Introduction
Hypothyroidism results from low levels of thyroid hormone with varied etiology and manifestations. Hypothyroidism is primarily categorized as primary and secondary (ie, central) hypothyroidism. In primary hypothyroidism, the thyroid gland cannot produce adequate thyroid hormone. The less commonly seen secondary or central hypothyroidism occurs when the thyroid gland functions normally; however, hypothyroidism results from the abnormal pituitary gland or hypothalamus function. Untreated hypothyroidism increases morbidity and mortality. In the United States, autoimmune thyroid disease (ie, Hashimoto thyroiditis) is the most common cause of hypothyroidism, but globally, lack of iodine in the diet is the most common cause.[1]
The presentation can vary from an asymptomatic patient in whom hypothyroidism is only recognized on routine blood work to myxedema coma, which is an extreme presentation of this condition. Classic clinical features, including cold intolerance, puffiness, decreased sweating, and skin changes, may not always be present.[2] A serum TSH level is typically used to assess for primary hypothyroidism in most patients initially.[2] Characteristic laboratory findings of hypothyroidism include elevated TSH levels and low free T4 levels. Today, the diagnosis of hypothyroidism is easily made by the use of simple blood tests and can be treated with exogenous thyroid hormone.[3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Hypothyroidism is primarily categorized as primary and secondary (ie, central) hypothyroidism. In primary hypothyroidism, the thyroid gland cannot produce adequate amounts of thyroid hormone. The less commonly seen secondary or central hypothyroidism occurs when the thyroid gland functions normally; however, hypothyroidism results from the abnormal pituitary gland or hypothalamus function.
The most prevalent etiology of primary hypothyroidism is an iodine deficiency in iodine-deficient geographic areas worldwide. Autoimmune thyroid diseases are the leading causes of hypothyroidism in the iodine-sufficient regions. Hashimoto thyroiditis is the most commonly seen etiology in the US and has a strong association with lymphoma. Hypothyroid etiology can be influenced locally by iodine fortification and the emergence of new iodine-deficient areas.[1]
Other conditions may also lead to primary hypothyroidism. Postpartum thyroiditis affects nearly 10% of women and often presents 8 to 20 weeks after the delivery of the infant. Only a few women require treatment with thyroid hormone. However, some women are at high risk for permanent hypothyroidism or recurrent postpartum thyroiditis in future pregnancies.[4] The use of radioactive iodine to manage Graves disease usually results in permanent hypothyroidism in about 80% to 90% of the patients within 8 to 20 weeks after treatment.[5][6] A relatively uncommon cause of primary hypothyroidism is subacute granulomatous thyroiditis, also known as de Quervain disease. Subacute granulomatous thyroiditis usually occurs in middle-aged women and is typically self-limited. Hypothyroidism can also be a part of the autoimmune polyendocrinopathy type-1 condition that results from a mutation in the AIRE gene. This condition is a constellation of Addison disease, hypoparathyroidism, and mucocutaneous candidiasis. Polyendocrinopathy type-2 includes hypothyroidism, Addison disease, and type 1 diabetes mellitus.[7][8] Other common causes of hypothyroidism include:
- Medications including amiodarone, thalidomide, oral tyrosine kinase inhibitors (eg, sunitinib, imatinib), stavudine, interferon, bexarotene, perchlorate, rifampin, ethionamide, phenobarbital, phenytoin, carbamazepine, interleukin-2, and lithium.[1][9]
- Thyroid surgery
- Radiotherapy of the head or neck area
- The use of immune checkpoint inhibitors (eg, anti-CTLA-4 and anti-PD-L1/PD-1 therapy) has been associated with both primary and secondary hypothyroidism.[10][11]
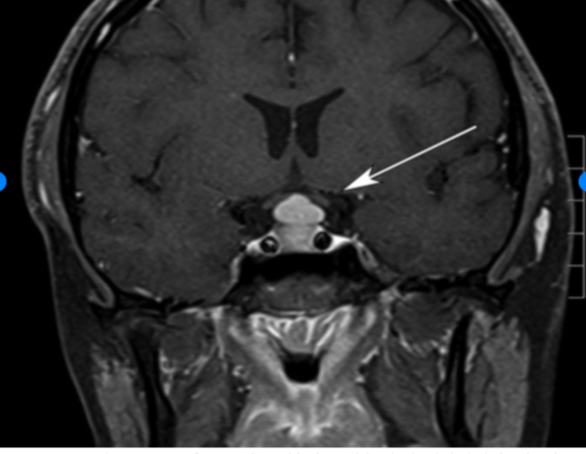
Secondary and tertiary hypothyroidism, also known as central hypothyroidism, is caused by a defect in the hypothalamic-pituitary axis secondary to any of the following (see Image. Pituitary Hyperplasia):
- Neoplastic, infiltrative, inflammatory, genetic, or iatrogenic disorders of the pituitary or hypothalamus.[2]
- Pituitary tumors
- Tumors compressing hypothalamus
- Sheehan syndrome
- Thyroid-releasing hormone (TRH) resistance [12]
- TRH deficiency
- Lymphocytic hypophysitis [13]
- Radiation therapy to the brain
- Medications (eg, dopamine, prednisone, or opioids) [14]
Epidemiology
The National Health and Nutrition Examination Survey (NHANESIII) study found the prevalence of overt hypothyroidism among individuals aged 12 years and older in the US to be 0.3% and subclinical hypothyroidism 4.3%. Female gender and increasing age are associated with a higher risk for thyroid-stimulating hormone (TSH) and an increased prevalence of antithyroid antibodies.[15] Hypothyroidism is more prevalent in women with small stature at birth and low body mass index in childhood.
Pathophysiology
The most common cause of hypothyroidism is the inability of the thyroid gland to produce a sufficient amount of thyroid hormone; however, less commonly, pituitary and hypothalamus impairment may also result in thyroid dysfunction. The hypothalamus secretes thyrotropin-releasing hormone (TRH) that stimulates the pituitary gland to produce thyroid-stimulating hormone (TSH). Thyroid-stimulating hormones stimulate the thyroid gland to produce and secrete mainly T4, approximately 100 to 125 nmol daily, and smaller quantities of T3. The half-life of T4 is 7 to 10 days, and eventually, T4 is converted to T3 peripherally by 5'-deiodination. Negative feedback on the production of TRH and TSH is exerted primarily by T3 and, to some extent, T4. Alteration in the structure and function of any of these organs or pathways can result in hypothyroidism.[16] Additionally, the decline in the production of T4 results in an increase in the secretion of TSH by the pituitary gland, causing hypertrophy and hyperplasia of the thyroid parenchyma, thereby leading to increased T3 production.
Histopathology
Autoimmune thyroiditis causes an increase in the turnover of iodine and impaired organification. Chronic inflammation of the parenchyma leads to predominant T-cell lymphocytic infiltration.[2] If this persists, the initial lymphocytic hyperplasia and vacuoles are replaced by dense fibrosis and atrophic thyroid follicles. Coexisting or associated malignancies, such as papillary thyroid cancer, can also be seen.[17]
History and Physical
Clinical History
A high index of suspicion for hypothyroidism should be maintained since the signs and symptoms can be mild and nonspecific. Furthermore, symptoms may vary widely in different patients. Classic clinical features, including cold intolerance, puffiness, decreased sweating, and skin changes, may not always be present. Therefore, a thorough inquiry into a patient's symptoms should be performed, including dry skin, voice changes, hair loss, constipation, fatigue, muscle cramps, cold intolerance, sleep disturbances, menstrual cycle abnormalities, weight gain, and galactorrhea.[2] Also, a complete medical, surgical, medication, and family history should be obtained. History of adverse pregnancy and neonatal outcomes should also be sought.[18]
Occasionally, patients with less common symptoms may be encountered. Symptoms of depression, anxiety, psychosis, and cognitive impairments (eg, memory loss) can be present.[19] In rare cases, patients may present with ascites, rhabdomyolysis, and pericardial effusion.[20][21] Patients can also present with carpal tunnel syndrome, sleep apnea, hyponatremia, hypercholesterolemia, congestive heart failure, and prolonged QT interval.[2] Hashimoto disease is difficult to differentiate clinically; however, some features specific to this condition include:
- Throat fullness
- Painless thyroid enlargement
- Episodic neck pains or sore throat
Physical Examination Findings
Most of the patients have normal thyroid examination findings. However, a careful physical examination may reveal some clues since the signs of hypothyroidism are very subtle. Physical examination may be significant for the following:
- Enlarged thyroid gland
- Weight gain
- Slowness of speech and movement
- Dry skin
- Coarse and brittle hair
- Pallor or jaundice
- Dull facial expressions
- Macroglossia
- Bradycardia
- Pericardial effusion
- Prolonged ankle reflex relaxation time [2]
Evaluation
Hyperthyroid Diagnostic Studies
A serum TSH level is typically used to assess for primary hypothyroidism in most patients initially. Characteristic laboratory findings of hypothyroidism include elevated TSH levels and low free T4 levels. In subclinical hypothyroidism, TSH levels are elevated, and free T4 levels are within normal limits.[2] Antithyroid antibodies (eg, thyroid peroxidase antibodies) should also be performed to evaluate for autoimmune thyroid diseases.[2] Patients with subclinical hypothyroidism and thyroid peroxidase antibody positivity have a greater risk of developing overt hypothyroidism.[2] Studies have shown that 50% of these patients will develop primary hypothyroidism within 20 years. The decision to follow up periodically with clinical evaluation and laboratory studies is based on clinical judgment, as there are no guidelines. Laboratory studies in patients with hypothyroidism may also reveal hyperlipidemia, elevated serum creatine kinase, elevated hepatic enzymes, and anemia.[2] Bun, creatinine, and uric acid levels can also be elevated in severe cases.[22]
Central hypothyroidism may be either of pituitary or of hypothalamic origin. In these cases, the TSH produced can be biologically inactive and affect the levels of bioactive TSH; therefore, the diagnosis of central hypothyroidism should be based on free T4 rather than TSH.[2] Imaging studies (eg, ultrasound) of the neck are not routinely recommended for hypothyroidism.
Hospitalized patients should undergo TSH testing only when thyroid dysfunction is suspected.[2] Slight abnormalities of TSH in sick patients during their hospital stay are suggestive of euthyroid sick syndrome. However, if the values of TSH are very high, hypothyroidism is likely. Reverse T3 is elevated when the patient has euthyroid sickness, though this is not routinely checked in clinical practice.
Hypothyroid Screening Recommendations
While there are no universal guidelines on screening the public for thyroid disease, the American Thyroid Association recommends that screening be performed every 5 years, beginning at the age of 35 years. Individuals at high risk for hypothyroidism include the following:
- Women older than 60 years
- Pregnant women
- Patients with a prior history of head and neck irradiation
- Patients with autoimmune disorders or type 1 diabetes
- Positive thyroid peroxidase antibodies
- Family history of thyroid disorders
Treatment / Management
Hypothyroidism is primarily treated with levothyroxine monotherapy.[3] The replacement levothyroxine dosage should be between 1.6 to 1.8 mcg/kg by mouth daily.[23] However, in patients who are older or with atrial fibrillation, reduction of the dose or starting at a low dosage and titrating up slowly as needed is important.[3] To help the absorption, levothyroxine should be taken 30 to 45 minutes before breakfast or at least 3 hours postprandially, whichever is most convenient for each patient. Moreover, elemental supplements or vitamins (eg, calcium and magnesium) can affect the absorption of levothyroxine; therefore, an interval of at least 4 hours should be placed between these agents and thyroid hormone administration. Furthermore, commonly used medications such as proton pump inhibitors also negatively impact levothyroxine absorption. Maintaining a consistent formulation or brand of levothyroxine might be essential for some patients as there might be slight variations in the dose of the generic formulations, which can have a clinical impact on a small subset of very sensitive hypothyroid patients.[3]
Switching to the intravenous (IV) form of thyroid hormone in the hospital is indicated when a patient is unable to take thyroid replacement orally or when myxedema coma is suspected. The levothyroxine dose is generally reduced to 50% to 75% of the oral dose.[24] The conversion is somewhat controversial regarding the exact dose, as different experts use different conversion percentages. The extreme difference in the cost between both formulations makes the intravenous use of levothyroxine the last resort in the rare cases of myxedema coma and patients with a strict NPO state.[24]
Liquid and gel capsule formulations of thyroid hormone replacements are used in malabsorption syndromes, as they do not require an acidic pH in the stomach.[25][26] The liquid formulation has been shown to achieve better control of hypothyroidism versus the tablet form, in general, as well as for special populations, like pregnancy and older patients.[27][28][29][30] Medications (eg, sucralfate, calcium preparations, and bile acid sequestrants) can interfere with the absorption of levothyroxine.[2] However, the levothyroxine absorption test can be done to prove if a patient can not absorb levothyroxine.[31][32][33](A1)
Based on the 2012 Clinical Practice Guidelines for Hypothyroidism in Adults by the American Association of Clinical Endocrinologists and the American Thyroid Association, therapy should be monitored and titrated based on serum TSH or free T4 measurements. Laboratory studies should be drawn every 4 to 8 weeks until target levels are achieved. Laboratory measurements should also be performed after starting treatment, after any dose change, after any change in the formulation or brand of levothyroxine, and after starting or stopping any medications that may affect the thyroid hormone levels.[2] If thyroid hormone levels are stable, then the monitoring interval can be extended to 6 months; if levels remain stable, then monitoring can be further extended to 12 months or done at shorter intervals on an individualized basis along with clinical evaluation.[2] Central hypothyroidism should be monitored based on free T4 rather than TSH.[2] Patients with cardiac disease should be monitored for the development of any symptoms of angina and atrial fibrillation.[2] If a patient is overly treated with thyroid replacement for an extended period, screening for osteoporosis is warranted.[3](A1)
A comprehensive workup for other differential diagnoses is recommended for unresolved symptoms in the presence of biochemical euthyroidism. Although there is a lack of strong evidence supporting the routine inclusion of triiodothyronine (T3) preparations with levothyroxine in the treatment of hypothyroidism, there might be a need to consider alternative combination T4 and T3 therapy in cases that do not respond to conventional T4 replacement.[34][35] The FDA has approved treatment options, including armor or nature thyroid; however, clinicians should understand that these formulations may increase cardiac arrhythmia risk. Moreover, these formulations are not approved for pregnant patients due to their T3 component or for patients with thyroid cancer, where strict TSH goals are required.
Effective treatment helps to improve hypothyroid signs and symptoms, improves patient well-being, and normalizes TSH and free T4 levels.[36] However, since the symptoms of hypothyroidism are nonspecific, a symptomatic patient with normalized labs while on thyroid replacement treatment may be indicative of another etiology. This is a difficult situation where the clinician must reevaluate a patient. Strong counseling skills are also of great help. Furthermore, thyroid replacement treatment can exacerbate coexisting adrenal insufficiency. Patients with known or suspected adrenal insufficiency should be tested and treated for adrenal insufficiency while awaiting the final test results.[2] Adrenal insufficiency can also be associated with subclinical hypothyroidism that is reversible with the treatment of adrenal insufficiency.[37] In patients who have confirmed adrenal insufficiency, physicians should consider a reassessment of thyroid tests following an adequate treatment of adrenal insufficiency. Ruling out or treating adrenal insufficiency when a patient has severe hypothyroidism (eg, myxedema coma) is essential. (A1)
Differential Diagnosis
Owing to the subtle signs and symptoms of hypothyroidism, the list of differential diagnoses is extensive. Differential diagnosis is based on signs and symptoms; for instance, fatigue can point to iron deficiency anemia, sleep apnea, depression, and rheumatological diseases.[35] The following disorders may have to be considered in the differential process.
- Euthyroid sick syndrome
- Goiter
- Myxedema coma
- Anemia
- Riedel thyroiditis
- Subacute thyroiditis
- Thyroid lymphoma
- Iodine deficiency
- Addison disease
- Chronic fatigue syndrome
- Depression
- Dysmenorrhea
- Erectile dysfunction
- Familial hypercholesterolemia
- Infertility
Prognosis
Without treatment, hypothyroidism may have a risk of high morbidity and mortality and can eventually lead to coma or even death. In children, failure to treat hypothyroidism can result in severe mental retardation. A leading cause of death in adults is heart failure. Most patients have a good prognosis with treatment, and the symptoms usually reverse in a few weeks or months.
Complications
Severe hypothyroidism may present as myxedema coma, and it is an endocrine emergency. Prompt recognition and early treatment in the intensive care unit (ICU) are essential, and even then, mortality may reach 25% to 60% of the affected cases.[38] Myxedema crisis should be suspected in cases where there is encephalopathy, hypothermia, seizures, hyponatremia, hypoglycemia, arrhythmias, cardiogenic shock, respiratory failure, and fluid retention.[38] Factors leading to an increased risk of myxedema crisis include inadequate doses of thyroid hormone, interruption in treatment, undiagnosed hypothyroidism, or the presence of acute illness such as sepsis [38], perhaps due to increased metabolic demands. Supportive therapy should be provided in the intensive care unit with fluid and electrolyte management, ventilator support, and vasopressors, as well as treatment of hypothermia and any underlying coexisting acute illnesses.[38]
Thyroid replacement treatment is initially provided by intravenous hydrocortisone at stress doses, followed by intravenous levothyroxine, then switched to oral levothyroxine when clinical improvement is achieved. The reason to give steroids is that these patients may have adrenal insufficiency, which can lead to an Addisonian crisis if the thyroid deficiency is replaced without addressing adrenal insufficiency due to creating a hypermetabolic state. It is recommended to check for adrenal insufficiency, but while waiting for the results of the tests, treatment should be started with steroids.
If the treatment is effective, cardiopulmonary and cognitive improvement should be observed.[3] An associated improvement in laboratory derangements should also be noted, including an up-trending of free T4, which should be measured every 1 to 2 days during the initial treatment period. Low-dose intravenous liothyronine (T3) can be considered until initial improvement.[3] TSH may not reflect changes in such cases as it can take up to 4 weeks to normalize; therefore, TSH may not be helpful. Endocrinology consultation should be considered at any time.
Enhancing Healthcare Team Outcomes
Hypothyroidism affects multiple organ systems across all age groups and affects patient well-being and ability to function daily. The primary care physician or endocrinologist best manages this disorder. Treatment is with levothyroxine monotherapy.[2] Effective treatment calls for a team-based and patient-centered approach. When patient symptoms are not adequately controlled despite normalization of thyroid labs, ruling out nonendocrine pathologies for the nonspecific symptoms is essential.
Endocrinology consultation is also recommended in complex scenarios such as preconception, pregnancy, congenital and pediatric hypothyroidism, failure of treatment, thyroid replacement absorption issues, coexisting cardiac or other endocrine disorders, difficulty in interpretation of thyroid test results, drug-induced hypothyroidism.[2] Other specialists that may be needed for the treatment of hypothyroidism under different situations are psychiatrists, obstetrician-gynecologists, pediatricians, cardiologists, and intensivists.
Pharmacists help provide advice on medication and food interactions, the effect of changes in levothyroxine formulations, and investigating the causes for the requirement of unusually high doses of levothyroxine or fluctuating TSH levels. Prompt notification to physicians of unusually high levels of TSH by laboratory personnel and close monitoring of vital signs and mental status by nurses can facilitate early treatment and better outcomes, especially in the inpatient setting (eg, myxedema coma). Rapid response teams can be effectively utilized when severe long-term hypothyroidism causes hemodynamic instability from myxedema coma. Close interprofessional communication with all the involved teams is essential to improve patient outcomes.
Media
(Click Image to Enlarge)
References
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nature reviews. Endocrinology. 2018 May:14(5):301-316. doi: 10.1038/nrendo.2018.18. Epub 2018 Mar 23 [PubMed PMID: 29569622]
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid : official journal of the American Thyroid Association. 2012 Dec:22(12):1200-35. doi: 10.1089/thy.2012.0205. Epub 2012 Nov 6 [PubMed PMID: 22954017]
Level 1 (high-level) evidenceJonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid : official journal of the American Thyroid Association. 2014 Dec:24(12):1670-751. doi: 10.1089/thy.2014.0028. Epub [PubMed PMID: 25266247]
Peng CC, Pearce EN. An update on thyroid disorders in the postpartum period. Journal of endocrinological investigation. 2022 Aug:45(8):1497-1506. doi: 10.1007/s40618-022-01762-1. Epub 2022 Feb 18 [PubMed PMID: 35181848]
Phowira J, Coffey KL, Bartholomew PH, Vennart N, Moreira M, Emerson H, Kennedy D, Weaver JU. Radioactive Iodine for the Treatment of Subclinical Thyrotoxicosis Grade 1 and 2: Outcome of up to 18-Year Follow Up. Frontiers in endocrinology. 2022:13():843857. doi: 10.3389/fendo.2022.843857. Epub 2022 Mar 9 [PubMed PMID: 35370990]
Xing YZ, Zhang K, Jin G. Predictive factors for the outcomes of Graves' disease patients with radioactive iodine (131I) treatment. Bioscience reports. 2020 Jan 31:40(1):. doi: 10.1042/BSR20191609. Epub [PubMed PMID: 31840740]
Paparella R, Menghi M, Micangeli G, Leonardi L, Profeta G, Tarani F, Petrella C, Ferraguti G, Fiore M, Tarani L. Autoimmune Polyendocrine Syndromes in the Pediatric Age. Children (Basel, Switzerland). 2023 Mar 19:10(3):. doi: 10.3390/children10030588. Epub 2023 Mar 19 [PubMed PMID: 36980146]
Bjørklund G, Pivin M, Hangan T, Yurkovskaya O, Pivina L. Autoimmune polyendocrine syndrome type 1: Clinical manifestations, pathogenetic features, and management approach. Autoimmunity reviews. 2022 Aug:21(8):103135. doi: 10.1016/j.autrev.2022.103135. Epub 2022 Jun 9 [PubMed PMID: 35690244]
Burch HB, Drug Effects on the Thyroid. The New England journal of medicine. 2019 Aug 22; [PubMed PMID: 31433922]
El Sabbagh R, Azar NS, Eid AA, Azar ST. Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. International journal of general medicine. 2020:13():1003-1009. doi: 10.2147/IJGM.S261433. Epub 2020 Nov 4 [PubMed PMID: 33177863]
Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. The Journal of clinical endocrinology and metabolism. 2021 Aug 18:106(9):e3704-e3713. doi: 10.1210/clinem/dgab263. Epub [PubMed PMID: 33878162]
Pappa T, Refetoff S. Resistance to Thyroid Hormone Beta: A Focused Review. Frontiers in endocrinology. 2021:12():656551. doi: 10.3389/fendo.2021.656551. Epub 2021 Mar 31 [PubMed PMID: 33868182]
Ju JS, Cui T, Zhao J, Chen JL, Ju HB. Clinical presentation and magnetic resonance imaging characteristics of lymphocytic hypophysitis: a systematic review with meta-analysis. Archives of medical science : AMS. 2023:19(4):976-986. doi: 10.5114/aoms/144628. Epub 2021 Dec 14 [PubMed PMID: 37560735]
Level 1 (high-level) evidenceKhoury T, Kadah A, Mari A, Sbeit W, Drori A, Mahamid M. Thyroid Dysfunction is Prevalent in Autoimmune Hepatitis: A Case Control Study. The Israel Medical Association journal : IMAJ. 2020 Feb:22(2):100-103 [PubMed PMID: 32043327]
Level 2 (mid-level) evidenceHollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism. 2002 Feb:87(2):489-99 [PubMed PMID: 11836274]
Level 3 (low-level) evidenceFeldt-Rasmussen U, Effraimidis G, Klose M. The hypothalamus-pituitary-thyroid (HPT)-axis and its role in physiology and pathophysiology of other hypothalamus-pituitary functions. Molecular and cellular endocrinology. 2021 Apr 5:525():111173. doi: 10.1016/j.mce.2021.111173. Epub 2021 Feb 4 [PubMed PMID: 33549603]
Anand A, Singh KR, Kushwaha JK, Hussain N, Sonkar AA. Papillary Thyroid Cancer and Hashimoto's Thyroiditis: An Association Less Understood. Indian journal of surgical oncology. 2014 Sep:5(3):199-204. doi: 10.1007/s13193-014-0325-4. Epub 2014 Jul 4 [PubMed PMID: 25419066]
Hou J, Yu P, Zhu H, Pan H, Li N, Yang H, Jiang Y, Wang L, Wang B, Wang Y, You L, Chen S. The impact of maternal hypothyroidism during pregnancy on neonatal outcomes: a systematic review and meta-analysis. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2016:32(1):9-13. doi: 10.3109/09513590.2015.1104296. Epub 2015 Nov 3 [PubMed PMID: 26527131]
Level 2 (mid-level) evidenceSamuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Current opinion in endocrinology, diabetes, and obesity. 2014 Oct:21(5):377-83. doi: 10.1097/MED.0000000000000089. Epub [PubMed PMID: 25122491]
Level 3 (low-level) evidenceZare-Khormizi MR, Rahmanian M, Pourrajab F, Akbarnia S. Massive pericardial effusion and rhabdomyolysis secondary to untreated severe hypothyroidism: the first report. Acta clinica Belgica. 2014 Oct:69(5):375-8. doi: 10.1179/2295333714Y.0000000049. Epub 2014 Jul 24 [PubMed PMID: 25056490]
Level 3 (low-level) evidenceKhalid S, Asad-Ur-Rahman F, Abbass A, Gordon D, Abusaada K. Myxedema Ascites: A Rare Presentation of Uncontrolled Hypothyroidism. Cureus. 2016 Dec 5:8(12):e912. doi: 10.7759/cureus.912. Epub 2016 Dec 5 [PubMed PMID: 28083456]
Saini V, Yadav A, Arora MK, Arora S, Singh R, Bhattacharjee J. Correlation of creatinine with TSH levels in overt hypothyroidism - a requirement for monitoring of renal function in hypothyroid patients? Clinical biochemistry. 2012 Feb:45(3):212-4. doi: 10.1016/j.clinbiochem.2011.10.012. Epub 2011 Oct 28 [PubMed PMID: 22061337]
Level 2 (mid-level) evidenceJonklaas J Optimal Thyroid Hormone Replacement. Endocrine reviews. 2022 Mar 9 [PubMed PMID: 34543420]
Barlow BT, Roberts RJ, Newman K, Harrison SK, Sin JH. Economic Evaluation of a Pharmacist-Led 5-Day Therapeutic Hold of IV Levothyroxine at an Academic Medical Center. Hospital pharmacy. 2022 Feb:57(1):20-25. doi: 10.1177/0018578720970457. Epub 2020 Nov 9 [PubMed PMID: 35521003]
Antonelli A, Elia G, Ragusa F, Paparo SR, Cavallini G, Benvenga S, Ferrari SM, Fallahi P. The Stability of TSH, and Thyroid Hormones, in Patients Treated With Tablet, or Liquid Levo-Thyroxine. Frontiers in endocrinology. 2021:12():633587. doi: 10.3389/fendo.2021.633587. Epub 2021 Mar 10 [PubMed PMID: 33790863]
Trimboli P, Scappaticcio L, De Bellis A, Maiorino MI, Knappe L, Esposito K, Bellastella G, Giovanella L. Different Formulations of Levothyroxine for Treating Hypothyroidism: A Real-Life Study. International journal of endocrinology. 2020:2020():4524759. doi: 10.1155/2020/4524759. Epub 2020 Jan 20 [PubMed PMID: 32184819]
Laurent I, Tang S, Astère M, Wang KR, Deng S, Xiao L, Li QF. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-analysis. Endocrine. 2018 Jul:61(1):28-35. doi: 10.1007/s12020-018-1574-8. Epub 2018 Mar 23 [PubMed PMID: 29572710]
Level 1 (high-level) evidenceTrimboli P, Virili C, Centanni M, Giovanella L. Thyroxine Treatment With Softgel Capsule Formulation: Usefulness in Hypothyroid Patients Without Malabsorption. Frontiers in endocrinology. 2018:9():118. doi: 10.3389/fendo.2018.00118. Epub 2018 Mar 21 [PubMed PMID: 29619010]
Cappelli C, Negro R, Pirola I, Gandossi E, Agosti B, Castellano M. Levothyroxine liquid solution versus tablet form for replacement treatment in pregnant women. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2016:32(4):290-2. doi: 10.3109/09513590.2015.1113518. Epub 2015 Nov 20 [PubMed PMID: 26585420]
Level 2 (mid-level) evidenceBenvenga S. Liquid and softgel capsules of l-thyroxine results lower serum thyrotropin levels more than tablet formulations in hypothyroid patients. Journal of clinical & translational endocrinology. 2019 Dec:18():100204. doi: 10.1016/j.jcte.2019.100204. Epub 2019 Aug 6 [PubMed PMID: 31844631]
Gonzales KM, Stan MN, Morris JC 3rd, Bernet V, Castro MR. The Levothyroxine Absorption Test: A Four-Year Experience (2015-2018) at The Mayo Clinic. Thyroid : official journal of the American Thyroid Association. 2019 Dec:29(12):1734-1742. doi: 10.1089/thy.2019.0256. Epub 2019 Dec 4 [PubMed PMID: 31680654]
Ghosh S, Pramanik S, Biswas K, Bhattacharjee K, Sarkar R, Chowdhury S, Mukhopadhyay P. Levothyroxine Absorption Test to Differentiate Pseudomalabsorption from True Malabsorption. European thyroid journal. 2020 Jan:9(1):19-24. doi: 10.1159/000504218. Epub 2019 Nov 20 [PubMed PMID: 32071898]
Caron P, Declèves X. The Use of Levothyroxine Absorption Tests in Clinical Practice. The Journal of clinical endocrinology and metabolism. 2023 Jul 14:108(8):1875-1888. doi: 10.1210/clinem/dgad132. Epub [PubMed PMID: 36916146]
Ettleson MD, Bianco AC. Individualized Therapy for Hypothyroidism: Is T4 Enough for Everyone? The Journal of clinical endocrinology and metabolism. 2020 Sep 1:105(9):e3090-104. doi: 10.1210/clinem/dgaa430. Epub [PubMed PMID: 32614450]
Hennessey JV, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. International journal of clinical practice. 2018 Feb:72(2):. doi: 10.1111/ijcp.13062. Epub 2018 Jan 30 [PubMed PMID: 29381251]
Guglielmi R, Frasoldati A, Zini M, Grimaldi F, Gharib H, Garber JR, Papini E. ITALIAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS STATEMENT-REPLACEMENT THERAPY FOR PRIMARY HYPOTHYROIDISM: A BRIEF GUIDE FOR CLINICAL PRACTICE. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Nov:22(11):1319-1326 [PubMed PMID: 27482609]
Abdullatif HD, Ashraf AP. Reversible subclinical hypothyroidism in the presence of adrenal insufficiency. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2006 Sep-Oct:12(5):572 [PubMed PMID: 17002934]
Level 3 (low-level) evidenceMathew V, Misgar RA, Ghosh S, Mukhopadhyay P, Roychowdhury P, Pandit K, Mukhopadhyay S, Chowdhury S. Myxedema coma: a new look into an old crisis. Journal of thyroid research. 2011:2011():493462. doi: 10.4061/2011/493462. Epub 2011 Sep 15 [PubMed PMID: 21941682]