():25-64. doi: 10.4137/PMC.S14459. Epub 2014 Aug 28 [PubMed PMID: 25232278]
Level 3 (low-level) evidenceKnutsson KA, Iovieno A, Matuska S, Fontana L, Rama P. Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. Journal of clinical medicine. 2021 Mar 11:10(6):. doi: 10.3390/jcm10061178. Epub 2021 Mar 11 [PubMed PMID: 33799843]
Level 2 (mid-level) evidenceJégou JP, Tromeur F. Superficial keratectomy for chronic corneal ulcers refractory to medical treatment in 36 cats. Veterinary ophthalmology. 2015 Jul:18(4):335-40. doi: 10.1111/vop.12153. Epub 2014 Feb 18 [PubMed PMID: 24548614]
Level 3 (low-level) evidenceAnshu A, Parthasarathy A, Mehta JS, Htoon HM, Tan DT. Outcomes of therapeutic deep lamellar keratoplasty and penetrating keratoplasty for advanced infectious keratitis: a comparative study. Ophthalmology. 2009 Apr:116(4):615-23. doi: 10.1016/j.ophtha.2008.12.043. Epub 2009 Feb 25 [PubMed PMID: 19243833]
Level 2 (mid-level) evidenceLekhanont K, Nonpassopon M, Nimvorapun N, Santanirand P. Treatment with intrastromal and intracameral voriconazole in 2 eyes with Lasiodiplodia theobromae keratitis: case reports. Medicine. 2015 Feb:94(6):e541. doi: 10.1097/MD.0000000000000541. Epub [PubMed PMID: 25674759]
Level 3 (low-level) evidenceIseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008 Jun:27(5):590-4. doi: 10.1097/ICO.0b013e318169d698. Epub [PubMed PMID: 18520510]
Level 3 (low-level) evidenceTabibian D, Richoz O, Riat A, Schrenzel J, Hafezi F. Accelerated photoactivated chromophore for keratitis-corneal collagen cross-linking as a first-line and sole treatment in early fungal keratitis. Journal of refractive surgery (Thorofare, N.J. : 1995). 2014 Dec:30(12):855-7. doi: 10.3928/1081597X-20141113-06. Epub [PubMed PMID: 25437486]
Level 3 (low-level) evidenceZhu Z, Zhang H, Yue J, Liu S, Li Z, Wang L. Antimicrobial efficacy of corneal cross-linking in vitro and in vivo for Fusarium solani: a potential new treatment for fungal keratitis. BMC ophthalmology. 2018 Mar 2:18(1):65. doi: 10.1186/s12886-018-0727-0. Epub 2018 Mar 2 [PubMed PMID: 29499665]
Uddaraju M, Mascarenhas J, Das MR, Radhakrishnan N, Keenan JD, Prajna L, Prajna VN. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. American journal of ophthalmology. 2015 Jul:160(1):131-4.e5. doi: 10.1016/j.ajo.2015.03.024. Epub 2015 Apr 1 [PubMed PMID: 25841317]
Level 1 (high-level) evidenceChee YE, Eliott D. The Role of Vitrectomy in the Management of Fungal Endophthalmitis. Seminars in ophthalmology. 2017:32(1):29-35. doi: 10.1080/08820538.2016.1228396. Epub 2016 Oct 28 [PubMed PMID: 27792412]
Gao Y, Chen N, Dong XG, Yuan GQ, Yu B, Xie LX. Surgical management of fungal endophthalmitis resulting from fungal keratitis. International journal of ophthalmology. 2016:9(6):848-53. doi: 10.18240/ijo.2016.06.10. Epub 2016 Jun 18 [PubMed PMID: 27366686]
De Smet MD, Carlborg EA. Managing severe endophthalmitis with the use of an endoscope. Retina (Philadelphia, Pa.). 2005 Dec:25(8):976-80 [PubMed PMID: 16340526]
Level 2 (mid-level) evidenceChatterjee S, Agrawal D. Recurrence of Infection in Corneal Grafts After Therapeutic Penetrating Keratoplasty for Microbial Keratitis. Cornea. 2020 Jan:39(1):39-44. doi: 10.1097/ICO.0000000000002044. Epub [PubMed PMID: 31259861]
Koenig SB, Harris GJ. Temporary suture tarsorrhaphy after penetrating keratoplasty. Cornea. 1991 Mar:10(2):121-2 [PubMed PMID: 2019120]
Cunha AM, Loja JT, Torrão L, Moreira R, Pinheiro D, Falcão-Reis F, Pinheiro-Costa J. A 10-Year Retrospective Clinical Analysis of Fungal Keratitis in a Portuguese Tertiary Centre. Clinical ophthalmology (Auckland, N.Z.). 2020:14():3833-3839. doi: 10.2147/OPTH.S268327. Epub 2020 Nov 12 [PubMed PMID: 33209016]
Level 2 (mid-level) evidenceGurnani B, Kaur K. Bacterial Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34662023]
Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. European journal of ophthalmology. 2022 Sep:32(5):NP87-NP91. doi: 10.1177/11206721211006564. Epub 2021 Mar 28 [PubMed PMID: 33779337]
Level 3 (low-level) evidenceGurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceGurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian journal of ophthalmology. 2022 Apr:70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. Epub [PubMed PMID: 35325996]
Gurnani B, Kaur K. Comment on: Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian journal of ophthalmology. 2022 Jun:70(6):2204. doi: 10.4103/ijo.IJO_345_22. Epub [PubMed PMID: 35648022]
Level 3 (low-level) evidenceGurnani B, Kaur K, Agarwal S, Lalgudi VG, Shekhawat NS, Venugopal A, Tripathy K, Srinivasan B, Iyer G, Gubert J. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmology and therapy. 2022 Oct:11(5):1629-1653. doi: 10.1007/s40123-022-00542-7. Epub 2022 Jul 5 [PubMed PMID: 35788551]
Gurnani B, Kaur K. Pythium Keratitis. StatPearls. 2024 Jan:(): [PubMed PMID: 34424645]
Song A, Deshmukh R, Lin H, Ang M, Mehta JS, Chodosh J, Said DG, Dua HS, Ting DSJ. Post-keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Frontiers in medicine. 2021:8():707242. doi: 10.3389/fmed.2021.707242. Epub 2021 Jul 7 [PubMed PMID: 34307431]
Kitazawa K, Wakimasu K, Yoneda K, Iliakis B, Sotozono C, Kinoshita S. A case of fungal keratitis and endophthalmitis post penetrating keratoplasty resulting from fungal contamination of the donor cornea. American journal of ophthalmology case reports. 2017 Apr:5():103-106. doi: 10.1016/j.ajoc.2016.12.015. Epub 2016 Dec 30 [PubMed PMID: 29503960]
Level 3 (low-level) evidenceYao YF, Zhang YM, Zhou P, Zhang B, Qiu WY, Tseng SC. Therapeutic penetrating keratoplasty in severe fungal keratitis using cryopreserved donor corneas. The British journal of ophthalmology. 2003 May:87(5):543-7 [PubMed PMID: 12714387]
Level 2 (mid-level) evidenceCoondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: A long overdue revisit. Indian dermatology online journal. 2014 Oct:5(4):416-25. doi: 10.4103/2229-5178.142483. Epub [PubMed PMID: 25396122]
Dhar S, Seth J, Parikh D. Systemic side-effects of topical corticosteroids. Indian journal of dermatology. 2014 Sep:59(5):460-4. doi: 10.4103/0019-5154.139874. Epub [PubMed PMID: 25284850]
Lievens CW, Cilimberg KC, Moore A. Contact lens care tips for patients: an optometrist's perspective. Clinical optometry. 2017:9():113-121. doi: 10.2147/OPTO.S139651. Epub 2017 Aug 11 [PubMed PMID: 30214367]
Level 3 (low-level) evidenceBui TH, Cavanagh HD, Robertson DM. Patient compliance during contact lens wear: perceptions, awareness, and behavior. Eye & contact lens. 2010 Nov:36(6):334-9. doi: 10.1097/ICL.0b013e3181f579f7. Epub [PubMed PMID: 20935569]
Fontana L, Moramarco A, Mandarà E, Russello G, Iovieno A. Interface infectious keratitis after anterior and posterior lamellar keratoplasty. Clinical features and treatment strategies. A review. The British journal of ophthalmology. 2019 Mar:103(3):307-314. doi: 10.1136/bjophthalmol-2018-312938. Epub 2018 Oct 24 [PubMed PMID: 30355718]
McElnea E, Power B, Murphy C. Interface Fungal Keratitis After Descemet Stripping Automated Endothelial Keratoplasty: A Review of the Literature With a Focus on Outcomes. Cornea. 2018 Sep:37(9):1204-1211. doi: 10.1097/ICO.0000000000001636. Epub [PubMed PMID: 29781928]