Introduction
Intraventricular meningiomas show similar characteristics to those found in the extra-axial space. Their cells of origin are the same; however, extra-axial meningiomas are derived from the arachnoid cap cells, naturally occurring near venous sinuses and dural edges. Intraventricular meningiomas originate from the choroid plexus' stroma and arise at the tela choroidea. Here, arachnoid cells are found secondary to the embryologic origin of the choroid plexus. Most meningiomas are considered to be benign World Health Organization (WHO) grade I, slow-growing lesions. However, some may progress to atypical or anaplastic histopathological types.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most meningiomas are sporadic, but some are associated with genetic syndromes or mutations. Most meningiomas, even sporadic, have a mutation in chromosome 22 (Merlin tumor suppressor gene, SIS oncogene, or INI1). This same mutation is seen in neurofibromatosis type 2, also called MISME syndrome due to multiple inherited schwannoma, meningiomas, and ependymomas.[1] A loss of chromosome 22q occurs in 89% of the intraventricular meningiomas and chromosome 1p in 44%.[1] NF2 mutations are the most frequent genetic alteration found in intraventricular meningiomas.[1]
Meningiomas have progesterone receptors in up to 72% to 90% of the tumors.[2][3] Female patients may present with worsening symptoms during pregnancy due to elevated blood progesterone levels.[4] The presence of this receptor may explain the significant female predilection seen in sporadic meningiomas.[3] Meningiomas also have estrogen (7%) and androgen (73%) receptors on their membranes.[3][4]
A well-validated factor for increased risk for meningioma development is exposure to ionizing radiation.[5]
Epidemiology
In general, meningiomas account for approximately 37% of all primary central nervous system tumors and 50% of all benign primary central nervous system tumors.[6][7] They have a female predilection with a 2.3 female/1.0 male ratio. An intraventricular location accounts for 5% of all meningiomas; convexity (35%), parasagittal (20%), sphenoid ridge (20%), tuberculum sellae (3%), infratentorial (13%), and others (4%).
Intraventricular meningiomas typically present between 30 and 60 years of age. The mean age at diagnosis is 42.2 +/- 8.2 years old.[8] They have a female predilection with 1.47 female/1.0 male ratio, lower than dural-based extra-axial meningiomas.[8] Intraventricular meningiomas account for 1% to 5% of all ventricular tumors.[8][9] Approximately 90% of them are WHO grade I.[8]
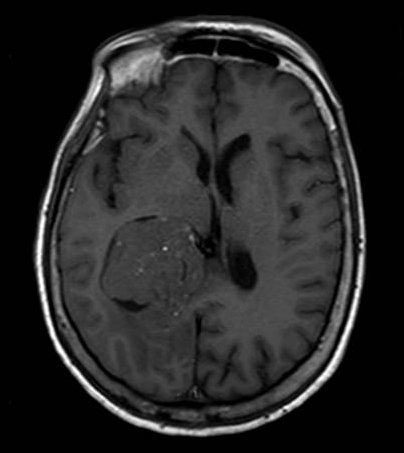
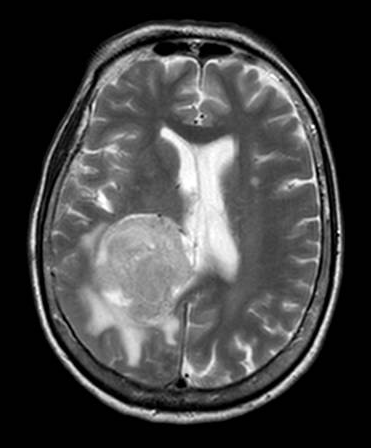
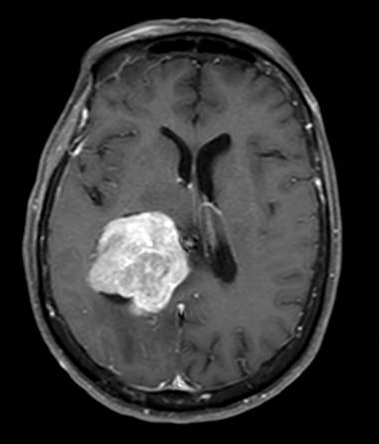
The lateral ventricle is the most common location (88.4%), with a minority found in the fourth ventricle (8.7%) or third ventricle (2.9%).[8] In most cases, they are located within the atrium.[10][11] However, they can be found in any ventricular space containing choroid plexus. The junction of the temporal horn, the occipital horn, and the lateral ventricle's posterior body is called the atrium. The trigone region is the floor of this area where the temporal and occipital horns unite. Some studies have reported that they are more common on the left side.[12][13]
In the pediatric population, intraventricular location is common, occurring in approximately 20% of the meningioma cases at a mean age of 12 to 14 years.[14][15] Pediatric cases have a male predilection with a 1.5 male/1.0 female ratio. About 93.3% of meningiomas are WHO grade I. Compared with adult intraventricular meningiomas, pediatric intraventricular meningiomas tend to have a higher incidence of benign subtypes.
Pathophysiology
Intraventricular meningiomas probably originate from embryological invagination of arachnoid cells into the choroid plexus.[8][16] Arachnoid cells are found in normal choroid plexus stroma.[16][17][18] During the sixth week of gestation, an invagination of mesenchyme in the myelencephalon's roof area forms the choroid plexus.[19] As the ventricular system invaginates, arachnoid cells are transported together with the choroid plexus.[16]
Intraventricular meningiomas originate from arachnoid cells seating within the choroid plexus.[20] Lateral ventricle meningiomas arise from mesenchymal stroma of the choroid plexus, which contains meningothelial inclusion bodies in the tela choroidea.[21] Third ventricle tumors originate from the tela of the velum interpositum and fourth ventricle meningiomas from the tela choroidea.[16][22][23][24]
Histopathology
Meningiomas exhibit a wide range of histological appearances. Of all the subtypes of the WHO classification, the most common are meningothelial, fibroblastic, and transitional meningiomas. Most of the subtypes have a benign behavior, but those categorized as WHO grade II and III are more likely to recur and follow a more aggressive clinical course.[25][26][27][28]
The majority of meningiomas immunostain for epithelial membrane antigen; however, it is less consistent in atypical and malignant lesions. Vimentin positivity is found in all meningiomas but is relatively nonspecific. Somatostatin receptor 2a is expressed strongly and diffusely in almost all cases, including anaplastic meningiomas, but can also be encountered in neuroendocrine neoplasms. Fibrous meningiomas commonly stain positive for S100. Other useful immunohistochemical markers include Ki-67 and progesterone receptors.[29]
Grade I
- Meningothelial
- Fibroblastic
- Transitional (mixed)
- Psammomatous
- Angiomatous
- Microcystic
- Secretory
- Lymphoplasmacyte-rich
- Metaplastic
Grade II
- Atypical
- Clear cell
- Chordoid
Grade III
- Rhabdoid
- Papillary
- Anaplastic/malignant
History and Physical
Most intraventricular meningiomas are benign, slow-growing lesions. They are typically found incidentally or become clinically evident when they obstruct the cerebrospinal fluid pathways and cause hydrocephalus. Three main clinical findings (headache, hydrocephalus, or visual field deficits) may be present.[10][11] Sometimes, cognitive deficits affecting attention and memory dominate the clinical findings.[10] Seizures are rare.[10]
Headache: The patient may present with a constant ipsilateral headache that does not resolve with oral medications. This is typically caused by adjacent parenchymal edema. If edema is present on imaging studies, surgical intervention is considered due to the risk of malignant presentation or a malignant lesion mimicking a meningioma.
Hydrocephalus: The patient complains of headache, nausea, blurry vision, sleepiness, lethargy, or coma. The patient can present with Cushing's triad with hypertension, bradycardia, and irregular respirations. Funduscopic examination typically shows bilateral optic nerve swelling. Unilateral or bilateral sixth nerve palsy can be present.
Visual field deficit: Contralateral homonymous quadrantanopia due to damage to adjacent optic radiations is found. Often, the patient is unaware of the quadrantanopia and is detected upon visual field evaluation in the ophthalmology clinic. Sometimes, the patient complains of difficulty visualizing objects in the upper corner of the visual field contralateral to the lesion's location.
Evaluation
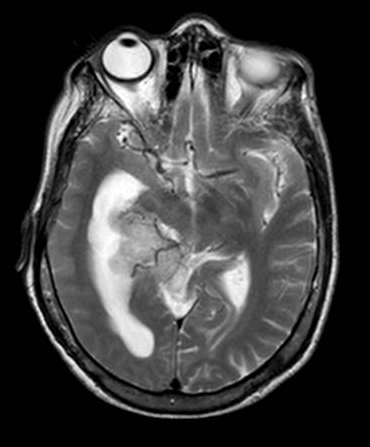
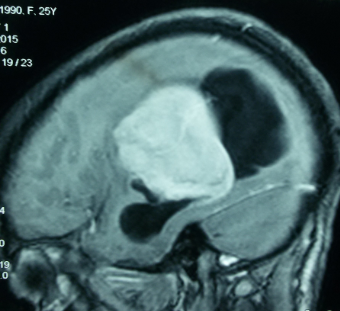
Brain magnetic resonance imaging (MRI) with and without contrast is the gold standard for evaluating intraventricular lesions. On the brain MRI with and without contrast, intraventricular meningioma appears as a solid, well-circumscribed mass, iso- or hypointense on T1 and T2-weighted images, and showing homogeneous contrast enhancement.
Head computed tomographic (CT) scans and brain digital subtraction angiography (DSA) may provide additional relevant information. Calcifications are easily seen on the head CT scan. DSA will show the tumors' specific feeders, and in large tumors, they can be embolized preoperatively. The vascular supply is usually from the anterior choroidal artery, but in larger lesions, the posterior choroidal artery also contributes. Typically, these tumors are highly vascularized. They drain into the deep ventricular veins.
Diffusion tensor imaging and functional MRI can help delineate and plan surgical approaches.[30] Visualization of the white matter fiber tracts allows the surgeon to select the best trajectory.[31][32]
Treatment / Management
Intraventricular meningiomas are usually WHO grade I slow-growing lesions. With the growing use of brain MRI, most of these tumors are found incidentally and followed in an outpatient manner. Surgery can prove difficult due to the deep location of these lesions, sometimes, with high morbidity and mortality.
Small asymptomatic lesions <3cm are closely followed with brain MRI every six months for 1-2 years, and then once per year.[11] If they become symptomatic or have an accelerated growth pattern, alternative treatment modalities are considered.
For large asymptomatic lesions >3cm in young good surgical candidates, gross total resection is recommended as it is curative in most cases. Surgery also provides and confirms the diagnosis. Neuronavigation can be used to plan and verify the exact trajectory of the surgery. Sometimes, complete resection can prove difficult due to vascular control being deep to the tumor, and pre-operative endovascular embolization should be attempted. If vascular control is not easily achieved, partial resection followed by adjuvant radiosurgery is a safe alternative. A needle biopsy is performed in poor surgical candidates if the diagnosis is unclear, followed by radiosurgery. If the diagnosis is clear, radiosurgery alone is sufficient.[11][33][34]
For symptomatic lesions due to obstructive hydrocephalus or adjacent parenchymal edema, gross total resection is recommended. An external ventricular drain is usually not needed as the approach will drain the ventricles. For patients who are acutely deteriorating, an external ventricular drain is placed until the surgery is performed.
Surgical approaches are based on which ventricle the tumor is located, laterality, and the size.[10][11][35][36]
Lateral ventricle:
Temporal horn of the lateral ventricle
- Transsylvian approach
- Transtemporal approach (through the posterior part of the middle temporal gyrus). Early visualization of the anterior choroidal artery. May cause visual field defects, and in the dominant hemisphere may cause aphasia.[37]
- Occipitotemporal sulcus approach. The occipitotemporal sulcus can be opened with a subtemporal craniotomy to allow access to the posterior temporal horn.[38] (B2)
Atrium
- Posterior interhemispheric transcingular approach (through dissection of the precuneus or cingulate gyrus).
- Interhemispheric transcallosal approach.[39] It can be performed from the contralateral side for better visualization.[11]
- Intraparietal sulcus approach. The intraparietal sulcus is opened with dissection of the parietal white matter toward the ventricle. It can produce visual field defects, apraxia, and acalculia.[38][40] The superior parietal lobule gyrus is preferred to reduce the complications.[36] A disadvantage is that the anterior choroidal supply is not identified until the end of the procedure.
- Transtemporal approach at the posterior part of the middle temporal gyrus. Early visualization of the anterior choroidal artery with devascularization of the tumor. The choroid plexus is followed until the tumor is reached and debulked.[11] (B2)
Occipital horn of the lateral ventricle
- Posterior interhemispheric transcingular approach.
- Anterior and posterior transcortical and transcallosal approaches. Care is taken to enter into the correct ventricle when using the transcallosal approach.[39]
Third ventricle:
- Anterior or posterior transcortical and transcallosal (interhemispheric) approach.
- Infratentorial supracerebellar approach. For tumors in the posterior area of the third ventricle.[11]
Fourth ventricle:
- Telovelar approach
- Transvermian midline approach
- Transcerebellar paramedian approach
Differential Diagnosis
- Choroid plexus papilloma
- Choroid plexus carcinoma
- Ependymoma
- Choroid plexus metastases
- Renal cell carcinoma metastasis
- High-grade astrocytoma
- Melanoma
- Primary central nervous system lymphoma
Prognosis
Intraventricular meningiomas have a favorable prognosis because 90% of them are WHO grade I.[8] If gross total resection is possible, then surgery is considered curative. However, in 10% of the patients, intraventricular meningiomas are WHO grade II and III and may require adjuvant radiosurgery. They have a mortality rate of 4%.[8] Recurrence occurs in 5.3% of the cases, but only 0.6% show an increasing tumor malignancy (from WHO grade I to II/III).[8] Progression-free survival at five years is 86%.[1]
For all types of meningiomas WHO grade I, the 10-year survival rate is 84%, and for WHO grade II & III, the 10-year survival rate of 62%.[7] For intraventricular meningiomas, the recurrence and mortality numbers are lower due to a better complete surgical resection rate.[8]
Complications
Postoperative epilepsy:
- It is caused by local cortical dysfunction secondary to parenchymal transgression during the surgical approach to the ventricle.
If the tumor is approached via dominant parietal lobe:
- Gerstmann syndrome (inferior parietal lobule)
- Agraphia
- Acalculia
- Finger agnosia
- Left to right confusion
If the tumor is approached via non-dominant parietal lobe:
- Alexia without agraphia
Contralateral homonymous hemianopsia or quadrantanopia:
- It is caused by damage to the optic radiations, located on the lateral ventricle's floor and roof.
Additional complications include:
- Intracerebral hemorrhage
- Vascular injury and stroke
- Hydrocephalus
- Pulmonary embolism
- Surgical infection
- Coma
- Death
Consultations
- Neurosurgeon
- Neurologist
- Ophthalmologist
- Physical medicine and rehabilitation
- Oncology
- Radiation oncology
Deterrence and Patient Education
There are no preventable causes of intraventricular meningiomas. Many of them are found incidentally and can be observed. They usually attain large sizes before symptoms commence. The age distribution is between 30 and 60 years of age, with a mean age at diagnosis is 42.2 years old. This is lower than the meningiomas in other locations. Histopathological distribution of intraventricular meningiomas is similar to those in other locations. Recurrence and mortality rates are lower than in other locations.
Surgery for intraventricular meningiomas is challenging. Complete surgical removal is frequent, and minimal recurrence rate is the rule. Some patients develop neurologic deficits primarily in visual fields. The use of functional brain mapping, diffusion tensor imaging, and tractography of white matter fiber tracts can help to select the best appropriate approach.
Enhancing Healthcare Team Outcomes
While the neurosurgeon is almost always involved in the care of patients with intraventricular meningiomas, it is important to consult with an interprofessional team of specialists that include a neurologist and ophthalmologist. The radiation oncologist evaluation is essential for those tumors that are treated with radiosurgery as a primary treatment or as an adjunct treatment. The nurses are also vital members of the team. They will evaluate the patient's vital signs and help with the education of the patient and family. In the postoperative period, physical therapy and rehabilitation may be needed for those with complications. It is essential to do preoperative visual field evaluation.
Many patients can present with deficits due to the tumor location. Postoperative reevaluation is needed as the surgical approach can cause new deficits. The neuroradiologist also plays a vital role in the differential diagnosis of the intraventricular mass. Interventional neuroradiologist may intervene with preoperative embolization of the tumor. Shared decision making and communication are essential elements for a good outcome.
The recurrence and mortality are lower for intraventricular meningiomas than in other localizations. A higher complete surgical resection rate is achieved than in other localizations. Gross total resection is the gold standard for the treatment of intraventricular meningiomas.[8] [Level 1]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Jungwirth G, Warta R, Beynon C, Sahm F, von Deimling A, Unterberg A, Herold-Mende C, Jungk C. Intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT. Acta neuropathologica communications. 2019 Aug 30:7(1):140. doi: 10.1186/s40478-019-0793-4. Epub 2019 Aug 30 [PubMed PMID: 31470906]
Connolly ID, Cole T, Veeravagu A, Popat R, Ratliff J, Li G. Craniotomy for Resection of Meningioma: An Age-Stratified Analysis of the MarketScan Longitudinal Database. World neurosurgery. 2015 Dec:84(6):1864-70. doi: 10.1016/j.wneu.2015.08.018. Epub 2015 Aug 28 [PubMed PMID: 26318633]
Portet S, Banor T, Bousquet J, Simonneau A, Flores M, Ingrand P, Milin S, Karayan-Tapon L, Bataille B. New Insights into Expression of Hormonal Receptors by Meningiomas. World neurosurgery. 2020 Aug:140():e87-e96. doi: 10.1016/j.wneu.2020.04.168. Epub 2020 May 1 [PubMed PMID: 32371078]
Gurcay AG, Bozkurt I, Senturk S, Kazanci A, Gurcan O, Turkoglu OF, Beskonakli E. Diagnosis, Treatment, and Management Strategy of Meningioma during Pregnancy. Asian journal of neurosurgery. 2018 Jan-Mar:13(1):86-89. doi: 10.4103/1793-5482.181115. Epub [PubMed PMID: 29492130]
Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro-oncology. 2012 Nov:14(11):1316-24. doi: 10.1093/neuonc/nos208. Epub 2012 Sep 5 [PubMed PMID: 22952197]
Level 1 (high-level) evidenceShibuya M. Pathology and molecular genetics of meningioma: recent advances. Neurologia medico-chirurgica. 2015:55(1):14-27. doi: 10.2176/nmc.ra.2014-0233. Epub 2014 Dec 20 [PubMed PMID: 25744347]
Level 3 (low-level) evidenceOstrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro-oncology. 2019 Nov 1:21(Suppl 5):v1-v100. doi: 10.1093/neuonc/noz150. Epub [PubMed PMID: 31675094]
Pereira BJA, de Almeida AN, Paiva WS, de Aguiar PHP, Teixeira MJ, Marie SKN. Natural history of intraventricular meningiomas: systematic review. Neurosurgical review. 2020 Apr:43(2):513-523. doi: 10.1007/s10143-018-1019-0. Epub 2018 Aug 15 [PubMed PMID: 30112665]
Level 1 (high-level) evidenceLapras C, Deruty R, Bret P. Tumors of the lateral ventricles. Advances and technical standards in neurosurgery. 1984:11():103-67 [PubMed PMID: 6536266]
Level 3 (low-level) evidenceLeśniewski K, Kunert P, Matyja E, Czernicki T, Wójtowicz K, Wojciechowski J, Marchel A. Trigone ventricular meningiomas - clinical characteristics, histopathology and results of surgical treatment. Neurologia i neurochirurgia polska. 2019:53(1):34-42. doi: 10.5603/PJNNS.a2019.0007. Epub 2019 Jan 10 [PubMed PMID: 30628049]
McDermott MW. Intraventricular meningiomas. Neurosurgery clinics of North America. 2003 Oct:14(4):559-69 [PubMed PMID: 15024801]
Fornari M, Savoiardo M, Morello G, Solero CL. Meningiomas of the lateral ventricles. Neuroradiological and surgical considerations in 18 cases. Journal of neurosurgery. 1981 Jan:54(1):64-74 [PubMed PMID: 7463122]
Level 3 (low-level) evidenceCriscuolo GR, Symon L. Intraventricular meningioma. A review of 10 cases of the National Hospital, Queen Square (1974-1985) with reference to the literature. Acta neurochirurgica. 1986:83(3-4):83-91 [PubMed PMID: 3492867]
Level 3 (low-level) evidenceLi Z, Li H, Jiao Y, Ma J, Wang S, Cao Y, Zhao J. Clinical features and long-term outcomes of pediatric intraventricular meningiomas: data from a single neurosurgical center. Neurosurgical review. 2018 Apr:41(2):525-530. doi: 10.1007/s10143-017-0884-2. Epub 2017 Aug 2 [PubMed PMID: 28766173]
Dash C, Pasricha R, Gurjar H, Singh PK, Sharma BS. Pediatric intraventricular meningioma: A series of six cases. Journal of pediatric neurosciences. 2016 Jul-Sep:11(3):193-196 [PubMed PMID: 27857785]
Level 3 (low-level) evidenceBhatoe HS, Singh P, Dutta V. Intraventricular meningiomas: a clinicopathological study and review. Neurosurgical focus. 2006 Mar 15:20(3):E9 [PubMed PMID: 16599425]
Kepes JJ. Presidential address: the histopathology of meningiomas. A reflection of origins and expected behavior? Journal of neuropathology and experimental neurology. 1986 Mar:45(2):95-107 [PubMed PMID: 3005518]
Shuangshoti S, Netsky MG. Histogenesis of choroid plexus in man. The American journal of anatomy. 1966 Jan:118(1):283-316 [PubMed PMID: 5915034]
Wannamaker GT. Intraventricular meningioma of the brain. Journal of the South Carolina Medical Association (1975). 1974 Aug:70(8):262-3 [PubMed PMID: 4546839]
Level 3 (low-level) evidenceGruss P, Engelhardt F, Kolmann HL, Völpel M. Ventricular meningiomas--report of 4 cases. Neurosurgical review. 1987:10(4):295-8 [PubMed PMID: 3506143]
Level 3 (low-level) evidenceMani RL, Hedgcock MW, Mass SI, Gilmor RL, Enzmann DR, Eisenberg RL. Radiographic diagnosis of meningioma of the lateral ventricle. Review of 22 cases. Journal of neurosurgery. 1978 Aug:49(2):249-55 [PubMed PMID: 671077]
Level 3 (low-level) evidenceLozier AP, Bruce JN. Meningiomas of the velum interpositum: surgical considerations. Neurosurgical focus. 2003 Jul 15:15(1):E11 [PubMed PMID: 15355013]
Level 3 (low-level) evidenceBehari S, Das KK, Kumar A, Mehrotra A, Srivastava AK, Sahu RN, Jaiswal AK. Large/giant meningiomas of posterior third ventricular region: falcotentorial or velum interpositum? Neurology India. 2014 May-Jun:62(3):290-5. doi: 10.4103/0028-3886.136934. Epub [PubMed PMID: 25033852]
Level 3 (low-level) evidenceChampagne PO, Bojanowski MW. Meningioma of the superior leaflet of the velum interpositum: A case report. Surgical neurology international. 2015:6(Suppl 3):S132-5. doi: 10.4103/2152-7806.155703. Epub 2015 Apr 22 [PubMed PMID: 25949856]
Level 3 (low-level) evidenceEom KS, Kim DW, Kim TY. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: a case report. Clinical neurology and neurosurgery. 2009 Sep:111(7):619-23. doi: 10.1016/j.clineuro.2009.05.002. Epub 2009 May 30 [PubMed PMID: 19482417]
Level 3 (low-level) evidenceCheng Z, Chao Q, Zhang H, Wang DW, Shu HS. Intraventricular cystic papillary meningioma: A case report and literature review. Medicine. 2020 Jul 31:99(31):e21514. doi: 10.1097/MD.0000000000021514. Epub [PubMed PMID: 32756190]
Level 3 (low-level) evidenceTao CY, Wang JJ, Li H, You C. Malignant intraventricular meningioma with craniospinal dissemination and concurrent pulmonary metastasis. World journal of surgical oncology. 2014 Jul 30:12():238. doi: 10.1186/1477-7819-12-238. Epub 2014 Jul 30 [PubMed PMID: 25073808]
Level 3 (low-level) evidenceLouis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun:131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9 [PubMed PMID: 27157931]
Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. International journal of clinical and experimental pathology. 2012:5(3):231-42 [PubMed PMID: 22558478]
Level 2 (mid-level) evidenceStaempfli P, Reischauer C, Jaermann T, Valavanis A, Kollias S, Boesiger P. Combining fMRI and DTI: a framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. NeuroImage. 2008 Jan 1:39(1):119-26 [PubMed PMID: 17931889]
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005:56(1):130-7; discussion 138 [PubMed PMID: 15617595]
Level 3 (low-level) evidenceWei CW, Guo G, Mikulis DJ. Tumor effects on cerebral white matter as characterized by diffusion tensor tractography. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2007 Feb:34(1):62-8 [PubMed PMID: 17352349]
Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. International journal of radiation oncology, biology, physics. 2003 Mar 15:55(4):1000-5 [PubMed PMID: 12605979]
Kim IY, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for intraventricular meningiomas. Acta neurochirurgica. 2009 May:151(5):447-52; discussion 452. doi: 10.1007/s00701-009-0273-x. Epub 2009 Apr 1 [PubMed PMID: 19337685]
Nayar VV, DeMonte F, Yoshor D, Blacklock JB, Sawaya R. Surgical approaches to meningiomas of the lateral ventricles. Clinical neurology and neurosurgery. 2010 Jun:112(5):400-5. doi: 10.1016/j.clineuro.2010.02.005. Epub 2010 Mar 1 [PubMed PMID: 20197209]
Nanda A, Bir SC, Maiti T, Konar S. Intraventricular Meningioma: Technical Nuances in Surgical Management. World neurosurgery. 2016 Apr:88():526-537. doi: 10.1016/j.wneu.2015.10.071. Epub 2015 Nov 5 [PubMed PMID: 26548837]
d'Avella D,Rossetto M,Denaro L,Sturiale CL, Lateral ventricle's choroid plexus tumors surgery in children: how I do it. Acta neurochirurgica. 2014 Jan; [PubMed PMID: 24170297]
D'Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lateral ventricle tumors: surgical strategies according to tumor origin and development--a series of 72 cases. Neurosurgery. 2005 Jan:56(1 Suppl):36-45; discussion 36-45 [PubMed PMID: 15799791]
Level 2 (mid-level) evidenceKempe LG, Blaylock R. Lateral-trigonal intraventricular tumors. A new operative approach. Acta neurochirurgica. 1976:35(4):233-42 [PubMed PMID: 998353]
D'Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lateral ventricle tumors: surgical strategies according to tumor origin and development--a series of 72 cases. Neurosurgery. 2008 Jun:62(6 Suppl 3):1066-75. doi: 10.1227/01.neu.0000333772.35822.37. Epub [PubMed PMID: 18695527]
Level 3 (low-level) evidence