Introduction
Insulin resistance is identified as the impaired biologic response of target tissues to insulin stimulation. All tissues with insulin receptors can become insulin resistant, but the tissues that primarily drive insulin resistance are the liver, skeletal muscle, and adipose tissue. Insulin resistance impairs glucose disposal, resulting in a compensatory increase in beta-cell insulin production and hyperinsulinemia. Recent studies have debated whether hyperinsulinemia precedes insulin resistance, as hyperinsulinemia itself is a driver of insulin resistance. This concept may be clinically valuable, suggesting that hyperinsulinemia associated with excess caloric intake may drive the metabolic dysfunction associated with insulin resistance. The metabolic consequences of insulin resistance include hyperglycemia, hypertension, dyslipidemia, hyperuricemia, elevated inflammatory markers, endothelial dysfunction, and a prothrombotic state. Progression of insulin resistance can lead to metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), and type 2 diabetes.[1][2][3][4][5]
Insulin resistance is primarily an acquired condition related to excess body fat, though genetic causes are also identified. The clinical definition of insulin resistance remains elusive, as there is no generally accepted test for insulin resistance. Clinically, insulin resistance is recognized via the metabolic consequences associated with insulin resistance as described in metabolic syndrome and insulin resistance syndrome.[6][7]
The gold standard for measurement of insulin resistance is the hyperinsulinemic-euglycemic glucose clamp technique. This research technique has limited clinical applicability; however, several clinically useful surrogate measures of insulin resistance include HOMA-IR, HOMA2, QUICKI, serum triglyceride, and triglyceride/HDL ratio. In addition, several measures assess insulin resistance based on serum glucose or insulin response to a glucose challenge.[8][9][10][11]
The predominant consequence of insulin resistance is type 2 diabetes (T2D). Insulin resistance is thought to precede the development of T2D by 10 to 15 years. The development of insulin resistance typically results in impaired glucose disposal into insulin-resistant tissues, especially skeletal muscle. Consequently, in the presence of excess calorie consumption, more insulin is required to traffic glucose into these tissues. The resultant hyperinsulinemia further contributes to insulin resistance. This vicious cycle continues until pancreatic beta-cell activity can no longer adequately meet the insulin demand created by insulin resistance, resulting in hyperglycemia. With a continued mismatch between insulin demand and insulin production, glycemic levels rise to those consistent with T2D. Weight gain usually occurs alongside hyperinsulinemia but may be related more to a chronic caloric excess than hyperinsulinemia. The anabolic effect of insulin decreases as tissues become more insulin-resistant, and weight gain eventually slows.[12][13][14][15]
Resistance to exogenous insulin has also been described. An arbitrary but clinically useful benchmark considers patients insulin-resistant when requiring more than 1 unit/kilogram/day of exogenous insulin to maintain glycemic control. Patients requiring greater than 200 units of exogenous insulin per day are considered severely insulin-resistant.[16]
In addition to T2D, the disease spectrum associated with insulin resistance includes obesity, cardiovascular disease, NAFLD, metabolic syndrome, and polycystic ovary syndrome (PCOS). These are all of great consequence in the United States, with a tremendous burden on the healthcare system to treat the direct and indirect conditions associated with insulin resistance. The microvascular complications of diabetes, such as neuropathy, retinopathy, and nephropathy, as well as the associated macrovascular complications of coronary artery disease [CAD], cerebral-vascular disease, and peripheral artery disease (PAD), will eventually consume the lion's share of the healthcare dollar as the disease progresses in severity.[17][18][19][20][21][22][23]
Lifestyle modifications should be the primary focus when treating insulin resistance. Nutritional intervention with calorie reduction and avoidance of carbohydrates that stimulate excessive insulin demand is a cornerstone of treatment. Physical activity helps to increase energy expenditure and improve skeletal muscle insulin sensitivity. Medications also can improve insulin response and reduce insulin demand.[24][25][26]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiologies of insulin resistance may be acquired, hereditary, or mixed. The great majority of people with insulin resistance fall have an acquired etiology.[25]
Acquired Etiologies of Insulin Resistance
- Increased visceral adiposity related to ectopic fat deposition and overflow from subcutaneous fat stores
- Aging process
- Physical inactivity
- Nutritional imbalance
- Medications (glucocorticoids, anti-adrenergic, protease inhibitors, selective serotonin reuptake inhibitors, atypical antipsychotics, and some exogenous insulins)
- High-sodium diets
- Glucose toxicity
- Lipotoxicity from excess circulating free fatty acids
In addition to the heritable components of the above etiologies of insulin resistance, there are several unrelated genetic syndromes with associated syndromic insulin resistance.[27]
Genetic Etiologies of Insulin Resistance
- Myotonic dystrophy
- Ataxia-telangiectasia
- Alstom syndrome
- Rabson-Mendenhall syndrome
- Werner syndrome
- Lipodystrophy
- Polycystic ovarian syndrome
- Type-A insulin resistance: Characterized by severe insulin resistance (abnormal glucose homeostasis, ovarian virialization, and acanthosis nigricans) caused by abnormalities of the insulin receptor gene
- Type-B insulin resistance: Characterized severe impairment of insulin action triggered by the presence of insulin receptor autoantibodies with resultant abnormal glucose homeostasis, ovarian hyperandrogenism, and acanthosis nigricans
An alternative classification of insulin resistance exists and is based on the site of dysfunction with respect to the insulin receptor. This classification system includes pre-receptor, receptor, and post-receptor etiologies.
Epidemiology
Epidemiologic assessment of insulin resistance is typically measured in relation to the prevalence of metabolic syndrome or insulin resistance syndrome. Criteria proposed by the National Cholesterol Education Program Adult Treatment Panel III national survey data suggest insulin resistance syndrome is widespread.
An analysis from 2003 of the National Health and Nutrition Examination Survey (NHANES) data showed insulin resistance affects about 22% of United States (US) adults older than 20 years. A more recent analysis of NHANES data from 2021 found that 40% of US adults aged 18 to 44 are insulin-resistant based on HOMA-IR measurements. While obesity rates have increased considerably over the past 2 decades, this rapid increase in prevalence was not only associated with increased adiposity. Hypertension, dyslipidemia, and limited physical activity also increased insulin resistance.
While there has been a rapid rise in pediatric obesity and type 2 diabetes, no consensus has been reached on the pediatric population's diagnostic criteria for insulin resistance. From a demographic standpoint, insulin resistance affects all races and ethnicities, with limited data on comparison between groups.[28][29]
Pathophysiology
The 3 primary sites of insulin resistance are the skeletal muscle, liver, and adipose tissue. In a state of chronic caloric surplus, the tissues in the body become resistant to insulin signaling. Skeletal muscle is a large reservoir for circulating glucose, accounting for up to 70% of glucose disposal as measured by the hyperinsulinemic-euglycemic clamp. The direct result of muscle insulin resistance is decreased glucose uptake by muscle tissue. Glucose is shunted from muscle to the liver, where de novo lipogenesis (DNL) occurs. With increased glucose substrate, the liver develops insulin resistance as well. Higher rates of DNL increase plasma triglyceride content and create an environment of excess energy substrate, which increases insulin resistance throughout the body, contributing to ectopic lipid deposition in and around visceral organs. [25]
Skeletal Muscle Tissue
After intake of a caloric load and conversion to glucose, muscle is the primary site for glucose disposal, accounting for up to 70% of tissue glucose uptake. In chronic caloric excess, muscle tissue accumulates intramyocellular fatty acids. Diacylglycerol is an intramyocellular fatty acid that signals energy excess within the cell. Diacylglycerol activates protein kinase C theta (PKC-theta), decreasing proximal insulin signaling. The direct result is decreased glucose transporter type 4 (GLUT4) translocation to the cell membrane and reduced glucose uptake by the muscle tissue. The excess glucose in the blood is shunted to the liver to be metabolized or stored.[25][30]
Hepatic Tissue
The liver is responsible for processing energy substrates. It packages, recirculates, and creates fatty acids and processes, stores, and creates glucose. If the liver becomes insulin-resistant, these processes are severely affected, resulting in significant metabolic consequences. When skeletal muscle develops insulin resistance, excess glucose in the blood is shunted to the liver. When the liver tissue senses an excess of energy substrate, particularly in the form of diacylglycerol, a process similar to that in skeletal muscle occurs. In the liver, the diacylglycerol content activates protein kinase C epsilon (PKC-epsilon), which decreases proximal insulin signaling. Excess glucose enters hepatocytes via insulin-independent pathways stimulating DNL via substrate push, creating more fatty acids from the glucose surplus. The excess fatty acid is deposited in the liver or as ectopic lipid throughout the viscera. Additionally, immune-mediated inflammatory changes contribute to excess lipolysis from adipose tissue, which is re-esterified by the liver and further adds to circulating fatty acid and ectopic lipid deposition. Finally, normal insulin-mediated suppression of gluconeogenesis is defective, and the liver continues to create more glucose, adding to the circulating glucose surplus.[25][30]
Adipose Tissue
Using the hyperinsulinemic-euglycemic clamp technique, researchers determined that lipolysis is sensitive to insulin. The failure of insulin to suppress lipolysis in insulin-resistant adipose tissue, especially visceral adipose tissue, increases circulating free fatty acids (FFAs). Higher levels of circulating FFAs directly affect both liver and muscle metabolism, further exacerbating insulin resistance in these tissues and contributing to lipotoxicity-induced beta-cell dysfunction.[25][30]
History and Physical
The clinical presentation of insulin resistance is variable concerning both history and physical examination findings. It depends on the subset of insulin resistance present, the duration of the condition, the level of beta-cell function, and the individual’s propensity for secondary illnesses due to insulin resistance. Common presentations include:
Associated Diseases
- Non-alcoholic fatty liver disease (NAFLD)
- Metabolic syndrome
- Prediabetes or type 2 diabetes
- Polycystic ovarian syndrome (PCOS)
- Obesity
- Microvascular disease (retinopathy, neuropathy, or nephropathy)
- Macrovascular disease (stroke, PAD, and CAD)
Associated Symptoms
- Hypertension
- Hyperlipidemia
- Gender and ethnicity-specific increased waist circumference
- The stigmata of PCOS (menstrual irregularities, hirsutism, acne, and alopecia)
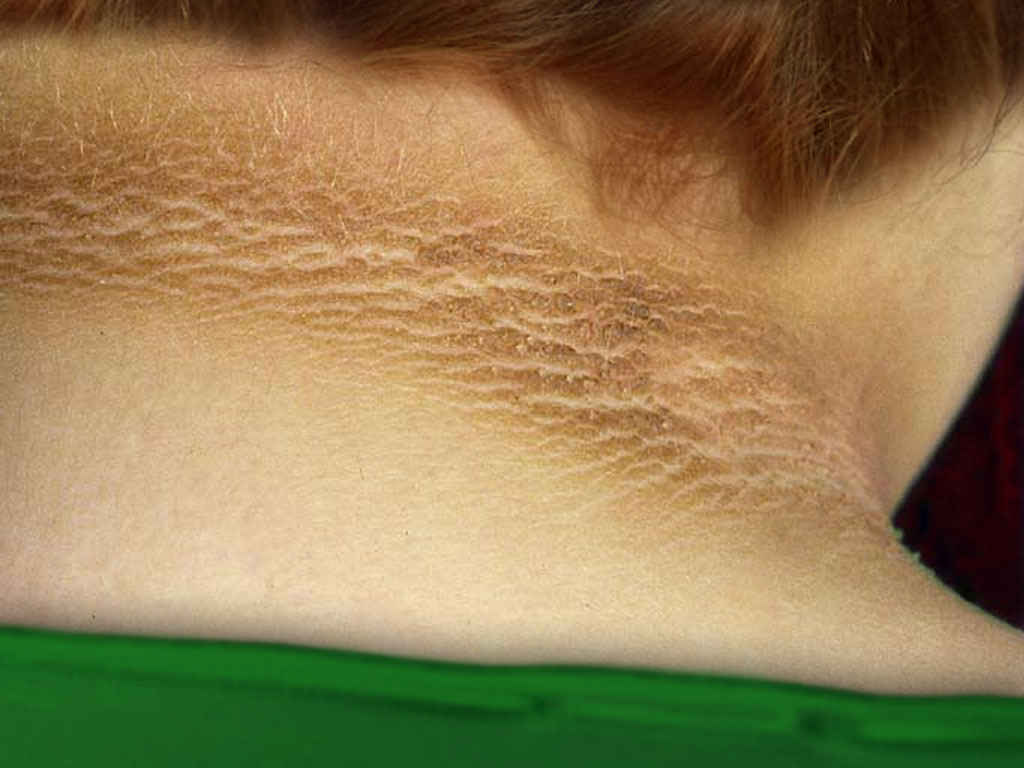
- Acanthosis nigricans (see Image. Acanthosis Nigricans)
- The stigmata of one of several genetic syndromes that include insulin resistance syndromes
- Type A or type B insulin resistance syndrome
Evaluation
The gold standard for measuring insulin resistance is the hyperinsulinemic-euglycemic glucose clamp technique. This is a research technique in which a fasting, non-diabetic patient is placed on a high-rate constant infusion of insulin to suppress hepatic glucose production; the blood glucose is frequently monitored while a concomitant 20% dextrose solution is given at varying rates to regulate the blood glucose in the euglycemic range. The amount of glucose required to reach a steady state reflects the exogenous glucose disposal needed to compensate for hyperinsulinemia. Insulin resistance calculation is based on whole-body glucose disposal and body size.[31]
The associated risks and complexity of the glucose clamp method limit its clinical usefulness. As a result, multiple surrogate markers for insulin resistance have been developed and tested. The homeostatic model assessment for insulin resistance (HOMA-IR), based on fasting glucose and fasting insulin levels, is a widely utilized measure of insulin resistance in clinical research. Other measures based on fasting insulin include HOMA2, the Glucose to Insulin Ratio (GIR), and the Quantitative Insulin Sensitivity Index (QUICKI). The McAuley Index utilizes fasting insulin and triglycerides. Post-glucose challenge tests, done after an overnight fast, measure insulin and glucose response to a 75-gram glucose load. Methods include the Matsuda Index and Insulin Sensitivity Index (ISI).[8][9][10][32][33][34][35][36]
Other surrogate markers involve triglycerides alone or in relation to HDL cholesterol. Patients with prediabetes and triglycerides greater than or equal to 150 g/dL were more likely to have insulin resistance. The triglyceride/HDL ratio is correlated with insulin resistance in individuals who identify as White. In general, a ratio greater than 3.0 is associated with insulin resistance. More specifically, a ratio greater than or equal to 3.5 in males and greater than or equal to 2.5 in females indicates insulin resistance. These correlations do not hold up in individuals who identify as Black.[11][37]
Measures of insulin resistance have not been integrated into clinical guidelines. As a result, the presence of insulin resistance is generally inferred from the clinical presentation. Metabolic syndrome (MetS) and insulin resistance syndrome (IRS) are considered to be clinical indicators of insulin resistance.
Multiple criteria for metabolic syndrome (MetS) exist. In 2009, a joint scientific statement harmonizing criteria for MetS was released.[38] MetS is identified by the presence of 3 or more of the following diagnostic cut points:
- A waist circumference of 32” to 40” based on gender and ethnicity
- Elevated triglycerides greater than or equal to 150 mg/dL or on medication to treat hypertriglyceridemia
- Reduced HDL less than 40 mg/dL in males or less than 50 mg/dL in females
- Elevated blood pressure greater than or equal to 130 mm Hg systolic or greater than or equal to 85 mm Hg diastolic or on antihypertensive medication
- Elevated fasting glucose greater than or equal to 100 mg/dL or on a glucose-lowering agent
The American College of Endocrinology identifies specific physiologic abnormalities that increase IRS risk.[39] These abnormalities include:
- Impaired glucose tolerance or impaired fasting glucose
- Abnormal uric acid metabolism
- Dyslipidemia (increased triglycerides, decreased HDL-C, or small, dense LDL)
- Hemodynamic changes such as elevated blood pressure
- Prothrombotic factors (PAI-1, fibrinogen)
- Markers of inflammation (eg, C-reactive protein, white blood cell count)
- Endothelial dysfunction
Other factors include the following:
- Body mass index (BMI) greater than or equal to 25 kg/m2
- Diagnosis of CVD, PCOS, NAFLD, or acanthosis nigricans
- A family history of T2D, hypertension, or CVD
- Sedentary lifestyle
- Non-white ethnicity
- Age older than 40 years
Treatment / Management
Intensive Lifestyle Intervention
Lifestyle intervention represents the cornerstone of treatment for insulin resistance. Dietary intervention should include a combination of calorie restriction and high glycemic index carbohydrate reduction. Physical activity improves both calorie expenditure and insulin sensitivity in muscle tissue.[40][41][42](A1)
Individuals with insulin resistance are at high risk of developing T2D. The Diabetes Prevention Program and its Outcomes Study (DPP & DPPOS) demonstrated that lifestyle intervention was a significant and cost-effective intervention for diabetes prevention in high-risk adults.[24][26] These interventions include:(A1)
- Dietary therapy with sodium reduction, fat reduction, calorie restriction, and attention to the glycemic index of foods
- Education, support, and personalized programs
- A 7% weight loss reduced the onset of T2D by 58%
- DPP included a metformin arm which reduced the onset of T2D by 31%
Specific Pharmacological Interventions for Blood Glucose Management
While no medications are FDA approved for the treatment of insulin resistance, general approaches include the following:
- Metformin is a common first-line therapy for medication treatment of T2D and is approved for use in PCOS. The DPP & DPPOS study showed that the combination of metformin and lifestyle interventions was medically useful and cost-effective. Despite the concerns about using metformin in mild to moderate renal dysfunction, several organizations, including the American Geriatric Society and the Kidney Disease Improving Global Outcomes guidelines, endorse use as long as the GFR exceeds 30.[24][26][43][44][45]
- Glucagon-like peptide one (GLP-1) receptor agonists stimulate the GLP-1 receptors in the pancreas, thereby increasing insulin release and inhibiting glucagon secretion. The use of GLP-1 agonists is associated with weight loss, which may reduce insulin resistance. Liraglutide and semaglutide are FDA-approved for the treatment of T2D and obesity. Another agent, tirzepitide, is a dual GLP-1 and gastric inhibitory polypeptide (GIP) agonist, has effects similar to semaglutide, and is also FDA-approved for treating T2D.[46][47][48]
- Sodium-glucose cotransporter 2 (SGLT2) inhibitors increase urinary glucose excretion, thereby reducing plasma glucose levels and exogenous insulin requirements. The use of SGLT2 inhibitors has also been associated with weight loss, which may reduce insulin resistance.[49][50]
- Thiazolidinediones improve insulin sensitivity and glucose control by increasing insulin-dependent glucose disposal in skeletal muscle and adipose tissue and decreasing hepatic glucose output. Though effective, associated secondary weight gain and fluid retention, with associated cardiovascular concerns, limit their use.[51][52]
- Dipeptidyl peptidase-4 (DPP-4) inhibitors prolong the activity of endogenous GLP-1 and GIP by preventing their breakdown.[4] (A1)
Surgery
Surgical intervention in the form of gastric sleeves, banding, and bypass is available for qualified individuals with obesity. The excess fat loss associated with bariatric surgery improves insulin sensitivity. The results of the STAMPEDE trial provide good evidence of the benefit of bariatric surgery on T2D.[53][54][55](B2)
Differential Diagnosis
- Lipodystrophy (acquired, localized or generalized): Loss of adipose tissue that results from either genetic or acquired causation and can result in the ectopic deposition of fat in either hepatic or muscular tissue[56]
- Polycystic ovarian syndrome (PCOS)
- Obesity: Excess body weight is categorized as overweight (BMI of 25 to 29.9), class I obesity (BMI of 30 to 34.9), class II obesity (BMI 35.0 to 39.9), and class III obesity (BMI greater than 40)[57]
- Hypertension: The most recent ACC/AHA guidelines for the diagnosis of hypertension include systolic pressure greater than or equal to 130 mm Hg or diastolic pressure greater than or equal to 80 mm Hg[58]
- Hypertriglyceridemia: Elevated triglyceride levels (greater than or equal to 150 mg/dL)
- Type 1 diabetes
- Type 2 diabetes
- Other forms of glucose intolerance (impaired fasting glucose, impaired glucose tolerance, and gestational diabetes)
Prognosis
The prognosis of insulin resistance depends on the subset of the disease, the severity of the disease, underlying pancreatic beta-cell function, the heritable susceptibility of the patient to the secondary complications from insulin resistance, and individual response to appropriate therapy. The outcomes range from mildly insulin-resistant, asymptomatic individuals to those with catastrophic cardiovascular or cerebrovascular events and their resulting morbidity and mortality.
Statistically, coronary artery disease is the leading cause of mortality in the US, with diabetes as seventh. The common basis for diabetes and much of the resultant vascular disease is insulin resistance. Additional mortality from insulin resistance occurs in the less common manifestations of the disease, including genetic syndromes and fatty deposition diseases. Finally, substantial morbidity manifests with the loss of reproductive function and associated features of PCOS.
Mitigation for the disease exists. Increased clinical awareness enables early diagnosis and treatment. Improved understanding of the disease process has resulted in more targeted, multi-faceted therapies. Efforts to attain and maintain a healthy weight through improved dietary intake and increased physical activity can reduce insulin resistance and prevent associated complications. More generalized lay recognition can increase the efficacy of preventative care, with the hope of an eventual downturn in epidemic obesity and resultant insulin resistance.[59]
Complications
Most of the complications from insulin resistance are related to the development of vascular complications.
The microvascular disease manifests as retinopathy, nephropathy, and peripheral neuropathy. In the central nervous system, dementia, stroke, mood disturbance, and gait instability may occur. Cardiac microvascular disease can manifest as angina, coronary artery spasm, and cardiomyopathy. Renal microvascular disease is a significant cause of chronic kidney disease, renal failure, and dialysis. Ophthalmological small vessel disease is a leading cause of retinopathy and visual impairment. Macrovascular disease, secondary to insulin resistance, causes PAD, CAD, and CVA.
Non-alcoholic fatty liver disease (NAFLD) is intricately related to insulin resistance and T2D. Patients with T2D have a 2-fold increased risk for NAFLD. With an increasing worldwide prevalence and incidence in children, NAFLD should be of great concern to clinicians treating patients with insulin resistance.[19][20]
Deterrence and Patient Education
Primary, secondary, and tertiary prevention have distinct roles in managing insulin resistance.
Primary prevention promotes public education regarding the importance of regular health monitoring. A healthy diet and increased activity level can prevent or delay the onset of insulin resistance, metabolic syndrome, and diabetes, along with the associated complications. The emphasis on behavior modification and a sustainable lifestyle is critical for long-term weight management.
Secondary prevention includes laboratory screening for insulin resistance, diabetes, and further subspecialist referral to manage the early intervention for insulin resistance. The DPP & DPPOS studies demonstrate the benefits of lifestyle change and the use of metformin to prevent progression from pre-diabetes to T2D. [24][26]
Public acceptance of tertiary prevention, such as intensive medical intervention and bariatric surgery for weight reduction, can lead to decreased morbidity and mortality associated with the consequent complications of insulin resistance.
Pearls and Other Issues
Intensive lifestyle intervention should be the first line of therapy for patients with metabolic syndrome or insulin resistance syndrome. The benefits of exercise cannot be understated in treating patients with insulin resistance. Barriers to exercise should be discussed, and a well-formulated plan, including moderate-intensity cardiovascular exercise like walking, should be provided in accordance with the physical activity guidelines. Discussion of dietary modification following the dietary guidelines should also be provided with individualization to the patient's preferences, with particular attention to reducing sugar, refined grain products, and high glycemic index carbohydrates.
For patients with T2D, insulin resistance, and hyperinsulinemia, consider treatment with agents to improve insulin sensitivity or contribute to weight loss, like metformin, GLP-1 receptor agonists, GLP-1/GIP receptor agonists, and SGLT2 inhibitors. [60][61][62]
Enhancing Healthcare Team Outcomes
Over the past few decades, the incidence of insulin resistance has skyrocketed primarily due to our lifestyle and the rising incidence of obesity. Without treatment, the condition is associated with numerous complications, including fatal cardiac events. Therefore, the management of insulin resistance is best done with an interprofessional team. The consultations and coordination of care most indicated for the treatment of insulin resistance include:
- Obesity medicine specialist: medical management for obesity treatment
- Bariatric surgeon: bariatric surgery is effective for obesity treatment in individuals who satisfy the criteria for surgery
- Endocrinology: early and aggressive management of T2D, hyperlipidemia, and PCOS
- Cardiology and cardiac surgery: management of the cardiovascular complications of insulin resistance
- Gastroenterology: early detection and treatment of NAFLD
- Neurology: management of the cerebrovascular and peripheral neurologic complications of insulin resistance
- Vascular surgery: surgical management of both carotid artery disease and PAD
- Nurse diabetic educator: assists the clinician in educating the patient on diabetes prevention
- Dietitian: educates the patient on a healthy diet, including low-carbohydrate approaches
- Physical therapist: educates the patient on how to increase physical activity safely
- Pharmacist: educates the patient on the importance of medication adherence, instructing the patient on the proper use of medications, potential drug-drug interactions, and side effects
- Social worker: assist the patient with the necessary support and finances to obtain treatment
- Psychologist: provide behavioral therapy support
There is limited evidence in favor of continuous glucose monitoring (CGM). Remote monitoring for healthcare teams shows benefits in the management of T2D. More research is needed to show the effects of CGM on those with prediabetes or insulin resistance without T2D.
The key to the management of insulin resistance is encouraging lifestyle changes. Dietary intervention should include a combination of calorie restriction and reduction of high glycemic index carbohydrates. Physical activity improves both calorie expenditure and insulin sensitivity in muscle tissue.[63][64]
Outcomes
The outcomes of well-managed insulin resistance are good for those who remain adherent to therapy. Unfortunately, many patients struggle with adherence to therapy, with consequential progression to T2D and subsequent risk of adverse cardiac or CNS events. Early identification and intervention with an interprofessional team approach are essential in managing these patients.[62][65]
Media
References
Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: a mechanistic review. Archives of pharmacal research. 2019 May:42(5):383-392. doi: 10.1007/s12272-019-01138-9. Epub 2019 Mar 5 [PubMed PMID: 30835074]
Brown JC, Harhay MO, Harhay MN. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis. Nutrition and metabolic insights. 2019:12():1178638819831712. doi: 10.1177/1178638819831712. Epub 2019 Feb 27 [PubMed PMID: 30833814]
Level 2 (mid-level) evidenceNolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes & vascular disease research. 2019 Mar:16(2):118-127. doi: 10.1177/1479164119827611. Epub 2019 Feb 15 [PubMed PMID: 30770030]
Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Frontiers in endocrinology. 2019:10():80. doi: 10.3389/fendo.2019.00080. Epub 2019 Feb 15 [PubMed PMID: 30828317]
Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. Journal of the Endocrine Society. 2019 Sep 1:3(9):1727-1747. doi: 10.1210/js.2019-00065. Epub 2019 Jul 24 [PubMed PMID: 31528832]
Hossan T, Kundu S, Alam SS, Nagarajan S. Epigenetic Modifications Associated with the Pathogenesis of Type 2 Diabetes Mellitus. Endocrine, metabolic & immune disorders drug targets. 2019:19(6):775-786. doi: 10.2174/1871530319666190301145545. Epub [PubMed PMID: 30827271]
Bothou C, Beuschlein F, Spyroglou A. Links between aldosterone excess and metabolic complications: A comprehensive review. Diabetes & metabolism. 2020 Feb:46(1):1-7. doi: 10.1016/j.diabet.2019.02.003. Epub 2019 Feb 27 [PubMed PMID: 30825519]
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul:28(7):412-9 [PubMed PMID: 3899825]
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes care. 1998 Dec:21(12):2191-2 [PubMed PMID: 9839117]
Level 3 (low-level) evidenceKatz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. The Journal of clinical endocrinology and metabolism. 2000 Jul:85(7):2402-10 [PubMed PMID: 10902785]
Level 1 (high-level) evidenceKim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism: clinical and experimental. 2010 Feb:59(2):299-304. doi: 10.1016/j.metabol.2009.07.027. Epub 2009 Sep 30 [PubMed PMID: 19796777]
Level 2 (mid-level) evidenceTobin GS, Cavaghan MK, Hoogwerf BJ, McGill JB. Addition of exenatide twice daily to basal insulin for the treatment of type 2 diabetes: clinical studies and practical approaches to therapy. International journal of clinical practice. 2012 Dec:66(12):1147-57. doi: 10.1111/ijcp.12032. Epub 2012 Oct 14 [PubMed PMID: 23061886]
Abdul-Ghani M, DeFronzo RA. Insulin Resistance and Hyperinsulinemia: the Egg and the Chicken. The Journal of clinical endocrinology and metabolism. 2021 Mar 25:106(4):e1897-e1899. doi: 10.1210/clinem/dgaa364. Epub [PubMed PMID: 33522574]
Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Grønbæk H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World journal of hepatology. 2019 Feb 27:11(2):138-149. doi: 10.4254/wjh.v11.i2.138. Epub [PubMed PMID: 30820265]
Pennings N, Jaber J, Ahiawodzi P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation. Diabetes/metabolism research and reviews. 2018 May:34(4):e2986. doi: 10.1002/dmrr.2986. Epub 2018 Mar 15 [PubMed PMID: 29392827]
Church TJ, Haines ST. Treatment Approach to Patients With Severe Insulin Resistance. Clinical diabetes : a publication of the American Diabetes Association. 2016 Apr:34(2):97-104. doi: 10.2337/diaclin.34.2.97. Epub [PubMed PMID: 27092020]
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrine reviews. 2012 Dec:33(6):981-1030. doi: 10.1210/er.2011-1034. Epub 2012 Oct 12 [PubMed PMID: 23065822]
Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Advances in experimental medicine and biology. 2017:960():1-17. doi: 10.1007/978-3-319-48382-5_1. Epub [PubMed PMID: 28585193]
Level 3 (low-level) evidenceTanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). Journal of diabetes research. 2020:2020():3920196. doi: 10.1155/2020/3920196. Epub 2020 Jul 31 [PubMed PMID: 32832560]
Nellaiappan K, Preeti K, Khatri DK, Singh SB. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Current diabetes reviews. 2022:18(1):e030821192146. doi: 10.2174/1573399817666210309104203. Epub [PubMed PMID: 33745424]
Reaven GM. The metabolic syndrome: is this diagnosis necessary? The American journal of clinical nutrition. 2006 Jun:83(6):1237-47 [PubMed PMID: 16762930]
McCormick N, O'Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, Choi HK. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis & rheumatology (Hoboken, N.J.). 2021 Nov:73(11):2096-2104. doi: 10.1002/art.41779. Epub 2021 Sep 26 [PubMed PMID: 33982892]
Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Physical therapy. 2008 Nov:88(11):1254-64. doi: 10.2522/ptj.20080020. Epub 2008 Sep 18 [PubMed PMID: 18801858]
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002 Feb 7:346(6):393-403 [PubMed PMID: 11832527]
Level 1 (high-level) evidenceSamuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. The Journal of clinical investigation. 2016 Jan:126(1):12-22. doi: 10.1172/JCI77812. Epub 2016 Jan 4 [PubMed PMID: 26727229]
Perreault L, Pan Q, Schroeder EB, Kalyani RR, Bray GA, Dagogo-Jack S, White NH, Goldberg RB, Kahn SE, Knowler WC, Mathioudakis N, Dabelea D, Diabetes Prevention Program Research Group. Regression From Prediabetes to Normal Glucose Regulation and Prevalence of Microvascular Disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes care. 2019 Sep:42(9):1809-1815. doi: 10.2337/dc19-0244. Epub 2019 Jul 18 [PubMed PMID: 31320445]
Ogawa W, Araki E, Ishigaki Y, Hirota Y, Maegawa H, Yamauchi T, Yorifuji T, Katagiri H. New classification and diagnostic criteria for insulin resistance syndrome. Endocrine journal. 2022 Feb 28:69(2):107-113. doi: 10.1507/endocrj.EJ21-0725. Epub 2022 Feb 1 [PubMed PMID: 35110500]
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Archives of internal medicine. 2003 Feb 24:163(4):427-36 [PubMed PMID: 12588201]
Level 3 (low-level) evidenceParcha V, Heindl B, Kalra R, Li P, Gower B, Arora G, Arora P. Insulin Resistance and Cardiometabolic Risk Profile Among Nondiabetic American Young Adults: Insights From NHANES. The Journal of clinical endocrinology and metabolism. 2022 Jan 1:107(1):e25-e37. doi: 10.1210/clinem/dgab645. Epub [PubMed PMID: 34473288]
Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiological reviews. 2018 Oct 1:98(4):2133-2223. doi: 10.1152/physrev.00063.2017. Epub [PubMed PMID: 30067154]
Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods in molecular biology (Clifton, N.J.). 2009:560():221-38. doi: 10.1007/978-1-59745-448-3_15. Epub [PubMed PMID: 19504253]
Level 3 (low-level) evidenceLegro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 1998 Aug:83(8):2694-8 [PubMed PMID: 9709933]
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes care. 2001 Mar:24(3):460-4 [PubMed PMID: 11289468]
Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes care. 2001 Apr:24(4):796-7 [PubMed PMID: 11315860]
Level 3 (low-level) evidenceMatsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999 Sep:22(9):1462-70 [PubMed PMID: 10480510]
Level 2 (mid-level) evidenceGutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes research and clinical practice. 2000 Mar:47(3):177-84 [PubMed PMID: 10741566]
Level 1 (high-level) evidenceSumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Archives of internal medicine. 2005 Jun 27:165(12):1395-400 [PubMed PMID: 15983289]
Level 2 (mid-level) evidenceAlberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20:120(16):1640-5. doi: 10.1161/CIRCULATIONAHA.109.192644. Epub 2009 Oct 5 [PubMed PMID: 19805654]
Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, Neufeld ND, Petak SM, Rodbard HW, Seibel JA, Smith DA, Wilson PW. American College of Endocrinology position statement on the insulin resistance syndrome. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2003 May-Jun:9(3):237-52 [PubMed PMID: 12924350]
Rácz O, Linková M, Jakubowski K, Link R, Kuzmová D. [Barriers of the initiation of insulin treatment in type 2 diabetic patients - conquering the "psychological insulin resistance"]. Orvosi hetilap. 2019 Jan:160(3):93-97. doi: 10.1556/650.2019.31269. Epub [PubMed PMID: 30640530]
Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. Journal of cellular physiology. 2019 Aug:234(8):12385-12392. doi: 10.1002/jcp.28066. Epub 2019 Jan 3 [PubMed PMID: 30605232]
He X, Wu D, Hu C, Xu T, Liu Y, Liu C, Xu B, Tang W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid : official journal of the American Thyroid Association. 2019 Mar:29(3):359-367. doi: 10.1089/thy.2017.0707. Epub 2019 Feb 1 [PubMed PMID: 30595105]
Level 1 (high-level) evidenceZhou J, Massey S, Story D, Li L. Metformin: An Old Drug with New Applications. International journal of molecular sciences. 2018 Sep 21:19(10):. doi: 10.3390/ijms19102863. Epub 2018 Sep 21 [PubMed PMID: 30241400]
Mottl AK, Alicic R, Argyropoulos C, Brosius FC, Mauer M, Molitch M, Nelson RG, Perreault L, Nicholas SB. KDOQI US Commentary on the KDIGO 2020 Clinical Practice Guideline for Diabetes Management in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2022 Apr:79(4):457-479. doi: 10.1053/j.ajkd.2021.09.010. Epub 2022 Feb 7 [PubMed PMID: 35144840]
Level 1 (high-level) evidenceAmerican Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. Journal of the American Geriatrics Society. 2013 Nov:61(11):2020-6. doi: 10.1111/jgs.12514. Epub [PubMed PMID: 24219204]
Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obesity science & practice. 2017 Mar:3(1):3-14. doi: 10.1002/osp4.84. Epub 2016 Dec 19 [PubMed PMID: 28392927]
Slomski A. Semaglutide's Weight-Loss Benefits Were Sustained in a 2-Year Study. JAMA. 2021 Dec 28:326(24):2464. doi: 10.1001/jama.2021.21717. Epub [PubMed PMID: 34962530]
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A, SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. The New England journal of medicine. 2022 Jul 21:387(3):205-216. doi: 10.1056/NEJMoa2206038. Epub 2022 Jun 4 [PubMed PMID: 35658024]
Zheng H, Liu M, Li S, Shi Q, Zhang S, Zhou Y, Su N. Sodium-Glucose Co-Transporter-2 Inhibitors in Non-Diabetic Adults With Overweight or Obesity: A Systematic Review and Meta-Analysis. Frontiers in endocrinology. 2021:12():706914. doi: 10.3389/fendo.2021.706914. Epub 2021 Aug 16 [PubMed PMID: 34484120]
Level 1 (high-level) evidenceNeeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, Broedl UC, Johansen OE. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diabetes & vascular disease research. 2016 Mar:13(2):119-26. doi: 10.1177/1479164115616901. Epub [PubMed PMID: 26873905]
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes care. 2004 Jan:27(1):256-63 [PubMed PMID: 14693998]
Level 3 (low-level) evidenceLebovitz HE. Thiazolidinediones: the Forgotten Diabetes Medications. Current diabetes reports. 2019 Nov 27:19(12):151. doi: 10.1007/s11892-019-1270-y. Epub 2019 Nov 27 [PubMed PMID: 31776781]
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR, STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. The New England journal of medicine. 2017 Feb 16:376(7):641-651. doi: 10.1056/NEJMoa1600869. Epub [PubMed PMID: 28199805]
McGlone ER, Carey I, Veličković V, Chana P, Mahawar K, Batterham RL, Hopkins J, Walton P, Kinsman R, Byrne J, Somers S, Kerrigan D, Menon V, Borg C, Ahmed A, Sgromo B, Cheruvu C, Bano G, Leonard C, Thom H, le Roux CW, Reddy M, Welbourn R, Small P, Khan OA. Bariatric surgery for patients with type 2 diabetes mellitus requiring insulin: Clinical outcome and cost-effectiveness analyses. PLoS medicine. 2020 Dec:17(12):e1003228. doi: 10.1371/journal.pmed.1003228. Epub 2020 Dec 7 [PubMed PMID: 33285553]
Level 2 (mid-level) evidencePurnell JQ, Dewey EN, Laferrère B, Selzer F, Flum DR, Mitchell JE, Pomp A, Pories WJ, Inge T, Courcoulas A, Wolfe BM. Diabetes Remission Status During Seven-year Follow-up of the Longitudinal Assessment of Bariatric Surgery Study. The Journal of clinical endocrinology and metabolism. 2021 Mar 8:106(3):774-788. doi: 10.1210/clinem/dgaa849. Epub [PubMed PMID: 33270130]
Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. The Journal of clinical endocrinology and metabolism. 2016 Dec:101(12):4500-4511 [PubMed PMID: 27710244]
Level 1 (high-level) evidenceAronne LJ. Classification of obesity and assessment of obesity-related health risks. Obesity research. 2002 Dec:10 Suppl 2():105S-115S [PubMed PMID: 12490659]
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex. : 1979). 2018 Jun:71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. Epub 2017 Nov 13 [PubMed PMID: 29133354]
Level 1 (high-level) evidenceWang B, Li F, Guo J, Wang C, Xu D, Li C. Effects of liver function, insulin resistance and inflammatory factors on vascular endothelial dilation function and prognosis of coronary heart disease patients complicated with NAFLD. Experimental and therapeutic medicine. 2019 Feb:17(2):1306-1311. doi: 10.3892/etm.2018.7043. Epub 2018 Dec 4 [PubMed PMID: 30680007]
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA. 2018 Nov 20:320(19):2020-2028. doi: 10.1001/jama.2018.14854. Epub [PubMed PMID: 30418471]
Mosher AL, Piercy KL, Webber BJ, Goodwin SK, Casavale KO, Olson RD. Dietary Guidelines for Americans: Implications for Primary Care Providers. American journal of lifestyle medicine. 2016 Jan-Feb:10(1):23-35. doi: 10.1177/1559827614521755. Epub 2014 Feb 6 [PubMed PMID: 30202257]
Hamdy O, Ganda OP, Maryniuk M, Gabbay RA, Members of the Joslin Clinical Oversight Committee. CHAPTER 2. Clinical nutrition guideline for overweight and obese adults with type 2 diabetes (T2D) or prediabetes, or those at high risk for developing T2D. The American journal of managed care. 2018 Jun:24(7 Spec No.):SP226-SP231 [PubMed PMID: 29938995]
Carlson AL, Mullen DM, Bergenstal RM. Clinical Use of Continuous Glucose Monitoring in Adults with Type 2 Diabetes. Diabetes technology & therapeutics. 2017 May:19(S2):S4-S11. doi: 10.1089/dia.2017.0024. Epub [PubMed PMID: 28541137]
Jackson MA, Ahmann A, Shah VN. Type 2 Diabetes and the Use of Real-Time Continuous Glucose Monitoring. Diabetes technology & therapeutics. 2021 Mar:23(S1):S27-S34. doi: 10.1089/dia.2021.0007. Epub [PubMed PMID: 33534631]
Dearborn JL, Viscoli CM, Inzucchi SE, Young LH, Kernan WN. Metabolic syndrome identifies normal weight insulin-resistant stroke patients at risk for recurrent vascular disease. International journal of stroke : official journal of the International Stroke Society. 2019 Aug:14(6):639-645. doi: 10.1177/1747493018816425. Epub 2018 Dec 3 [PubMed PMID: 30507360]