Introduction
Intracranial hypertension is a condition characterized by elevated pressure within the skull. The increase in pressure can exert significant stress on the brain and other intracranial structures, potentially leading to a range of neurological symptoms and complications. Intracranial hypertension's clinical manifestations vary depending on the underlying cause, severity of pressure elevation, and individual patient factors. Common symptoms may include severe headaches, visual disturbances, nausea, vomiting, tinnitus, and, in severe cases, seizures or coma.
Diagnosing intracranial hypertension typically involves a combination of clinical evaluation, results of neuroimaging studies like computed tomography (CT) and magnetic resonance imaging (MRI), and intracranial pressure (ICP) measurement via invasive or noninvasive techniques. Early recognition and prompt management of this condition are essential to prevent potential complications, including permanent neurological damage and even death. Managing ICH typically involves addressing the underlying cause, optimizing cerebral perfusion, and sometimes surgical interventions to relieve pressure on the brain.
Cerebrospinal Fluid and Intracranial Pressure
The human skull has a fixed volume of approximately 1400 to 1700 mL. Intracranial content volume comprises 80% brain parenchyma, 10% cerebrospinal fluid, and 10% blood.
The choroid plexus is the main CSF producer and regulator, secreting around 20 mL per hour, averaging 450 mL per day. Arachnoid granulations reabsorb CSF and drain it into the venous system at similar rates. Normal cerebrospinal fluid (CSF) production varies by age, with typically high production during infancy that declines and stabilizes in childhood and adulthood. CSF pressures greater than 250 mm H20 in adults and 200 mm H20 in children generally signify increased ICP.[1][2][3][4]
Intracranial volume is more or less constant once the sutures completely ossify. Intracranial tissue or fluid volume elevation can raise intracranial pressure, which can occur in the presence of intracranial masses, ventricular stenosis, and hematomas. A large part of treating intracranial hypertension involves mitigating the risk of increased ICP and making timely clinical decisions to prevent adverse consequences.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The brain parenchyma's physiologic volume is relatively constant in adults but may change due to mass lesions or cerebral edema. Cerebral edema can occur following acute hypoxic encephalopathy, sizeable cerebral infarctions, and severe traumatic brain injury (TBI). CSF and blood volume in the intracranial space vary regularly as these are the primary ICP regulators.
Neurological injuries such as stroke or trauma can damage the control mechanisms, maintaining appropriate CSF volumes. Increased CSF production, as in the presence of a choroid plexus papilloma, can make the CSF secretion rate exceed the reabsorption rate. Impaired CSF reabsorption, seen in arachnoid granulation adhesions after bacterial meningitis, can also raise the ICP. CSF drainage interruption and hydrocephalus may arise from conditions such as intraventricular masses, congenital aqueductal stenosis, and acute intraventricular hemorrhage.
Cerebral blood flow (CBF) is the primary intracranial blood volume regulator. Diseases obstructing venous outflow, such as venous sinus thrombosis, jugular vein compression, and neck surgery-associated structural changes, may cause intracranial blood congestion and intracranial hypertension. Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri, is a term for chronic ICP increase due to unknown causes with no known structural change.[5][6]
Intracranial hypertension may be divided into primary and secondary causes, some of which are listed below.
Primary or Intracranial Causes
- Trauma, producing epidural hematoma, subdural hematoma, intracerebral hemorrhage, subarachnoid hemorrhage, or contusions
- Brain tumors
- Ischemic stroke
- Nontraumatic intracerebral hemorrhage from aneurysm rupture or hypertensive hemorrhage
- Idiopathic or benign intracranial hypertension
- Hydrocephalus
- Meningitis
- Congenital malformations, including aqueductal stenosis, Dandy-Walker malformation, and Chiari malformation
Secondary or Extracranial Causes
- Hypoventilation, causing hypoxia or hypercarbia
- Hypertension
- Airway obstruction
- Metabolic, usually drug-related
- Seizures
- Hyperpyrexia
- High-altitude cerebral edema
- Cervical structural venous outflow obstruction
- Polypharmacy
Epidemiology
Intracranial hypertension epidemiology depends on the etiology. Conditions presenting with acute ICP elevation are distributed in the population differently from pathologies causing chronic ICP increase. For example, about 60% of spontaneous hemorrhages arise from intracranial bleeding secondary to systemic hypertension.[7] Up to a third of hypertensive hemorrhages occur in patients aged over 80. Amyloid angiopathy is another common etiology of spontaneous intracranial hemorrhage, more common in older patients' cerebral cortices.[8] Subarachnoid hemorrhage occurs with an annual incidence of up to 91 cases out of 100,000, 85% of which are due to aneurysmal rupture.[9] In 2019, 27 million new cases of TBI occurred worldwide, ranging from mild to severe.[10]
Meanwhile, up to 90% of individuals with chronic IIH are women of childbearing age. People with chronic hypertension or obesity have an increased risk of developing intracranial hypertension. The occurrence frequency is 1.0 in 100,000 in the general population, 1.6 to 3.5 in 100,000 among women, and 7.9 to 20 in 100,000 among women who are overweight.
Pathophysiology
The total volume within the intracranial and spinal canals remains constant with only minute fluctuations. A volumetric increase can elevate ICP.[11] Normal ICP in adults ranges from 10 to 20 cm H20. ICP elevation increases the risk of neural injury from direct compression or CBF reduction. Clinically, CBF is determined indirectly from the measurable parameters, cerebral perfusion pressure (CPP) and mean arterial pressure (MAP), based on the following equation:
Cerebral perfusion pressure (CPP) = Mean arterial pressure (MAP) - Intracranial pressure (ICP)
Inflow Dynamics
CPP is the blood flow pressure to the brain, the force driving oxygen delivery necessary for neuronal functioning. This value remains within the 50 to 100 mm Hg range due to autoregulation. ICP elevation reduces CPP, diminishing blood flow pressure to the brain. The physiologic autoregulatory response to reduced CPP is to increase the MAP systemically and dilate cerebral blood vessels. Consequently, cerebral blood volume rises, further increasing ICP.
However, CPP drops paradoxically, producing a feedback cycle that reduces cerebral flow and perfusion. This feedback loop can cause cerebral ischemia and brain infarction with neuronal death.[12] In intracranial hemorrhage cases, increased blood pressure may worsen intracranial bleeding. A minimum CPP of 60 mm Hg is recommended to maintain adequate cerebral perfusion.
Outflow Dynamics
Continuous CSF and blood venous drainage also regulate total intracranial volume. Acute ventricular CSF flow obstruction without decreasing the choroid plexus' production rate results in CSF accumulation. Increased CSF volume elevates ventricular wall pressure, leading to transependymal flow and a potentially rapidly fatal ICP rise.[13] Chronic CSF reabsorption reduction can occur secondary to ventricular wall changes following pathologies like meningitis and intraventricular hemorrhage. Conditions like acute thrombosis, traumatic occlusion from epidural hematoma, depressed skull fracture, and chronic stenosis can obstruct venous drainage pathways. Outflow occlusion produces intracranial intravascular volume elevation with subsequent ICP elevation.[14]
History and Physical
History
Patients with intracranial hypertension may present unconscious, apneic, and pulseless, which are signs of cardiorespiratory arrest. Resuscitative measures must be started immediately for all patients in cardiorespiratory arrest, regardless of cause. A primary survey must be performed promptly to address airway, breathing, and circulatory problems. A more thorough investigation may be pursued once the patient is stable.
History provides valuable insights into the onset, progression, and nature of intracranial hypertension symptoms. The most commonly reported manifestations include headaches, visual changes, nausea, and vomiting. Additional symptoms such as cranial nerve palsies and mental status changes further underscore the condition's neurological impact.
A thorough review of past medical history, including comorbid conditions and medication use, provides essential context for understanding the underlying etiology of intracranial hypertension. Conditions such as hypertension, obesity, thyroid disorders, and prior head trauma may predispose individuals to elevated ICP. Medications with potential neurotoxic effects or those associated with fluid retention warrant consideration as possible contributors to intracranial hypertension. Specific cases are considered below.
Idiopathic Intracranial Hypertension
Chronic ICP elevation often presents as nonspecific headaches likely mediated by the trigeminal nerve's dura and blood vessel pain fibers. Pain is generally diffuse and worse in the mornings or after a Valsalva maneuver. Nausea and vomiting are also commonly reported.
The 2 most frequent IIH symptoms are chronic headache and progressive visual deterioration secondary to papilledema. About 20% to 40% of patients have double vision, most frequently with horizontal diplopia associated with abducens nerve compression and palsy.[15] Transient visual abnormalities occur frequently, often described as a gradual dimming in 1 or both eyes. Visual abnormalities worsen with postural changes. Peripheral visual loss may be reported, most commonly beginning in the nasal inferior quadrant with subsequent central visual field loss. Visual acuity alterations with blurring or distortion may occur.
Variable degrees of loss of color distinction may be reported. Visual loss is permanent in up to 40% of cases after treatment.[16] In more severe or chronic cases, a sudden visual loss can occur due to intraocular hemorrhage. Tinnitus with a pulsing rhythm can occur, exacerbated by supine or bending positions and Valsalva maneuvers. Neurological findings are indications of severe disease.
Acute Intracranial Hypertension
Acute ICP elevation is most often due to traumatic injury, giving rise to mechanical parenchymal or anoxic injury and subsequent cytotoxic edema. Other possible etiologies include intraparenchymal hemorrhage, subdural hematoma, epidural hematoma, or hydrocephalus secondary to acute obstruction or subarachnoid hemorrhage. Initial symptoms of acute ICP elevation include nausea, vomiting, lethargy, confusion, and sometimes irritability. These symptoms' underlying causes can be challenging to identify in the complex inpatient setting with many other metabolic, psychological, and systemic pathologic cofactors.
Brain herniation can occur, producing decreased consciousness or responsiveness. The sites most vulnerable to herniation include the cerebral surface and central transtentorial, uncal, transtentorial, cerebellar tonsillar or foramen magnum, and transcalvarial routes. Focal neurological symptoms depend on the location of pressure-induced irritation and injury. Unconsciousness results from pressure on the midbrain's reticular formations. Respiratory drive and effort changes may occur, leading to respiration and oxygenation failure.
Physical Examination
Physical examination complements the history by providing objective findings indicative of intracranial hypertension. Fundoscopic examination assesses for papilledema, a hallmark sign of elevated ICP. The characteristic findings include optical disc swelling, disc margin blurring, and venous congestion. Cranial nerve examination evaluates for abnormalities in visual acuity, pupillary reactions, facial symmetry, and hearing, providing further insights into neurological function. Motor and sensory assessments aid in identifying focal neurological deficits, while gait and coordination evaluations assess cerebellar function and balance.
A mental status change or depressed sensorium should be promptly evaluated. A complete neurological assessment is essential whenever intracranial hypertension is suspected. Cranial nerve assessment is particularly important for identifying lesions. Pupillary reflex blunting with fixed dilation of one pupil and "down and out" ocular position are highly indicative of pressure irritation or ipsilateral oculomotor nerve injury.[17] Spontaneous periorbital bruising may also be present. Cushing triad classically presents with bradycardia, respiratory depression, and hypertension and is highly indicative of intracranial hypertension.
Infants can have widening cranial sutures and bulging fontanelles. Infants do not display papilledema when ICP is elevated on ocular examination due to the fontanelle's compliance features.
Evaluation
Diagnostic testing for intracranial hypertension aims to confirm ICP elevation and identify potential underlying causes. The diagnostic workup typically involves a combination of imaging studies, lumbar puncture, and ophthalmologic evaluation.
Neuroimaging studies assess brain anatomy, identify structural abnormalities, and detect ICP elevation signs. Common imaging modalities include CT and MRI.
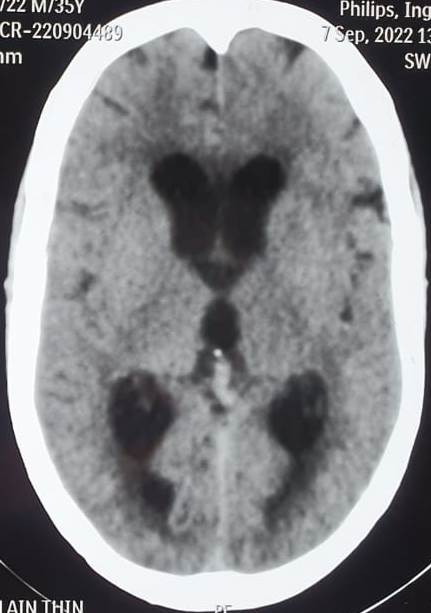
CT scans can identify acute conditions, such as hemorrhage, tumors, or hydrocephalus (see Image. Communicating Hydrocephalus Computed Tomography). CT can also detect ventricular enlargement or cerebral sulci effacement. CT venography assesses venous sinus patency.
MRI allows for superior soft tissue contrast and is particularly valuable for evaluating subtle structural abnormalities, such as small tumors or Chiari malformations, that may contribute to intracranial hypertension. MRI can also detect abnormalities in CSF flow dynamics and assess for complications such as venous sinus thrombosis. MR venography evaluates venous sinus patency and identifies abnormalities such as stenosis or thrombosis, which can contribute to intracranial hypertension.
A lumbar puncture measures ICP directly and assesses CSF composition. An elevated opening pressure (>20 mm Hg in adults) suggests intracranial hypertension. CSF analysis may reveal abnormally increased protein levels or evidence of underlying etiologies such as infection or inflammation.
Additional studies may be conducted based on clinical suspicion. Cerebral angiography evaluates for vascular abnormalities, such as arteriovenous malformations or dural arteriovenous fistulas. CSF flow studies evaluate CSF dynamics and identify CSF circulation abnormalities. Endocrine tests screen for hormonal disorders, such as hypothyroidism or adrenal insufficiency, which can contribute to intracranial hypertension.
Correctly identifying the etiology of intracranial hypertension depends on the combination of a good clinical evaluation and diagnostic testing. Approaches to specific intracranial hypertension cases are explained below.
Idiopathic Intracranial Hypertension
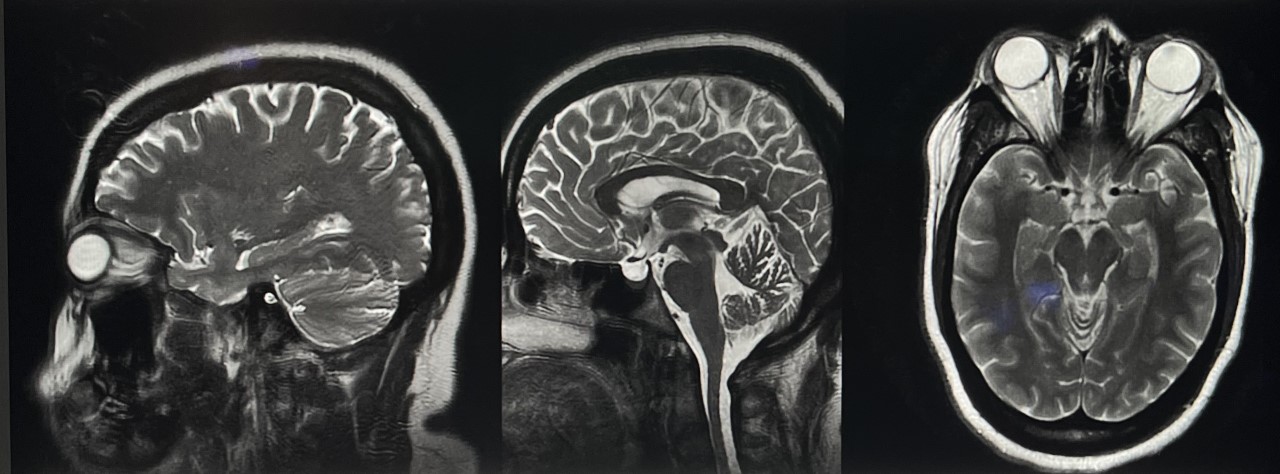
A brain MRI, with and without contrast, provides a detailed intracranial view when evaluating chronic causes of intracranial hypertension (see Image. Idiopathic Intracranial Hypertension MRI). A lumbar puncture is recommended for diagnosis, allowing for measuring opening pressures and evaluating infectious and inflammatory etiologies. However, an intracranial mass should be ruled out via imaging before performing this procedure to avoid the risk of downward herniation. Invasive ICP measurement may be considered if papilledema evaluation yields negative results despite ongoing clinical suspicion.
Progressive visual deterioration is a common papilledema complication. Thus, a neuro-ophthalmology referral is recommended to examine the visual field in detail and monitor symptoms (see Image. Idiopathic Intracranial Hypertension Fundus Examination). Symptom progression is a strong consideration for procedural intervention.[18]
Acute Intracranial Hypertension
The initial evaluation of acute intracranial hypertension should include a head CT. Cerebral edema-associated CT scan findings indicating intracranial hypertension include compressed basal cisterns, herniation patterns, cortical sulcal effacement, and midline shift. However, the absence of these findings does not rule out the condition. Complete blood count and metabolic panel are usually checked in all patients with suspected intracranial hypertension to evaluate for infection, anemia, and electrolyte abnormalities. A thorough neurologic examination, followed by serial examinations during management, is critical in evaluating patients with suspected acute ICP elevation.
A ventriculostomy catheter is the preferred ICP monitoring device and may be used for therapeutic CSF drainage to lower ICP. When ventricles cannot be cannulated, intraparenchymal devices using microsensors and fibreoptic transducers may be used. Subdural and epidural monitors are not as accurate as ventriculostomy and parenchymal monitors.[19][20][21][22]
Treatment / Management
Management of Acute Intracranial Hypertension
A sudden significant ICP increase is a neurosurgical emergency requiring close intensive care unit (ICU) monitoring. The initial management goals are airway protection, hemodynamic stability, and progression arrest. The heart rate, blood pressure, body temperature, ventilation and oxygenation, blood glucose, input and output, and cardiac rhythms must be closely monitored. Patients with suspected intracranial hypertension, especially individuals with severe TBI, should also have ICP monitoring.[23][24][25]
Intracranial hypertension management has 4 tiers. Implementation should begin at Tier 0 and progress stepwise if the patient does not improve.
- Tier 0:
- Elevate the head of the bed to 30° with a neutral position to minimize venous outflow resistance and maximize CSF flow dynamics.
- If a cervical collar is in place for possible cervical spine injuries, ensure the collar is loosened to prevent venous outflow obstruction.
- Hypoxia and hypercapnia can increase ICP, making respiratory management crucial. A normal carbon dioxide partial pressure (PaCO2 = 35-45 mm Hg) and adequate oxygenation (PaO2 > 94%) should be maintained by control ventilation without increasing the PEEP.
- Agitation and pain can increase blood pressure and ICP. Adequate sedation and analgesia are thus necessary adjunctive treatments. Medications with a minimal hypotensive effect are preferred. Hypovolemia can precipitate these medications' hypotensive side effects and should be treated before administration. Shorter-acting agents may allow for interrupting sedation to evaluate neurological status.
- Fever can increase the brain's metabolic rate and is a potent vasodilator. Higher body temperatures can increase cerebral blood flow and ICP. Fever should be controlled with antipyretics and cooling blankets. Infectious causes must be ruled out.
- Blood pressure elevation is commonly seen in patients with intracranial hypertension, especially due to TBI. Higher blood pressures also maintain cerebral perfusion in patients with untreated intracranial mass lesions. Systemic hypertension in these patients may be allowed, in some cases, to optimize cerebral perfusion without compromising overall cardiovascular status. Meanwhile, treating systemic hypertension in the absence of an intracranial mass lesion requires an individualized approach.
- The preferred antihypertensive drugs when managing intracranial pressure include β-blockers, such as labetalol and esmolol, and calcium channel blockers—agents that reduce blood pressure without affecting the ICP. Short-acting agents are favored. However, sodium nitroprusside, nitroglycerin, and nifedipine are generally avoided due to their potential to decrease systemic vascular resistance further and cause cerebral vasodilation.
- Seizures can complicate intracranial hypertension and should be prevented by prophylactic medications, especially in severe TBI.
- Tier 1:
- Hyperosmolar therapy may be initiated to decrease cerebral edema. Serum sodium concentration elevation induces osmotic fluid diffusion from the cerebral parenchyma into the serum. Baseline sodium levels should be monitored every 4 to 6 hours during therapy. Hypertonic saline can be given as a bolus of 3%, 7%, or 23.4% or as a continuous infusion of 3% saline if a more progressive increase in sodium level is desired.
- Mannitol is commonly used as a hyperosmolar agent, usually given as a bolus of 0.25 to 1 g/kg body weight. However, serum osmolality should be kept below 320 mOsm to avoid renal failure, hypokalemia, and hypoosmolarity.
- An external ventricular catheter may be placed for ICP monitoring and lowering by CSF drainage. In cases where ICP elevation is due to a CSF obstructive pathology, external ventricular drainage is the primary therapeutic method. External ventricular drainage may also be used as needed in diffuse cerebral edema cases for close pressure measurement and CSF drainage.
- Tier 2:
- Temporary hyperventilation with a goal PaCO2 of 30 to 35 mm Hg may be administered, though only up to 24 hours. More extended treatment periods have no benefit and may even be deleterious.
- Tier 3:
- Barbiturate therapy may be initiated to suppress electroencephalogram bursts and intracranial stimulation. Patients must be on continuous electroencephalogram when administering this treatment, and a general neurology service or neurointensive clinician must be consulted.
- Emergent surgical management should be considered if intracranial hypertension is refractory to medical management.
- Hypothermia may also be induced as part of management to reduce brain activity.
Surgical interventions
Intracranial mass lesions producing elevated ICP should be removed as soon as possible. CSF drainage reduces intracranial volume and pressure immediately. This modality may be used as an adjunct treatment for lowering ICP. However, CSF drainage has limited utility when the brain is diffusely swollen, and the ventricles are collapsed.
Decompressive craniectomy is used for treating severe uncontrolled intracranial hypertension. This procedure surgically removes part of the calvaria to create a window in the skull, allowing the swollen brain to herniate through the bone window, thus relieving pressure.
Management of Idiopathic Intracranial Hypertension
IIH management focuses on alleviating suspected CSF or venous outflow pathologic dynamics. Primary interventions are determined based on the severity of symptoms observed on initial evaluation.
Patients with BMI >30 kg/m² should be counseled on weight loss on initial evaluation, as weight loss of 15% can lead to IIH remission.[18] Acetazolamide therapy may be initiated to decrease CSF production volume if the patient is not at risk of immediate vision loss. The starting dose is 250 to 500 mg/day, titrated to a maximum of 4 g/day, though 1 g/day is generally tolerated. Topiramate at 5 to 50 mg BID is another option to reduce CSF production. However, women should be counseled that the medication may decrease anticontraceptive medications' effectiveness.(B3)
Surgical interventions
Surgical interventions are typically reserved for patients at risk of vision loss. A high-volume lumbar puncture can be performed to decrease ocular pressures and temporize until a more definitive surgical intervention can be achieved. Possible surgical interventions include ventriculoperitoneal shunt and venous sinus stenting. Lateral ventricle volumes in patients with IIH are often small, and intraoperative image guidance can assist in accurate placement.
A 2015 meta-analysis of patients with refractory IIH undergoing CSF diversion demonstrated headache improvement in 80% of patients after CSF diversion, papilledema improvement in 70%, and visual acuity in 45%. Headache improvement was noted in 83% of patients, papilledema improved in 97%, and visual symptoms improved in 78%.[26](A1)
Ventriculoperitoneal shunting complications most commonly present as shunt malfunction (proximal or distal obstruction) requiring shunt revision procedures. Venous sinus stenting is indicated only if dural venous stenosis is observed in vascular imaging studies.
Optin nerve sheath fenestration is reserved for patients with refractory papilledema, which is often asymmetric and has visual symptoms as the primary complaint. Less improvement in headaches was reported following this procedure, though an overall lower procedural complication rate than CSF diversion was documented.
Differential Diagnosis
Intracranial hypertension can arise from various causes. The conditions below are frequently encountered and must be considered in the differential diagnosis. A thorough clinical assessment and judicious diagnostic evaluation can help differentiate these conditions.
- Acute nerve injury
- Benign intracranial hypertension (Pseudotumor cerebri)
- Cerebrovascular ischemia or hemorrhage
- Hydrocephalus
- Intracranial epidural abscess
- Intracranial hemorrhage
- Leptomeningeal carcinoma
- Low-grade astrocytoma
- Lyme disease
- Meningioma
- Meningitis
- Migraine headache
- Papilledema
- Subarachnoid hemorrhage
- Venous sinus thrombosis
Prognosis
Depending on the etiology, prognosis is highly variable, ranging from lethal to benign. Children usually can tolerate higher ICP for a more extended period.
IIH is not associated with any specific mortality risk, but surgical treatments influence morbidity and mortality. The prognosis of this condition depends on visual function. Untreated disc edema can cause irreversible optic neuropathy and loss of color vision.
Short-lived acute intracranial hypertension has a good prognosis when treated promptly. However, treatment delays and the presence of a malignant etiology are associated with a poor prognosis. Many patients who survive develop permanent neurological deficits.[27][28]
Complications
Complications of intracranial hypertension vary, depending on the underlying etiology. These complications include:
- Stroke
- Seizures
- Optic neuropathy
- Loss of vision
- Stupor
- Coma
- Respiratory arrest
Patients with preexisting conditions must be counseled to seek medical attention promptly if symptoms appear and persist despite appropriate initial treatment.
Consultations
Evaluation and management of intracranial hypertension often require interdisciplinary collaboration. The interprofessional approach optimizes outcomes for patients with this condition. The following specialties are involved in the care of patients with intracranial hypertension:
- Neurologist
- Neurosurgeon
- Interventional Radiologist
- Intensivist
- Neuro-ophthalmologist
- Emergency clinician
Deterrence and Patient Education
Preventing intracranial hypertension involves addressing modifiable risk factors and promoting overall brain and eye health. General preventive measures include the following:
- Maintaining a healthy weight to reduce IIH risk
- Regular eye examinations to detect papilledema and other eye abnormalities early
- Managing medications like corticosteroids and oral contraceptives that can give rise to benign intracranial hypertension
- Managing comorbid conditions that may give rise to disorders that cause intracranial hypertension, such as stroke
- Avoiding risky behaviors to prevent TBI
- Genetic counseling for people with a family history of congenital anomalies with associated intracranial hypertension
Patients with IIH should also be educated regarding the condition's potential for disabling blindness. These individuals should consult an ophthalmologist for any visual disturbance.
Pearls and Other Issues
Intracranial hypertension presents a multifaceted challenge in clinical practice, requiring a nuanced approach to diagnosis and management. Early recognition of symptoms such as severe headaches, visual disturbances, nausea, and vomiting is paramount, prompting a comprehensive evaluation that includes thorough history-taking, physical examination, and diagnostic testing. Once the etiology is identified, treatment strategies for intracranial hypertension should aim to reduce intracranial pressure, alleviate symptoms, and address the underlying cause. Treatment approaches often include a combination of lifestyle modifications, medication management, and, in some cases, surgical interventions. Interprofessional collaboration is essential in navigating the complexities of intracranial hypertension management.
Enhancing Healthcare Team Outcomes
In the hospital setting, acute intracranial hypertension is best managed by an interprofessional team consisting of a neurologist, neurosurgeon, intensivist, ICU nurses, internist, and pulmonologist. Management is mainly focused on treating and reversing the etiology. Patients often need intensive care and continuous monitoring. Additionally, parameters such as heart rate, blood pressure, body temperature, ventilation, oxygenation, blood glucose, fluid input and output, and electrocardiogram should be monitored. Patients with suspected intracranial hypertension, especially in the context of severe TBI, should also have ICP monitoring.
The primary care clinician counsels patients on IIH's risk factors and weight loss in the outpatient setting. A neurologist should be consulted for headache management. A neuro-ophthalmologist's formal evaluation and monitoring of visual acuity is recommended, as visual function deterioration indicates disease progression and possibly requires surgical intervention.
Collaboration and communication among interprofessional team members are essential for optimizing patient outcomes and providing comprehensive care for individuals with intracranial hypertension. All team members bring unique perspectives and skills to the table, contributing to a comprehensive approach to management that addresses the complex needs of patients with intracranial hypertension from various angles.
Media
(Click Image to Enlarge)
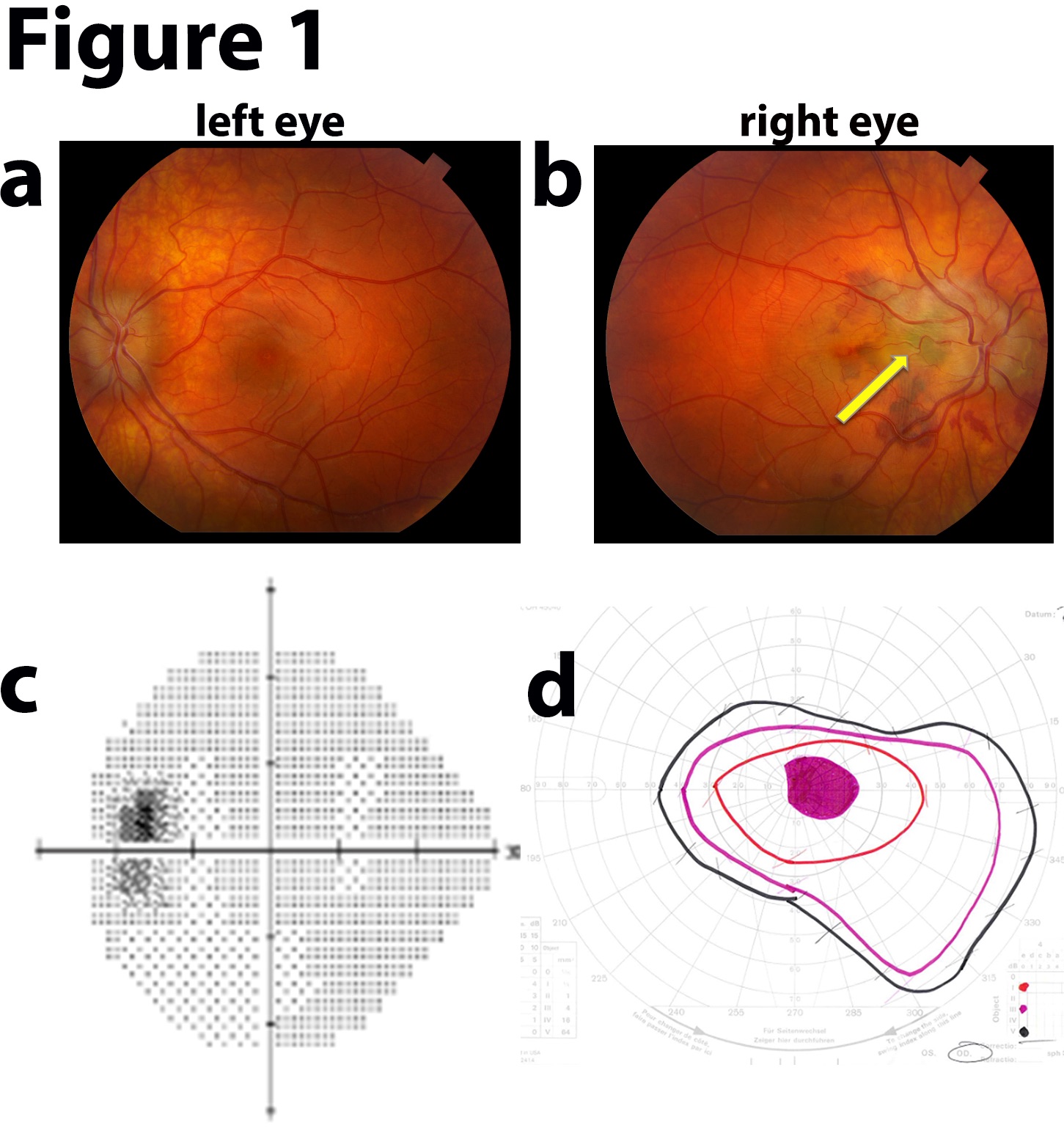
Idiopathic Intracranial Hypertension Fundus Examination. Vision loss in the right eye with bilateral disc swelling may indicate idiopathic intracranial hypertension. Fundus photographs a and b reveal bilateral disc edema. The right eye (image b) shows peripapillary subretinal blood and a choroidal neovascular membrane (yellow arrow). Humphrey visual field shows an enlarged blind spot in the left eye (image d). Goldman visual field reveals a right-eye central scotoma.
Contributed by Prof. Bhupendra C. K. Patel MD, FRCS with permission from MoranCore at https://morancore.utah.edu/section-12-retina-and-vitreous/iih-associated-choroidal-neovascular-membranes/
(Click Image to Enlarge)
(Click Image to Enlarge)
References
O'Reilly MW, Westgate CS, Hornby C, Botfield H, Taylor AE, Markey K, Mitchell JL, Scotton WJ, Mollan SP, Yiangou A, Jenkinson C, Gilligan LC, Sherlock M, Gibney J, Tomlinson JW, Lavery GG, Hodson DJ, Arlt W, Sinclair AJ. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI insight. 2019 Mar 21:4(6):. pii: 125348. doi: 10.1172/jci.insight.125348. Epub 2019 Mar 21 [PubMed PMID: 30753168]
Zanon E, Pasca S. Intracranial haemorrhage in children and adults with haemophilia A and B: a literature review of the last 20 years. Blood transfusion = Trasfusione del sangue. 2019 Sep:17(5):378-384. doi: 10.2450/2019.0253-18. Epub 2018 Feb 4 [PubMed PMID: 30747705]
Mondragon J, Klovenski V. Pseudotumor Cerebri. StatPearls. 2024 Jan:(): [PubMed PMID: 30725609]
Stevens SM, McClelland CM, Chen JJ, Lee MS. Idiopathic Intracranial Hypertension in a Mother and Pre-pubertal Twins. Neuro-ophthalmology (Aeolus Press). 2019 Feb:43(1):49-52. doi: 10.1080/01658107.2018.1480047. Epub 2018 Jun 26 [PubMed PMID: 30723525]
Baracchini C, Farina F, Pieroni A, Palmieri A, Kulyk C, Viaro F, Gabrieli JD, Cester G, Causin F, Manara R. Ultrasound Identification of Patients at Increased Risk of Intracranial Hemorrhage After Successful Endovascular Recanalization for Acute Ischemic Stroke. World neurosurgery. 2019 May:125():e849-e855. doi: 10.1016/j.wneu.2019.01.198. Epub 2019 Feb 8 [PubMed PMID: 30743030]
Ma YH, Leng XY, Dong Y, Xu W, Cao XP, Ji X, Wang HF, Tan L, Yu JT. Risk factors for intracranial atherosclerosis: A systematic review and meta-analysis. Atherosclerosis. 2019 Feb:281():71-77. doi: 10.1016/j.atherosclerosis.2018.12.015. Epub 2018 Dec 23 [PubMed PMID: 30658194]
Level 1 (high-level) evidenceWilkinson CM, Kalisvaart ACJ, Kung TFC, Abrahart AH, Khiabani E, Colbourne F. Tissue Compliance and Intracranial Pressure Responses to Large Intracerebral Hemorrhage in Young and Aged Spontaneously Hypertensive Rats. Hypertension (Dallas, Tex. : 1979). 2024 Jan:81(1):151-161. doi: 10.1161/HYPERTENSIONAHA.123.21628. Epub 2023 Nov 1 [PubMed PMID: 37909235]
Cozza M, Amadori L, Boccardi V. Exploring cerebral amyloid angiopathy: Insights into pathogenesis, diagnosis, and treatment. Journal of the neurological sciences. 2023 Nov 15:454():120866. doi: 10.1016/j.jns.2023.120866. Epub 2023 Nov 2 [PubMed PMID: 37931443]
Rotim A, Raguž M, Gajski D, Vrban F, Jurilj M, Orešković D, Hrabar J, Kalousek V, Sajko T, Rotim K. METEOROLOGICAL VARIABLES ASSOCIATED WITH SUBARACHNOID HEMORRHAGE: A SINGLE CENTER STUDY. Acta clinica Croatica. 2022 Dec:61(4):673-680. doi: 10.20471/acc.2022.61.04.14. Epub [PubMed PMID: 37868170]
Guan B, Anderson DB, Chen L, Feng S, Zhou H. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. BMJ open. 2023 Oct 6:13(10):e075049. doi: 10.1136/bmjopen-2023-075049. Epub 2023 Oct 6 [PubMed PMID: 37802626]
Level 1 (high-level) evidenceKim DJ, Czosnyka Z, Kasprowicz M, Smieleweski P, Baledent O, Guerguerian AM, Pickard JD, Czosnyka M. Continuous monitoring of the Monro-Kellie doctrine: is it possible? Journal of neurotrauma. 2012 May 1:29(7):1354-63. doi: 10.1089/neu.2011.2018. Epub 2011 Nov 4 [PubMed PMID: 21895518]
Tadevosyan A, Kornbluth J. Brain Herniation and Intracranial Hypertension. Neurologic clinics. 2021 May:39(2):293-318. doi: 10.1016/j.ncl.2021.02.005. Epub 2021 Mar 31 [PubMed PMID: 33896520]
Jirlow U, Arvidsson L, Magneli S, Cesarini K, Rostami E. Evaluation of Miethke M.scio Device Implantation for Intracranial Pressure Monitoring in Patients with Cerebrospinal Fluid Disorders. World neurosurgery. 2023 Nov:179():e63-e74. doi: 10.1016/j.wneu.2023.07.102. Epub 2023 Jul 26 [PubMed PMID: 37506838]
Park JH, Yoon SH. New concept of cerebrospinal fluid dynamics in cerebral venous sinus thrombosis. Medical hypotheses. 2008:70(1):143-7 [PubMed PMID: 17570605]
Raoof N, Hoffmann J. Diagnosis and treatment of idiopathic intracranial hypertension. Cephalalgia : an international journal of headache. 2021 Apr:41(4):472-478. doi: 10.1177/0333102421997093. Epub 2021 Feb 25 [PubMed PMID: 33631966]
Chen JJ, Thurtell MJ, Longmuir RA, Garvin MK, Wang JK, Wall M, Kardon RH. Causes and Prognosis of Visual Acuity Loss at the Time of Initial Presentation in Idiopathic Intracranial Hypertension. Investigative ophthalmology & visual science. 2015 Jun:56(6):3850-9. doi: 10.1167/iovs.15-16450. Epub [PubMed PMID: 26070058]
Patel S, Maria-Rios J, Parikh A, Okorie ON. Diagnosis and management of elevated intracranial pressure in the emergency department. International journal of emergency medicine. 2023 Oct 13:16(1):72. doi: 10.1186/s12245-023-00540-x. Epub 2023 Oct 13 [PubMed PMID: 37833652]
Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, Hemmings K, Williamson M, Burdon MA, Hassan-Smith G, Digre K, Liu GT, Jensen RH, Sinclair AJ. Idiopathic intracranial hypertension: consensus guidelines on management. Journal of neurology, neurosurgery, and psychiatry. 2018 Oct:89(10):1088-1100. doi: 10.1136/jnnp-2017-317440. Epub 2018 Jun 14 [PubMed PMID: 29903905]
Level 3 (low-level) evidenceSun S, Li Y, Zhang H, Wang X, She L, Yan Z, Lu G. The effect of mannitol in the early stage of supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. World neurosurgery. 2018 Dec 18:():. pii: S1878-8750(18)32818-3. doi: 10.1016/j.wneu.2018.11.249. Epub 2018 Dec 18 [PubMed PMID: 30576817]
Level 1 (high-level) evidenceMcHugh DC, Fiore SM, Strong N, Egnor MR. Bifrontal Biparietal Cruciate Decompressive Craniectomy in Pediatric Traumatic Brain Injury. Pediatric neurosurgery. 2019:54(1):6-11. doi: 10.1159/000495067. Epub 2019 Jan 3 [PubMed PMID: 30605902]
Sheikh MF, Unni N, Agarwal B. Neurological Monitoring in Acute Liver Failure. Journal of clinical and experimental hepatology. 2018 Dec:8(4):441-447. doi: 10.1016/j.jceh.2018.04.013. Epub 2018 May 5 [PubMed PMID: 30568346]
Godoy DA, Núñez-Patiño RA, Zorrilla-Vaca A, Ziai WC, Hemphill JC 3rd. Intracranial Hypertension After Spontaneous Intracerebral Hemorrhage: A Systematic Review and Meta-analysis of Prevalence and Mortality Rate. Neurocritical care. 2019 Aug:31(1):176-187. doi: 10.1007/s12028-018-0658-x. Epub [PubMed PMID: 30565090]
Level 1 (high-level) evidenceJha RM, Kochanek PM. A Precision Medicine Approach to Cerebral Edema and Intracranial Hypertension after Severe Traumatic Brain Injury: Quo Vadis? Current neurology and neuroscience reports. 2018 Nov 7:18(12):105. doi: 10.1007/s11910-018-0912-9. Epub 2018 Nov 7 [PubMed PMID: 30406315]
Hoffmann J, Mollan SP, Paemeleire K, Lampl C, Jensen RH, Sinclair AJ. European headache federation guideline on idiopathic intracranial hypertension. The journal of headache and pain. 2018 Oct 8:19(1):93. doi: 10.1186/s10194-018-0919-2. Epub 2018 Oct 8 [PubMed PMID: 30298346]
Alali AS, Temkin N, Barber J, Pridgeon J, Chaddock K, Dikmen S, Hendrickson P, Videtta W, Lujan S, Petroni G, Guadagnoli N, Urbina Z, Chesnut RM. A clinical decision rule to predict intracranial hypertension in severe traumatic brain injury. Journal of neurosurgery. 2018 Sep 28:131(2):612-619. doi: 10.3171/2018.4.JNS173166. Epub [PubMed PMID: 30265194]
Satti SR, Leishangthem L, Chaudry MI. Meta-Analysis of CSF Diversion Procedures and Dural Venous Sinus Stenting in the Setting of Medically Refractory Idiopathic Intracranial Hypertension. AJNR. American journal of neuroradiology. 2015 Oct:36(10):1899-904. doi: 10.3174/ajnr.A4377. Epub 2015 Aug 6 [PubMed PMID: 26251432]
Level 1 (high-level) evidenceSacco TL, Davis JG. Management of Intracranial Pressure Part II: Nonpharmacologic Interventions. Dimensions of critical care nursing : DCCN. 2019 Mar/Apr:38(2):61-69. doi: 10.1097/DCC.0000000000000341. Epub [PubMed PMID: 30702474]
Pal A, Sengupta P, Biswas D, Sen C, Mukherjee A, Pal S. Pattern of Idiopathic Intracranial Hypertension in Indian Population. Annals of Indian Academy of Neurology. 2019 Jan-Mar:22(1):47-51. doi: 10.4103/aian.AIAN_116_18. Epub [PubMed PMID: 30692759]