Introduction
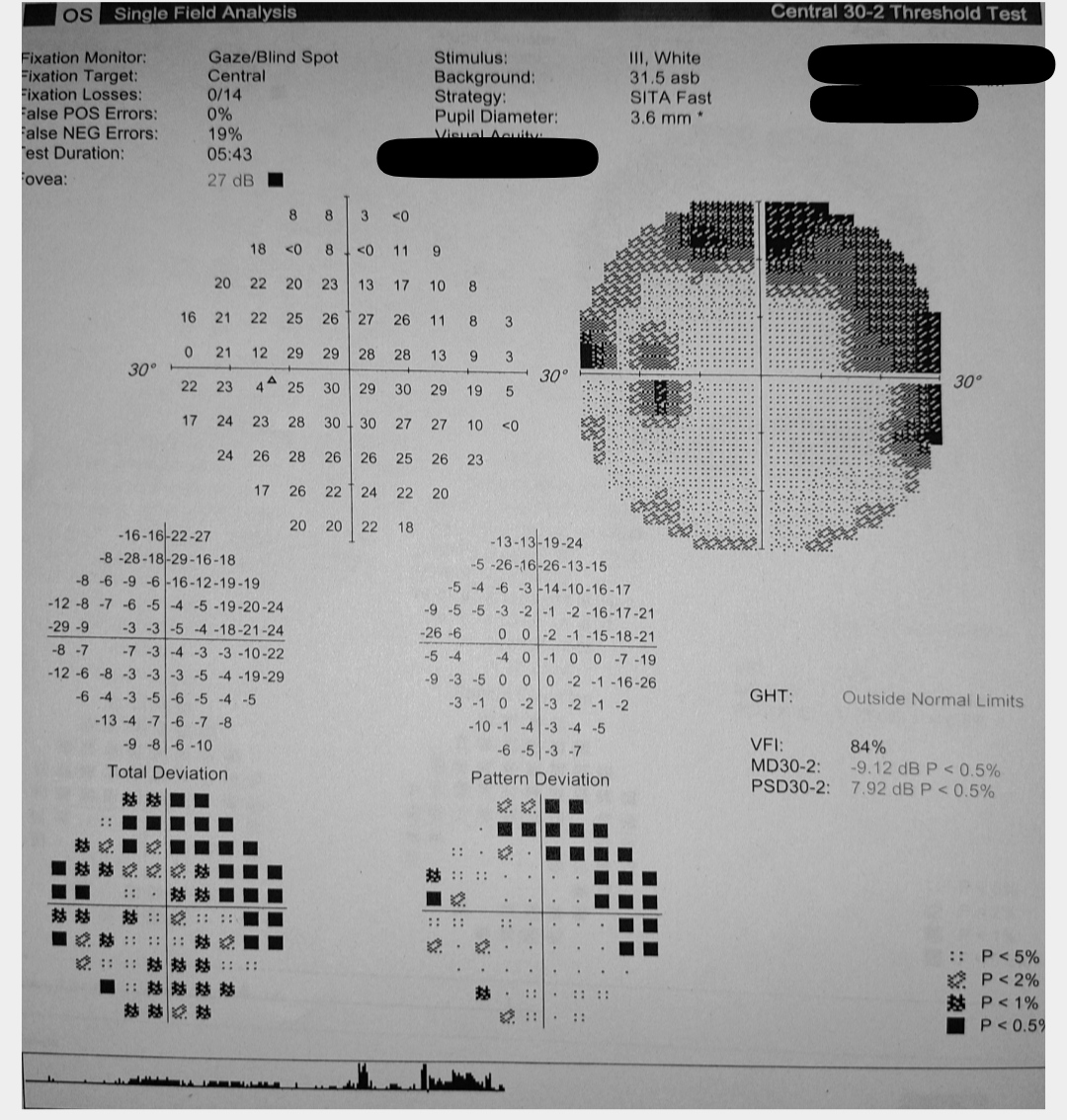
Glaucoma is a neurodegenerative disorder characterized by progressive optic disc degeneration and visual field loss (see Image. Left Eye Glaucomatous Visual Field Changes).[1] Intraocular pressure (IOP) is the chief modifiable risk factor; periodic tonometric measurements are thus fundamental in managing this disease.[2][3][4] Glaucoma is the most common etiology of irreversible blindness.[5][6] The condition affects over 80 million people globally and is expected to surpass 110 million by 2040.[7]
Glaucoma has 2 main subtypes: open-angle and angle-closure.[8] Juvenile open-angle glaucoma (JOAG) is an uncommon form of primary open-angle glaucoma (POAG) with earlier onset (age 3-40 years), typically with a higher IOP and more severe visual field loss compared with adult-onset POAG.[9] Juvenile glaucoma is often hereditary and significantly influenced by genetic factors, distinguishing it from other forms of glaucoma that primarily affect older individuals.[10][11][12] The condition can manifest without an apparent cause and is generally more severe than adult-onset glaucoma. Prompt diagnosis and timely treatment are crucial, as the disease can progress rapidly—leading to significant vision loss.
Many studies report that JOAG typically demonstrates an autosomal dominant inheritance pattern.[13][14] Mutations in the myocilin (MYOC) gene are well established as part of the genetic etiology of this condition.[15] The MYOC gene regulates the production of the myocilin protein found in the eye's trabecular meshwork; MYOC mutations result in the synthesis of atypical myocilin protein, which builds up in the trabecular meshwork cells. This accumulation hampers the performance of the cells and diminishes aqueous humor drainage, ultimately raising IOP.[16][17] Other gene mutations reported in this type of glaucoma include CYP1B1, PITX2, and FOXC1.[18][19][20]
Anatomy of the Eye
Understanding the etiology of juvenile glaucoma requires knowledge of the anatomical structures involved. The eye is subdivided into anterior and posterior regions (see Image. Schematic of Eye Anatomy). The anterior part is in front of the lens and divides into anterior and posterior chambers. In the posterior chamber, the eye's ciliary body produces a fluid called "aqueous humor." This fluid flows from the posterior chamber into the anterior chamber through the pupil and exits the eye via the trabecular and uveoscleral pathways. Most aqueous humor outflow flows through the trabecular meshwork and the canal of Schlemm, which are parts of the trabecular pathway.[21] In the uveoscleral pathway, aqueous humor passes into the supraciliary and suprachoroidal spaces through the ciliary muscle.[22] Both routes drain the aqueous humor into the venous circulation.[23]
In JOAG, a trabecular meshwork abnormality or deformity frequently reduces the ability of the aqueous humor to flow out, thus increasing IOP. Juvenile glaucoma affects the optic nerve head (optic disc). Elevated IOP leads to gradual optic nerve damage, defined by a reduction in the retinal nerve fiber layer (RNFL) and the loss of optic disc fibers. The damage is permanent and cannot be reversed. If left untreated, the condition can result in visual field abnormalities, eventually leading to total vision loss.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
JOAG often results from the abnormal development of the trabecular meshwork. Aqueous humor drainage may be inadequate, increasing IOP and damaging the optic nerve. The etiology of juvenile glaucoma is typically genetically based. The disorder frequently exhibits an autosomal dominant inheritance pattern characterized by varied expressivity and incomplete penetrance. Multiple significant genetic alterations influence JOAG, each playing a role in the disease through distinct mechanisms. Research has shown several genetic factors, which include MYOC, CYP1B1, PITX2, and FOXC1 gene mutations.[24]
Myocilin gene (MYOC) mutations are well established as part of the genetic etiology of JOAG.[25] Myocilin protein is found in the eye's trabecular meshwork, and the MYOC gene controls its synthesis. MYOC mutations result in the synthesis of atypical myocilin protein, which builds up in the trabecular meshwork cells. This accumulation hampers the cells' performance and diminishes aqueous humor drainage, ultimately elevating IOP. Mutations in the CYP1B1 gene can also cause abnormalities in the trabecular meshwork and other structures in the front part of the eye.[26] These anomalies can increase IOP and injure the optic nerve. Studies have also shown that PITX2 and FOXC1 gene mutations are linked to Axenfeld-Rieger syndrome, which can manifest as JOAG.[27] The proper functioning of these genes is essential for normal eye development, and any interference with their activity can produce a condition called "anterior segment dysgenesis," raising the likelihood of developing glaucoma.
Anatomical and developmental anomalies in the trabecular meshwork and Schlemm canal are key factors in juvenile glaucoma, affecting the regulation of aqueous humor flow. Trabecular meshwork dysgenesis refers to developmental trabecular meshwork abnormalities that impede normal aqueous humor drainage in JOAG.[28] These anomalies may involve an insufficiently formed or dysfunctional trabecular meshwork, leading to increased resistance in draining aqueous fluid and elevated IOP. Angle dysgenesis refers to structural anomalies in the anterior chamber angle, specifically in the location of the trabecular meshwork.[29] These abnormalities may hinder the proper passage of aqueous humor from the eye. These defects may involve abnormalities in the angle recess or aberrant tissue, further increasing IOP. Some individuals with JOAG have a noticeable and forwardly shifted Schwalbe line, a condition known as posterior embryotoxon.[30] This anatomical abnormality may be linked to other irregularities in the angle of the eye and have a role in glaucoma development.
The physiological mechanisms responsible for juvenile glaucoma are intricately linked to the dynamics of the formation and drainage of aqueous humor.[31] While less frequent, specific JOAG cases may entail an amplified generation of aqueous humor, which can overpower the outflow capacity of the trabecular meshwork, resulting in elevated IOP. The prominent physiological disruption in JOAG is the hindered flow of aqueous humor through the trabecular meshwork. This limitation might arise from structural problems, genetic changes impacting outflow routes, or a combination of these factors. Even within the normal range, IOP variations might harm the optic nerve in at-risk individuals.[32] The variability in these changes may be more noticeable in younger individuals because of the dynamic nature of their developing eye structure.
Genetic and anatomical factors are the leading causes of JOAG, although environmental and lifestyle variables may also influence when and how the disease develops. A previous eye injury, especially during childhood, can make persons more susceptible to additional factors that can worsen JOAG or cause similar symptoms.[33] Extended corticosteroid use, whether taken internally or applied externally, can increase IOP in cortisone responders and potentially increase susceptibility to JOAG.[34]
Epidemiology
Adult-onset glaucoma is more prevalent but represents a substantial proportion of glaucomatous conditions among younger individuals. The incidence of JOAG varies among different populations. JOAG has been reported to be responsible for 3.4% of newly diagnosed glaucomas in Nigeria, compared to 1.9% in Saudi Arabia, 3.3% in India, and 0.7% in Caucasians. JOAG's prevalence in the United States has been reported to be about 1 in 50,000. Study results have reported this condition's prevalence of 0.38 per 100,000, affecting those between 4 and 20 years. A Dallas Glaucoma Registry survey of 376 eyes from 239 children with glaucoma revealed that 4% of patients had JOAG.
JOAG's exact incidence in the United States is difficult to determine because of its rarity and the possibility of inaccurate or delayed diagnosis. JOAG is categorized within the broader classifications of childhood and adult-onset POAG. Thus, JOAG's prevalence varies across studies using different criteria to define cases. Differences in age ranges among cohorts in various studies have led to heterogeneous prevalence rates that may not be directly comparable. For example, results from several studies have reported a prevalence rate of about 15% in Ohio and South India.[35][36] These studies established a maximum age range of 16 to 18 years, reporting a greater prevalence rate. A study conducted in hospitals in Egypt had results that revealed that JOAG accounted for only 1% of childhood glaucomas.[37] A screening study conducted in West Africa identified that 16% of the individuals diagnosed with POAG were between 20 and 40 years old.
Although age ranges may differ, most generally consider JOAG a subtype of POAG that manifests before age 40.[38] The reported age range for this diagnosis in the literature varies widely, with studies citing ranges from 10 to 30 years, 5 to 35 years, 10 to 35 years, and 10 to 40 years.[39] The lower age limit is usually set at a minimum of 3 years.
The Childhood Glaucoma Research Network proposed an International Consensus Classification to resolve the inconsistency in naming childhood glaucoma.[40] According to this classification, the youngest age at which JOAG can occur is 4 years, and the disease can continue to develop until age 30 or 40. The condition may be categorized as adult-onset POAG beyond age 40; however, the disparity in age-based categorization persists. Diagnosis requires open angles, elevated IOP (greater than 21 mm Hg on at least 2 occasions), and evidence of visual field defects or glaucomatous optic neuropathy.
JOAG has several risk factors. Studies results indicate a marginally greater occurrence of JOAG in men than women. In a study based on 25 eyes with JOAG, 64% of the patients were men. Researchers have reported 87% of individuals with JOAG to be myopic in a study of 23 affected patients.[41] The incidence of JOAG has been reported to be slightly higher among people from West Asia, South Asia, and Africa than among individuals from Europe.[42] Juvenile glaucoma frequently exhibits familial aggregation, with the condition affecting numerous family members across different generations. Mutations in genes such as MYOC, CYP1B1, PITX2, and FOXC1 play a crucial role in developing JOAG. Genetic markers may be utilized to identify individuals at risk and provide guidance for implementing early intervention programs.
Pathophysiology
JOAG's pathophysiology is not entirely understood.[43] Primary JOAG is a multifaceted condition marked by increased pressure within the eye, damage to the optic nerve, and consequent loss of vision in the field of view. The pathogenesis of JOAG is characterized by the interplay of genetic, anatomical, and physiological variables that collectively contribute to the initiation and advancement of the disease.[44]
The underlying mechanism of juvenile glaucoma centers on the disruption of the normal regulation of fluid flow in the eye, known as aqueous humor dynamics. This disruption results in increased IOP and consequent damage to the optic nerve. Several crucial mechanisms are involved in the development of JOAG. Genetic mutations include those involving the MYOC, CYP1B1, PITX2, and FOXC1 genes.[45] These genetic mutations are associated with anterior segment dysgenesis syndromes, including Axenfeld-Rieger syndrome, characterized by certain traits that make individuals more susceptible to JOAG. Abnormalities in these specific genes result in developmental anomalies in the anterior eye structures, reducing aqueous humor flow.
Structural abnormalities that can alter aqueous outflow include trabecular meshwork, angle dysgeneses, and other anterior chamber angle anomalies—such as angle constriction and aberrant tissues. Elevated IOP exerts pressure on the lamina cribrosa, impairing optic nerve function, which leads to retinal ganglion cell death and optic nerve degeneration over time.[46] The optic cup becomes more prominent with time, and the neuroretinal rim becomes thinner.[47]
Glaucomatous damage increases the cup-to-disc ratio.[48] Optical coherence tomography (OCT) reveals reduced retinal nerve fiber layer thickness, particularly in the superior and inferior quadrants, correlating directly with optic nerve damage severity.[49] Visual field testing frequently uncovers distinctive glaucomatous abnormalities, including arcuate scotomas, nasal steps, and paracentral scotomas. These abnormalities merge as the disease advances, resulting in broader sensitivity loss in the outer areas.
Patients with JOAG often exhibit lower corneal hysteresis, indicating reduced corneal resilience to mechanical stress and suggesting fundamental structural problems contributing to glaucoma development. Variations in central corneal thickness (CCT) can impact IOP measurements.[50] Although not a direct cause of JOAG, CCT is crucial in interpreting IOP measurements and evaluating the risk of developing glaucoma.[51]
Gonioscopy can detect anterior chamber angle anomalies, including elevated iris insertion, presence of iris processes, or angle recession from prior trauma, which can complicate the evaluation and treatment of JOAG.[52] Myopia is also frequently linked to JOAG. The anatomical and mechanical characteristics of nearsighted eyes can make individuals more prone to higher IOP and optic nerve injury.[53]
History and Physical
JOAG and adult-onset POAG have similar findings. A thorough and systematic approach to history-taking is essential when evaluating patients with suspected juvenile glaucoma. Many individuals diagnosed with JOAG have no symptoms, particularly during the initial phases of the condition. Nevertheless, some patients may experience visual impairments such as blurry vision, halos appearing around lights, or occasional eye discomfort. Others may exhibit more noticeable impairments in their visual field if the illness has advanced.[54] Several visual complaints, like nyctalopia (difficulty adjusting to darkness), frequent corrective eyewear prescription changes, or peripheral vision loss, may suggest increasing optic nerve deterioration and visual field loss. Other symptoms may develop, including an enlarged eye, tearing, blinking, and glare.
A comprehensive family history is crucial when evaluating for glaucoma, as JOAG often involves genetic factors and should be distinguished from secondary forms. Requesting information about relatives with a medical history of glaucoma, blindness, or other eye-related disorders is critical. A family history of glaucoma in either parents or siblings significantly increases suspicion of JOAG.[55][56]
The clinician should evaluate for prior eye conditions, operations, or injuries.[57] Previous eye injuries can increase the likelihood of developing secondary glaucoma, which can have symptoms similar to JOAG; previous instances of increased IOP or optic nerve anomalies must also be investigated.
Systemic conditions that may impact ocular health include diabetes, hypertension, and autoimmune disorders. These diseases are typically linked to the development of glaucoma in adults but may offer significant background information.[58] Information must be elicited regarding the use of drugs, specifically corticosteroids, which can potentially increase IOP. Prolonged steroid use, whether systemic, topical, or inhalational, can contribute to the development of secondary glaucoma.[59]
Evaluation
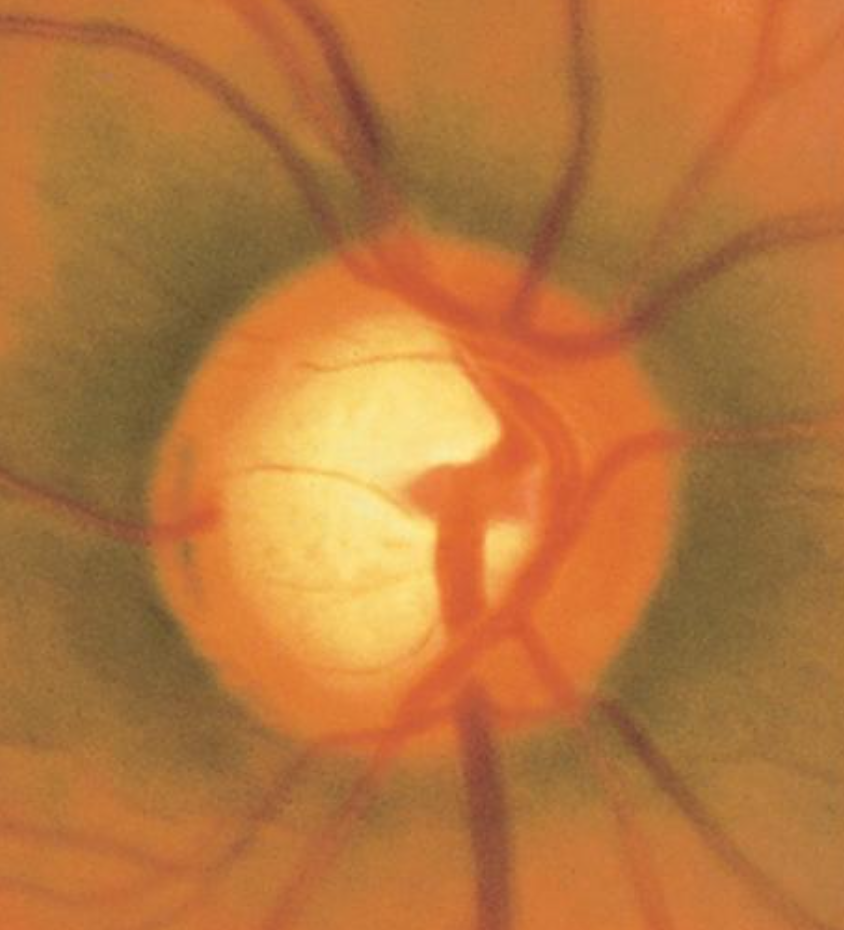
An extensive ocular examination is essential for assessing juvenile glaucoma (see Image. Optic Nerve Cup-to-Disc Ratio of 0.75). The process comprises the following stages:
- Visual acuity assessment: Assess the visual acuity in both eyes using a Snellen chart or a comparable tool. This test establishes a standard for evaluating the effect of glaucoma on a patient's ability to see.
- IOP measurement: Goldmann applanation tonometry is the most reliable method for measuring IOP and should be conducted in all suspected JOAG cases.[60][61] Alternative techniques like rebound tonometry, tonopen, or noncontact tonometry can be employed in uncooperative individuals or infants.
- Slit-lamp examination: A comprehensive slit-lamp examination can help evaluate the anterior eye. Look for angle, iris, or structural irregularities. Assess the cornea, iris, and lens for any related observations.
- Gonioscopy: This essential diagnostic procedure examines the angle of the eye where the cornea meets the iris. Perform gonioscopy to assess the anterior chamber angle and ascertain the trabecular meshwork's structure. This test distinguishes between open-angle and angle-closure glaucoma and detects any structural abnormalities.[62]
- Optic nerve head assessment: Inspect the optic nerve head using direct or indirect ophthalmoscopy. Record the cup-to-disc ratio, the neuroretinal rim's thinning, and any optic nerve impairment signs.[63]
- Fundus assessment: Examine the macula and peripheral retina for abnormalities and concurrent retinal disorders.
Similar to other forms of glaucoma, several diagnostic tests, in addition to the basic ocular examination, are necessary for the diagnosis and management of juvenile glaucoma. These modalities include the following:
- Visual field testing: This diagnostic procedure assesses the extent and quality of a person's peripheral vision. Automated perimetry, such as the Humphrey Visual Field (HVF) test, is essential for identifying and measuring visual field abnormalities. Look for distinctive glaucomatous patterns, such as arcuate scotomas, nasal steps, and paracentral abnormalities.[64][65]
- OCT: This medical imaging technique can capture images of the retinal nerve fiber layer (RNFL) and the optic nerve head with a high level of resolution. Utilize RNFL thickness measurement to identify initial signs of glaucomatous alterations and track the advancement of the disease. Macular OCT can also evaluate the thickness of the ganglion cell layer. OCT may also assess the anterior segment to obtain information about pachymetry and the angle parameters.[66]
- Measurement of corneal parameters: Corneal pachymetry measures the thickness of the CCT. IOP measurements taken with Goldmann applanation tonometry and other tonometers may be slightly influenced by corneal thickness. Thinner corneas are prone to developing JOAG.[67] Corneal hysteresis provides information on the cornea's biomechanical characteristics; reduced hysteresis levels are linked to a higher likelihood of glaucoma development.
- Ultrasonic biomicroscopy: This modality can provide information on anterior chamber structures. Ultrasonic biomicroscopy is particularly beneficial for detecting modest anatomical abnormalities that impact the flow of aqueous humor.[68]
- Electrophysiological testing: This diagnostic procedure measures and records the electrical activity of cells and tissues in the body. Visual evoked potentials or electroretinography tests are typically not used in routine clinical settings. However, these tests may be considered if significant optic nerve or retinal impairment is suspected.[69]
- Genetic testing and counseling: Genetic testing is recommended for juvenile glaucoma because of its hereditary nature. The test is particularly important in patients with a positive family history or early symptom onset. Genetic testing can identify gene mutations frequently linked to JOAG, including MYOC, CYP1B1, PITX2, and FOXC1. Determining precise mutations can validate the diagnosis and offer insights into the hereditary origins of the disease.[70][71]
Treatment / Management
As in other forms of glaucoma, JOAG treatment aims to halt or slow down further optic nerve degeneration and visual field loss by decreasing the IOP. Imaging of the optic nerve is often necessary to assess disease progression. JOAG is often resistant to medical therapy, presents with severe symptoms, and progresses faster. Thus, surgical treatment is usually warranted.[72](B3)
Medical Therapy
Medical therapy is the first-line treatment for JOAG and is often a bridge to surgical intervention. Results from a study of 23 patients revealed that 83% of patients with juvenile glaucoma required filtration surgery (trabeculectomy). Medical therapy for JOAG includes β-blockers, topical carbonic anhydrase inhibitors, prostaglandin analogs, Rho kinase inhibitors, and α-adrenergic agonists. If no contraindications such as asthma exist, β-blockers are often the first-line therapy for JOAG. Topical carbonic anhydrase inhibitors are less effective than β-blockers but useful as additional therapy or alternatives when β-blockers are contraindicated.
Prostaglandins are more effective in adult glaucoma than childhood glaucoma. α-adrenergic agonists are effective but have potential systemic side effects requiring close observation when prescribed to young patients.[73] Combining 2 or more drugs in fixed combinations, such as timolol/dorzolamide, brimonidine/timolol, and netarsudil/latanoprost, can enhance compliance by lowering the number of eye drops needed.(B3)
Surgery
Multiple surgical options are available, including trabeculectomy, drainage implantation, angle procedures (goniotomy and trabeculotomy), and cycloablative modalities.[74] Trabeculectomy is the backbone of surgical therapy in JOAG, as the procedure produces good outcomes.[75] Results from a study in India that included 60 eyes of 41 patients revealed that 50% to 87% of individuals who received this therapy reported good IOP control without requiring medications in the first 3 years after the operation.[76](B2)
Mitomycin C (MMC) and other types of antifibrotics are used to prevent fibrosis associated with the procedure. Adding MMC to trabeculectomy results in lower IOP. However, this additional step increases the risk of developing hypotony maculopathy and bleb-related infection.[77] Glaucoma drainage implantation (GDI) is an alternative to trabeculectomy for JOAG. GDI uses various implant types, such as the Ahmed glaucoma valve, the Baerveldt implant, and the Molteno implant; GDI is preferable when conjunctival scarring is a risk after trabeculectomy.[78](B2)
A retrospective study's results found that the Ahmed glaucoma valve achieved a 100% success rate in pediatric glaucoma at the 1-year follow-up. The final IOP was between 6 and 18 mm Hg, and none of the patients experienced complete visual loss.[79] A prospective study of 52 eyes with juvenile glaucoma demonstrated that the Molteno implant produced IOPs less than 21 mm Hg.[80] Reports show successful angle procedures in JOAG, but these procedures are often used for managing congenital glaucoma.
Minimally invasive glaucoma surgery (MIGS) may be considered in select patients without a problematic target IOP.[81] Techniques like iStent, Xen Gel Stent, and Hydrus Microstent are less intrusive alternatives that reduce IOP by improving fluid flow through the trabecular meshwork or the uveoscleral pathway. MIGS is gaining popularity because of its favorable safety profile and shorter recovery periods. However, MIGS is typically not intended for treating severe or complex glaucoma cases requiring significant and lasting IOP reductions.[82](B3)
Laser
Laser trabeculoplasty is another glaucoma treatment option. Selective laser trabeculoplasty (SLT) is one modality, and it uses a Q-switched, frequency-doubled Nd:YAG (532 nm) laser. SLT enhances aqueous outflow through the trabecular meshwork to decrease IOP. The procedure induces less coagulative damage and structural change than older laser trabeculoplasty techniques. SLT typically does not provide the same IOP-lowering effect as surgical therapy, and due to goniodysgenesis in JOAG, it is often not as effective as in other forms of POAG.[83] Argon laser trabeculoplasty (ALT) employs heat energy to augment fluid outflow via the trabecular meshwork, similar to SLT. However, ALT is used less frequently because of the increased likelihood of posttreatment complications and reduced reproducibility compared to SLT.(A1)
Management
Consistent surveillance and subsequent evaluation are essential for effectively managing JOAG and averting vision deterioration. Close monitoring of patients with JOAG is recommended, with regular follow-up appointments scheduled every 3 to 12 months. The frequency of these visits may vary depending on the target IOP and the condition's severity and rate of progression.
Individuals with quickly advancing glaucoma may require more frequent appointments. Routine visual field tests, OCT imaging, and IOP measurements help monitor disease advancement and modify treatment strategies as needed.[84] Patients and their families should be provided with pertinent information regarding the significance of treatment adherence, attending regular follow-up visits, and making lifestyle changes to control IOP and avoid disease advancement effectively.(B3)
Differential Diagnosis
Other conditions that may also be mistaken for JOAG due to similarities in presentation include the following:
- Congenital glaucoma [85]
- Steroid-induced glaucoma [86]
- Pseudoexfoliation glaucoma [87]
- Pigmentary glaucoma [88]
- Uveitic glaucoma [89]
- Traumatic glaucoma [90]
- Inflammatory glaucoma [91]
- Other forms of primary open-angle glaucoma [92]
- Aniridia [93]
- Sturge-Weber syndrome [94]
- Megalopapilla [95]
- Optic disc cupping secondary to prematurity [96]
- Chandler syndrome [97]
A thorough clinical evaluation combined with appropriate diagnostic testing can distinguish these conditions from JOAG and guide management.
Staging
Evaluating the stage of JOAG is essential for assessing the condition's seriousness, informing therapy choices, and tracking disease advancement. The staging process usually relies on a mix of clinical observations, such as IOP, the optic nerve head's appearance, RNFL thickness, and the presence of visual field abnormalities.
Prognosis
The prognosis for JOAG is generally favorable if diagnosed and treated early. In a Japanese study of 47 eyes with juvenile glaucoma, the results revealed that 21 eyes (45%) had normal visual fields, whereas 13 eyes (28%) progressed to an advanced stage, mostly due to delayed diagnosis; even patients requiring a trabeculectomy can have reduced IOPs to halt visual field loss.[98] The prognosis of JOAG is determined by various factors, including the age of onset, severity at the time of diagnosis, treatment effectiveness, and patient compliance with follow-up care. Prompt identification and proper treatment are essential for enhancing long-term results and safeguarding eyesight. Regarding the age of onset, the occurrence of juvenile glaucoma at a younger age, particularly before age 10, is frequently linked to a more severe illness progression and a less favorable outlook. Onset during late adolescence or early adulthood tends to lead to a more favorable outlook due to the eye structures being more developed at these stages and slower disease progression.
The initial disease severity is crucial for prognosis. Early-stage disease, with minimal optic nerve damage and no significant vision loss, often has a more favorable outlook. Patients who successfully reach and sustain the target IOP through medicinal or surgical interventions generally experience a more positive outlook.[99] A robust familial background of glaucoma may suggest a more severe illness progression, necessitating more frequent surveillance and earlier treatment. Certain genetic mutations, such as those of the MYOC gene, can impact disease severity and treatment response. Compliance with treatment and follow-up enhances the likelihood of a good prognosis over time.
Complications
Significant delays in glaucoma treatment can lead to visual impairment and blindness. However, diagnosis is often delayed because glaucoma may be asymptomatic until it becomes advanced. Other complications depend on the type of medication used and the surgery performed. These complications include the following:
- Eye redness or pain
- Irritation of the cornea
- Persistent high intraocular pressure
- Hypotony
- Choroidal effusions
- Suprachoroidal hemorrhages
- Infection
- Cataract development or progression [100]
Families at risk for JOAG must be advised to have regular eye examinations and comply with treatment to slow disease progression.
Deterrence and Patient Education
At-risk families must receive education about JOAG. These individuals should understand the condition's genetic origins, symptoms, and treatment options. Families at risk must also be advised about factors that can delay or hasten disease progression. Genetic counseling is important for individuals with JOAG to make informed family planning decisions.
Early diagnosis and treatment increase the chance of preserving vision and preventing permanent visual field loss. Offering patients access to counseling and support groups can assist them in managing the emotional and psychological difficulties associated with living with glaucoma. Participating in clinical trials and research studies offers the opportunity to obtain state-of-the-art treatments and contribute to an enhanced comprehension of the disease.
Pearls and Other Issues
JOAG impairs vision and reduces patients' quality of life. Issues arise from IOP elevation, optic nerve damage, and the consequences of prolonged glaucoma therapy. Understanding this condition's complexities is essential for successful management and enhancing outcomes. Timely identification, aggressive treatment, and thorough monitoring are crucial in maintaining vision or reducing visual impairment in individuals with this complex condition.
Enhancing Healthcare Team Outcomes
Patients with JOAG may initially present to the emergency department physician or primary care clinician. Communication between healthcare professionals is necessary to improve visual health outcomes. Patients should receive a referral to an ophthalmologist for definitive diagnosis and treatment planning. Regular ophthalmic examinations involving IOP, optic nerve, and visual field assessments are fundamental to identifying early glaucoma and delaying disease progression. The treatment options for juvenile glaucoma, including medicines, laser treatments, and surgical procedures, must be discussed.
Genetic counseling is necessary to provide affected individuals and families with information regarding the genetic aspects of JOAG. Clinical protocols should be developed and followed to recognize this disease early, preventing irreversible vision loss. Effective deterrence methods should be implemented, and comprehensive patient education should be provided to manage juvenile glaucoma and control its progression.
Caring for patients with JOAG necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes. Taking a proactive stance in deterring and educating patients is crucial in reducing the consequences of juvenile glaucoma, preserving vision, and improving the overall quality of life for those affected and their families. Achieving these aims requires continued focus on public awareness, genetic counseling, and comprehensive patient-centered care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
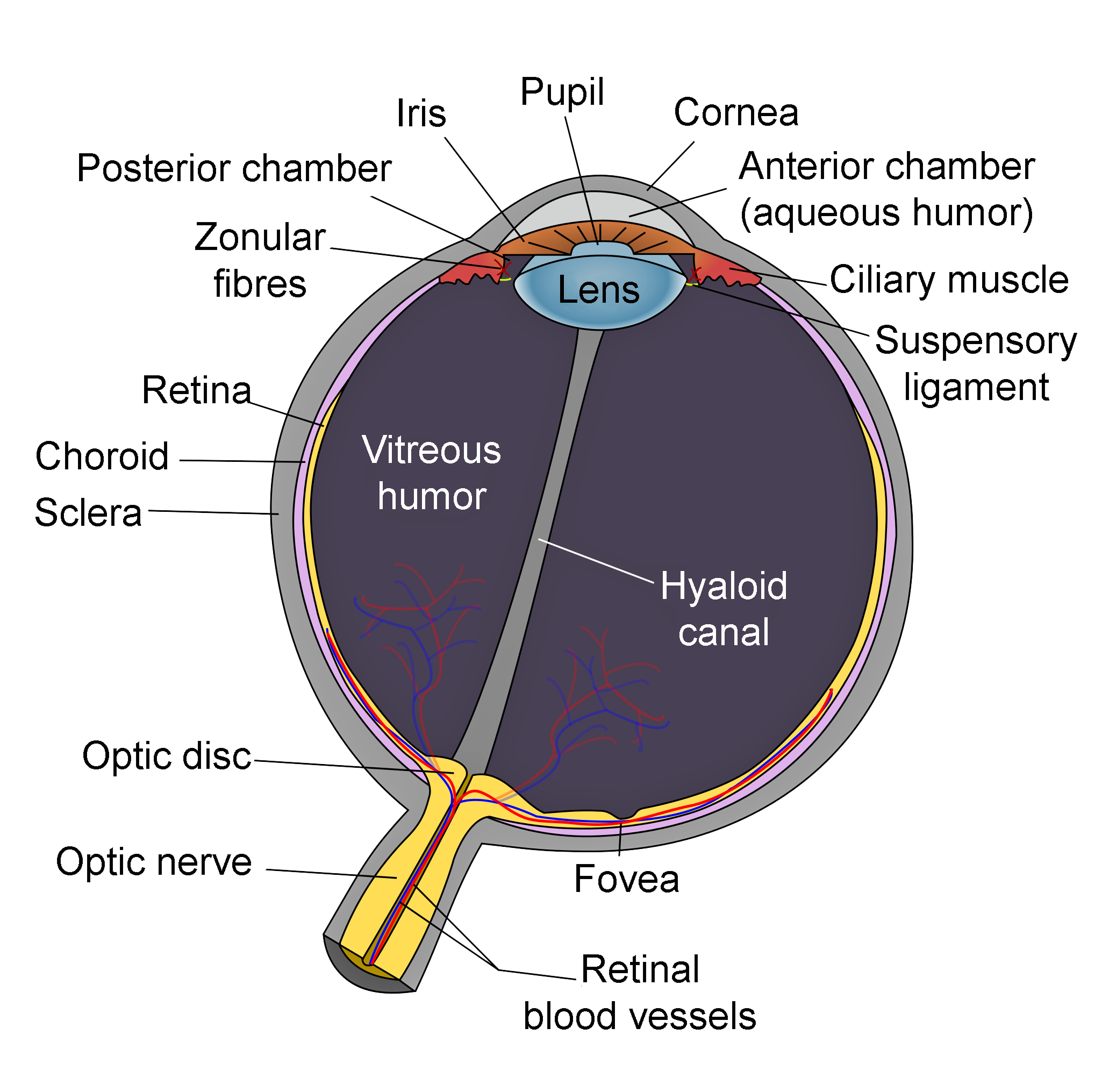
Schematic of Eye Anatomy. This image illustrates the anatomic relationships between the optic disc, optic nerve, fovea, sclera, choroid, vitreous humor, hyaloid canal, retina, retinal blood vessels, zonular fibers, iris, pupil, cornea, anterior chamber (aqueous humor), lens, posterior chamber, ciliary muscle, and suspensory ligament.
Contributed by R.H. Castilhos and Jordi March i Nogué (CC by SA-3.0 https://creativecommons.org/licenses/by-sa/3.0/deed.en)
References
Sekhar GC. Glaucoma definition: Implications for equitable care. Indian journal of ophthalmology. 2021 May:69(5):1025-1026. doi: 10.4103/ijo.IJO_3771_20. Epub [PubMed PMID: 33913824]
Orzalesi N, Fogagnolo P. An innovative approach to the management of IOP in glaucoma. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2024 Feb:262(2):365. doi: 10.1007/s00417-023-06253-4. Epub 2023 Sep 26 [PubMed PMID: 37750954]
Bader J, Zeppieri M, Havens SJ. Tonometry. StatPearls. 2024 Jan:(): [PubMed PMID: 29630277]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27 [PubMed PMID: 34501306]
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. The Lancet. Global health. 2017 Dec:5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5. Epub 2017 Oct 11 [PubMed PMID: 29032195]
Level 1 (high-level) evidenceJonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet (London, England). 2017 Nov 11:390(10108):2183-2193. doi: 10.1016/S0140-6736(17)31469-1. Epub 2017 May 31 [PubMed PMID: 28577860]
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014 Nov:121(11):2081-90. doi: 10.1016/j.ophtha.2014.05.013. Epub 2014 Jun 26 [PubMed PMID: 24974815]
Level 1 (high-level) evidenceDietze J, Blair K, Havens SJ. Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 30855805]
Selvan H, Gupta S, Wiggs JL, Gupta V. Juvenile-onset open-angle glaucoma - A clinical and genetic update. Survey of ophthalmology. 2022 Jul-Aug:67(4):1099-1117. doi: 10.1016/j.survophthal.2021.09.001. Epub 2021 Sep 16 [PubMed PMID: 34536459]
Level 3 (low-level) evidenceMantravadi AV, Vadhar N. Glaucoma. Primary care. 2015 Sep:42(3):437-49. doi: 10.1016/j.pop.2015.05.008. Epub 2015 Jul 29 [PubMed PMID: 26319348]
Gupta V, Somarajan BI, Gupta S, Chaurasia AK, Kumar S, Dutta P, Gupta V, Sharma A, Tayo BO, Nischal K. The inheritance of juvenile onset primary open angle glaucoma. Clinical genetics. 2017 Aug:92(2):134-142. doi: 10.1111/cge.12906. Epub 2017 Feb 16 [PubMed PMID: 27779752]
Arora S, Maeda M, Francis B, Maeda M, Sit AJ, Mosaed S, Nazarali S, Damji KF. Efficacy and safety of ab interno trabeculectomy in juvenile open-angle glaucoma. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Oct:53(5):482-486. doi: 10.1016/j.jcjo.2017.12.013. Epub 2018 Mar 14 [PubMed PMID: 30340716]
Svidnicki PV, Braghini CA, Costa VP, Schimiti RB, de Vasconcellos JPC, de Melo MB. Occurrence of MYOC and CYP1B1 variants in juvenile open angle glaucoma Brazilian patients. Ophthalmic genetics. 2018 Dec:39(6):717-724. doi: 10.1080/13816810.2018.1546405. Epub [PubMed PMID: 30484747]
Kwun Y, Lee EJ, Han JC, Kee C. Clinical Characteristics of Juvenile-onset Open Angle Glaucoma. Korean journal of ophthalmology : KJO. 2016 Apr:30(2):127-33. doi: 10.3341/kjo.2016.30.2.127. Epub 2016 Mar 25 [PubMed PMID: 27051261]
Liuska PJ, Harju M, Kivelä TT, Turunen JA. Prevalence of MYOC risk variants for glaucoma in different populations. Acta ophthalmologica. 2021 Nov:99(7):e1090-e1097. doi: 10.1111/aos.14738. Epub 2021 Jan 9 [PubMed PMID: 33421356]
Gupta V, Gupta S, Dhawan M, Sharma A, Kapoor KS, Sihota R. Extent of asymmetry and unilaterality among juvenile onset primary open angle glaucoma patients. Clinical & experimental ophthalmology. 2011 Sep-Oct:39(7):633-8. doi: 10.1111/j.1442-9071.2011.02522.x. Epub 2011 Feb 7 [PubMed PMID: 21631667]
Level 2 (mid-level) evidenceGoldwyn R, Waltman SR, Becker B. Primary open-angle glaucoma in adolescents and young adults. Archives of ophthalmology (Chicago, Ill. : 1960). 1970 Nov:84(5):579-82 [PubMed PMID: 5478882]
Pan Y, Iwata T. Exploring the Genetic Landscape of Childhood Glaucoma. Children (Basel, Switzerland). 2024 Apr 9:11(4):. doi: 10.3390/children11040454. Epub 2024 Apr 9 [PubMed PMID: 38671671]
Michels K, Bohnsack BL. Ophthalmological Manifestations of Axenfeld-Rieger Syndrome: Current Perspectives. Clinical ophthalmology (Auckland, N.Z.). 2023:17():819-828. doi: 10.2147/OPTH.S379853. Epub 2023 Mar 10 [PubMed PMID: 36926528]
Level 3 (low-level) evidenceReis LM, Amor DJ, Haddad RA, Nowak CB, Keppler-Noreuil KM, Chisholm SA, Semina EV. Alternative Genetic Diagnoses in Axenfeld-Rieger Syndrome Spectrum. Genes. 2023 Oct 17:14(10):. doi: 10.3390/genes14101948. Epub 2023 Oct 17 [PubMed PMID: 37895297]
Buffault J, Labbé A, Hamard P, Brignole-Baudouin F, Baudouin C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal francais d'ophtalmologie. 2020 Sep:43(7):e217-e230. doi: 10.1016/j.jfo.2020.05.002. Epub 2020 Jun 16 [PubMed PMID: 32561029]
van Zyl T, Yan W, McAdams A, Peng YR, Shekhar K, Regev A, Juric D, Sanes JR. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2020 May 12:117(19):10339-10349. doi: 10.1073/pnas.2001250117. Epub 2020 Apr 27 [PubMed PMID: 32341164]
Marshall LL, Hayslett RL, Stevens GA. Therapy for Open-Angle Glaucoma. The Consultant pharmacist : the journal of the American Society of Consultant Pharmacists. 2018 Aug 1:33(8):432-445. doi: 10.4140/TCP.n.2018.432. Epub [PubMed PMID: 30068436]
Miller MA, Fingert JH, Bettis DI. Genetics and genetic testing for glaucoma. Current opinion in ophthalmology. 2017 Mar:28(2):133-138. doi: 10.1097/ICU.0000000000000344. Epub [PubMed PMID: 27898466]
Level 3 (low-level) evidenceVergaro A, Rezková L, Fichtl M, Jedličková J, Ďuďáková Ľ, Růžičková E, Lišková P. PRIMARY OPEN-ANGLE GLAUCOMA DUE TO MUTATIONS IN THE MYOC GENE. Ceska a slovenska oftalmologie : casopis Ceske oftalmologicke spolecnosti a Slovenske oftalmologicke spolecnosti. 2022 Summer:78(5):242-248. doi: 10.31348/2022/25. Epub [PubMed PMID: 36220364]
Abu-Amero KK, Morales J, Aljasim LA, Edward DP. CYP1B1 Mutations are a Major Contributor to Juvenile-Onset Open Angle Glaucoma in Saudi Arabia. Ophthalmic genetics. 2015 Jun:36(2):184-7. doi: 10.3109/13816810.2013.841961. Epub 2013 Oct 7 [PubMed PMID: 24099281]
Reis LM, Maheshwari M, Capasso J, Atilla H, Dudakova L, Thompson S, Zitano L, Lay-Son G, Lowry RB, Black J, Lee J, Shue A, Kremlikova Pourova R, Vaneckova M, Skalicka P, Jedlickova J, Trkova M, Williams B, Richard G, Bachman K, Seeley AH, Costakos D, Glaser TM, Levin AV, Liskova P, Murray JC, Semina EV. Axenfeld-Rieger syndrome: more than meets the eye. Journal of medical genetics. 2023 Apr:60(4):368-379. doi: 10.1136/jmg-2022-108646. Epub 2022 Jul 26 [PubMed PMID: 35882526]
Singh P, Gupta A, Tripathy K. Iridocorneal Dysgenesis. StatPearls. 2024 Jan:(): [PubMed PMID: 36251848]
Gupta V, Birla S, Varshney T, Somarajan BI, Gupta S, Gupta M, Panigrahi A, Singh A, Gupta D. In vivo identification of angle dysgenesis and its relation to genetic markers associated with glaucoma using artificial intelligence. Indian journal of ophthalmology. 2024 Mar 1:72(3):339-346. doi: 10.4103/IJO.IJO_1456_23. Epub 2023 Dec 26 [PubMed PMID: 38146977]
Alwadani S, Alward WLM, Syed NA, Bouhenni RA, Brownstein S, Edward DP. Posterior Embryotoxon Revisited: An Immunohistologic Study. Ophthalmology. Glaucoma. 2022 Jul-Aug:5(4):396-401. doi: 10.1016/j.ogla.2022.01.003. Epub 2022 Feb 4 [PubMed PMID: 35131519]
Yan X, Wu S, Liu Q, Li Y, Zhu W, Zhang J. Accumulation of Asn450Tyr mutant myocilin in ER promotes apoptosis of human trabecular meshwork cells. Molecular vision. 2020:26():563-573 [PubMed PMID: 32818018]
Park SC, Kee C. Large diurnal variation of intraocular pressure despite maximal medical treatment in juvenile open angle glaucoma. Journal of glaucoma. 2007 Jan:16(1):164-8 [PubMed PMID: 17224768]
Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma: results from the Dallas Glaucoma Registry. Clinical ophthalmology (Auckland, N.Z.). 2013:7():1739-46. doi: 10.2147/OPTH.S45480. Epub 2013 Aug 28 [PubMed PMID: 24039394]
Terris M. Toward a new, "independent-cooperative model" of international health. Journal of public health policy. 1993 Autumn:14(3):265-75 [PubMed PMID: 8254004]
Bouhenni RA, Ricker I, Hertle RW. Prevalence and Clinical Characteristics of Childhood Glaucoma at a Tertiary Care Children's Hospital. Journal of glaucoma. 2019 Jul:28(7):655-659. doi: 10.1097/IJG.0000000000001259. Epub [PubMed PMID: 30950965]
Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, Garudadri C, Mandal AK. Profile of childhood glaucoma at a tertiary center in South India. Indian journal of ophthalmology. 2019 Mar:67(3):358-365. doi: 10.4103/ijo.IJO_786_18. Epub [PubMed PMID: 30777953]
Mokbel TH, El Hefney EM, Hagras SM, ALNagdy AA, Badawi AE, Kasem MA, El Shaer SM. Childhood glaucoma profile in Dakahelia, Egypt: a retrospective study. International journal of ophthalmology. 2018:11(4):674-680. doi: 10.18240/ijo.2018.04.23. Epub 2018 Apr 18 [PubMed PMID: 29675390]
Level 2 (mid-level) evidenceGupta V, Somarajan BI, Gupta S, Walia GK, Singh A, Sofi R, Chaudhary RS, Sharma A. The mutational spectrum of Myocilin gene among familial versus sporadic cases of Juvenile onset open angle glaucoma. Eye (London, England). 2021 Feb:35(2):400-408. doi: 10.1038/s41433-020-0850-z. Epub 2020 Apr 16 [PubMed PMID: 32300215]
Level 3 (low-level) evidenceElgin U, Şen E, Uzel M, Yılmazbaş P. Comparison of Refractive Status and Anterior Segment Parameters of Juvenile Open-Angle Glaucoma and Normal Subjects. Turkish journal of ophthalmology. 2018 Dec 27:48(6):295-298. doi: 10.4274/tjo.68915. Epub [PubMed PMID: 30605935]
Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin AV. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Current opinion in ophthalmology. 2018 Sep:29(5):385-394. doi: 10.1097/ICU.0000000000000516. Epub [PubMed PMID: 30096087]
Level 3 (low-level) evidenceWiggs JL, Del Bono EA, Schuman JS, Hutchinson BT, Walton DS. Clinical features of five pedigrees genetically linked to the juvenile glaucoma locus on chromosome 1q21-q31. Ophthalmology. 1995 Dec:102(12):1782-9 [PubMed PMID: 9098278]
Birla S, Gupta D, Somarajan BI, Gupta S, Chaurasia AK, Kishan A, Gupta V. Classifying juvenile onset primary open angle glaucoma using cluster analysis. The British journal of ophthalmology. 2020 Jun:104(6):827-835. doi: 10.1136/bjophthalmol-2019-314660. Epub 2019 Sep 28 [PubMed PMID: 31563868]
Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR. Primary open-angle glaucoma. Nature reviews. Disease primers. 2016 Sep 22:2():16067. doi: 10.1038/nrdp.2016.67. Epub 2016 Sep 22 [PubMed PMID: 27654570]
Karaconji T, Zagora S, Grigg JR. Approach to childhood glaucoma: A review. Clinical & experimental ophthalmology. 2022 Mar:50(2):232-246. doi: 10.1111/ceo.14039. Epub 2022 Jan 25 [PubMed PMID: 35023613]
Balikov DA, Jacobson A, Prasov L. Glaucoma Syndromes: Insights into Glaucoma Genetics and Pathogenesis from Monogenic Syndromic Disorders. Genes. 2021 Sep 11:12(9):. doi: 10.3390/genes12091403. Epub 2021 Sep 11 [PubMed PMID: 34573386]
Gupta V, Chaurasia AK, Gupta S, Gorimanipalli B, Sharma A, Gupta A. In Vivo Analysis of Angle Dysgenesis in Primary Congenital, Juvenile, and Adult-Onset Open Angle Glaucoma. Investigative ophthalmology & visual science. 2017 Nov 1:58(13):6000-6005. doi: 10.1167/iovs.17-22695. Epub [PubMed PMID: 29183046]
Sihota R, Sidhu T, Dada T. The role of clinical examination of the optic nerve head in glaucoma today. Current opinion in ophthalmology. 2021 Mar 1:32(2):83-91. doi: 10.1097/ICU.0000000000000734. Epub [PubMed PMID: 33470671]
Level 3 (low-level) evidenceGupta V, James MK, Singh A, Kumar S, Gupta S, Sharma A, Sihota R, Kennedy DJ. Differences in Optic Disc Characteristics of Primary Congenital Glaucoma, Juvenile, and Adult Onset Open Angle Glaucoma Patients. Journal of glaucoma. 2016 Mar:25(3):239-43. doi: 10.1097/IJG.0000000000000154. Epub [PubMed PMID: 25265002]
Level 2 (mid-level) evidenceAbdelrahman AM, Eltanamly RM, Elsanabary Z, Hassan LM. Optical coherence tomography angiography in juvenile open angle glaucoma: correlation between structure and perfusion. International ophthalmology. 2021 Mar:41(3):883-889. doi: 10.1007/s10792-020-01643-7. Epub 2020 Nov 13 [PubMed PMID: 33185822]
Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. European journal of ophthalmology. 2005 Mar-Apr:15(2):196-201 [PubMed PMID: 15812759]
Khalil AK. The Changing Face of the Cornea in a Case of Juvenile Glaucoma and Subclinical Keratoconus. Klinische Monatsblatter fur Augenheilkunde. 2022 Aug 4:():. doi: 10.1055/a-1819-1412. Epub 2022 Aug 4 [PubMed PMID: 35388451]
Level 3 (low-level) evidenceBoese EA, Alward WLM, Fingert JH. Gonioscopy-Assisted Transluminal Trabeculotomy for Myocilin Juvenile Glaucoma. Ophthalmology. Glaucoma. 2022 May-Jun:5(3):369-370. doi: 10.1016/j.ogla.2021.07.003. Epub 2021 Jul 14 [PubMed PMID: 34273563]
Marques AM, Ananina G, Costa VP, de Vasconcellos JPC, de Melo MB. Estimating the age of the p.Cys433Arg variant in the MYOC gene in patients with primary open-angle glaucoma. PloS one. 2018:13(11):e0207409. doi: 10.1371/journal.pone.0207409. Epub 2018 Nov 16 [PubMed PMID: 30444892]
Jayaram H, Kolko M, Friedman DS, Gazzard G. Glaucoma: now and beyond. Lancet (London, England). 2023 Nov 11:402(10414):1788-1801. doi: 10.1016/S0140-6736(23)01289-8. Epub 2023 Sep 21 [PubMed PMID: 37742700]
Geyer O, Mathalone N, Wolf A, Melamud A. [CHILDHOOD GLAUCOMA]. Harefuah. 2019 Feb:158(2):115-120 [PubMed PMID: 30779490]
Micheal S, Hogewind BF, Khan MI, Siddiqui SN, Zafar SN, Akhtar F, Qamar R, Hoyng CB, den Hollander AI. Variants in the PRPF8 Gene are Associated with Glaucoma. Molecular neurobiology. 2018 May:55(5):4504-4510. doi: 10.1007/s12035-017-0673-5. Epub 2017 Jul 13 [PubMed PMID: 28707069]
Achigbu EO, Oguego NC, Achigbu K. Spectrum of Eye Disorders Seen in a Pediatric Eye Clinic South East Nigeria. Nigerian journal of surgery : official publication of the Nigerian Surgical Research Society. 2017 Jul-Dec:23(2):125-129. doi: 10.4103/njs.NJS_37_16. Epub [PubMed PMID: 29089738]
Lopes NL, Gracitelli CPB, Rolim-de-Moura C. Childhood Glaucoma Profile in a Brazilian Tertiary Care Center Using Childhood Glaucoma Research Network Classification. Journal of glaucoma. 2021 Feb 1:30(2):129-133. doi: 10.1097/IJG.0000000000001712. Epub [PubMed PMID: 33086262]
Roberti G, Oddone F, Agnifili L, Katsanos A, Michelessi M, Mastropasqua L, Quaranta L, Riva I, Tanga L, Manni G. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Survey of ophthalmology. 2020 Jul-Aug:65(4):458-472. doi: 10.1016/j.survophthal.2020.01.002. Epub 2020 Feb 11 [PubMed PMID: 32057761]
Level 3 (low-level) evidenceZeppieri M, Gurnani B. Applanation Tonometry. StatPearls. 2024 Jan:(): [PubMed PMID: 35881737]
Salvetat ML, Zeppieri M, Tosoni C, Brusini P. Comparisons between Pascal dynamic contour tonometry, the TonoPen, and Goldmann applanation tonometry in patients with glaucoma. Acta ophthalmologica Scandinavica. 2007 May:85(3):272-9 [PubMed PMID: 17488456]
Pasaoglu I, Basarir B. Comparison of anterior chamber angle parameters and iris structure of juvenile open-angle glaucoma and pigmentary glaucoma. Indian journal of ophthalmology. 2022 Feb:70(2):558-563. doi: 10.4103/ijo.IJO_2012_21. Epub [PubMed PMID: 35086237]
Umfress AC, Mawn LA, Joos KM. Short-term Optic Disc Cupping Reversal in a Patient With Mild Juvenile Open-angle Glaucoma Due to Early Idiopathic Intracranial Hypertension. Journal of glaucoma. 2019 Apr:28(4):e53-e57. doi: 10.1097/IJG.0000000000001151. Epub [PubMed PMID: 30531192]
Gupta V, Ganesan VL, Kumar S, Chaurasia AK, Malhotra S, Gupta S. Visual Disability Among Juvenile Open-angle Glaucoma Patients. Journal of glaucoma. 2018 Apr:27(4):e87-e89. doi: 10.1097/IJG.0000000000000887. Epub [PubMed PMID: 29394204]
Gupta S, Singh A, Mahalingam K, Selvan H, Gupta P, Pandey S, Somarajan BI, Gupta V. Myopia and glaucoma progression among patients with juvenile onset open angle glaucoma: A retrospective follow up study. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2021 May:41(3):475-485. doi: 10.1111/opo.12805. Epub 2021 Apr 7 [PubMed PMID: 33826775]
Level 2 (mid-level) evidenceNadeem S. Choroidal thickness in juvenile open angle glaucoma: insights from a south asian case-control study. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2024 May 14:():. doi: 10.1007/s00417-024-06495-w. Epub 2024 May 14 [PubMed PMID: 38743094]
Level 2 (mid-level) evidenceStangos AN, Whatham AR, Sunaric-Megevand G. Primary viscocanalostomy for juvenile open-angle glaucoma. American journal of ophthalmology. 2005 Sep:140(3):490-6 [PubMed PMID: 16084786]
Urbak SF. Ultrasound biomicroscopical study of the irido-corneal angle in dominant juvenile open-angle glaucoma, in POAG, and in normal eyes. Acta ophthalmologica Scandinavica. 1999 Apr:77(2):160-4 [PubMed PMID: 10321531]
Senger C, Moreto R, Watanabe SES, Matos AG, Paula JS. Electrophysiology in Glaucoma. Journal of glaucoma. 2020 Feb:29(2):147-153. doi: 10.1097/IJG.0000000000001422. Epub [PubMed PMID: 31809397]
Verma IC, Paliwal P, Singh K. Genetic Testing in Pediatric Ophthalmology. Indian journal of pediatrics. 2018 Mar:85(3):228-236. doi: 10.1007/s12098-017-2453-7. Epub 2017 Oct 2 [PubMed PMID: 28971364]
Kumar A, Han Y, Oatts JT. Genetic changes and testing associated with childhood glaucoma: A systematic review. PloS one. 2024:19(2):e0298883. doi: 10.1371/journal.pone.0298883. Epub 2024 Feb 22 [PubMed PMID: 38386645]
Level 1 (high-level) evidenceJohnson AT, Drack AV, Kwitek AE, Cannon RL, Stone EM, Alward WL. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology. 1993 Apr:100(4):524-9 [PubMed PMID: 8479711]
Level 3 (low-level) evidenceTalbot AW, Russell-Eggitt I. Pharmaceutical management of the childhood glaucomas. Expert opinion on pharmacotherapy. 2000 May:1(4):697-711 [PubMed PMID: 11249511]
Level 3 (low-level) evidenceIkeda H, Ishigooka H, Muto T, Tanihara H, Nagata M. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Archives of ophthalmology (Chicago, Ill. : 1960). 2004 Aug:122(8):1122-8 [PubMed PMID: 15302651]
Level 2 (mid-level) evidenceGupta V, Ghosh S, Sujeeth M, Chaudhary S, Gupta S, Chaurasia AK, Sihota R, Gupta A, Kapoor KS. Selective laser trabeculoplasty for primary open-angle glaucoma patients younger than 40 years. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Feb:53(1):81-85. doi: 10.1016/j.jcjo.2017.07.023. Epub 2017 Sep 25 [PubMed PMID: 29426447]
Pathania D, Senthil S, Rao HL, Mandal AK, Garudadari CS. Outcomes of trabeculectomy in juvenile open angle glaucoma. Indian journal of ophthalmology. 2014 Feb:62(2):224-8. doi: 10.4103/0301-4738.101074. Epub [PubMed PMID: 23571250]
Level 2 (mid-level) evidenceTsai JC, Chang HW, Kao CN, Lai IC, Teng MC. Trabeculectomy with mitomycin C versus trabeculectomy alone for juvenile primary open-angle glaucoma. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2003 Jan-Feb:217(1):24-30 [PubMed PMID: 12566869]
Level 2 (mid-level) evidenceIshida K, Mandal AK, Netland PA. Glaucoma drainage implants in pediatric patients. Ophthalmology clinics of North America. 2005 Sep:18(3):431-42, vii [PubMed PMID: 16055000]
Balekudaru S, Vadalkar J, George R, Vijaya L. The use of Ahmed glaucoma valve in the management of pediatric glaucoma. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2014 Aug:18(4):351-6. doi: 10.1016/j.jaapos.2014.03.013. Epub [PubMed PMID: 25173898]
Ah-Chan JJ, Molteno AC, Bevin TH, Herbison P. Otago Glaucoma Surgery Outcome Study: follow-up of young patients who underwent Molteno implant surgery. Ophthalmology. 2005 Dec:112(12):2137-42 [PubMed PMID: 16325709]
Dhingra D, Bhartiya S. Evaluating glaucoma surgeries in the MIGS context. Romanian journal of ophthalmology. 2020 Apr-Jun:64(2):85-95 [PubMed PMID: 32685772]
Oseni J, Laroche D. Cataract surgery and Hydrus stent implantation in juvenile open-angle glaucoma: A case report. Journal of the National Medical Association. 2022 Dec:114(6):584-588. doi: 10.1016/j.jnma.2022.09.004. Epub 2022 Sep 24 [PubMed PMID: 36167750]
Level 3 (low-level) evidenceLamoureux EL, Mcintosh R, Constantinou M, Fenwick EK, Xie J, Casson R, Finkelstein E, Goldberg I, Healey P, Thomas R, Ang GS, Pesudovs K, Crowston J. Comparing the effectiveness of selective laser trabeculoplasty with topical medication as initial treatment (the Glaucoma Initial Treatment Study): study protocol for a randomised controlled trial. Trials. 2015 Sep 11:16():406. doi: 10.1186/s13063-015-0924-6. Epub 2015 Sep 11 [PubMed PMID: 26362541]
Level 1 (high-level) evidenceMohan N, Chakrabarti A, Nazm N, Mehta R, Edward DP. Newer advances in medical management of glaucoma. Indian journal of ophthalmology. 2022 Jun:70(6):1920-1930. doi: 10.4103/ijo.IJO_2239_21. Epub [PubMed PMID: 35647957]
Level 3 (low-level) evidenceMandal AK, Chakrabarti D, Gothwal VK. Approach to primary congenital glaucoma: A perspective. Taiwan journal of ophthalmology. 2023 Oct-Dec:13(4):451-460. doi: 10.4103/tjo.TJO-D-23-00104. Epub 2023 Oct 19 [PubMed PMID: 38249492]
Level 3 (low-level) evidenceFeroze KB, Zeppieri M, Khazaeni L. Steroid-Induced Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28613653]
Zeppieri M, Musa M. Beyond the Dusty Fog: Local Eye Drop Therapy and Potentially New Treatment Alternatives in Pseudoexfoliative Glaucoma. Current medicinal chemistry. 2024:31(13):1608-1619. doi: 10.2174/0109298673255220231010073215. Epub [PubMed PMID: 37855339]
Zeppieri M. Pigment dispersion syndrome: A brief overview. Journal of clinical and translational research. 2022 Oct 31:8(5):344-350 [PubMed PMID: 36518550]
Level 3 (low-level) evidencevan Meerwijk CLLI, Jansonius NM, Los LI. Uveitic glaucoma in children: a systematic review on surgical outcomes. Journal of ophthalmic inflammation and infection. 2022 Nov 7:12(1):35. doi: 10.1186/s12348-022-00313-2. Epub 2022 Nov 7 [PubMed PMID: 36344704]
Level 1 (high-level) evidenceNg JK, Lau O. Traumatic Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 36251842]
Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Progress in retinal and eye research. 2021 Jul:83():100916. doi: 10.1016/j.preteyeres.2020.100916. Epub 2020 Oct 17 [PubMed PMID: 33075485]
Quigley HA. Glaucoma. Lancet (London, England). 2011 Apr 16:377(9774):1367-77. doi: 10.1016/S0140-6736(10)61423-7. Epub 2011 Mar 30 [PubMed PMID: 21453963]
Tripathy K, Salini B. Aniridia. StatPearls. 2024 Jan:(): [PubMed PMID: 30844160]
Yeom S, Comi AM. Updates on Sturge-Weber Syndrome. Stroke. 2022 Dec:53(12):3769-3779. doi: 10.1161/STROKEAHA.122.038585. Epub 2022 Oct 20 [PubMed PMID: 36263782]
Lee HS, Park SW, Heo H. Megalopapilla in children: a spectral domain optical coherence tomography analysis. Acta ophthalmologica. 2015 Jun:93(4):e301-5. doi: 10.1111/aos.12545. Epub 2014 Sep 1 [PubMed PMID: 25178150]
Kletke SN, Mills MD, Tomlinson LA, Yu Y, Ying GS, Binenbaum G. Pediatric glaucoma suspects: characteristics and outcomes. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2022 Oct:26(5):236.e1-236.e6. doi: 10.1016/j.jaapos.2022.05.010. Epub 2022 Sep 14 [PubMed PMID: 36113699]
Ichhpujani P, Kaushik S, Gupta A, Pandav SS. Bilateral Chandler's syndrome: Uncommon entity diagnosed by ultrasound biomicroscopy and confocal microscopy. Indian journal of ophthalmology. 2020 Mar:68(3):528-529. doi: 10.4103/ijo.IJO_1123_19. Epub [PubMed PMID: 32057025]
Koraszewska-Matuszewska B, Samochowiec-Donocik E, Filipek E. [Prognosis in juvenile glaucoma after trabeculectomy]. Klinika oczna. 2002:104(2):115-8 [PubMed PMID: 12174451]
Seresirikachorn K, Thiamthat W, Annopawong K, Wanichwecharungruang B, Friedman DS, Vu DM. Treatment Outcomes for Juvenile Open Angle Glaucoma in Thailand. Journal of glaucoma. 2023 Nov 1:32(11):976-982. doi: 10.1097/IJG.0000000000002309. Epub 2023 Sep 12 [PubMed PMID: 37725790]
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14:311(18):1901-11. doi: 10.1001/jama.2014.3192. Epub [PubMed PMID: 24825645]