Introduction
According to Centers for Disease Control data, the incidence of diabetes mellitus in the United States in 2015 was 30.3 million and is predicted to increase yearly with an already prediabetic population of 84 million.[1] The prevalence of diabetes is highest in the Alaska Native and Native American populations, followed by African Americans, Hispanics, Asians, and Whites.[1] There are different types of diabetes mellitus described in the latest American Diabetes Association (ADA) classification, but the two most commonly seen are type 1 diabetes and type 2 diabetes.[2]
Type 1 diabetes is an immune-mediated destruction of the insulin-producing beta cells in the pancreas. Due to this insulin deficiency, individuals with type 1 diabetes must rely on lifelong exogenous insulin administration to maintain normal blood glucose levels. Often referred to in the past as juvenile diabetes, type 1 diabetes typically manifests in younger individuals, commonly being diagnosed during childhood, teenage years, or early adulthood. Many times, the diagnosis of type 1 diabetes is made when a patient is admitted to the hospital for diabetic ketoacidosis, a life-threatening condition characterized by hyperglycemia, metabolic acidosis, and ketosis.[2]
Type 2 diabetes, once known as adult-onset diabetes, was traditionally seen in older individuals. However, due to rising obesity rates, type 2 diabetes now affects a broad age range, from children to older individuals. Type 2 diabetes arises either from peripheral insulin resistance or a reduction in insulin secretion. Although the exact mechanism is not universally agreed upon, it's hypothesized that increased intracellular fatty acid metabolites may activate a serine kinase cascade, impairing insulin signaling.[1] Typically, by the time of diagnosis, more than 60% of pancreatic beta cells may have lost their function.[3] Complete beta cell destruction may occur as the disease progresses, necessitating reliance on exogenous insulin. Initially, type 2 diabetes is typically managed with oral hypoglycemic agents and lifestyle modifications. Studies have demonstrated that caloric restriction and weight loss can improve glycemic control.[4]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Surgery and acute illness are stressors that alter homeostasis and lead to hyperglycemia. Stress conditions raise counter-regulatory hormones such as glucagon, epinephrine, cortisol, and growth hormone. This process leads to insulin resistance, increased hepatic glucose production, impaired peripheral glucose utilization, and relative insulin deficiency.[5]
Epinephrine stimulates glucagon secretion and inhibits insulin secretion by the pancreatic beta cells.[6] Additionally, the increased level of stress hormones leads to enhanced lipolysis and high free fatty acid (FFA) concentrations. Increased FFA correlates with inhibiting insulin-stimulated glucose uptake and limits the intracellular signaling cascade in skeletal muscle responsible for glucose transport activity.[5]
Hyperglycemia leads to the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha, interleukin 6, and interleukin 1B.[7] In addition, hyperglycemia has also been shown to impair leukocyte function, phagocytosis, chemotaxis, and bacterial destruction.[8] Increased reactive oxygen species due to hyperglycemia can result in direct cellular damage and further impair the vascular and immune systems. This oxidative stress contributes to increased platelet aggregation and a prothrombotic state.[9]
Indications
Diagnosis of diabetes is made when a patient meets one of the following criteria:
- A glucose level equal to, or greater than, 126 mg/dL after fasting for at least 8 hours
- Random venous plasma glucose equal to, or greater than, 200 mg/dL in a patient with classic symptoms of hyperglycemia
- Plasma glucose equal to, or greater than, 200 mg/dL measured two hours after a glucose load of 75 g during an oral glucose tolerance test
- Hemoglobin A1C equal to, or greater than, 6.5% [10]
Equipment
Intraoperative management of diabetes requires access to the following:
- Alaris pump and tubing
- 100 mL bag of normal saline
- Insulin
- Point of care glucose testing strips (alternative arterial line or venous blood gas analysis)
- 50% dextrose
Personnel
Different providers may be involved in the overall care of patients with diabetes, from primary care providers to specialists like endocrinologists who manage diabetes regularly. When a patient is scheduled for a surgical procedure, all providers contributing to everyday patient care should be involved in preparing the patient before the operation. Instructions can be provided by the provider who knows the patient the best and would feel comfortable giving the appropriate advice for the patient to get ready for the surgery. In most cases, the surgical team should reach out to the patient's medical team and ask for advice to optimize the patient's diabetes status. The anesthesia team responsible for the surgical procedure should also try to reach out and obtain accurate information on the patient's outpatient treatments to decide the best approach for perioperative glycemic control.
Technique or Treatment
There are no strict guidelines for canceling a surgical case due to hyperglycemia. The Society for Ambulatory Anesthesia (SAMBA) recommends canceling a case if a patient is experiencing diabetic ketoacidosis, hyperglycemic hyperosmolar nonketotic state, or severe dehydration.[11]
On the day of surgery, if a patient has a blood sugar over 140 mg/dL, blood glucose should be monitored every 2 hours intraoperatively with a point of care glucose via arterial line or venous blood from a peripheral intravenous line.
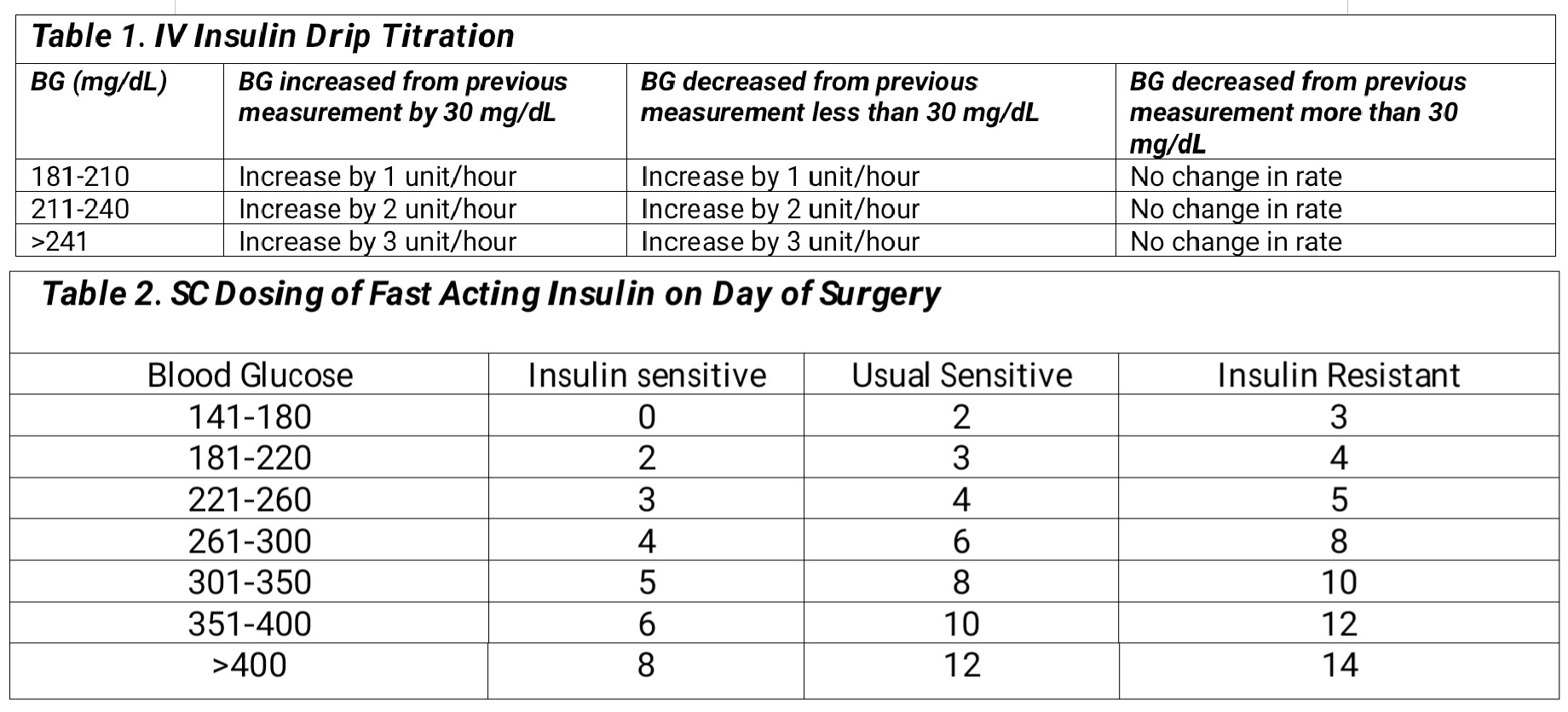
If a patient has a blood glucose level greater than or equal to 180 mg/dL and is critically ill, an intravenous insulin drip should be initiated.[12] The starting dose of the insulin drip (units/hour) can be calculated with blood glucose/100 and titrated according to Table 1.[5] The half-life of insulin is 35 minutes, so it is titrated easily, and blood sugar monitoring should occur hourly.
For those who are not critically ill and have a short procedure (less than 4 hours), which does not usually anticipate large fluid shifts, it is preferable to use rapid-acting subcutaneous (SC) insulin. Table 2 below gives guidance on how to dose SC insulin on the day of surgery for those who are insulin sensitive (no history of diabetes), those who are insulin resistant (total daily dose (TDD) of insulin exceeding 80 units, BMI greater than 35, prednisone dose greater than 20 mg), and whose who require usual amounts of insulin (all other categories).[5]
Complications
Control of hyperglycemia intraoperatively is of great importance because there is a 50% increase in morbidity and mortality in patients with diabetes mellitus compared to patients without diabetes mellitus.[13]
Research correlates perioperative hyperglycemia with increased hospital and intensive care unit length of stay, poor healing progression[14], and higher numbers of postoperative cases of pneumonia, systemic blood infection, urinary tract infection, acute renal failure, and acute myocardial infarction.[13]
Hypokalemia is an associated complication of hyperglycemia treatment with insulin, especially with coinciding diabetic ketoacidosis. Insulin drives potassium extracellular and hydrogen ions intracellular. Excess potassium is lost in urine due to osmotic diuresis. Other derangements include hypocalcemia, hypomagnesemia, and QT prolongation.[15]
Other life-threatening complications of insulin use include hypoglycemia. Those treated with tight glycemic control were five times more likely to experience severe hypoglycemia (blood glucose less than 40 mg/dL) postoperatively in comparison to liberal glucose treatment.[16] Symptoms of hypoglycemia include tremors, sweating, dizziness, light-headedness, seizures, and loss of consciousness. Intraoperatively hypoglycemia can cause a delay in emergence from anesthesia until exogenous glucose is administered to normalize blood sugar.[17]
Clinical Significance
There is no agreement among the anesthesiology community on the standard of care for blood glucose levels. However, there is a consensus on aiming for blood glucose levels below 180 mg/dL, as more aggressive goals may cause hypoglycemia. Recent metanalysis revealed that there is no difference in mortality in patients treated with very tight glucose control (blood glucose <110 mg/dL), tight glucose control (111-150 mg/dL), and liberal blood glucose control (blood glucose <220 mg/dL).[16] There was a decrease in adverse events in the tight glucose control group for surgical site infection, acute kidney injury, sepsis, and atrial fibrillation.
Enhancing Healthcare Team Outcomes
Each institution should develop a standardized protocol for hyperglycemia to be used in the preoperative area, intraoperative, and postanesthesia care units to ensure optimal perioperative glucose control. Communication amongst an interprofessional group is necessary as surgeons, anesthesiologists, intensivists, internal medicine providers, endocrinologists, pharmacists, and nurses are usually closely involved in patient care. Most patients will come from the main floor or the intensive care unit, so there should be a good handoff.
After the operation, prioritizing optimal communication among the care teams is crucial for the patient's recovery. This approach ensures continuity of care until the patient fully recovers. If any complications or adverse effects arise, they should be promptly addressed in a multidisciplinary team approach. Any changes in the patient's diabetes management must be communicated to their primary care physicians or endocrinologists. These healthcare providers will follow up with the patient to adjust medications or implement other measures for maintaining optimal diabetes control at all times. An interprofessional team strategy is critical in preventing long-term complications associated with diabetes.
Nursing, Allied Health, and Interprofessional Team Interventions
Before undergoing surgery, patients need to consult with their regular healthcare providers (eg, primary care physicians, nurse practitioners, or endocrinologists) who manage their diabetes. This consultation will allow patients to tailor their diabetes management for the upcoming procedure. Intraoperatively, the anesthesia team assumes responsibility for diabetes treatment decisions. The anesthesia team should provide a detailed account of the diabetes management during the surgery to the healthcare team caring for the patients postoperatively. This continuity of care will ensure there are no gaps in diabetes management, from surgery to full recovery and eventual discharge from the hospital.
Media
References
Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World journal of diabetes. 2010 Jul 15:1(3):68-75. doi: 10.4239/wjd.v1.i3.68. Epub [PubMed PMID: 21537430]
Glaser N, Fritsch M, Priyambada L, Rewers A, Cherubini V, Estrada S, Wolfsdorf JI, Codner E. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric diabetes. 2022 Nov:23(7):835-856. doi: 10.1111/pedi.13406. Epub [PubMed PMID: 36250645]
Level 3 (low-level) evidenceCerf ME. Beta cell dysfunction and insulin resistance. Frontiers in endocrinology. 2013:4():37. doi: 10.3389/fendo.2013.00037. Epub 2013 Mar 27 [PubMed PMID: 23542897]
Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1985 Nov:61(5):917-25 [PubMed PMID: 4044780]
Duggan EW, Carlson K, Umpierrez GE. Perioperative Hyperglycemia Management: An Update. Anesthesiology. 2017 Mar:126(3):547-560. doi: 10.1097/ALN.0000000000001515. Epub [PubMed PMID: 28121636]
McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinology and metabolism clinics of North America. 2012 Mar:41(1):175-201. doi: 10.1016/j.ecl.2012.01.001. Epub 2012 Feb 17 [PubMed PMID: 22575413]
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994 May 24:91(11):4854-8 [PubMed PMID: 8197147]
Level 3 (low-level) evidenceDelamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic medicine : a journal of the British Diabetic Association. 1997 Jan:14(1):29-34 [PubMed PMID: 9017350]
Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001 Jun:50(6):1491-4 [PubMed PMID: 11375352]
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes care. 2018 Jan:41(Suppl 1):S13-S27. doi: 10.2337/dc18-S002. Epub [PubMed PMID: 29222373]
Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, Merrill DG, Twersky R, Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesthesia and analgesia. 2010 Dec:111(6):1378-87. doi: 10.1213/ANE.0b013e3181f9c288. Epub 2010 Oct 1 [PubMed PMID: 20889933]
Level 1 (high-level) evidenceDuggan EW, Klopman MA, Berry AJ, Umpierrez G. The Emory University Perioperative Algorithm for the Management of Hyperglycemia and Diabetes in Non-cardiac Surgery Patients. Current diabetes reports. 2016 Mar:16(3):34. doi: 10.1007/s11892-016-0720-z. Epub [PubMed PMID: 26971119]
Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes care. 2010 Aug:33(8):1783-8. doi: 10.2337/dc10-0304. Epub 2010 Apr 30 [PubMed PMID: 20435798]
Level 2 (mid-level) evidenceMichel M, Lucke-Wold B. Diabetes management in spinal surgery. Journal of clinical images and medical case reports. 2022 Jun:3(6):. pii: 1906. Epub 2022 Jun 22 [PubMed PMID: 35795240]
Level 3 (low-level) evidenceJohansen NJ, Christensen MB. A Systematic Review on Insulin Overdose Cases: Clinical Course, Complications and Treatment Options. Basic & clinical pharmacology & toxicology. 2018 Jun:122(6):650-659. doi: 10.1111/bcpt.12957. Epub 2018 Feb 23 [PubMed PMID: 29316226]
Level 1 (high-level) evidenceKang ZQ, Huo JL, Zhai XJ. Effects of perioperative tight glycemic control on postoperative outcomes: a meta-analysis. Endocrine connections. 2018 Dec 1:7(12):R316-R327. doi: 10.1530/EC-18-0231. Epub [PubMed PMID: 30120204]
Level 1 (high-level) evidenceMisal US, Joshi SA, Shaikh MM. Delayed recovery from anesthesia: A postgraduate educational review. Anesthesia, essays and researches. 2016 May-Aug:10(2):164-72. doi: 10.4103/0259-1162.165506. Epub [PubMed PMID: 27212741]