Introduction
Acute stroke is the acute onset of focal neurological deficits in a vascular territory affecting the brain, retina, or spinal cord due to underlying cerebrovascular diseases.[1] Stroke is prevalent across patient populations and can significantly cause morbidity and mortality. Strokes are categorized as ischemic and hemorrhagic. Hemorrhagic strokes can further be classified as intracerebral and subarachnoid hemorrhage. Among ischemic strokes, the Trial Org 10172 in Acute Stroke Treatment (TOAST) classification is used to subdivide the categories that include the following:
- Cardioembolism
- Small vessel occlusion
- Large artery atherosclerosis
- Stroke of undetermined etiology
- Stroke of other determined etiology (possible or probable depending on the results of ancillary studies) [2]
A crucial aspect of classification involves the physician's ability to designate a specific subtype diagnosis as probable or possible, depending on the level of certainty. A diagnosis is considered "probable" when clinical findings, neuroimaging data, and results of diagnostic studies align with a particular subtype, and other potential causes have been ruled out. On the other hand, a diagnosis is labeled as "possible" when clinical findings and neuroimaging data suggest a specific subtype, but additional studies have not been conducted. Given that many patients undergo a limited number of diagnostic tests, the categorizations of probable and possible allow the physician to establish as precise a subgroup diagnosis as possible.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of ischemic stroke is a thrombotic or embolic event that causes an impairment of blood flow to an area of the brain. In a thrombotic event, the blood flow to the brain is obstructed within the blood vessel due to a thrombus (clot) within the vessel itself, usually secondary to atherosclerotic disease, arterial dissection, fibromuscular dysplasia, or inflammatory conditions. In an embolic event, debris from elsewhere in the body blocks blood flow through the affected vessel. The source of emboli can be the proximal artery, such as an atherosclerotic plaque in the internal carotid artery, causing an artery-to-artery embolic stroke distally from any proximal source, commonly from the heart. Occasionally, the source may be from the right side of the circulation, going through a right-to-left shunt, such as a patent foramen ovale, towards the cerebral arterial system. The etiology of stroke affects both prognosis and outcomes.[3][4]
Cardioembolism: This group comprises patients with arterial blockages likely caused by an embolism originating in the heart. Cardiac sources are categorized into high-risk and medium-risk groups based on their propensity for embolism. At least 1 cardiac source of an embolus must be identified to consider a possible or probable diagnosis of cardioembolic stroke. Clinical and brain imaging findings resemble those described for large-artery atherosclerosis. Evidence of a previous transient ischemic attack (TIA) or stroke in more than 1 vascular territory or systemic embolisms supports a clinical diagnosis of cardiogenic stroke. Possible large artery atherosclerotic sources of thrombosis or embolism should be ruled out. A stroke occurring in a patient with a medium-risk cardiac source of embolism and no other apparent cause of stroke is categorized as a possible cardioembolic stroke.[5]
Large artery atherosclerosis: These patients will exhibit clinical and brain imaging findings indicating either significant (> 50%) narrowing or complete blockage of a major brain artery or branch cortical artery, likely due to atherosclerosis. Clinical manifestations may include symptoms of cerebral cortical impairment (eg, aphasia, neglect, or limited motor function) or dysfunction of the brainstem or cerebellum. The presence of intermittent claudication history, TIAs in the same vascular territory, a carotid bruit, or weakened pulses can aid in confirming the clinical diagnosis. Lesions in the cortex, cerebellum, brainstem, or subcortical hemispheric infarctions larger than 1.5 cm in diameter observed on computed tomography (CT) or magnetic resonance imaging (MRI) indicate potential large artery atherosclerotic origin. Additional supportive evidence through duplex imaging or arteriography demonstrating more than 50% stenosis of a relevant intracranial or extracranial artery is necessary. Diagnostic evaluations should rule out potential sources of cardiogenic embolism. A diagnosis of stroke attributed to large artery atherosclerosis cannot be established if duplex or arteriographic studies appear normal or only reveal minimal changes.[6]
Small vessel occlusion: This category encompasses patients with strokes commonly classified as lacunar infarcts in other systems. Patients in this group should present with 1 of the typical clinical lacunar syndromes and should not exhibit signs of cerebral cortical dysfunction. A history of diabetes mellitus or hypertension supports the clinical diagnosis. Additionally, patients should have normal CT scan or MRI findings or demonstrate a relevant brainstem or subcortical hemisphere lesion with a diameter of <1.5 cm. The absence of potential cardiac sources for embolism is expected, and evaluation of the major extracranial arteries should not reveal stenosis >50% in an artery on the same side.[7]
Stroke of undetermined etiology: In numerous cases, determining the cause of a stroke proves challenging. Some patients undergo extensive evaluation yet yield no likely etiology. Others undergo only a cursory evaluation, resulting in an undetermined cause. This category also encompasses patients with 2 or more potential causes of stroke, making it difficult for physicians to reach a definitive diagnosis. For instance, a patient presenting with a medium-risk cardiac source of embolism alongside another potential cause of stroke would be classified as having a stroke of undetermined etiology. Similarly, a patient with atrial fibrillation and ipsilateral carotid stenosis of 50%, or one with a traditional lacunar syndrome and ipsilateral carotid stenosis of 50%, would fall into this category.[8]
Stroke of other determined etiology: This group consists of patients with uncommon stroke causes, such as nonatherosclerotic vasculopathies, hypercoagulable states, or hematologic disorders. Patients in this category should exhibit clinical symptoms and CT or MRI findings indicative of acute ischemic stroke, regardless of the size or location of the lesion. Diagnostic tests, such as blood tests or arteriography, should reveal 1 of these unusual stroke causes. Other studies should be conducted to rule out cardiac sources of embolism and large artery atherosclerosis.[9]
Epidemiology
In 2021, stroke accounted for 1 in 6 deaths from cardiovascular disease, with someone in the US experiencing a stroke every 40 seconds, resulting in a stroke-related death every 3 minutes and 14 seconds.[10] Annually, over 795,000 Americans suffer from a stroke, of which approximately 610,000 are initial occurrences.[10] Nearly a quarter of these strokes, roughly 185,000 cases, occur in individuals with a history of previous strokes. Ischemic strokes, blocking blood flow to the brain, constitute about 87% of all strokes. According to the Framingham Heart Study, stroke incidence has declined. However, the cohort was predominantly a White population.[11][12][13]
The financial burden of stroke in the US amounted to nearly $56.5 billion between 2018 and 2019, covering healthcare expenses, medication, and lost productivity due to missed workdays. Stroke stands as a primary contributor to severe long-term disability, particularly affecting mobility in over half of stroke survivors aged 65 and older.[14]
Disparities in stroke incidence and outcomes exist across racial and ethnic groups, with non-Hispanic Black adults facing nearly twice the risk of a first stroke compared to White adults, and both non-Hispanic Black and Pacific Islander adults exhibiting the highest stroke-related mortality rates. Moreover, the death rate attributed to stroke rose from 38.8 per 100,000 in 2020 to 41.1 per 100,000 in 2021.[15]
Pathophysiology
In thrombosis, an obstructive process prevents blood flow to regions of the brain. The most common risk factor is large vessel atherosclerosis. Other risk factors include vasculitides and arterial dissection.
Embolic events occur when a clot originates from another location in the body. Most commonly, the clot's source is the heart's valve or chambers, for example, when a clot forms within the atria in atrial fibrillation and dislodges into the arterial vascular supply. Less frequent sources include venous, septic, air, or fat emboli.
Lacunar infarcts are usually seen in the subcortical areas of the brain supplied by small penetrating or perforating arteries, usually without collaterals. These include the lenticulostriate arteries from the middle cerebral artery, the thalamic perforators from the posterior cerebral artery, and the paramedian branches from the basilar artery. The underlying pathology of these penetrating arteries is small vessel arteriolosclerosis caused by hypertension, aging, smoking, diabetes, and other conventional vascular risk factors.[16]
Cerbral Autoregulation
Under physiological conditions, cerebral blood flow is primarily regulated by the resistance within the cerebral blood vessels, which correlates directly with their diameter. Vasodilation results in increased blood volume within the brain and heightened cerebral blood flow, while vasoconstriction produces the opposite effect.[17] Additionally, cerebral blood flow is influenced by fluctuations in cerebral perfusion pressure.
Cerebral autoregulation denotes the ability to maintain relatively stable cerebral blood flow despite moderate shifts in perfusion pressure.[17][18] The precise mechanisms underlying autoregulation remain incompletely understood and likely involve multiple pathways. Evidence suggests that the smooth muscle in cerebral vessels reacts directly to changes in perfusion pressure by contracting with pressure elevation and relaxing with pressure reduction. Furthermore, decreases in cerebral blood flow may prompt blood vessel dilation by releasing vasoactive substances, although the specific molecules responsible have yet to be identified. Nitric oxide released by endothelial cells also appears to contribute to autoregulation.
Ordinarily, cerebral blood flow regulation through autoregulation operates within a mean arterial pressure (MAP) range of 60 to 150 mm Hg, albeit with individual variations in upper and lower limits. Beyond this range, the brain's ability to compensate for perfusion pressure changes diminishes, causing cerebral blood flow to rise or fall passively in response to pressure fluctuations. This passive response poses a risk of ischemia at low pressures and edema at high pressures.
During certain pathological conditions, such as ischemic stroke, cerebral autoregulation becomes impaired. As cerebral perfusion pressure declines, cerebral blood vessels dilate to augment cerebral blood flow.[19] However, if the decrease in perfusion pressure exceeds the brain's compensatory capacity, cerebral blood flow diminishes. Initially, an increase in the oxygen extraction fraction occurs to sustain oxygen delivery to the brain. Subsequently, as cerebral blood flow continues to decrease, additional mechanisms come into play.
Protein synthesis is inhibited below a cerebral blood flow rate of 50 mL/100 g/min. Protein synthesis ceases altogether at 35 mL/100 g/min, and there is a transient increase in glucose utilization. When cerebral blood flow drops to 25 mL/100 g/min, glucose utilization declines significantly, and anaerobic glycolysis ensues, resulting in tissue acidosis due to lactic acid accumulation. Neuronal electrical failure occurs at a cerebral blood flow of 16 to 18 mL/100 g/min, followed by failure of membrane ion homeostasis at 10 to 12 mL/100 g/min.[19] This threshold typically marks the onset of infarction.
In hypertensive individuals, autoregulation adapts to operate at higher arterial pressures. Lowering blood pressure to normal levels in such individuals could exacerbate autoregulation dysfunction during stroke, leading to further reductions in cerebral blood flow.
Concept of the Ischemic Penumbra
During an acute ischemic stroke, the brain tissue that relies exclusively on 1 artery for its blood supply will suffer an infarct, forming what is known as the infarct core.[20] Surrounding this core is an area of brain tissue called the ischemic penumbra, which maintains some blood supply through collateral circulation. However, as swelling from the infarction grows, the penumbra diminishes, and the infarct core expands. Under normal conditions, cerebral perfusion is approximately 50 mL/100 g/min. Brain cells begin to die when perfusion drops below 30%, equivalent to <15 mL/100 g/min. Hence, when blood flow is reduced but remains above 30% of the normal rate, the brain tissue is ischemic but not infarcted, highlighting the critical principle that "time is brain." This principle underscores the importance of timely revascularization treatments in acute ischemic stroke, given the distinct time windows for intervention based on these physiological insights.[21][22][23]
Ischemic Stroke Syndromes
Ischemic strokes can present in predetermined syndromes due to decreased blood flow to particular areas of the brain that correlate to exam findings—allowing clinicians to predict the site of the brain vasculature that can be affected.
Middle Cerebral Artery (MCA) Infarction
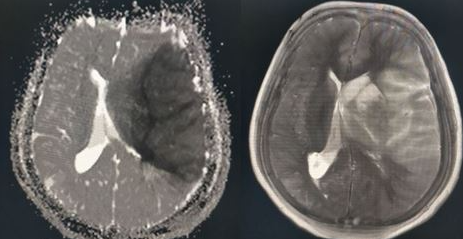
The MCA is the most common artery involved in stroke (see Images. Left MCA Territory Infarction and Right-Sided MDA 'Cord Sign' Harbingering Acute Infection). The MCA is divided into 4 segments (M1, M2, M3, and M4) and supplies a large area of the lateral surface of the brain, part of the basal ganglia, and the internal capsule. The M1 (horizontal) segment gives off the lenticulostriate arteries, which supply the basal ganglia and internal capsule. The M1 segment continues to the M2 (Sylvian) segment and supplies the insula, superior temporal lobe, parietal lobe, and inferolateral frontal lobe.[24]
The MCA distribution involves the lateral cerebral cortex. MCA syndrome is best explained by understanding the homunculus of the cerebral cortex, in which the lateral portion contains motor and sensory functions that involve the face and upper extremities. MCA infarctions classically present with contralateral hemiparesis, facial paralysis, and sensory loss in the face and upper extremities. The lower extremities may be involved, especially when the deep brain structures are involved, but upper extremity symptoms usually predominate. In addition, gaze preferences towards the side of the lesion may be seen. Additional symptoms include the following:
- Dysarthria is characterized by difficulty phonating due to the physical weakness of the facial muscles. Dysarthria does not have much localizing value in acute stroke, as both cortical and subcortical infarcts can cause dysarthria. Dominant and nondominant hemisphere strokes can also cause dysarthria. The most severe form of dysarthria is anarthria, during which the patient will have no speech output. Dysarthria is often misinterpreted as aphasia.
- Neglect is when the patient seems to "ignore" a hemisphere of their world due to an inability to see that area. Neglect is a nondominant cortical sensory dysfunction. Extinction or inability to perceive double simultaneous stimuli is a bedside test commonly performed for a nondominant cortical lesion.
- Visual field loss occurs in MCA infarcts because the branches of the MCA supply the optic radiations. A more limited ischemic stroke affecting the parietal lobe may cause contralateral inferior quadrantanopia. In contrast, a limited temporal lobe infarct will result in a contralateral superior quadrantanopia (pie-in-the-sky).
- Aphasia, or the inability to produce or understand language, is caused by injury to the language areas of the dominant hemisphere of the brain, which are supplied by the dominant MCA.
Anterior Cerebral Artery (ACA) Infarction
The ACA supplies blood to the medial areas of the frontal, prefrontal, primary motor, primary sensory, and supplemental motor cortices. These are areas corresponding to the lower extremities in the cortical homunculus. Pure ACA infarcts are uncommon because of the good collateral blood supply. The sensory and motor cortices receive sensory information and control the contralateral lower extremity movement. The ACA distribution involves the medial cerebral cortex. The clinical presentation of an ACA infarction includes contralateral sensory and motor deficits in the lower extremity. The upper extremity and face are spared.[25]
Posterior Cerebral Artery (PCA) Infarction
The superficial PCA supplies the occipital lobe and the medial portion of the temporal lobe. The deep PCA supplies the thalamus and other posterior deep structures of the brain. The occipital lobe is the location of the visual cortex. The thalamus is the main relaying center for ascending and descending nervous networks. The most common cause of a PCA infarct is an atherothrombotic lesion in the vertebral artery.[26]
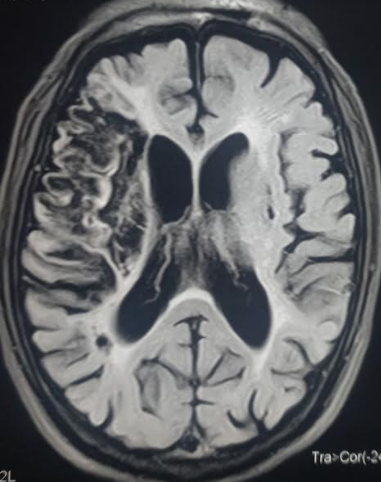
PCA infarctions can be divided into deep and superficial categories based on the PCA supply. If the deep segments of the PCA are involved, the symptoms are mainly related to thalamic dysfunction. The symptoms may include hypersomnolence, cognitive deficits, ocular findings, hypoesthesia, and ataxia. Larger infarcts that involve the deep structures can lead to hemisensory loss and hemiparesis due to the involvement of the thalamus and the adjacent internal capsule. Superficial infarcts present mainly with contralateral homonymous hemianopia, often with macular sparing. Rarely, bilateral PCA infarcts present with amnesia and cortical blindness.[27][28] In many posterior circulation strokes, symptomatology can be subtle; therefore, healthcare providers should have a low index of suspicion and obtain imaging and neurology consultations earlier (see Image. Axial Section on Noncontrast CT Head Shows Left PCA Ischemic Stroke and No Hemorrhage).[29]
Vertebrobasilar Infarction
The vertebrobasilar region of the brain is supplied by the 2 vertebral arteries and the basilar artery as they course the anterior surface of the pons. The vertebral arteries arise from their respective subclavian artery, course through the transverse foramina of the upper 6 cervical vertebrae, and enter the skull through the foramen magnum. The main branches of each vertebral artery are the posterior cerebellar artery (PICA) and the anterior spinal artery. The vertebral arteries terminate by merging to form the basilar artery. The basilar artery bifurcates into 2 posterior cerebral arteries, which join the circle of Willis. In addition to the paramedian penetrating branches, the basilar artery gives rise to the anterior inferior cerebellar artery and the superior cerebellar artery. These arteries supply the cerebellum and brainstem.[30]
The clinical presentation includes ataxia, vertigo, headache, vomiting, oropharyngeal dysfunction, visual field deficits, and abnormal oculomotor findings. Patterns of clinical presentation vary depending on the location and the infarction pattern of embolism or atherosclerosis. [31][32]
Cerebellar Infarction
Patients may present with ataxia, nausea, vomiting, headache, dysarthria, and vertigo symptoms. In addition, edema and rapid clinical deterioration can complicate cerebellar infarction.[31]
Lacunar Infarction
Lacunar infarcts result from the occlusion of small perforating or penetrating arteries. By definition, these infarcts are smaller than 1 centimeter in diameter. See StatPearls' companion reference, "Lacunar Stroke," for more information.[33] Lacunar infarction can present with a pure motor or sensory loss, sensorimotor deficit, or ataxic-hemiparesis.[34][35][36]
History and Physical
Ischemic strokes manifest suddenly, making it crucial to determine the time when symptoms first appeared. If the exact moment of symptom onset is unknown, clinicians use the time the patient was last observed in their usual state of health, free from new neurological symptoms, as a reference point. This established timeline is critical for deciding the appropriateness of administering intravenous (IV) thrombolytics. Another vital aspect of the clinical assessment involves investigating potential underlying causes to anticipate the stroke's mechanism. Factors to consider include common vascular risk factors such as hypertension, a history of stroke or TIAs, smoking, and diabetes. Additionally, a history of cardiac diseases, notably atrial fibrillation, recent myocardial infarction, and cardiomyopathy, should be ascertained by the treating clinician. Factors like a history of neck injury, recent chiropractic manipulations, and signs of hypercoagulopathy also play a significant role in the evaluation.[37]
A neurological examination is crucial for all patients suspected of having a stroke. Monitoring vital signs and heart rhythms is essential, as is listening for a neck bruit, which can indicate vascular abnormalities. The National Institutes of Health Stroke Scale (NIHSS) [See NIH Stroke Scale] is the standard tool for assessing stroke severity, featuring 11 categories and scores ranging from 0 to 42.[38][39] These categories include the level of consciousness (LOC) instructions, LOC questions, LOC commands, gaze direction, vision, facial symmetry, arm and leg motor skills, limb coordination, sensory perception, language abilities, speech clarity, and attention to both sides of the body (see Table 1. National Institutes of Health Stroke Scale). The stroke scale should be performed in a specified order, basing each score on the patient's performance during the examination rather than on predicted abilities.
Table 1. National Institutes of Health Stroke Scale
| Category | Score Meaning | Score |
| 1a. LOC Instructions | 0: Fully alert; 1: Not fully alert, but not drowsy; 2: Obtunded or requires minor stimulation to stay alert; 3: Unresponsive or requires repeated stimulation | |
| 1b. LOC Questions | 0: Answers both questions correctly; 1: Answers 1 question correctly; 2: Answers neither question correctly | |
| 1c. LOC Commands | 0: Performs both tasks correctly; 1: Performs 1 task correctly; 2: Performs neither task correctly | |
| 2. Best Gaze | 0: Normal; 1: Partial gaze palsy; 2: Forced deviation | |
| 3. Visual Field Testing | 0: No visual loss; 1: Partial hemianopia; 2: Complete hemianopia; 3: Bilateral hemianopia (blind including cortical blindness) | |
| 4. Facial Palsy | 0: Normal symmetrical movements; 1: Minor paralysis; 2: Partial paralysis; 3: Complete paralysis of 1 or both sides | |
| 5. Motor Arm (score both left and right) | 0: No drift; 1: Drifts down; 2: Some effort against gravity; 3: No effort against gravity; 4: No movement | |
| 6. Motor Leg (score both left and right) | 0: No drift; 1: Drifts down; 2: Some effort against gravity; 3: No effort against gravity; 4: No movement | |
| 7. Limb Ataxia | 0: Absent; 1: Present in 1 limb; 2: Present in 2 limbs | |
| 8. Sensory | 0: Normal; 1: Mild to moderate sensory loss; 2: Severe to total sensory loss | |
| 9. Best Language | 0: Normal; 1: Mild to moderate aphasia; 2: Severe aphasia; 3: Mute or global aphasia | |
| 10. Dysarthria | 0: Normal; 1: Mild to moderate dysarthria; 2: Severe dysarthria or anarthria | |
| 11. Extinction and Inattention | 0: No abnormality; 1: Visual, tactile, auditory, spatial, or personal inattention; 2: Profound hemi-inattention or neglect to more than 1 modality | |
| Total: |
Evaluation
An organized stroke protocol is highly recommended to expedite evaluation.[38] The door-to-needle time of 60 minutes is recommended for acute ischemic stroke patients who qualify for thrombolytics.[38]
The goals in the initial phase include the following:
- Ensuring medical stability, with particular attention to airway, breathing, and circulation
- Quickly reversing any conditions that are contributing to the patient's problem
- Determining if the patient is a candidate for IV thrombolytic therapy or endovascular thrombectomy
- Moving toward uncovering the pathophysiologic basis of the patient's neurologic symptoms
The initial evaluation of any patient is airway, breathing, circulation, and vital signs. Patients may present with respiratory abnormalities from elevated intracranial pressure (ICP) and are at risk of aspiration and asphyxiation. Endotracheal intubation may be necessary to ensure adequate oxygenation and ventilation.
A fingerstick glucose check should be performed, as hypoglycemia can easily be ruled out as a cause of neurological abnormalities.
A plain CT head is recommended for patients within 20 minutes of presentation to rule out hemorrhage. In stroke centers or hospitals that can provide emergency care, vascular imaging should be considered for possible endovascular intervention. However, endovascular intervention should not delay the administration of thrombolytics.[38]
Diffusion-weighted imaging (DWI) is a specialized MRI technique that measures the diffusion of water molecules within tissue. DWI is particularly sensitive for detecting acute ischemic strokes due to its ability to reveal cytotoxic edema—a hallmark of acute infarction—within minutes after stroke onset. DWI can indicate a brain infarction much earlier than other MRI sequences, such as Fluid-Attenuated Inversion Recovery (FLAIR). While a DWI scan can show abnormalities within minutes of a stroke, a FLAIR sequence might take approximately 4.5 hours to reveal signs of a brain infarction. If DWI shows signs of a stroke while the FLAIR sequence does not, it suggests that the ischemic stroke occurred <4.5 hours ago. This timing is crucial because patients with strokes <4.5 hours old may be eligible for early IV thrombolysis, potentially reversing the neurological deficits.[40]
Other diagnostic tests include an electrocardiogram (ECG), troponin levels, complete blood count (CBC), electrolytes, blood urea nitrogen (BUN), creatinine (Cr), and coagulation factors. The healthcare provider should evaluate an ECG and troponin because stroke is often associated with coronary artery disease. A CBC may reveal anemia or indicate infection. Healthcare providers should correct electrolyte abnormalities, which can cause altered mental status and could cloud the diagnosis of ischemic stroke. BUN and Cr should be monitored as contrast studies may worsen kidney function. Coagulation factors, including PT, PTT, and INR, should also be drawn as elevated levels may suggest a cause of hemorrhagic stroke.[38]
For institutions without expert imaging interpretation, the US Food and Drug Administration (FDA) highly recommends using a teleradiology system for image interpretation for suspected stroke patients. Rapid imaging interpretation assists with the decision to administer IV alteplase. A discussion and agreement between telestroke neurologists and radiologists is highly recommended. In areas that do not have an in-house stroke team or telestroke protocol, a telephone consultation may be considered to administer thrombolytics. The level of evidence for this recommendation is limited.[41][42][43]
Treatment / Management
The goal of therapy in acute ischemic stroke is to preserve tissue in areas where perfusion is decreased but sufficient to avoid infarction. Tissue in this area of oligemia is preserved by restoring blood flow to the compromised regions and improving collateral flow. Recanalization strategies include IV recombinant tissue-type plasminogen activator and mechanical thrombectomy. Restoring blood flow can minimize the effects of ischemia only if performed quickly. The use of endovascular techniques has been used successfully in selected patients to treat acute ischemic stroke. Another consideration is neuroprotective agents, but none have been shown to improve clinical outcomes.
Essential treatments that have been shown in controlled trials to be efficacious in acute ischemic stroke treatment in different patient groups include the following:
Acute Reperfusion Therapy
- IV alteplase (within 4.5 hours of stroke onset): The American Heart Association (AHA)/American Stroke Association (ASA) recommends IV alteplase (TPA) for patients who satisfy inclusion criteria and have symptom onset or last known baseline within 3 hours.[44] IV TPA should be administered at 0.9 mg/kg, with a maximum dose of 90 mg. The first 10% of the dose is given as a bolus over the first minute, and the remainder is given over the next 60 minutes. The time has been extended to 4.5 hours for selected candidates.
Inclusion criteria include diagnosis of ischemic stroke with "measurable neurological deficit," symptom onset within 3 hours before treatment, and age 18 years or older.[44]
Healthcare providers should review the exclusion criteria for thrombolytics before administering TPA. According to the FDA, the contraindications to IV thrombolysis include active internal bleeding, recent intracranial surgery or severe head trauma, intracranial conditions that may increase the risk of bleeding, bleeding diathesis, severe uncontrolled hypertension, current intracranial hemorrhage, subarachnoid hemorrhage, and a history of a recent stroke.
Healthcare providers must consider the treatment benefits and risks for patients who present between 3 hours and 4.5 hours from symptom onset. Additional relative exclusion criteria for this patient category include age older than 80 years, NIHSS >25, oral anticoagulant use, and a history of diabetes and prior ischemic stroke.[44]
- MRI-guided thrombolysis for stroke with unknown time of onset: Most of these patients wake up with an acute stroke. The patients will be ineligible for IV thrombolytic therapy because their last known normal will be at bedtime. The WAKE-UP Stroke Trial used the mismatch between a positive DWI MRI sequence showing an acute ischemic infarction and a negative FLAIR MRI sequence indicating that the infarct occurs within 4.5 hours of the MRI. DWI is positive within 30 minutes of an acute infarct. FLAIR sequence will not be positive until about 4.5 hours after an acute infarct. The mismatch indicates that the stroke occurred within 4.5 hours, and therefore, IV thrombolytic will be indicated. The WAKE-UP Stroke Trial confirmed the positive result.[40][45]
- IV tenecteplase (within 4.5 hours of stroke onset): Another fibrinolytic agent, tenecteplase (TNK), may be considered an alternative to alteplase. TNK has advantages over TPA with its longer half-life and can be administered as a single intramuscular (IM) dose rather than a 1-hour IV infusion of TPA. TNK can be given at a dose of 30 to 50 mg IV bolus over 5 sec once (based on weight). TNK is also cheaper to deliver because it is given as a single IM dose. TNK has become the fibrinolytic agent of choice for many stroke centers, especially during the COVID-19 pandemic. Recent studies have shown that TNK appeared to have efficacy and safety profiles similar to TPA.[46][47] The 2023 AHA guidelines state that choosing TNK over TPA in patients without contraindications for IV fibrinolytic who are also eligible to undergo mechanical thrombectomy may be reasonable.[38] A dose of 0.4 mg/kg, compared to 0.25 mg/kg, showed no advantage. (A1)
- Mechanical thrombectomy (within 6 hours of stroke onset): Mechanical thrombectomy should be considered in all patients, even those who received fibrinolytic therapy. The AHA/ASA guidelines do not recommend observation for a response after IV TPA in patients considered for mechanical thrombectomy.[44]
In recent years, significant advancements have been made in acute stroke care. Multiple stroke trials in 2015 showed that endovascular thrombectomy in the first 6 hours is much better than standard medical care in patients with large vessel occlusion (LVO) in the arteries of the proximal anterior circulation. These benefits are sustained irrespective of geographical location and patient characteristics.[48][49]
(B3)
- Mechanical thrombectomy with perfusion study (within 16 to 24 hours of stroke onset): Perfusion imaging studies (CT perfusion or MR perfusion) can define the areas of the brain that are ischemic but not infarcted, the ischemic penumbra. Depending on the size of the penumbra relative to the ischemic core, good amounts of brain tissue can be salvaged by restoring blood flow in cases of LVO identified on CT or MR angiogram, resulting in better clinical outcomes.
In 2018, a significant paradigm shift occurred in stroke care. The DAWN trial showed significant benefits of endovascular thrombectomy in patients with LVO in the arteries of the proximal anterior circulation. This trial extended the stroke window to 24 hours in selected patients using perfusion imaging. Subsequently, more patients can be treated, even up to 24 hours.[50]
Mechanical thrombectomy is recommended within 6 to 16 hours of the last known normal in selected patients with LVO with acute ischemic stroke in the anterior circulation and meets other DAWN and DEFUSE 3 criteria. In selected patients who meet the DAWN criteria, mechanical thrombectomy is reasonable within 24 hours of the last known normal.[50][51]
- Endovascular therapy (thrombectomy) for acute ischemic stroke with large infarct: Two large trials published in 2023, the ANGEL ASPECT Trial and the SELECT 2 Trial, showed positive results with endovascular thrombectomy therapy for patients with large ischemic strokes due to LVO and an Alberta Stroke Program Early CT Score (ASPECTS) of 3 to 5. The patients presented within 24 hours of the last known normal. Their NIHSS score is >6. The outcomes are better among patients treated with thrombectomy than those on standard medical therapy.[52][53]
Definition of LVO: Intracranial internal carotid artery, middle cerebral artery (M1), basilar artery, and posterior cerebral artery (P1) occlusion [54]
Determining ASPECTS: This is a 10-point score system for acute middle cerebral artery territory ischemic stroke. One point will be deducted from 10 with an area of the brain infarcted, as shown on CT. Therefore, the smaller the number, the larger the infarct size. These regions are the caudate, putamen, internal capsule, insular cortex, M1, M2, M3, M4, M5, and M6.[55]
Basilar artery occlusion: This particular type of rare stroke, with about 80% of patients ending up with poor outcomes. AHA guidelines recommend mechanical thrombectomy within 6 hours of onset. Two large trials published in 2022 demonstrate the benefits of endovascular treatment over conventional therapy. The ATTENTION trial showed the benefits within 12 hours.[56] The BAOCHE trial showed the benefits of thrombectomy 6 to 24 hours after strokes due to basilar artery occlusion.[57] In conclusion, thrombectomy should be considered in the treatment of these patients for up to 24 hours.
(B2)
Acute Hospital Management [38]
- Blood pressure: The guidelines suggest blood pressure (BP) management of <180/105 mm Hg for the first 24 hours after IV TPA. The 2023 AHA guidelines also recommended that in patients for whom mechanical thrombectomy is planned and who have not received IV fibrinolytic therapy, it is reasonable to maintain BP ≤185/110 mm Hg before the procedure.[44] A new recommendation is lowering BP initially by 15% in patients with comorbid conditions such as acute heart failure or aortic dissection. Antihypertensive management does not prevent death or dependency in patients with BP <220/120 mm Hg who did not receive IV TPA and have no comorbid conditions requiring blood pressure reduction. The theoretical risk of BP lowering is the potential reduction of perfusion to the ischemic areas, which is pressure-dependent. The risk applies to the first 48 to 72 hours after an acute ischemic stroke. For patients with a BP ≥220/120 mm Hg who did not receive IV TPA, the guideline suggests it may be reasonable to reduce BP by 15% in the first 24 hours, although the benefit is uncertain. A large trial recently published showed a U-shaped curve correlating acute MAP and stroke clinical outcomes. The ideal mean systolic BP is 135 mm Hg to 150 mm Hg.[44]
Antihypertensive options include the following:
- Labetalol 10 to 20 mg IV; may repeat once
- Nicardipine 5 mg/hour IV. Increase 2.5 mg/hour every 5 to 15 minutes. The maximum dose is 15 mg/hour.
- Clevidipine 1 to 2 mg/hour IV. Double dose every 15 minutes. Maximum 21 mg/hour.
- Hydralazine and enalaprilat may be considered.
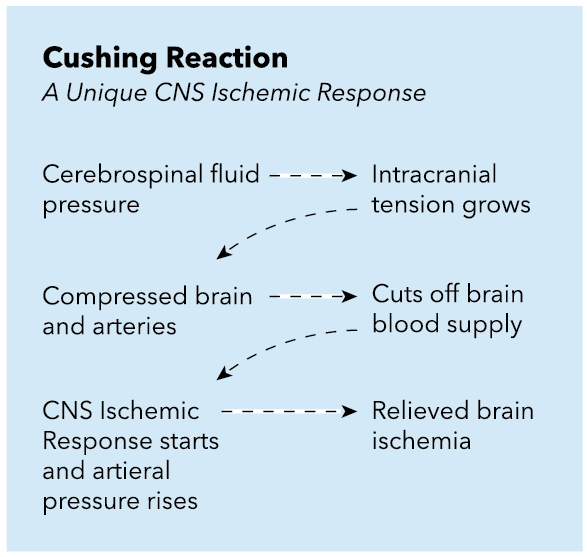
Hypotension and hypovolemia should be avoided because cerebral perfusion pressure is dependent on the maintenance of an elevated MAP as ICP increases due to an ischemic event (see Image. Cushing Reaction: CNS Ischemic Response).
- Temperature: Hyperthermia >38 °C should be avoided and treated appropriately. Antipyretics such as acetaminophen may be used. Common sources of infection, such as pneumonia and urinary tract infections, should be ruled out. There is insufficient data to support therapeutic hypothermia in acute ischemic strokes currently. A retrospective study recently demonstrated an association between a peak temperature >39 °C (100.4 °F) in the first 24 hours and an increased risk of in-hospital mortality.
- Glucose: Maintain glucose in the 140 to 180 mg/dL range in the first 24 hours. Healthcare providers should treat blood glucose <60 mg/dL to achieve normoglycemia. The brain is dependent on oxidative pathways that require glucose for metabolism, and the metabolic demand of the brain is high; therefore, hypoglycemic episodes can decrease the brain's ability to repair. However, hyperglycemia is hypothesized to decrease reperfusion due to the oxidation of nitric oxide-dependent mechanisms and subsequent loss of vascular tone. Moreover, increased acidosis also plays a part, possibly due to injury to lactic acid-sensing channels. Capes et al showed that hyperglycemia in ischemic stroke patients increases 30-day mortality and is an independent risk factor for hemorrhagic stroke conversion.[58] (A1)
- Nutrition: Early enteric feeding should be encouraged. For patients with dysphagia, use a nasogastric tube to promote enteric feeding. If there is concern that the patient may have swallowing difficulties for a prolonged period (more than 2 to 3 weeks), placing a percutaneous gastrostomy tube is recommended. Early feeding has been demonstrated to have an absolute reduction in the risk of death.[59] (A1)
- DVT prophylaxis: Intermittent pneumatic compression is recommended for all immobile patients unless contraindications exist. The European Stroke Organization recommended acutely intermittent pneumatic compression for all immobile stroke patients. They also recommended low-dose heparin or low molecular weight heparin for DVT prophylaxis if the benefit outweighs the risk of bleeding.[60][61] (A1)
- Depression screening: Screening for depression should be considered after an acute ischemic stroke. Rates of post-stroke depression range from 18% to 33%. Risk factors are female sex, large strokes, a stroke affecting the frontal areas, and poor social support. Selective serotonin reuptake inhibitors are the best medications for post-stroke depression.[62][63] (A1)
- Cerebellar/Cerebral edema: Cerebral edema occurs with acute ischemic stroke, first due to cytotoxic edema with cell swelling, followed by vasogenic edema when the blood-brain barrier is lost. The degree and volume of cerebral edema correlate well with the size of the stroke. Cerebral edema is not significant clinically in lacunar infarction. However, cerebral edema may become symptomatic, resulting in worsening of the stroke symptoms and worse in impairment of consciousness due to herniation. Cerebral edema peaks in 3 to 5 days after an ischemic stroke.[64]
Cerebellar edema complicates cerebellar infarctions, and clinicians must know that these patients can rapidly decompensate. The increased ICP can cause obstructing hydrocephalus on the fourth ventricle or cause transtentorial herniation of the superior vermis and downward cerebellar tonsillar herniation. Signs include change or worsening mental status, decreased level of consciousness, respiratory abnormalities, change in pupillary size, posturing, and death.
Early recognition and diagnosis of intracranial hypertension due to cerebral edema are essential in caring for acute stroke patients to improve outcomes. Obtain neurosurgical consultation early. A ventriculostomy is indicated in the setting of obstructive hydrocephalus after cerebellar infarct. A decompressive suboccipital craniectomy is highly recommended in cerebellar edema with mass effect cases.[65][66]
- Seizures: Post-stroke seizures occur in about 10% of patients, mainly hemorrhagic strokes or cortical infarcts. If the patient experiences a seizure within the first 2 weeks, antiepileptic drugs are indicated for a short period, generally 1 month. Long-term anticonvulsant therapy will be indicated if the seizure occurs later, for weeks or months after a stroke. However, the routine prophylactic use of antiepileptic drugs is not recommended.[67][68]
- Cardiac evaluation: Cardiac monitoring for atrial fibrillation or other arrhythmias is recommended in the first 24 hours. The benefit of further monitoring after that is unclear. An initial troponin is recommended because there is an association between stroke and coronary artery disease.
- Antiplatelet treatment: Aspirin is recommended within 24 to 48 hours of symptom onset. A Cochrane review concluded that aspirin given within 48 hours of symptom onset for ischemic strokes prevented the recurrence of ischemic strokes and improved long-term outcomes. In addition, there was no significant risk of early intracranial hemorrhage with aspirin.[69] (A1)
- Antithrombotic treatment: Full-dose anticoagulation is not recommended in acute stroke. The main exception is low-dose anticoagulation for DVT prophylaxis.
In patients with atrial fibrillation, the guidelines state it is reasonable to initiate oral anticoagulation within 4 to 14 days after neurological symptoms onset. When to start anticoagulation in patients with atrial fibrillation after acute stroke is always a dilemma; initiation usually depends on factors like stroke size and other comorbidities. Usually, if the stroke size is small to moderate, anticoagulation is started in 7 to 14 days.[70]
Sometimes, there are patients with small hemorrhagic transformation after acute stroke, and in this scenario, delay of anticoagulation is warranted. Delay of anticoagulation after hemorrhagic transformation is not associated with excessive stroke recurrence.[71]
(B3)
Differential Diagnosis
The differential diagnosis of ischemic stroke includes the following:
- Complicated migraine
- Drug toxicity
- Intracranial abscess
- Intracranial hemorrhage
- Intracranial tumor
- Hyperglycemia
- Hypoglycemia
- Hypertensive encephalopathy
- Metabolic abnormalities
- Movement disorders
- Multiple sclerosis
- Seizure
- Sepsis
- Syncope
- Wernicke encephalopathy
Prognosis
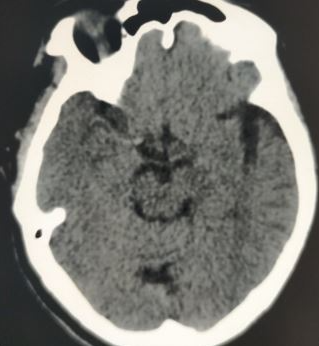
Prognosis in stroke is pivotal in guiding treatment decisions and informing patients and caregivers about potential outcomes. The prognosis involves assessing various factors such as the type of stroke, its severity, the extent of neurological deficits, comorbidities, and response to treatment. Predictive tools, including clinical scales and imaging modalities, aid in predicting outcomes like functional impairment, mortality, and risk of recurrence (see Image. Encephalomalacia Following Ischemic Stroke). Early intervention and rehabilitation significantly influence prognosis, highlighting the importance of prompt medical attention and personalized care. While some individuals may achieve full recovery, others might experience long-term disabilities or complications. The prognosis in stroke emphasizes the need for multidisciplinary approaches, ongoing monitoring, and support to optimize outcomes and enhance the quality of life for affected individuals and their families.[73][74]
Complications
The complications of acute ischemic stroke are many and common.[75] These include but are not limited to the following:
- DVT and pulmonary embolism: DVT prophylaxis is indicated.
- Aspiration and pneumonia: A swallowing evaluation before feeding is always indicated and is part of stroke center accreditation.
- Seizures
- Depression
- Cerebral edema and increased intracranial pressure
Postoperative and Rehabilitation Care
Early rehabilitation for stroke patients is beneficial, although very early rehabilitation, within 24 hours, should be avoided. The AVERT trial randomized patients to receive very early rehabilitation within 24 hours of stroke compared to usual stroke-unit care, and early mobilization demonstrated less favorable outcomes using the modified Rankin score.[76]
Deterrence and Patient Education
Deterrence and prevention strategies play a vital role in reducing the incidence and impact of ischemic stroke. By addressing modifiable risk factors such as hypertension, diabetes, high cholesterol, and smoking through lifestyle modifications and pharmacological interventions, individuals can significantly decrease their likelihood of experiencing a stroke. Additionally, raising awareness about the warning signs of stroke and promoting timely access to medical care for symptoms such as sudden weakness, numbness, or difficulty speaking can expedite treatment and minimize damage. Community-based education campaigns emphasizing healthy behaviors, regular exercise, and balanced diets further contribute to stroke prevention efforts. Through comprehensive efforts targeting both individual behaviors and societal factors, it is possible to mitigate the burden of ischemic stroke and enhance overall public health.
Pearls and Other Issues
Clinical pearls that offer valuable insights into the management and care of patients with ischemic stroke include the following:
- Have a low threshold for evaluation of stroke, especially in at-risk populations. TIME IS BRAIN!
- Stroke symptoms depend on the ischemic area of the brain and, therefore, vary.
- Noncontrast head CT is the first imaging indicated for acute stroke, mainly to rule out hemorrhage.
- Consider TPA or TNK if thrombotic CVA is identified within 4.5 hours of symptom onset.
- When indicated, an early CT angiogram or MR angiogram with perfusion study should be obtained to look for LVO and initiate endovascular thrombectomy therapy within 6 to 24 hours.
- Be aware of presenting blood pressure. Blood pressure management recommendations may include aggressive blood pressure management or permissive hypertension, depending on the type of stroke and whether IV fibrinolytic is indicated.
- Start an antiplatelet agent within 24 hours of presentation.
- Consider other risk factor management, including addressing hyperlipidemia, hyperglycemia, and cardiac arrhythmias that may increase the risk of vascular disease or a thrombotic event.
- Consider early and aggressive physical and occupational therapy after the onset of CVA.
Enhancing Healthcare Team Outcomes
The effective management of ischemic stroke demands a cohesive and interprofessional healthcare team dedicated to delivering patient-centered care, improving outcomes, and maximizing safety and team performance. This approach includes physicians, advanced care practitioners, nurses, pharmacists, neuroimaging technicians, rehabilitation therapists, and stroke specialists working together.
Prompt recognition and treatment of ischemic stroke is critical. Emergency medicine providers must be adept at identifying early stroke symptoms to initiate immediate care. Neuroimaging technicians and radiologists are essential for quickly obtaining and interpreting scans such as CTs and MRIs, which are vital for confirming the diagnosis of ischemic stroke. Neurointeventionalists may be needed to provide interventions such as thrombectomies. Pharmacists play a crucial role in ensuring the timely administration of thrombolytic agents when appropriate and in managing the patient's medication regimen to prevent complications and secondary strokes. Nurses monitor patients closely for changes in condition and manage care protocols. Rehabilitation therapists are vital to the patient's recovery process, starting in the acute phase and continuing through long-term rehabilitation.
The prognosis for patients treated with TPA is good, but the outcomes are guarded for those who do not receive thrombolytic medication.[77] Effective communication among all team members is paramount for the rapid diagnosis, decision-making, and implementation of treatment plans. This collaboration facilitates a comprehensive approach to care, from acute management to rehabilitation and secondary prevention. All care decisions should be based on ethical considerations, including informed consent and respect for patient autonomy. The team prioritizes shared decision-making, respecting patient preferences while ensuring beneficence and non-maleficence.
An interprofessional healthcare team approach is essential for a timely and effective response, minimizing complications, and prioritizing patient safety and quality of care in managing ischemic stroke. Education and ongoing professional development are essential. They ensure the healthcare team remains knowledgeable about the latest evidence-based practices in stroke care, from acute management to prevention of recurrence. Through dedicated collaboration, the healthcare team will provide patient-centered care from emergency care to rehabilitation, ultimately enhancing outcomes and quality of life for affected patients.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Jul:44(7):2064-89. doi: 10.1161/STR.0b013e318296aeca. Epub 2013 May 7 [PubMed PMID: 23652265]
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan:24(1):35-41 [PubMed PMID: 7678184]
Level 1 (high-level) evidenceNtaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2020 Jan 28:75(3):333-340. doi: 10.1016/j.jacc.2019.11.024. Epub [PubMed PMID: 31976872]
Pierik R, Algra A, van Dijk E, Erasmus ME, van Gelder IC, Koudstaal PJ, Luijckx GR, Nederkoorn PJ, van Oostenbrugge RJ, Ruigrok YM, Scheeren TWL, Uyttenboogaart M, Visser MC, Wermer MJH, van den Bergh WM, on behalf of the Parelsnoer Institute-Cerebrovascular Accident Study Group. Distribution of Cardioembolic Stroke: A Cohort Study. Cerebrovascular diseases (Basel, Switzerland). 2020:49(1):97-104. doi: 10.1159/000505616. Epub 2020 Jan 21 [PubMed PMID: 31962331]
Spence JD. Cardioembolic stroke: everything has changed. Stroke and vascular neurology. 2018 Jun:3(2):76-83. doi: 10.1136/svn-2018-000143. Epub 2018 Mar 9 [PubMed PMID: 30022801]
Cole JW. Large Artery Atherosclerotic Occlusive Disease. Continuum (Minneapolis, Minn.). 2017 Feb:23(1, Cerebrovascular Disease):133-157. doi: 10.1212/CON.0000000000000436. Epub [PubMed PMID: 28157748]
Li Q, Yang Y, Reis C, Tao T, Li W, Li X, Zhang JH. Cerebral Small Vessel Disease. Cell transplantation. 2018 Dec:27(12):1711-1722. doi: 10.1177/0963689718795148. Epub 2018 Sep 25 [PubMed PMID: 30251566]
Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the Approach to Embolic Stroke of Undetermined Source: A Review. JAMA neurology. 2019 Jul 1:76(7):855-861. doi: 10.1001/jamaneurol.2019.0591. Epub [PubMed PMID: 30958521]
Kim H, Kim JT, Lee JS, Kim BJ, Kang J, Lee KJ, Park JM, Kang K, Lee SJ, Kim JG, Cha JK, Kim DH, Park TH, Lee KB, Lee J, Hong KS, Cho YJ, Park HK, Lee BC, Yu KH, Oh MS, Kim DE, Ryu WS, Choi JC, Kwon JH, Kim WJ, Shin DI, Yum KS, Sohn SI, Hong JH, Lee SH, Park MS, Choi KH, Lee J, Bae HJ. Stroke of Other Determined Etiology: Results From the Nationwide Multicenter Stroke Registry. Stroke. 2022 Aug:53(8):2597-2606. doi: 10.1161/STROKEAHA.121.037582. Epub 2022 May 9 [PubMed PMID: 35531778]
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023 Feb 21:147(8):e93-e621. doi: 10.1161/CIR.0000000000001123. Epub 2023 Jan 25 [PubMed PMID: 36695182]
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):447-54. doi: 10.1161/CIR.0000000000000366. Epub [PubMed PMID: 26811276]
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 7:135(10):e146-e603. doi: 10.1161/CIR.0000000000000485. Epub 2017 Jan 25 [PubMed PMID: 28122885]
White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005 Mar 15:111(10):1327-31 [PubMed PMID: 15769776]
Level 2 (mid-level) evidenceJackson G, Chari K. National Hospital Care Survey Demonstration Projects: Stroke Inpatient Hospitalizations. National health statistics reports. 2019 Nov:(132):1-11 [PubMed PMID: 32510306]
Level 3 (low-level) evidenceJaiswal V, Hanif M, Ang SP, Suresh V, Ruchika F, Momi NK, Naz S, Rajak K, Halder A, Kumar T, Naz H, Alvarez VHA. The Racial Disparity Among the Clinical Outcomes Post Stroke and its Intervention Outcomes: A Systematic Review and Meta-analysis. Current problems in cardiology. 2023 Sep:48(9):101753. doi: 10.1016/j.cpcardiol.2023.101753. Epub 2023 Apr 21 [PubMed PMID: 37088178]
Level 1 (high-level) evidenceMarkus HS, de Leeuw FE. Cerebral small vessel disease: Recent advances and future directions. International journal of stroke : official journal of the International Stroke Society. 2023 Jan:18(1):4-14. doi: 10.1177/17474930221144911. Epub [PubMed PMID: 36575578]
Level 3 (low-level) evidenceMarkus HS. Cerebral perfusion and stroke. Journal of neurology, neurosurgery, and psychiatry. 2004 Mar:75(3):353-61 [PubMed PMID: 14966145]
Atkins ER, Brodie FG, Rafelt SE, Panerai RB, Robinson TG. Dynamic cerebral autoregulation is compromised acutely following mild ischaemic stroke but not transient ischaemic attack. Cerebrovascular diseases (Basel, Switzerland). 2010 Feb:29(3):228-35. doi: 10.1159/000267845. Epub 2009 Dec 18 [PubMed PMID: 20029195]
Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010 Nov:41(11):2697-704. doi: 10.1161/STROKEAHA.110.594168. Epub 2010 Oct 7 [PubMed PMID: 20930158]
Chalet L, Boutelier T, Christen T, Raguenes D, Debatisse J, Eker OF, Becker G, Nighoghossian N, Cho TH, Canet-Soulas E, Mechtouff L. Clinical Imaging of the Penumbra in Ischemic Stroke: From the Concept to the Era of Mechanical Thrombectomy. Frontiers in cardiovascular medicine. 2022:9():861913. doi: 10.3389/fcvm.2022.861913. Epub 2022 Mar 9 [PubMed PMID: 35355966]
Desowska A, Turner DL. Dynamics of brain connectivity after stroke. Reviews in the neurosciences. 2019 Jul 26:30(6):605-623. doi: 10.1515/revneuro-2018-0082. Epub [PubMed PMID: 30768425]
Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. Journal of experimental stroke & translational medicine. 2010 Mar 15:3(1):47-55 [PubMed PMID: 20607107]
Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981 Nov-Dec:12(6):723-5 [PubMed PMID: 6272455]
Nogles TE, Galuska MA. Middle Cerebral Artery Stroke. StatPearls. 2024 Jan:(): [PubMed PMID: 32310592]
Matos Casano HA, Tadi P, Ciofoaia GA. Anterior Cerebral Artery Stroke. StatPearls. 2024 Jan:(): [PubMed PMID: 30726018]
Kuybu O, Tadi P, Dossani RH. Posterior Cerebral Artery Stroke. StatPearls. 2024 Jan:(): [PubMed PMID: 30335329]
Brandt T, Steinke W, Thie A, Pessin MS, Caplan LR. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovascular diseases (Basel, Switzerland). 2000 May-Jun:10(3):170-82 [PubMed PMID: 10773642]
Cereda C, Carrera E. Posterior cerebral artery territory infarctions. Frontiers of neurology and neuroscience. 2012:30():128-31. doi: 10.1159/000333610. Epub 2012 Feb 14 [PubMed PMID: 22377879]
Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Frontiers in neurology. 2014:5():30. doi: 10.3389/fneur.2014.00030. Epub 2014 Apr 7 [PubMed PMID: 24778625]
Carvalho V, Cruz VT. Clinical presentation of vertebrobasilar stroke. Porto biomedical journal. 2020 Nov-Dec:5(6):e096. doi: 10.1097/j.pbj.0000000000000096. Epub 2020 Nov 24 [PubMed PMID: 33283066]
Jensen MB, St Louis EK. Management of acute cerebellar stroke. Archives of neurology. 2005 Apr:62(4):537-44 [PubMed PMID: 15824250]
Level 3 (low-level) evidenceAldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: etiology, diagnosis, and prognosis. Annals of neurology. 1987 Feb:21(2):149-58 [PubMed PMID: 3827223]
Venkataraman P, Tadi P, Lui F. Lacunar Syndromes (Archived). StatPearls. 2024 Jan:(): [PubMed PMID: 30480945]
Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA neurology. 2018 Oct 1:75(10):1273-1281. doi: 10.1001/jamaneurol.2018.1073. Epub [PubMed PMID: 30167649]
Level 3 (low-level) evidenceWardlaw JM. What causes lacunar stroke? Journal of neurology, neurosurgery, and psychiatry. 2005 May:76(5):617-9 [PubMed PMID: 15834013]
Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988 Sep:19(9):1074-82 [PubMed PMID: 3046071]
Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005 May 18:293(19):2391-402 [PubMed PMID: 15900010]
Level 1 (high-level) evidencePowers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Kothari R, Hall K, Brott T, Broderick J. Early stroke recognition: developing an out-of-hospital NIH Stroke Scale. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 1997 Oct:4(10):986-90 [PubMed PMID: 9332632]
Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Günther M, Guibernau J, Häusler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C, WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. The New England journal of medicine. 2018 Aug 16:379(7):611-622. doi: 10.1056/NEJMoa1804355. Epub 2018 May 16 [PubMed PMID: 29766770]
Cassella CR, Jagoda A. Ischemic Stroke: Advances in Diagnosis and Management. Emergency medicine clinics of North America. 2017 Nov:35(4):911-930. doi: 10.1016/j.emc.2017.07.007. Epub [PubMed PMID: 28987436]
Level 3 (low-level) evidenceDemaerschalk BM, Bobrow BJ, Raman R, Ernstrom K, Hoxworth JM, Patel AC, Kiernan TE, Aguilar MI, Ingall TJ, Dodick DW, Meyer BC, Stroke Team Remote Evaluation Using a Digital Observation Camera (STRokE DOC) in Arizona—The Initial Mayo Clinic Experience (AZ TIME) Investigators. CT interpretation in a telestroke network: agreement among a spoke radiologist, hub vascular neurologist, and hub neuroradiologist. Stroke. 2012 Nov:43(11):3095-7. doi: 10.1161/STROKEAHA.112.666255. Epub 2012 Sep 13 [PubMed PMID: 22984007]
Level 1 (high-level) evidenceJohnston KC, Worrall BB, Teleradiology Assessment of Computerized Tomographs Online Reliability Study. Teleradiology Assessment of Computerized Tomographs Online Reliability Study (TRACTORS) for acute stroke evaluation. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2003 Fall:9(3):227-33 [PubMed PMID: 14611689]
Level 3 (low-level) evidenceHoh BL, Ko NU, Amin-Hanjani S, Chou SH-Y, Cruz-Flores S, Dangayach NS, Derdeyn CP, Du R, Hänggi D, Hetts SW, Ifejika NL, Johnson R, Keigher KM, Leslie-Mazwi TM, Lucke-Wold B, Rabinstein AA, Robicsek SA, Stapleton CJ, Suarez JI, Tjoumakaris SI, Welch BG. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2023 Jul:54(7):e314-e370. doi: 10.1161/STR.0000000000000436. Epub 2023 May 22 [PubMed PMID: 37212182]
Zhang J, Ta N, Fu M, Tian FH, Wang J, Zhang T, Wang B. Use of DWI-FLAIR Mismatch to Estimate the Onset Time in Wake-Up Strokes. Neuropsychiatric disease and treatment. 2022:18():355-361. doi: 10.2147/NDT.S351943. Epub 2022 Feb 21 [PubMed PMID: 35228801]
Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, Jenssen KN, Tobro H, Rörholt DM, Kaur K, Eltoft A, Evensen K, Haasz J, Singaravel G, Fromm A, Thomassen L. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. The Lancet. Neurology. 2022 Jun:21(6):511-519. doi: 10.1016/S1474-4422(22)00124-7. Epub 2022 May 4 [PubMed PMID: 35525250]
Level 1 (high-level) evidenceRehman AU, Mohsin A, Cheema HA, Zahid A, Ebaad Ur Rehman M, Ameer MZ, Ayyan M, Ehsan M, Shahid A, Aemaz Ur Rehman M, Shah J, Khawaja A. Comparative efficacy and safety of tenecteplase and alteplase in acute ischemic stroke: A pairwise and network meta-analysis of randomized controlled trials. Journal of the neurological sciences. 2023 Feb 15:445():120537. doi: 10.1016/j.jns.2022.120537. Epub 2022 Dec 29 [PubMed PMID: 36630803]
Level 1 (high-level) evidenceTadi P, Lui F. Acute Stroke. StatPearls. 2024 Jan:(): [PubMed PMID: 30570990]
Widimsky P, Snyder K, Sulzenko J, Hopkins LN, Stetkarova I. Acute ischaemic stroke: recent advances in reperfusion treatment. European heart journal. 2023 Apr 7:44(14):1205-1215. doi: 10.1093/eurheartj/ehac684. Epub [PubMed PMID: 36477996]
Level 3 (low-level) evidenceNogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11 [PubMed PMID: 29129157]
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. The New England journal of medicine. 2018 Feb 22:378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24 [PubMed PMID: 29364767]
Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, Zhang J, Zhou Z, Yao X, Wang G, Song W, Cai X, Nan G, Li D, Wang AY, Ling W, Cai C, Wen C, Wang E, Zhang L, Jiang C, Liu Y, Liao G, Chen X, Li T, Liu S, Li J, Gao F, Ma N, Mo D, Song L, Sun X, Li X, Deng Y, Luo G, Lv M, He H, Liu A, Zhang J, Mu S, Liu L, Jing J, Nie X, Ding Z, Du W, Zhao X, Yang P, Liu L, Wang Y, Liebeskind DS, Pereira VM, Ren Z, Wang Y, Miao Z, ANGEL-ASPECT Investigators. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. The New England journal of medicine. 2023 Apr 6:388(14):1272-1283. doi: 10.1056/NEJMoa2213379. Epub 2023 Feb 10 [PubMed PMID: 36762852]
Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, Chen M, Blackburn S, Sitton CW, Churilov L, Sundararajan S, Hu YC, Herial NA, Jabbour P, Gibson D, Wallace AN, Arenillas JF, Tsai JP, Budzik RF, Hicks WJ, Kozak O, Yan B, Cordato DJ, Manning NW, Parsons MW, Hanel RA, Aghaebrahim AN, Wu TY, Cardona-Portela P, Pérez de la Ossa N, Schaafsma JD, Blasco J, Sangha N, Warach S, Gandhi CD, Kleinig TJ, Sahlein D, Elijovich L, Tekle W, Samaniego EA, Maali L, Abdulrazzak MA, Psychogios MN, Shuaib A, Pujara DK, Shaker F, Johns H, Sharma G, Yogendrakumar V, Ng FC, Rahbar MH, Cai C, Lavori P, Hamilton S, Nguyen T, Fifi JT, Davis S, Wechsler L, Pereira VM, Lansberg MG, Hill MD, Grotta JC, Ribo M, Campbell BC, Albers GW, SELECT2 Investigators. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. The New England journal of medicine. 2023 Apr 6:388(14):1259-1271. doi: 10.1056/NEJMoa2214403. Epub 2023 Feb 10 [PubMed PMID: 36762865]
Nicholls JK, Ince J, Minhas JS, Chung EML. Emerging Detection Techniques for Large Vessel Occlusion Stroke: A Scoping Review. Frontiers in neurology. 2021:12():780324. doi: 10.3389/fneur.2021.780324. Epub 2022 Jan 6 [PubMed PMID: 35095726]
Level 2 (mid-level) evidencePop NO, Tit DM, Diaconu CC, Munteanu MA, Babes EE, Stoicescu M, Popescu MI, Bungau S. The Alberta Stroke Program Early CT score (ASPECTS): A predictor of mortality in acute ischemic stroke. Experimental and therapeutic medicine. 2021 Dec:22(6):1371. doi: 10.3892/etm.2021.10805. Epub 2021 Sep 27 [PubMed PMID: 34659517]
Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, Wen C, Zhou P, Chen W, Zeng G, Li Y, Ma Z, Yu C, Su J, Zhou Z, Chen Z, Liao G, Sun Y, Ren Y, Zhang H, Chen J, Yue X, Xiao G, Wang L, Liu R, Liu W, Liu Y, Wang L, Zhang C, Liu T, Song J, Li R, Xu P, Yin Y, Wang G, Baxter B, Qureshi AI, Liu X, Hu W, ATTENTION Investigators. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. The New England journal of medicine. 2022 Oct 13:387(15):1361-1372. doi: 10.1056/NEJMoa2206317. Epub [PubMed PMID: 36239644]
Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, Shi Z, Gao Z, Song C, Chen W, Peng Y, Yao C, Wei M, Li T, Wei L, Xiao G, Yang H, Ren M, Duan J, Liu X, Yang Q, Liu Y, Zhu Q, Shi W, Zhu Q, Li X, Guo Z, Yang Q, Hou C, Zhao W, Ma Q, Zhang Y, Jiao L, Zhang H, Liebeskind DS, Liang H, Jadhav AP, Wen C, Brown S, Zhu L, Ye H, Ribo M, Chang M, Song H, Chen J, Ji X, BAOCHE Investigators. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. The New England journal of medicine. 2022 Oct 13:387(15):1373-1384. doi: 10.1056/NEJMoa2207576. Epub [PubMed PMID: 36239645]
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001 Oct:32(10):2426-32 [PubMed PMID: 11588337]
Level 1 (high-level) evidenceDennis M, Lewis S, Cranswick G, Forbes J, FOOD Trial Collaboration. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health technology assessment (Winchester, England). 2006 Jan:10(2):iii-iv, ix-x, 1-120 [PubMed PMID: 16409880]
Level 1 (high-level) evidenceAVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet (London, England). 2015 Jul 4:386(9988):46-55. doi: 10.1016/S0140-6736(15)60690-0. Epub 2015 Apr 16 [PubMed PMID: 25892679]
Level 1 (high-level) evidenceDennis M, Caso V, Kappelle LJ, Pavlovic A, Sandercock P, European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. European stroke journal. 2016 Mar:1(1):6-19. doi: 10.1177/2396987316628384. Epub 2016 Mar 1 [PubMed PMID: 31008263]
Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. General hospital psychiatry. 2020 Sep-Oct:66():70-80. doi: 10.1016/j.genhosppsych.2020.06.011. Epub 2020 Jun 27 [PubMed PMID: 32717644]
Legg LA, Rudberg AS, Hua X, Wu S, Hackett ML, Tilney R, Lindgren L, Kutlubaev MA, Hsieh CF, Barugh AJ, Hankey GJ, Lundström E, Dennis M, Mead GE. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. The Cochrane database of systematic reviews. 2021 Nov 15:11(11):CD009286. doi: 10.1002/14651858.CD009286.pub4. Epub 2021 Nov 15 [PubMed PMID: 34780067]
Level 1 (high-level) evidenceDostovic Z, Dostovic E, Smajlovic D, Ibrahimagic OC, Avdic L. Brain Edema After Ischaemic Stroke. Medical archives (Sarajevo, Bosnia and Herzegovina). 2016 Oct:70(5):339-341 [PubMed PMID: 27994292]
Neugebauer H, Witsch J, Zweckberger K, Jüttler E. Space-occupying cerebellar infarction: complications, treatment, and outcome. Neurosurgical focus. 2013 May:34(5):E8. doi: 10.3171/2013.2.FOCUS12363. Epub [PubMed PMID: 23634927]
Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M, American Heart Association Stroke Council. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Apr:45(4):1222-38. doi: 10.1161/01.str.0000441965.15164.d6. Epub 2014 Jan 30 [PubMed PMID: 24481970]
Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Archives of neurology. 2002 Feb:59(2):195-201 [PubMed PMID: 11843689]
Xu MY. Poststroke seizure: optimising its management. Stroke and vascular neurology. 2019 Mar:4(1):48-56. doi: 10.1136/svn-2018-000175. Epub 2018 Dec 9 [PubMed PMID: 31105979]
Sandercock PA, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. The Cochrane database of systematic reviews. 2014 Mar 26:2014(3):CD000029. doi: 10.1002/14651858.CD000029.pub3. Epub 2014 Mar 26 [PubMed PMID: 24668137]
Level 1 (high-level) evidencePaciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, Chondrogianni M, Bovi P, Carletti M, Cappellari M, Zedde M, Ntaios G, Karagkiozi E, Athanasakis G, Makaritsis K, Silvestrelli G, Lanari A, Ciccone A, Putaala J, Tomppo L, Tatlisumak T, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Becattini C, Mosconi MG, Cimini LA, Soloperto R, Masotti L, Vannucchi V, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Sohn SI, Marcheselli S, Mumoli N, De Lodovici ML, Bono G, Furie KL, Tadi P, Yaghi S, Toni D, Letteri F, Tassinari T, Kargiotis O, Lotti EM, Flomin Y, Mancuso M, Maccarrone M, Giannini N, Bandini F, Pezzini A, Poli L, Padovani A, Scoditti U, Denti L, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Gourbali V, Orlandi G, Giuntini M, Chiti A, Giorli E, Gialdini G, Corea F, Ageno W, Bellesini M, Colombo G, Monaco S, Maimone Baronello M, Karapanayiotides T, Caso V. Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non-Vitamin-K Oral Anticoagulants (RAF-NOACs) Study. Journal of the American Heart Association. 2017 Nov 29:6(12):. doi: 10.1161/JAHA.117.007034. Epub 2017 Nov 29 [PubMed PMID: 29220330]
Level 3 (low-level) evidencePaciaroni M, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, Tadi P, Becattini C, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Mosconi MG, Cimini LA, Altavilla R, Volpi G, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn SI, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Athanasakis G, Makaritsis K, Karagkiozi E, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Colombo G, Silvestrelli G, Ciccone A, Lanari A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Ulivi L, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D'Anna S, Toni D, Letteri F, Giuntini M, Lotti EM, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Maimone Baronello M, Csiba L, Szabó L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen LP, Barlinn J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Caso V. Hemorrhagic Transformation in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. Journal of the American Heart Association. 2018 Nov 20:7(22):e010133. doi: 10.1161/JAHA.118.010133. Epub [PubMed PMID: 30571487]
Level 3 (low-level) evidenceChou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, Ferencik M. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2022 Aug 23:328(8):754-771. doi: 10.1001/jama.2022.12138. Epub [PubMed PMID: 35997724]
Level 1 (high-level) evidenceSeshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006 Feb:37(2):345-50 [PubMed PMID: 16397184]
Level 2 (mid-level) evidenceCarandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006 Dec 27:296(24):2939-46 [PubMed PMID: 17190894]
Level 2 (mid-level) evidenceChohan SA, Venkatesh PK, How CH. Long-term complications of stroke and secondary prevention: an overview for primary care physicians. Singapore medical journal. 2019 Dec:60(12):616-620. doi: 10.11622/smedj.2019158. Epub [PubMed PMID: 31889205]
Level 3 (low-level) evidenceConnell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical rehabilitation. 2008 Aug:22(8):758-67. doi: 10.1177/0269215508090674. Epub [PubMed PMID: 18678576]
Puri I, Bhatia R, Vibha D, Singh MB, Padma MV, Aggarwal P, Prasad K. Stroke-related education to emergency department staff: An acute stroke care quality improvement initiative. Neurology India. 2019 Jan-Feb:67(1):129-133. doi: 10.4103/0028-3886.253636. Epub [PubMed PMID: 30860110]
Level 2 (mid-level) evidence