Introduction
The concept of contrast is the foundation upon which imaging rests. Contrast is simply the ability to distinguish 2 objects. Medical imaging allows adjacent substances or tissue to be distinguishable and visualized. Contrast allowed Marie Curie to identify her bones from the outline of her hand.[1] The ability to distinguish target tissue from the surrounding structures is how anatomy is defined, pathology is identified, and diagnoses are made.[2] While specific anatomical structures have inherent contrast due to their physical properties, others are naturally poorly delineated. See Image. Computed Tomography With Contrast. Radiographs easily define bones against a background of muscle and fat, but identifying early hepatocellular carcinoma from a background of a nodular cirrhotic liver is limited using routine computed tomography imaging.[3] To increase the contrast of these very similar tissues, an agent can change the appearance of the target tissue, the background tissue, or both.[4]
The optimal use of contrast depends on the modality and physics of the imaging system. Radiographs and computed tomography reflect how a target tissue depletes an energy signal as it passes through the tissue. The variables contributing to the signal loss include the energy beam’s and the target’s physical properties.[5] By changing the beam’s wavelength, amplitude, and frequency, inherent contrast can be maximized.[2] Different techniques are utilized to evaluate a rib fracture versus pneumonia despite both studies covering the same anatomical structures. The physical density, atomic structure, and location of lung parenchyma differ from the bones.[5] While the technique can be manipulated to maximize some of the tissue’s characteristics, the pathology or tissue of concern may be beyond the system's contrast resolution unless outside variables are introduced. Contrast media is a material added to delineate or better discern these otherwise subtle findings.
Various types of contrast media are useful in medical imaging. The 2 largest groups include computed tomography and magnetic resonance imaging (MRI) agents. The contrast groups are not interchangeable. Additional, less commonly utilized contrast agents, including fluoroscopy-based air or CO2, and molecular imaging (nuclear imaging), are not discussed in depth here.[6] A contrast medium can be utilized internally or externally to a patient, and administration is frequently enterally or intravascularly. Intravascular phases can be arterial, venous, or lymphatic. This topic is limited to administration intravenously.
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The injection of intravenous contrast typically occurs through peripherally inserted catheters. Larger catheters in larger veins allow a faster rate of administration and larger volumes of contrast to be delivered. The most common site for intravenous catheter placement is the antecubital fossa. This location tolerates sizes adequate for the use of power injectors. Power injectors are machines often built into or controlled by the computed tomography scanner that inject the contrast at a preselected rate, time, and volume to meet needed imaging requirements for the desired clinical question. The utilization of power injectors depends on the gauge of the catheter and the flow limits. More distal intravenous locations often do not tolerate 14 to 20-gauge needles and, if utilized, see a higher rate of contrast extravasation and associated complications.[7] More peripheral intravenous catheters also have a longer time delay before contrast reaches the desired location, an essential consideration for the timing of image acquisition. Centrally placed catheters are becoming more frequently used and, when correctly placed, are tolerated well.[8] Peripherally inserted central catheters can be used but may have flow limitations, inhibiting higher power injection rates.[9] Exceeding a catheter's maximum flow rate can cause catheter failure and fracture. While power injectors do a vast majority of injections, some technologists utilize hand injection, which is subject to user variance. Hand injection is often an option in interventional procedures.
In contrast to injection, a technologist can manually select the timing of image acquisition.[10] When considering the timing of a study, multiple factors come into play. The foremost consideration is when (arterial, venous, capillary) in the blood pool cycle, followed by where (central versus peripheral) contrast would maximize the sensitivity and specificity of the desired pathology. The timing of the study to evaluate for pulmonary embolus is different than that of assessing for deep venous thrombus. Once the desired timing and location are known, patient factors such as height, weight, cardiac output, vascular disease, and portal hypertension come into play.
Homogenous distal venous opacification is more variable than central arterial opacification because more patient factors are involved. Most studies take place at a set time interval after contrast administration. An alternative method with lower variability sets at trigger once a targeted area is enhanced beyond the selected threshold and the imaging starts.[10] For this, a small amount of radiation is pulsed over a selected target, such as the pulmonary artery. Once there is a measurable increase in attenuation, the computed tomography scanner is triggered to scan at the selected time interval. While this does reduce some variance, the placement of the target gate is subject to human error and can result in inadequate timing. A common example of a mishap occurs when the target overlies the aorta instead of the pulmonary artery, resulting in the incorrect timing of a pulmonary embolus study.[11]
For pathology in the upper extremity, contrast should be injected in the contralateral side to prevent confounding venous enhancement or streak artifact.
Plain Films
Intravenous contrast is no longer routinely used to evaluate plain films (radiographs). Intravenous pyelograms were previously used to evaluate the urinary collecting system but are now considered inferior to their computed tomography equivalent.[12] One potential use for plain films is identifying the location of extravasated contrast if there is clinical uncertainty; however, most cases can be managed based on clinical symptoms alone.
Computed Tomography
Computed Tomography Intravenous Contrast
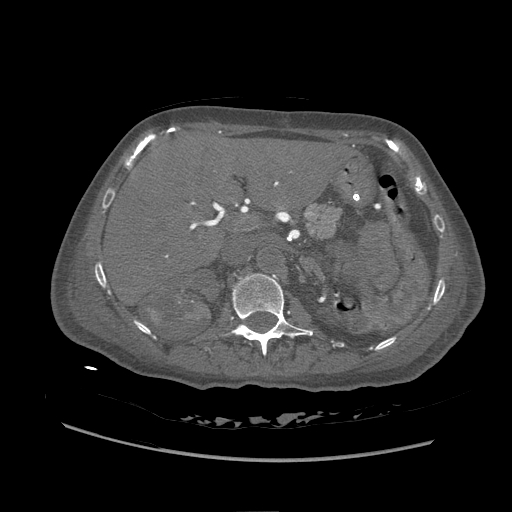
Intravenous contrast for computed tomography is the most commonly used contrast agent overall (see Image. Contrasted Computed Tomography of the Abdomen, Aortic Thrombosis). X-ray beams rely on energy passing through tissue, with some energy being deflected or absorbed. The difference in the resulting rays or shadows creates an amplitude-dependent picture. The tissue modifies the energy beam or x-ray through scattering and absorption. Iodine is an element used in contrast media that utilizes both methods and subsequently changes the X-ray.[13] It is both physically dense, causing scatter, and having outer electrons with binding energy at just the right level to absorb x-ray energy, which is eventually released in another direction or converted into heat. While iodine is naturally found in human bodies, the quantities of it needed to cause a signal change in targeted tissues would be lethal. For this reason, iodine is bound into a larger molecular structure, so it can be less biologically active and predominantly filtered and secreted with minimum disassociation.[13]
Early binding agents used for iodine were high osmolality agents, with their osmolality often exceeding 1500 mosm/kg H2O, approaching 5 to 8 times that of normal human serum, 290 mosm/kg H2O. Due to multiple side effects, by the mid-1990s, these agents had declined in use in favor of low-osmolality (less than 3 times the osmolality of normal human serum) or even iso-osmolar agents.[14] In addition to reducing osmolality, altering the ion levels and viscosity has decreased the incidence of adverse reactions and side effects.[15]
The addition of intravenous contrast media increases the density and, thus, attenuation of the blood with which it mixes. The appearance of intravenous contrast depends on the timing and contrast concentration. The iodinated blood results in signal loss or opacification. Early timing reveals undiluted or minimally diluted medium as it gets injected through the veins, and the attenuation of energy may be so great as to cause streak artifact. As it moves centrally, more blood mixes with the medium. As time passes, the contrast becomes progressively diluted as it passes into the arteries, tissues, and distal peripheral veins. It obtains equilibration within several minutes before being filtered and then secreted, predominantly through the urinary system. Modern contrast agents diffuse quickly, and mixing iodinated and non-iodinated blood is more related to blood flow than diffusion properties.[16]
The appearance of tissues in a contrast-enhanced study depends on the timing of image acquisition relative to the contrast bolus. This timing depends on the pathology of interest or study indication.[16] A chest computed tomogram used to evaluate a chest wall mass needs contrast in a different area (capillary beds) than when assessing for a pulmonary embolism in the pulmonary artery. Increasing the contrast of pathology requires understanding the pathophysiology related to its blood supply. For example, a large vessel dissection may be most visible with dense contrast in the arterial phase, but a slow bleed may be better appreciated with the gradual accumulation of hyperdense products in a delayed image.[16] Another example is how hepatocellular carcinoma is hypervascular compared to surrounding parenchyma, but most colonic metastases to the liver are hypo-vascular; hence, there is a need for images from multiple time points evaluating for liver disease.[17] Patient variables, such as patient size, weight, vascular disease, and cardiac function, further complicate the contrast timing. A young pregnant female opacity her pulmonary arteries much quicker than a patient with heart failure. The radiologist determines the timing of contrast boluses and the infusion rate in conjunction with the technologist and gets tailored for the patient, the indication, and the equipment.
Contrast-Induced Nephropathy
Early studies of contrast safety and efficacy quickly established links to contrast use and a decline in renal function (see Image. Transitional Cell Carcinoma of the Bladder). This association, once widespread, has come under scrutiny in the last few decades.[18] The American College of Radiology now describes that contrast-induced nephropathy (CIN) is not as prevalent as once estimated, and what was often previously called CIN may better be classified as post-contrast acute kidney injury (PC-AKI) due to many renal injuries being associated and not caused by the contrast. While often an AKI after contrast can be attributed to other risk factors, not all PC-AKI can be accounted for consistently with a real risk of CIN, even if lower than once thought. The exact physiology of CIN is not known. PC-AKI and CIN definitions vary slightly; however, most use the following criteria within 48 hours of contrast administration.[19][20][21]
- Serum creatinine increase 0.3 mg/dl
- Increase in serum creatinine 50%
- Urine output of less than 0.5 ml/kg/hr for at least 6 hours.
Many early study design flaws that overrepresented CIN are now being corrected. Inadequate risk factor identification and subsequent control groups have been the most cited cause of misleading associations. The early studies had their basis on high osmolality contrast, a contrast medium no longer used with a higher side effect profile. Many of the early studies predominantly utilized patients undergoing heart catheterization, a procedure associated with significant embolic and nephrotoxic risk in addition to the contrast use.[22] Furthermore, fluoroscopic studies would utilize contrast volume, concentrations, and viscosities different from computed tomography. Most CIN studies were based on hospitalized patients who had numerous additional causes for AKI beyond contrast administration and procedural risk.[23] The definition of AKI also varied, with creatinine most often defining AKI. Creatinine levels do not always correlate with renal injury or may be delayed.[24][18][25] The estimated glomerular filtration rate (eGFR) has proven more effective for identifying CIN and PC-AKI.[26]
Creating a randomized controlled study to describe CIN has proven difficult, if not impossible, to employ.[18] As studies began to have more robust control groups and employed propensity score matching, the calculated AKI risk related to contrast administration dropped significantly.[27][28] Guidelines now suggest that CIN risk is highest in those with baseline impaired renal function based on eGFR.[29] Levels equal to or above 45 ml/min/1.73m2 are considered the normal risk, with no precautions recommended. An eGFR below 30 ml/min/1.73m2 is considered a higher risk, and a risk-benefit analysis discussion and documentation is necessary. An eGFR between 30 and 45 ml/min/1.73m2 is borderline; however, the ACR advocates that they are not at increased risk.[30]
Estimates are that it takes approximately 20 hours for normally functioning kidneys to clear contrast. The concern that increased contrast levels may have a nephrotoxic effect has led to the idea of waiting 24 hours between contrasted studies; however, no studies have adequately addressed this concept. In a patient with no renal function, no CIN is possible; however, for those in late-stage renal disease still producing urine, the patient may still be at increased risk. While the true risk of CIN or PC-AKI is fully delineated, contrast use likely increases as contrast agents improve, and researchers continue to report low levels of CIN.[31]
Contrast Allergy
Like CIN, the rates of contrast allergy have dynamically varied.[32] The early hyperosmolar agents had high rates of allergic and physiologic reactions; some reported as high as 15%.[19][33] These agents are no longer in use, and adverse reactions are much lower with current agents. In addition to the previously higher reaction rates, the idea that shellfish allergy is related to iodine once pervaded the public mind, and the erroneous link of shellfish allergy to contrast allergy is still often reported by patients. There is no correlation between shellfish allergy and iodinated allergy.[34]
The American College of Radiology has classified reactions into 2 basic categories: physiologic and allergic-like reactions and each category is subdivided into mild, moderate, and severe. The physiologic reactions are often secondary to pain, vasovagal, ionotropic, infusion sensation, and neurologic. While often seen as benign and dependent on the dose, these physiologic reactions can be deadly, with seizures or life-threatening hypotension and arrhythmia.[35] Physiologic reactions include but are not limited to nausea, vomiting, flushing, chills, warmth, headaches, dizziness, anxiety, metallic taste, hypertension, arrhythmia, and seizures.
While a type 1 or IgE response mediates most allergies, only 50% of severe contrast have a corresponding skin test; this suggests an alternative or histamine-dependent pathway.[36] Allergy-like reactions can be severe, with anaphylactic reactions requiring immediate care, and are independent of dose once above threshold limits.[37] The differentiation of physiologic and allergic-like reactions guides treatment and pretreatment recommendations. Allergy-like reactions are well-defined and described in the American College of Radiology manual on contrast media. Mild reactions are self-limiting. Moderate symptoms can progress if therapy is not initiated. Mild to moderate allergic reactions include diffuse edema, facial edema without dyspnea, pruritus, urticaria, itchy throat, nasal congestion, diffuse erythema, conjunctivitis, bronchospasm, wheezing, or mild hypoxia. Severe reactions require intervention and can be life-threatening if not treated appropriately. Severe reactions include diffuse edema or facial edema with dyspnea, erythema with hypotension, laryngeal edema with stridor, wheezing, or bronchospasm with significant hypoxia or anaphylactic shock.[33]
The treatment for acute contract reactions depends on the presenting symptom, and radiologists and emergency physicians are typically well-versed. Treatment paradigms should include but not be limited to bronchospasm, laryngeal edema, hypotension, anaphylactic reactions, pulmonary edema, hypertensive crisis, seizures, hypoglycemia, and anxiety. Example treatment paradigms are in the ACR Manual on Contrast Media.[38]
The combination of allergic and physiologic reactions associated with low osmolality contrast mediums is low, with reports varying between 0.2% and 0.7%.[19] A prior allergic-like reaction is the greatest risk factor, with an increased risk of 5-fold to 6-fold.[33][39] Patients with increased risk from prior reactions should merit consideration for pretreatment. Pretreatment targets patients with mild to moderate reactions, with limited data to show the efficacy of pretreating patients with prior severe reactions.
Pretreatment Algorithms
Pretreatment algorithms are focused on multiple doses of steroids with a small period to permit steroid efficacy and an additional dose of antihistamine before contrast injection. An estimated 4 to 6 hours are required before steroids can mitigate allergic-type reactions, and the most cited algorithm has a 13-hour protocol.[40] A 5-hour protocol has been established, but the efficacy of a shorter duration has yet to be proven in large cohort studies, so many institutions prefer the 13-hour protocol for routine studies. Protocols 1 and 2 below are for routine studies where a 13-hour treatment is feasible. Protocols 3 and 4 can be utilized in a 5-hour protocol when a 13-hour protocol compromises patient care.[41][42]
- Prednisone 50 mg PO, 13, 7, and 1 hour before the scan. Diphenhydramine 50 mg PO/IV/IM 1 hour before the scan.
- Methylprednisolone 32 mg PO 13 and 2 hours before the scan. Diphenhydramine 50 mg PO/IV/IM 1 hour before the scan.
- Methylprednisolone 40 mg intravenously or hydrocortisone 200mg intravenously every 4 hours for at least 2 doses of diphenhydramine 50 mg intravenously 1 hour before the scan.
- Dexamethasone 7.5 mg intravenously or betamethasone 6 mg intravenously every 4 hours for at least 2 doses. Diphenhydramine 50 mg intravenously 1 hour before the scan.
Even with pretreatment, estimates are that 12% of patients with prior reactions have breakthrough reactions; however, the severity is typically similar or less than previous responses.[43] The number needed to treat for mild and moderate reactions was estimated at 69, with the NNT for severe reactions much higher at 569 in a study of 1051 pretreated patients.[41]
Metformin Use
Metformin is a medication commonly used to manage diabetes. Metformin use is associated with lactic acidosis, a potential side effect exacerbated by poor renal function. No special precautions are warranted if patients are appropriately screened for contraindications to include renal function. Since there is a risk of CIN or PC-AKI with contrast use, the development of new or worsened renal dysfunction can merit altering a patient’s metformin use until such dysfunction is ruled out to prevent lactic acidosis.[44] The ACR recommends for patients with normal renal function without suspected AKI and a baseline eGFR equal to or greater than 30 mL/min/1.73 m2, there is no need to suspend metformin use or test post-contrast renal function. For patients with an eGFR below 30 mL/min/1.73m2, suspected of AKI, or for a procedure that increases renal embolic risk, the ACR recommends that metformin is held for 48 hours and restarted after evaluating renal function.[45]
Other Intravenous Contrast Complications and Considerations
Contrast extravasation occurs in 0.1% to 1% of intravenous contrast administrations, with the most common correlating risk factor being peripheral wrist or distal leg intravenous injection site.[46] Extravasation complications are typically mild, with supportive care, including brief observation, usually sufficient.[47] Needle aspiration has not proven therapeutic. The risk of extravasation is not well correlated with volume; however, compartment syndrome correlates with larger volumes. Surgical consultation should be emergently sought if there are compartment syndrome or vascular compromise indications. Indications of compartment syndrome include altered tissue perfusion, change in sensation, progressive pain, progressive loss in the range of motion (passive and active), or paresthesia.[48] The swelling may increase but should peak within 48 hours, and patients should be given appropriate return instructions before discharge.
Myasthenia gravis exacerbations have correlated with contrast administrations.[49] This topic is under debate in the literature, and the ACR considers it a relative contraindication for contrast administration. Active thyroid storm and patients undergoing thyroid ablation are relative contraindications to contrast administration. There is insufficient evidence for the ACR to suggest special precautions for sickle cell disease, pheochromocytoma, beta-blocker use, or non-thyroid storm thyrotoxicosis.[38]
Intravenous contrast does cross the placenta and is detectable within the fetus.[19] While the levels are low and transient, the FDA has classified them as category B medications without adequate findings to suggest an increased risk to the mother or fetus. Due to the unknown risk, contrast use is rare in a pregnant woman. The most common scenario that utilizes contrast in pregnancy is the evaluation of pulmonary embolus. There is insufficient evidence to suggest that iodinated contrast is a risk to the mother or fetus, including thyroid function.[19]
Similarly, contrast is secreted in breast milk in low doses, with only a small amount of ingested contrast absorbed.[50] Breastfeeding can continue after intravenous contrast without increased risk to the baby; however, if parents are concerned about excreted contrast, breast milk for up to 24 hours may be pumped and discarded. There is no benefit to discarding milk beyond 24 hours. There has been no documented perinatal hypothyroidism from LCOM intravenous administration.
Magnetic Resonance
MRI Contrast
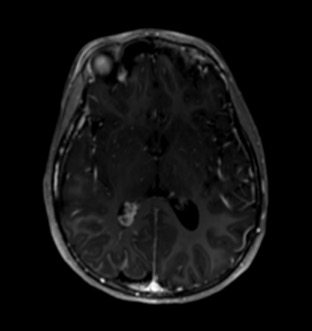
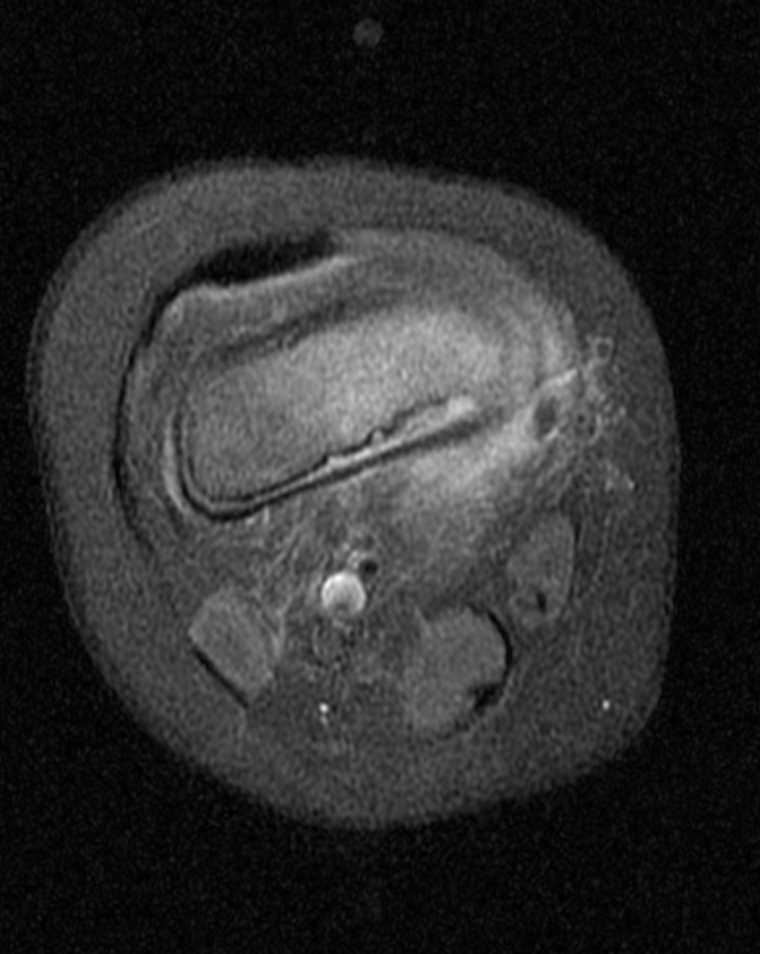
While iodine-based contrast directly affects the passage of the signal through the target material, gadolinium indirectly changes the signal measured in the surrounding water. MRI signal is detected by subtle variances of the magnetic fields created by the orientation of water molecules. A large magnetic field aligns these molecules; then, they are subject to radio pulses, modulating the orientation, angulation, and measurable signal. After the radio pulse, the water molecules start to realign themselves with the MRI magnet field, and the T1 and T2 signals can be measured, which gradually tapers through a period known as relaxation time.[51] Gadolinium is paramagnetic, which distorts the magnetic field established by the MRI machine. This metallic structure can cause shortened relaxation time on the water molecules within the immediate vicinity, causing increased signal.[52] See Images. Axial T1 Postcontrast Brain MRI, MRI T1-Weighted Postcontrast Knee Osteomyelitis.
Like iodine, gadolinium is toxic with properties similar to calcium. It is attached to a chelating molecule that mitigates its harmful effect while allowing it to alter the MRI signal of the surrounding water.[53] The combined gadolinium and chelators are quickly filtered from the bloodstream and excreted. There are 2 forms of chelating molecules, a linear and a cage-like macrocyclic complex, with the newer macrocyclic forms having lower dissociation rates. The disassociation from these chelators and empty chelators themselves are believed to be the source of most of the adverse effects. Experts once thought that gadolinium levels were only transient in a patient; post-mortem studies have shown detectable levels years after administration, and the long-term effects of that are not understood.[54] The long-term effects of gadolinium deposition will likely be a topic of study for years. The most well-known reaction to gadolinium-based contrast is that of nephrogenic systemic fibrosis.
Nephrogenic Systemic Fibrosis
In the late 1990s, gadolinium-based intravenous contrast MRI studies were on the rise, and similarly, reports of a strange skin disease arose that confounded researchers. While it baffled many scientists for years, eventually, an association with gadolinium-based contrast and poor renal function was made.[55] Nephrogenic systemic fibrosis (NSF) is not entirely understood but is believed to arise from free gadolinium. Pathophysiology is not defined, but it is suspected that once freed from its carrier molecule, gadolinium causes inflammation and fibrosis, which manifests as a skin condition. It involves muscles and visceral organs and can progress to induce fibrosis and possibly death. The initial presentation is often vague, with weakness, pruritus, and skin papules. The skin has a woody, firm, anchored feeling as it progresses or may even resemble a peau d'orange texture.[56] The diagnosis of NSF is a combination of a physical exam, histological findings, and a critical history of gadolinium exposure.
NSF was eventually found to be associated with reduced renal function, and the FDA put a black box warning on gadolinium-based drugs.[57] Since then, the rate of NSF has significantly dropped and has almost become a historical diagnosis. The risk of NSF depends on the type of gadolinium and the amount of renal dysfunction. Types of gadolinium fall into 3 groups, with group 1 agents having a higher risk of NSF warranting specific recommendations by the ACR. These recommendations for group 1 agents reflect how the risk of NSF is inversely related to renal function. The ACR recommends against utilizing group 1 agents with eGFR at or below 30 mL/min/1.73m2. If group 1 agents are required, a documented discussion of the risk-benefit ratio with the patient is recommended.[38] Group 2 agents have correlated with such a low rate of NSF that the ACR makes no special precautions. Group 3 agents are relatively new and do not have sufficient evidence to warrant special precautions; however, the risk is suspected to be low, especially with hepatically cleared agents.
Additional factors have been shown to increase the risk of NSF, including AKI in the setting of chronic renal dysfunction, concomitant hepatic dysfunction, repeated boluses of contrast, a high volume of contrast, hypertension requiring medical therapy, diabetes, and patients undergoing dialysis.[58] For those undergoing dialysis, it is recommended to coordinate dialysis in close approximation to reduce prolonged levels of circulating gadolinium. In all cases, contrast boluses are recommended to use low doses and avoid unnecessary repeated contrast-enhanced studies.[59]
Gadolinium Allergy
Like iodinated contrast, intravenous gadolinium injections correlate with allergic-like and physiologic-like reactions, with most constituting physiologic reactions. Allergic reactions are believed to be equal or less than those of iodinated contrast, with rates published between 0.004% and 0.7%.[60] The most significant risk factor for having an allergic-like reaction is a prior allergic reaction to gadolinium-based contrast. There is no cross-reactivity of iodine contrast-based allergies with gadolinium-based allergies. Treatment paradigms and pretreatment therapies are identical to those for iodinated contrast allergies.[59]
The rate of contrast extravasation is less than that of computed tomography, with supportive care being adequate for almost all patients.[61] An often-overlooked risk factor a responder must consider is the patient's location as it relates to the MRI magnet. To prevent magnet-related accidents and deaths, moving the patient out of the scanning room is highly encouraged before beginning treatment. MRI facilities typically have specific MRI-safe resuscitation tools to prevent metallic objects from becoming deadly projectiles. There have been cases of patient deaths from oxygen bottles, patient gurneys, and wheelchairs getting caught in the powerful MRI magnet. The mantra "the magnet is always on" does not change when emergent treatment is needed.[62]
Gadolinium in Pregnancy and Breastfeeding
Gadolinium is detectable in the fetus and breast milk after intravenous injection. The effects of intrauterine gadolinium administration are unknown; thus, gadolinium-based contrast is generally avoided whenever possible while pregnant.[63] Like iodinated contrast, gadolinium-based contrast is present in breast milk at trace levels. The levels are so low that the ACR recommends that breastfeeding habits do not require change for lactating mothers. The effects of trace gadolinium in an infant are unknown; thus, mothers who wish to suspend breastfeeding are advised that pumping and dumping for 12 to 24 hours is appropriate, and there is little to no benefit to doing this beyond 24 hours.[19]
Ultrasonography
Current interest in the imaging capabilities of contrast-enhanced ultrasound is mostly limited to academic institutions. One of the most significant advantages is the lack of renal injury associated with contrast use compared to computed tomography or MRI-based contrast.[64] This characteristic allowed increased sensitivity when screening for masses and increased characterization regardless of renal function. The principle of ultrasound contrast depends on the resonance of air bubbles to sound waves, causing an increase in the backscatter signal up to 30dB.[65] This signal, combined with harmonic waveforms, allows for high resolution of the intravascular contrast. Ultrasound contrast comprises small phospholipid or albumin-coated microspheres with small pockets of air. The air eventually diffuses out of the microsphere, and the empty coat is then filtered out by the kidneys and endoreticular system.
Contrast-enhanced ultrasound permits real-time evaluation of the enhancement characteristics, allowing targeted assessment of the wash-in and washout kinetic patterns. There is ongoing research to determine the imaging characteristics of different masses; many parallel enhancement patterns are seen in computed tomography and MRI. While initially used as a screening tool, requiring computed tomography or MRI for complete characterization, contrast-enhanced ultrasound has the potential to diagnose more pathologies in the future definitively.[66]
Nuclear Medicine
The image acquisition of nuclear medicine is vastly different from computed tomography and MRI. While contrast mediums act upon the imaging signal, injected radiopharmaceuticals are the imaging source and are not acted upon by intravenous mediums. Nuclear medicine utilizes computed tomography and MRI imaging for SPECT-CT, PET-CT, and PET-MRI; each may be contrast-enhanced to improve the specificity of selected pathologies.[67][68] Consultation with a nuclear medicine-trained radiologist may be warranted to discuss adding contrast enhancement to these studies.
Angiography
Classically, angiography has referred to studies done under fluoroscopy; however, the advent of rapid computed tomography image acquisition has led to computed tomography angiography. Classic angiography is now predominantly found in the interventional radiology suite and the cardiac catheter lab. Computed tomography angiography is often utilized because it is faster, costs less, and is less user-dependent. The sensitivity of computed tomography vs. fluoroscopic evaluation depends on the indication. The spatial resolution is higher with fluoroscopic angiography; however, the ability to define surrounding structures or contrast resolution is far superior to computed tomography. While many studies came from the fluoroscopy suite, computed tomography angiography has become the standard in many situations.
The use of intravenous contrast in computed tomography angiography relies on the combination of contrast enhancement curves and the timing of the study. Fluoroscopic evaluation utilizes small intravascular boluses administered in real time by a physician. The real-time evaluation allows the physician to adapt the study to the findings, allowing targeted evaluation of multiple areas and more judicious use of contrast. Computed tomography temporal resolution is limited to a few time points that can be assessed but can cover larger areas with the larger boluses utilized.[69] Both interventional radiology and cardiology have been acutely aware of the CIN risk they expose their patients to and strive to utilize better tolerated reduced osmolality agents. While concentrations may vary, a total volume not to exceed 3.7 to 5 ml/kg is the recommendation.[70][71]
MRI angiography, like computed tomography angiography, is quickly becoming clinically advantageous. A classic scenario is the MRI of the head and neck to evaluate vasculature following a transient ischemic attack. One advantage of MRI is that some vasculature can be evaluated without contrast, utilizing the flow properties of moving blood.[72] While this method is useful when renal function prohibits computed tomography or MRI contrast, it may be riddled with artifacts and is not the diagnostic image of choice. The MRI evaluation of the neck can be done with and without contrast, with some institutions utilizing both methods to account for various forms of artifacts that can confound 1 method versus another.[73] MRI angiography for pulmonary embolus without contrast is another area that is being developed and may soon become more widespread.[74]
Patient Positioning
The positioning of patients in contrast-enhanced computed tomography or MRIs is limited to the obstruction of vascular flow and the layering of extravascular contrast. The positioning of arms is often changed to prevent signal loss and increased doses. Technicians must take care when positioning the arms to prevent the compression of vascular structures, especially on the limb through which injection occurs. Compressed limbs cause increased extravasation and poor contrast bolus formation.
Evaluation of the urological system utilizes extracted contrast to evaluate the ureters and bladder. The normal peristalsis limits the evaluation of the ureters, and multiple time points may be an option before the complete pacification of the ureters. As contrast fills the bladder, there can be mixing artifacts and possible layering of dense contrast. To minimize mixing artifacts and allow the most sensitive evaluation of the bladder wall, the patient is often asked to roll several times. The inability to mix contrast within the bladder in non-mobile patients may lead to inconclusive or false-positive studies.[75]
Clinical Significance
Using contrast media has increased the sensitivity and specificity of many exams. While not all conditions merit contrast, ordering physicians should know the risks. The risk of PC-AKI (CIN) and NSF is most evident for those with an eGFR at or below 30, and caution is advised. While undergoing contrast administration, nurses, technologists, and physicians who know how to identify and treat potentially life-threatening reactions should monitor patients for reactions. The history of a prior reaction may merit pretreatment of at least 5, or possibly 13 hours. The use of contrast in a pregnant population is limited, but in lactating women, no pause in breastfeeding is required.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
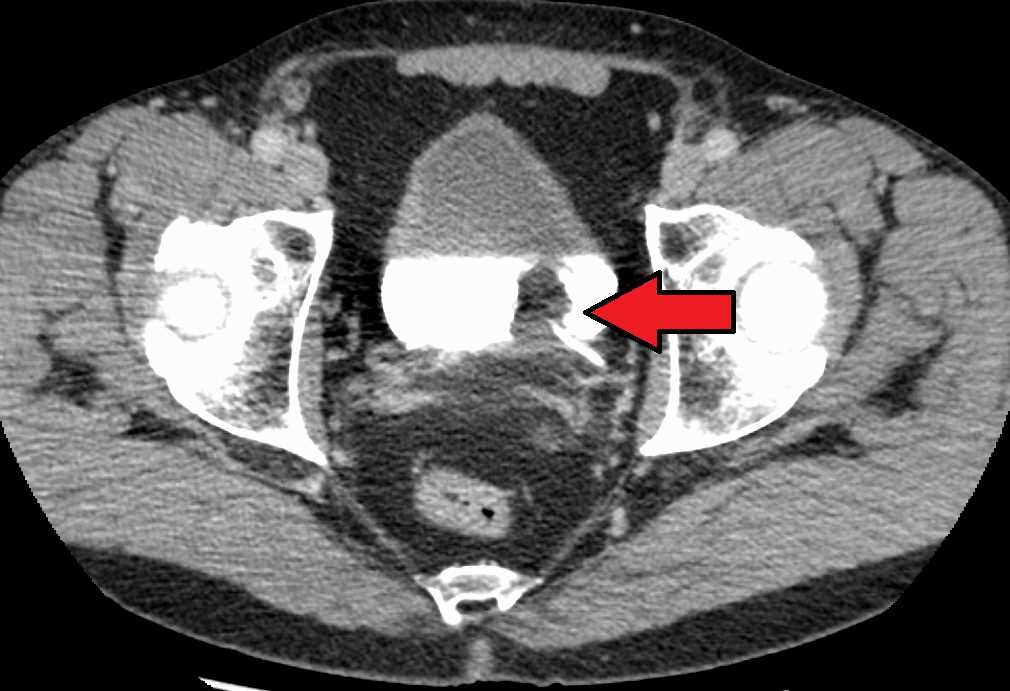
Transitional Cell Carcinoma of the Bladder. The white area in the bladder is contrast.
James Heilman, MD, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
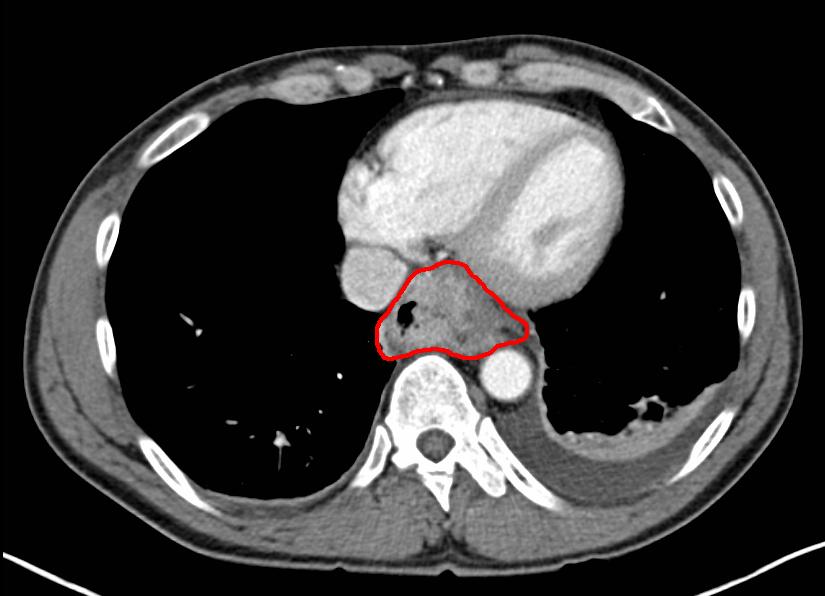
Computed Tomography With Contrast. Computed tomography with contrast axial image showing cancer of the esophagus.
Tdvorak, Public Domain, via Wikimedia Commons
References
Kułakowski A. The contribution of Marie Skłodowska-Curie to the development of modern oncology. Analytical and bioanalytical chemistry. 2011 Jun:400(6):1583-6. doi: 10.1007/s00216-011-4712-1. Epub [PubMed PMID: 21331492]
Mazonakis M, Damilakis J. Computed tomography: What and how does it measure? European journal of radiology. 2016 Aug:85(8):1499-504. doi: 10.1016/j.ejrad.2016.03.002. Epub 2016 Mar 10 [PubMed PMID: 26995675]
Willatt J, Ruma JA, Azar SF, Dasika NL, Syed F. Imaging of hepatocellular carcinoma and image guided therapies - how we do it. Cancer imaging : the official publication of the International Cancer Imaging Society. 2017 Mar 4:17(1):9. doi: 10.1186/s40644-017-0110-z. Epub 2017 Mar 4 [PubMed PMID: 28259177]
Burrowes DP, Medellin A, Harris AC, Milot L, Wilson SR. Contrast-enhanced US Approach to the Diagnosis of Focal Liver Masses. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Sep-Oct:37(5):1388-1400. doi: 10.1148/rg.2017170034. Epub [PubMed PMID: 28898188]
Seeram E. Computed Tomography: A Technical Review. Radiologic technology. 2018 Jan:89(3):279CT-302CT [PubMed PMID: 29298954]
Hahn ST, Pfammatter T, Cho KJ. Carbon dioxide gas as a venous contrast agent to guide upper-arm insertion of central venous catheters. Cardiovascular and interventional radiology. 1995 May-Jun:18(3):146-9 [PubMed PMID: 7648588]
Level 1 (high-level) evidenceSchaverien MV, Evison D, McCulley SJ. Management of large volume CT contrast medium extravasation injury: technical refinement and literature review. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2008:61(5):562-5; discussion 565 [PubMed PMID: 17459795]
Level 3 (low-level) evidenceBuijs SB, Barentsz MW, Smits MLJ, Gratama JWC, Spronk PE. Systematic review of the safety and efficacy of contrast injection via venous catheters for contrast-enhanced computed tomography. European journal of radiology open. 2017:4():118-122. doi: 10.1016/j.ejro.2017.09.002. Epub 2017 Sep 29 [PubMed PMID: 29034281]
Level 1 (high-level) evidenceCoyle D, Bloomgarden D, Beres R, Patel S, Sane S, Hurst E. Power injection of contrast media via peripherally inserted central catheters for CT. Journal of vascular and interventional radiology : JVIR. 2004 Aug:15(8):809-14 [PubMed PMID: 15297584]
Hinzpeter R, Eberhard M, Gutjahr R, Reeve K, Pfammatter T, Lachat M, Schmidt B, Flohr TG, Kolb B, Alkadhi H. CT Angiography of the Aorta: Contrast Timing by Using a Fixed versus a Patient-specific Trigger Delay. Radiology. 2019 May:291(2):531-538. doi: 10.1148/radiol.2019182223. Epub 2019 Mar 5 [PubMed PMID: 30835189]
Chaturvedi A, Oppenheimer D, Rajiah P, Kaproth-Joslin KA, Chaturvedi A. Contrast opacification on thoracic CT angiography: challenges and solutions. Insights into imaging. 2017 Feb:8(1):127-140. doi: 10.1007/s13244-016-0524-3. Epub 2016 Nov 17 [PubMed PMID: 27858323]
Hale Z, Hanna E, Miyake M, Rosser CJ. Imaging the urologic patient: the utility of intravenous pyelogram in the CT scan era. World journal of urology. 2014 Feb:32(1):137-42. doi: 10.1007/s00345-013-1085-4. Epub 2013 Apr 25 [PubMed PMID: 23615746]
Level 2 (mid-level) evidenceSpampinato MV, Abid A, Matheus MG. Current Radiographic Iodinated Contrast Agents. Magnetic resonance imaging clinics of North America. 2017 Nov:25(4):697-704. doi: 10.1016/j.mric.2017.06.003. Epub 2017 Aug 23 [PubMed PMID: 28964459]
Baerlocher MO, Asch M, Myers A. The use of contrast media. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2010 Apr 20:182(7):697. doi: 10.1503/cmaj.090118. Epub 2010 Mar 15 [PubMed PMID: 20231343]
Costa N. Understanding contrast media. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2004 Sep-Oct:27(5):302-12 [PubMed PMID: 15385894]
Level 3 (low-level) evidenceBae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010 Jul:256(1):32-61. doi: 10.1148/radiol.10090908. Epub [PubMed PMID: 20574084]
Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A. Liver metastases of colorectal cancer: US, CT or MR? Cancer imaging : the official publication of the International Cancer Imaging Society. 2005 Nov 23:5 Spec No A(Spec No A):S149-56 [PubMed PMID: 16361131]
Luk L, Steinman J, Newhouse JH. Intravenous Contrast-Induced Nephropathy-The Rise and Fall of a Threatening Idea. Advances in chronic kidney disease. 2017 May:24(3):169-175. doi: 10.1053/j.ackd.2017.03.001. Epub [PubMed PMID: 28501080]
Level 3 (low-level) evidenceBeckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015 Oct:35(6):1738-50. doi: 10.1148/rg.2015150033. Epub [PubMed PMID: 26466182]
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS. Post-contrast acute kidney injury - Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. European radiology. 2018 Jul:28(7):2845-2855. doi: 10.1007/s00330-017-5246-5. Epub 2018 Feb 9 [PubMed PMID: 29426991]
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. European radiology. 2018 Jul:28(7):2856-2869. doi: 10.1007/s00330-017-5247-4. Epub 2018 Feb 7 [PubMed PMID: 29417249]
Wyman RM, Safian RD, Portway V, Skillman JJ, McKay RG, Baim DS. Current complications of diagnostic and therapeutic cardiac catheterization. Journal of the American College of Cardiology. 1988 Dec:12(6):1400-6 [PubMed PMID: 2973480]
Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 May:39(5):930-6 [PubMed PMID: 11979336]
Mervak BM, Cohan RH, Ellis JH, Khalatbari S, Davenport MS. Intravenous Corticosteroid Premedication Administered 5 Hours before CT Compared with a Traditional 13-Hour Oral Regimen. Radiology. 2017 Nov:285(2):425-433. doi: 10.1148/radiol.2017170107. Epub 2017 Jul 26 [PubMed PMID: 28745940]
Badrick T, Turner P. The Uncertainty of the eGFR. Indian journal of clinical biochemistry : IJCB. 2013 Jul:28(3):242-7. doi: 10.1007/s12291-012-0280-1. Epub 2012 Dec 28 [PubMed PMID: 24426218]
Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013 Sep:268(3):719-28. doi: 10.1148/radiol.13122276. Epub 2013 Apr 11 [PubMed PMID: 23579046]
Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean journal of radiology. 2015 Mar-Apr:16(2):286-96. doi: 10.3348/kjr.2015.16.2.286. Epub 2015 Feb 27 [PubMed PMID: 25741190]
McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014 Apr:271(1):65-73. doi: 10.1148/radiol.13130775. Epub 2014 Jan 16 [PubMed PMID: 24475854]
Level 2 (mid-level) evidenceRao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006 May:239(2):392-7 [PubMed PMID: 16543592]
Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The Controversy of Contrast-Induced Nephropathy With Intravenous Contrast: What Is the Risk? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2020 Jan:75(1):105-113. doi: 10.1053/j.ajkd.2019.05.022. Epub 2019 Aug 28 [PubMed PMID: 31473019]
Level 3 (low-level) evidenceLameire NH. Contrast-induced nephropathy--prevention and risk reduction. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006 Jun:21(6):i11-23 [PubMed PMID: 16723348]
Fakhran S, Alhilali L, Kale H, Kanal E. Assessment of rates of acute adverse reactions to gadobenate dimeglumine: review of more than 130,000 administrations in 7.5 years. AJR. American journal of roentgenology. 2015 Apr:204(4):703-6. doi: 10.2214/AJR.14.13430. Epub [PubMed PMID: 25794059]
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR. American journal of roentgenology. 2008 Aug:191(2):409-15. doi: 10.2214/AJR.07.3421. Epub [PubMed PMID: 18647910]
Level 2 (mid-level) evidenceBoehm I. Seafood allergy and radiocontrast media: are physicians propagating a myth? The American journal of medicine. 2008 Aug:121(8):e19. doi: 10.1016/j.amjmed.2008.03.035. Epub [PubMed PMID: 18691465]
Level 3 (low-level) evidenceBoyd B, Zamora CA, Castillo M. Managing Adverse Reactions to Contrast Agents. Magnetic resonance imaging clinics of North America. 2017 Nov:25(4):737-742. doi: 10.1016/j.mric.2017.06.008. Epub [PubMed PMID: 28964463]
Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, Chang YS, Goo JM, Cho SH. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015 Jun:70(6):625-37. doi: 10.1111/all.12589. Epub 2015 Mar 20 [PubMed PMID: 25649510]
Level 1 (high-level) evidenceBush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR. American journal of roentgenology. 1991 Dec:157(6):1153-61 [PubMed PMID: 1950858]
Kodzwa R. ACR Manual on Contrast Media: 2018 Updates. Radiologic technology. 2019 Sep:91(1):97-100 [PubMed PMID: 31471485]
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990 Jun:175(3):621-8 [PubMed PMID: 2343107]
Level 3 (low-level) evidenceLasser EC, Berry CC, Talner LB, Santini LC, Lang EK, Gerber FH, Stolberg HO. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. The New England journal of medicine. 1987 Oct 1:317(14):845-9 [PubMed PMID: 3627208]
Level 1 (high-level) evidenceDavenport MS, Mervak BM, Ellis JH, Dillman JR, Dunnick NR, Cohan RH. Indirect Cost and Harm Attributable to Oral 13-Hour Inpatient Corticosteroid Prophylaxis before Contrast-enhanced CT. Radiology. 2016 May:279(2):492-501. doi: 10.1148/radiol.2015151143. Epub 2015 Nov 4 [PubMed PMID: 26536404]
Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs. changing the contrast medium. European radiology. 2016 Jul:26(7):2148-54. doi: 10.1007/s00330-015-4028-1. Epub 2015 Oct 1 [PubMed PMID: 26427700]
Kim YS, Choi YH, Cho YJ, Lee S, Yoon SH, Park CM, Kang HR. Incidence of Breakthrough Reaction in Patients with Prior Acute Allergic-Like Reactions to Iodinated Contrast Media according to the Administration Route. Korean journal of radiology. 2018 Mar-Apr:19(2):352-357. doi: 10.3348/kjr.2018.19.2.352. Epub 2018 Feb 22 [PubMed PMID: 29520194]
Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clément O, Heinz-Peer G, Reimer P, Stacul F, van der Molen AJ, Webb JA. Metformin and contrast media. Radiology. 2010 Aug:256(2):672-3; author reply 673. doi: 10.1148/radiol.100566. Epub [PubMed PMID: 20656850]
Level 3 (low-level) evidenceBailey CJ, Turner RC. Metformin. The New England journal of medicine. 1996 Feb 29:334(9):574-9 [PubMed PMID: 8569826]
Sinan T, Al-Khawari H, Chishti FA, Al Saeed OM, Sheikh M. Contrast media extravasation: manual versus power injector. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2005 Mar-Apr:14(2):107-10 [PubMed PMID: 15785103]
Williamson EE, McKinney JM. Assessing the adequacy of peripherally inserted central catheters for power injection of intravenous contrast agents for CT. Journal of computer assisted tomography. 2001 Nov-Dec:25(6):932-7 [PubMed PMID: 11711806]
Cohan RH, Ellis JH, Garner WL. Extravasation of radiographic contrast material: recognition, prevention, and treatment. Radiology. 1996 Sep:200(3):593-604 [PubMed PMID: 8756899]
Mehrizi M, Pascuzzi RM. Complications of radiologic contrast in patients with myasthenia gravis. Muscle & nerve. 2014 Sep:50(3):443-4. doi: 10.1002/mus.24254. Epub 2014 Aug 5 [PubMed PMID: 24677227]
Level 2 (mid-level) evidenceBettmann MA. Frequently asked questions: iodinated contrast agents. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Oct:24 Suppl 1():S3-10 [PubMed PMID: 15486247]
Plewes DB, Kucharczyk W. Physics of MRI: a primer. Journal of magnetic resonance imaging : JMRI. 2012 May:35(5):1038-54. doi: 10.1002/jmri.23642. Epub [PubMed PMID: 22499279]
Hendrick RE, Haacke EM. Basic physics of MR contrast agents and maximization of image contrast. Journal of magnetic resonance imaging : JMRI. 1993 Jan-Feb:3(1):137-48 [PubMed PMID: 8428081]
Ramalho J, Ramalho M, Jay M, Burke LM, Semelka RC. Gadolinium toxicity and treatment. Magnetic resonance imaging. 2016 Dec:34(10):1394-1398. doi: 10.1016/j.mri.2016.09.005. Epub 2016 Sep 28 [PubMed PMID: 27693607]
Bussi S, Coppo A, Botteron C, Fraimbault V, Fanizzi A, De Laurentiis E, Colombo Serra S, Kirchin MA, Tedoldi F, Maisano F. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. Journal of magnetic resonance imaging : JMRI. 2018 Mar:47(3):746-752. doi: 10.1002/jmri.25822. Epub 2017 Jul 21 [PubMed PMID: 28730643]
Endrikat J, Dohanish S, Schleyer N, Schwenke S, Agarwal S, Balzer T. 10 Years of Nephrogenic Systemic Fibrosis: A Comprehensive Analysis of Nephrogenic Systemic Fibrosis Reports Received by a Pharmaceutical Company from 2006 to 2016. Investigative radiology. 2018 Sep:53(9):541-550. doi: 10.1097/RLI.0000000000000462. Epub [PubMed PMID: 29547493]
Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magnetic resonance imaging. 2016 Dec:34(10):1383-1390. doi: 10.1016/j.mri.2016.07.016. Epub 2016 Aug 13 [PubMed PMID: 27530966]
Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR. American journal of roentgenology. 2007 Feb:188(2):586-92 [PubMed PMID: 17242272]
Level 2 (mid-level) evidenceAbu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Advances in chronic kidney disease. 2011 May:18(3):188-98. doi: 10.1053/j.ackd.2011.03.001. Epub [PubMed PMID: 21531325]
Level 3 (low-level) evidenceNamasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emergency radiology. 2006 Jul:12(5):210-5 [PubMed PMID: 16688432]
Granata V, Cascella M, Fusco R, dell'Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo A, Petrillo A. Immediate Adverse Reactions to Gadolinium-Based MR Contrast Media: A Retrospective Analysis on 10,608 Examinations. BioMed research international. 2016:2016():3918292. doi: 10.1155/2016/3918292. Epub 2016 Aug 29 [PubMed PMID: 27652261]
Level 2 (mid-level) evidenceHeshmatzadeh Behzadi A, Farooq Z, Newhouse JH, Prince MR. MRI and CT contrast media extravasation: A systematic review. Medicine. 2018 Mar:97(9):e0055. doi: 10.1097/MD.0000000000010055. Epub [PubMed PMID: 29489663]
Level 1 (high-level) evidencePanych LP, Madore B. The physics of MRI safety. Journal of magnetic resonance imaging : JMRI. 2018 Jan:47(1):28-43. doi: 10.1002/jmri.25761. Epub 2017 May 19 [PubMed PMID: 28543948]
Puac P, Rodríguez A, Vallejo C, Zamora CA, Castillo M. Safety of Contrast Material Use During Pregnancy and Lactation. Magnetic resonance imaging clinics of North America. 2017 Nov:25(4):787-797. doi: 10.1016/j.mric.2017.06.010. Epub 2017 Sep 8 [PubMed PMID: 28964468]
Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F, Cantisani V, Choi B, Chammas MC, Clevert DA, Claudon M, Correas JM, Cui XW, Cosgrove D, D'Onofrio M, Dong Y, Eisenbrey J, Fontanilla T, Gilja OH, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lyshchik A, Franca Meloni M, Moriyasu F, Nolsøe C, Piscaglia F, Radzina M, Saftoiu A, Sidhu PS, Sporea I, Schreiber-Dietrich D, Sirlin CB, Stanczak M, Weskott HP, Wilson SR, Willmann JK, Kim TK, Jang HJ, Vezeridis A, Westerway S. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound international open. 2018 Jan:4(1):E2-E15. doi: 10.1055/s-0043-123931. Epub 2018 Feb 7 [PubMed PMID: 29423461]
Wang S, Hossack JA, Klibanov AL. Targeting of microbubbles: contrast agents for ultrasound molecular imaging. Journal of drug targeting. 2018 Jun-Jul:26(5-6):420-434. doi: 10.1080/1061186X.2017.1419362. Epub 2018 Jan 9 [PubMed PMID: 29258335]
Furrer MA, Spycher SCJ, Büttiker SM, Gross T, Bosshard P, Thalmann GN, Schneider MP, Roth B. Comparison of the Diagnostic Performance of Contrast-enhanced Ultrasound with That of Contrast-enhanced Computed Tomography and Contrast-enhanced Magnetic Resonance Imaging in the Evaluation of Renal Masses: A Systematic Review and Meta-analysis. European urology oncology. 2020 Aug:3(4):464-473. doi: 10.1016/j.euo.2019.08.013. Epub 2019 Sep 27 [PubMed PMID: 31570270]
Level 1 (high-level) evidenceBasu S, Hess S, Nielsen Braad PE, Olsen BB, Inglev S, Høilund-Carlsen PF. The Basic Principles of FDG-PET/CT Imaging. PET clinics. 2014 Oct:9(4):355-70, v. doi: 10.1016/j.cpet.2014.07.006. Epub 2014 Aug 5 [PubMed PMID: 26050942]
Dhull VS, Rana N, Nazar AH. Contrast Media in PET/Computed Tomography Imaging. PET clinics. 2016 Jan:11(1):85-94. doi: 10.1016/j.cpet.2015.07.007. Epub 2015 Sep 16 [PubMed PMID: 26590446]
Haubenreisser H, Bigdeli A, Meyer M, Kremer T, Riester T, Kneser U, Schoenberg SO, Henzler T. From 3D to 4D: Integration of temporal information into CT angiography studies. European journal of radiology. 2015 Dec:84(12):2421-4. doi: 10.1016/j.ejrad.2015.06.014. Epub 2015 Jun 18 [PubMed PMID: 26152869]
Gupta RK, Bang TJ. Prevention of Contrast-Induced Nephropathy (CIN) in Interventional Radiology Practice. Seminars in interventional radiology. 2010 Dec:27(4):348-59. doi: 10.1055/s-0030-1267860. Epub [PubMed PMID: 22550376]
Goldfarb S, McCullough PA, McDermott J, Gay SB. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clinic proceedings. 2009 Feb:84(2):170-9. doi: 10.4065/84.2.170. Epub [PubMed PMID: 19181651]
de Havenon A, Mossa-Basha M, Shah L, Kim SE, Park M, Parker D, McNally JS. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology. 2017 Dec:59(12):1193-1202. doi: 10.1007/s00234-017-1925-9. Epub 2017 Sep 23 [PubMed PMID: 28942481]
Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke. European journal of radiology. 2017 Nov:96():162-172. doi: 10.1016/j.ejrad.2017.08.014. Epub 2017 Aug 24 [PubMed PMID: 29054448]
Johns CS, Swift AJ, Hughes PJC, Ohno Y, Schiebler M, Wild JM. Pulmonary MR angiography and perfusion imaging-A review of methods and applications. European journal of radiology. 2017 Jan:86():361-370. doi: 10.1016/j.ejrad.2016.10.003. Epub 2016 Oct 4 [PubMed PMID: 28341390]
Cheng K, Cassidy F, Aganovic L, Taddonio M, Vahdat N. CT urography: how to optimize the technique. Abdominal radiology (New York). 2019 Dec:44(12):3786-3799. doi: 10.1007/s00261-019-02111-2. Epub [PubMed PMID: 31317210]