Evaluation and Management of Perioperative Hypertension
Evaluation and Management of Perioperative Hypertension
Introduction
Hypertension (HTN) is the most common medical diagnosis. It results in end-organ damage in the vasculature, heart, brain, kidneys, and eyes. It is associated with more cardiovascular disease (CVD) deaths than any other modifiable disease, accounting for an estimated 50% of deaths from coronary artery disease and stroke in one large study.[1] It is responsible for the deaths of approximately nine million people annually worldwide, is present in more than 60% of people 60 years of age and older, and is controlled in under 20% of patients globally.[2][3] Since most patients are asymptomatic, and associated complications are serious, HTN has been labeled the silent killer. The silent nature of the disease is especially concerning as later onset of treatment is associated with cardiac and renal pathophysiologic changes and a higher risk of CVD, compared with the normal population, even among treated hypertensives who achieve the same blood pressure (BP) values as the normal population.[4]
Although generally managed by primary care providers such as internists, family practitioners, and nurse practitioners, severe perioperative HTN may result in excess surgical bleeding, myocardial ischemia and/or infarction, congestive heart failure (CHF) and acute pulmonary edema (APE). Therefore, it is vital that anesthesiologists, nurses, and all healthcare professionals who manage patients in preparation for surgery, and during the perioperative period, are knowledgeable regarding the care of patients with HTN.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
It has long been taught that BP increases as a normal consequence of aging. Recent work indicates this may not be so. The Yanomami, who live in the Amazon rainforest and ingest minimal salt, have the lowest BP of any society in the world.[5] In contrast, the Yekwana, who live nearby and have had some exposure to salt, have higher levels of BP, albeit far lower than modern societies.[5] Therefore, HTN may be a result of salt intake and/or a modern lifestyle. Additional risk factors for HTN include family history, increased weight, sedentary lifestyle, cigarette smoking, and a diet low in potassium.
Epidemiology
Systolic blood pressure (SBP) increases progressively into the ninth decade of life. Diastolic blood pressure (DBP) increases into the mid-50s, after which it begins to decline, resulting in increased pulse pressure. The decrease in DBP seems due to large artery stiffness, resulting in less aortic blood volume and less elastic recoil in diastole.[6] HTN affects approximately 29% of the population, 30% of males, and 28.1% of females. It is more prevalent in males until about the sixth decade, after which it is more prevalent in females.[7] It is more prevalent among African Americans than whites, affecting 40.8% of African American males and 41.5% of African American females. Prevalence is 25.9% among Hispanic Americans and 24.9% among non-Hispanic Asians. Prevalence is higher in Europe than the United States, at 38% in Italy, and 55% in Germany likely due to the fact that European practice guidelines have recommended higher BP values at which to initiate pharmacologic treatment.[8]
Due to the aging population, obesity epidemic, and increasing adoption of sedentary lifestyles, the prevalence of HTN will continue to rise. By 2025, it is estimated that the number of people with HTN will increase to nearly 1.5 billion people worldwide.[9] It is increasingly affecting children as well, due to increasing obesity and sedentary behavior in the young.
Pathophysiology
HTN results in the deposition of atherosclerotic plaque in arteries. Coronary artery disease (CAD), sometimes combined with plaque rupture, leads to myocardial ischemia and infarction. Increased afterload due to HTN increases the works of the heart, resulting in left ventricular hypertrophy (LVH) and CHF. The hypertrophied heart requires more oxygen, but the flow of oxygenated blood is impaired in CAD by luminal narrowing of the coronary arteries. Myocardial infarction results in decreased contractility, which decreases blood flow to the systemic circulation in systole. Coronary blood flow occurs in diastole, and coronary perfusion is decreased in the presence of hypertension-induced LVH because LVH results in decreased compliance and increased left ventricular end-diastolic pressure (LVEDP). Examining the equation for coronary perfusion pressure (CPP), CPP = DBP – LVEDP, makes it readily apparent that increased LVEDP results in decreased coronary blood flow (CBF) in diastole. One way to improve coronary perfusion is to slow the heart rate, which increases the proportion of cardiac cycle time spent in diastole. This explains why tachycardia can result in myocardial ischemia. Although CBF can also be increased by increasing DBP, excessive increases in DBP, whether achieved with volume infusion and/or vasoconstrictors, can result in increased lung water and even APE.
Diastolic dysfunction (DD) may be present for years, with structural and physiologic changes, before symptoms develop. Older literature refers to diastolic heart failure. This language has been replaced with the term heart failure with preserved ejection fraction (HFpEF). DD that leads to HFpEF is most commonly caused by HTN. The underlying pathophysiology relates to the increased wall thickness and collagen, resulting in a stiff LV with decreased compliance, impaired relaxation, a smaller cavity, and increased LVEDP. LV filling is impaired in diastole, and elevated left atrial pressure may be transmitted to the alveoli, resulting in fatigue and/or dyspnea.[10] Since tachycardia decreases diastolic filling time, left-sided pressures rise in the face of exercise, or any cause of tachycardia such as surgical stress. Like systolic heart failure, diastolic heart failure is associated with severely decreased exercise capacity, as well as neurohumoral activation with increased norepinephrine levels, increased brain natriuretic peptide, and decreased quality of life.[11] HFpEF is predominantly a disorder of older adults, with women more often afflicted than men, especially those with longstanding HTN.[12] DD causes slightly more than half the cases of CHF and explains the occurrence of CHF in patients with normal systolic function.
Atherosclerosis in cerebral vessels can impair blood flow to the brain, causing an ischemic stroke. Atherosclerotic vessels in the brain may also burst, resulting in a hemorrhagic stroke. Hemorrhagic stroke on the surface of the brain causes a subarachnoid hemorrhage, while a ruptured vessel within the brain results in intracerebral bleeding. HTN may also result in embolic stroke when atherosclerotic plaque in the ascending aorta dislodges and travels to the brain where it occludes a blood vessel. Embolic stroke may also occur if atherosclerotic emboli originate in the venous circulation, but subsequently, enter the arterial circulation via a patent foramen ovale.
Atherosclerosis of arteries to the kidneys impairs oxygen delivery to the nephrons. In time, the glomerular filtration rate decreases, and damaged kidneys lose the ability to filter the blood. In fact, HTN is the second most common cause of end-stage renal disease (ESRD) after diabetes. Damaged kidneys also result in an impaired renin-angiotensin-aldosterone pathway, further elevating BP.
History and Physical
Most patients with HTN report their diagnosis when presenting for a preoperative visit. Still, in a study of patients manifesting hypertensive crises, more than a third were unaware they had HTN.[13] Therefore, the importance of measuring BP at the preoperative visit cannot be overemphasized. Furthermore, it should be measured in both arms. Despite published guidelines for BP measurement and a review of BP measurement for anesthesiologists, inaccurate BP measurement is common. BP should be measured in a quiet room, with the patient sitting.[14][15] Undersized cuffs result in erroneously elevated BP, and this is the most common cause of erroneously reported HTN. A properly sized cuff has a bladder cuff length that is 80% to 100% the circumference of the arm, while the width of the cuff should be 40% of the upper mid-arm circumference. The cuff should be centered over the brachial artery, directly on the skin. Too large a cuff usually results in an accurate reading. BP cuffs measure mean arterial pressure (MAP), with SBP and DBP determined by algorithms that vary between manufacturers. Although cuff BP is most often measured in the upper arm, it can be measured in the legs. BP is 10 to 20 mm Hg higher in the legs as compared with the arms. If BP is greater than 130/80 mm Hg but less than 160/90 mmHg, and a patient states they have white-coat HTN, it should be confirmed that white-coat HTN has been properly diagnosed with ambulatory or home BP monitoring. One to five percent of patients with white-coat hypertension converts to sustained HTN annually. The incidence is greater with older age, obesity, higher initial BP, and Blacks. White-coat HTN has been associated with slightly increased CVD risk and all-cause mortality risk.
History should focus on symptoms associated with end-organ damage. Hypertensive heart disease may result in coronary artery disease or LVH with associated DD. The clinician should inquire about symptoms, including chest pain or pressure, dyspnea on exertion, orthopnea, or paroxysmal nocturnal dyspnea. The distance a patient can walk on level ground, and the number of flights of stairs they can climb before the onset of symptoms should be documented. Symptoms of stroke or transient ischemic attacks should be sought. Symptoms of kidney disease, such as those related to fluid overload, are only present in patients with severe kidney disease. Recent changes in visual acuity may reflect hypertensive retinopathy.
Signs of HTN on physical examination reflect changes to end organs. An S3 gallop may indicate CHF in patients with a dilated LV, but may also be present in healthy pregnant women and athletes. An S4 is heard when blood flow from atrial contraction enters a non-compliant LV. Therefore, it may be heard in the presence of LVH or diastolic heart failure. Distended neck veins may indicate fluid overload or CHF, though this may also occur in isolated pulmonary hypertension. Rales, also called fine crackles, suggest CHF or APE, though they may also be caused by infection.[16] With a history of a prior stroke, a neurologic examination should document related deficits. A fundoscopic examination can detect changes secondary to HTN. These may include retinal arteriolar changes and/or hemorrhage, and papilledema may be seen with hypertensive urgencies and emergencies. However, a fundoscopic examination is not routinely performed as part of preoperative evaluation.
Evaluation
Initial Evaluation of a Hypertensive Patient
Ideally, treatment for HTN should be initiated by primary care providers electively, not immediately prior to surgery. The initiation of treatment should be made after several measurements of BP on at least two different occasions. Diet in overweight patients, exercise, and decreased salt intake should be a mainstay of treatment. The goal of treatment should be to reduce both BP and risk of CVD. Primary agents to treat HTN include thiazide diuretics, angiotensin-converting enzyme inhibitors (ACE), angiotensin receptor blockers (ARB), and calcium channel blockers. Secondary agents include loop diuretics, potassium-sparing diuretics, aldosterone antagonists, beta-blockers, alpha-1 blockers, centrally acting drugs, and direct vasodilators. Description of initiation, and use of these agents, has been described.[17]
Preoperative Evaluation
Patients with prior medical history should be assessed in a preoperative anesthesia clinic a minimum of one week prior to surgery. This affords time for management changes to optimize the patient’s state of health, including BP. A new concept, the perioperative surgical home, has been described, using a team approach to both optimize the patient preoperatively, and guide care during the postoperative period.[18]
If BP is well controlled, and history and physical examination are unremarkable, further testing may be unnecessary for uncomplicated surgery or procedures, but is appropriate if history or physical are concerning, and for larger and invasive surgeries. Electrocardiography (ECG), transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) detect the presence of LVH. Echocardiography can also measure its severity. Wall motion abnormalities and left ventricular ejection fraction (LVEF) can be detected with TTE and TEE. DD by Doppler-echo, in combination with a normal LVEF and a history of CHF, suggests a diagnosis of HFpEF. Referral to a cardiologist may be advisable to determine appropriate preoperative tests, assess perioperative risk, and make focused recommendations for perioperative care. Cardiac catheterization is usually only performed when indicated by symptoms, and the cardiologist believes preoperative intervention may be indicated, such as percutaneous coronary intervention in a patient with worsening angina. A neurologist should assess neurologic signs or symptoms before elective surgery. Serum creatinine can indicate impaired renal function, though it needs to be appreciated that approximately 50% of kidney function may be lost before creatinine begins to rise. Electrolytes should be performed if patients are on antihypertensives that impact electrolytes, such as diuretics. Complete blood count and platelet count are indicated if the procedure may be associated with significant blood loss. Still, many preoperative clinics will perform a complete blood count prior to all but minor procedures. In patients with HTN, a basic metabolic panel should be performed to document the preoperative state of kidney function with serum creatinine.
Treatment / Management
Preoperative Medications
Most patients with HTN are treated initially with a diuretic, although calcium channel blockers (CCB), ACE and ARB can be first-line drugs in non-black patients. In Blacks, first-line treatment should be initiated with a diuretic or CCB, as there is less cardiovascular and cerebrovascular morbidity and mortality with these agents as compared with ACE. All patients should be treated with an ACE or ARB if they have stage 3 kidney disease or chronic kidney disease with greater than 300 mg/day of albuminuria. Despite these guidelines, patients may be receiving various combinations of antihypertensive medications based upon their cardiovascular risk and the presence of end-organ damage.
There is some disagreement regarding antihypertensive medications on the day of surgery (DOS). In general, patients should be instructed to take their oral antihypertensive medications the DOS with a sip of water. It is widely accepted to withhold diuretics, due to the overnight fast. Still, in patients with severe CHF, a reduced dose of diuretic, or even the usual dose, might be considered. Perhaps this decision should be made by the anesthesiologist in the preoperative area, after measurement of BP and auscultation of the lungs. Patients on chronic beta-blocker therapy should receive their beta-blocker on the DOS. However, beta-blockade therapy should not be initiated immediately before surgery, for although it has been shown to decrease the incidence of cardiac events, it also increases the risk of bradycardia, stroke, and death.[19] Some practitioners withhold ACE before noncardiac surgery, based on a prospective cohort study that found a higher incidence of intraoperative hypotension, and the primary composite outcome of all-cause death, stroke, or myocardial injury. Still, the effect size was small, and while a meta-analysis of 6022 patients undergoing noncardiac surgery supports the association between the continuation of ACE and ARB on the DOS and intraoperative hypotension, it found no differences in mortality, cardiac events, stroke, acute kidney injury (AKI), or length of stay between the groups.[20][21] Some continue ACE and ARB in patients with CHF.(A1)
Preoperative Evaluation on the Day of Surgery (DOS)
Patients who present for anesthesia should have normal BP on the DOS, although it may be somewhat increased above their usual level due to anxiety. Once SBP reaches 170 mm Hg or DBP reaches 100 mm Hg, it is likely the patient will manifest BP gyrations in the perioperative period. These can usually be managed safely with appropriate administration of anesthetics, analgesics, and antihypertensives. If a patient presents with SBP of 180 or DBP of 110 and has no prior history of HTN, or manifests these BP measurements despite having taken their BP medications the DOS, elective surgery should be postponed until BP is better controlled. If SBP is 180 or DBP is 110 and the patient has not taken their antihypertensives that morning, they should be given with a sip of water, or a comparable intravenous antihypertensive administered. A small dose of an anxiolytic such as midazolam could be administered as well. Changing the order of cases should be considered to afford these agents time to work prior to inducing anesthesia. The 2017 American College of Cardiology/American Heart Association guidelines label SBP of 180 mm Hg as hypertensive crisis, so a decision to proceed with surgery with SBP of 180 mm Hg should be made with caution, considering the patient’s overall state of health and urgency of the procedure. If SBP is 200 mm Hg, elective surgery should be canceled, as a large retrospective study has shown that patients who receive anesthesia with an SBP of 200 mm Hg have twice the risk of a postoperative rise in troponin, and twice the risk of death, compared with patients with lower BP.[22] However, DBP of 110 is itself a risk factor for cardiovascular complications. It has been associated with ECG changes of myocardial ischemia, especially if blood pressure drops to <50% of awake MAP and isolated diastolic HTN is associated with increased risk of both CHF and death in the general population.[23](B2)
If surgery is emergent and must proceed despite poorly controlled BP, precautions should be taken. A recent ECG and echocardiogram should be reviewed. In patients in whom such information is not available, a brief delay to obtain a STAT ECG and echocardiogram may be appropriate. An increasing number of anesthesiologists are becoming proficient in point-of-care TTE, as the ability to assess cardiac function rapidly is invaluable for the assessment and management of critically ill patients. If point-of-care TTE suggests significant pathology, a formal echocardiogram should be performed and interpreted by a cardiologist. If surgery is of an emergent nature, careful monitoring of BP with an arterial line is advised, and pharmacologic therapies should be immediately available to treat HTN. Such treatment may need to be continued into the post-anesthesia care unit (PACU) and/or intensive care unit (ICU).
Intraoperative Management
One of the anesthesiologist’s primary responsibilities is to ensure safe levels of BP. This may be achieved with anesthetics, analgesics, and antihypertensive agents, with the choice of specific techniques and drugs tailored to the specific patient’s comorbidities. Poor management of BP in the perioperative period may cause end-organ complications. However, assuming BP is carefully managed during anesthesia, it is more likely the anesthesiologist will be tailoring management of the patient based upon preexisting end-organ complications of HTN, or the measured BP on the DOS.
Regional nerve blocks provide surgical anesthesia with minimal hemodynamic changes. Spinal and epidural techniques similarly permit maintenance of spontaneous ventilation, but BP may drop significantly. Such decreases may be ameliorated with volume infusion and/or vasoconstrictors. Rigid adherence to certain anesthetics for specific procedures is not recommended, as each patient’s comorbidities need to be considered. Still, the application of regional techniques and patient-controlled analgesia help minimize postoperative pain and the stress response. In addition, postoperative mobilization may be more rapid after regional techniques.
Acute complications of HTN can be minimized by maintaining BP in an appropriate range, both intraoperatively and in the PACU. Since patients with poorly controlled BP may have DD, it is important to prevent tachycardia. Beta-blockers are appropriate for this purpose. CCB, ACE inhibitors, and ARB not only lower BP but, in some studies, have been found to result in regression of LVH. It is important to maintain normal sinus rhythm (NSR) in the presence of DD. If atrial fibrillation (AFIB) develops, especially if the ventricular response is rapid, stroke volume may decrease, and pulmonary venous pressures may rise substantially. Rapid cardioversion or, at minimum, rate control may be necessary to stabilize these patients. To prevent the recurrence of AFIB, beta-blockers and amiodarone may be useful.
Target values for SBP, DPB, and MAP have been recommended. One group reported that variability and area under the curve for SBP outside the range of 105 to 130 mm Hg is associated with increased mortality after coronary artery bypass graft surgery.[24] In patients with stable CAD, SBP <120 mm Hg and DBP <70 mm Hg were each associated with adverse cardiovascular outcomes, including death.[25] Of note, these authors reported a J-shaped curve so that SBP of 140 and DBP of 80 was associated with an increased risk of cardiovascular events. In other words, tightly controlled BP may be important to minimize cardiovascular risk in patients with CAD. If 130 mm Hg seems an ideal SBP for the patient with CAD, it seems likely to be safe for patients without CAD as well, although these patients could likely tolerate SBP lower than 130 mm Hg under anesthesia, based upon their age, overall state of health, and the surgical procedure.(B2)
Recent work indicates that patients with HFpEF have a greater risk of heart failure with DBP <60 mm Hg, and a greater risk of death and cardiovascular death with DBP of ≥90 and <60 mm Hg.[26] This work also found that the hazard ratio for hospitalization for CHF and death increased significantly between DBP of 100 mm Hg and DBP of 110 mm Hg. Others reported a greater risk of death with DBP <70 mm Hg, which may be due to subclinical myocardial injury.[27][28] Therefore, DBP should be maintained between 70 to 90 mm Hg perioperatively.(A1)
MAP should be maintained 60 to 65 mm Hg, as a large retrospective study found that, during noncardiac surgery, short periods of MAP <55 mm Hg are associated with increased risk of adverse cardiac events and AKI.[29] Not only was the risk of these outcomes increased with the increasing length of time that MAP was <55 mm Hg, but MAP below 55 mm Hg, even for just a few minutes, was associated with myocardial injury and AKI.[29] This is important for perioperative AKI is associated with a shortened lifespan.[30](B2)
Poorly controlled HTN may lead to large reductions in BP during the administration of anesthesia, and both treated and untreated hypertensive patients often display a lower BP nadir than normotensive patients. Severe HTN and significant hypotension are both associated with increased risk of perioperative complications. Maintenance of appropriate intraoperative BP targets is important to minimize the risk of CHF. This is especially important, for in patients undergoing noncardiac surgery, non-ischemic heart failure is associated with a 9.3% 30-day mortality, and ischemic heart failure is associated with a 9.2% 30-day mortality, vs. 2.9% for CAD, and the risk of postop 30-day mortality is only marginally lower for minor surgery.[31] (B2)
Intravenous agents that may be used to treat severe intraoperative HTN under anesthesia include sodium nitroprusside, nicardipine, and nitroglycerin. Infusion of these agents, with appropriate adjustments, can provide control of even severely elevated BP. Rapid dose adjustments can be facilitated with beat-to-beat arterial line monitoring. Once a steady-state is reached, hydralazine may be used to provide longer-term control, and to facilitate weaning from such infusions. Reports of hypotension after hydralazine are generally associated with doses of 10 mg or more. Under anesthesia, doses of just 5 mg may decrease SBP up to 25 mm Hg or more.
Furthermore, onset may be as long as 7 to 10 minutes when used in small doses, and the peak effect may not occur for 20 to 25 minutes. Practitioners who do not appreciate this may administer a second dose before the first dose has peaked. Still, if one appreciates these pharmacologic properties, titrating hydralazine in doses of 2.5, 5, or 10 mg can be effective, both to decrease continuous antihypertensive infusions and to transition patients to eventual oral therapy. Beta-blockers such as labetalol and metoprolol have been used to treat intraoperative HTN. Since metoprolol is primarily a beta 1-selective agent, its use might result in unopposed alpha action. Esmolol is an ultra-short-acting beta 1-selective antagonist. With onset after injection of one minute, it is useful to prevent acute rises in BP, such as that associated with intubation or other acute stimuli. Its nine-minute half-life makes it less practical for ongoing BP control than the other agents described as infusion may require very frequent adjustments. Though rare for undiagnosed pheochromocytoma to present during an unrelated surgery, acute and severe intraoperative increases in BP should raise suspicion, especially if such severe HTN manifests in relation to abdominal pressure or manipulation. Treatment of suspected pheochromocytoma should be initiated with phentolamine or sodium nitroprusside, as beta-blockers can result in unopposed alpha action.
In summary, in patients with HTN and/or significant cardiovascular disease, overall appropriate targets for intraoperative BP can be summarized as SBP approximately 130 mm Hg, MAP 60 to 65 mm Hg, and DBP 70 to 90 mm Hg. While elevated BP should be decreased, care should be taken to reduce BP cautiously, as reset autoregulation in the brain and kidneys could result in organ injury if BP is reduced dramatically and/or quickly. Therefore, some recommend that intraoperative SBP not be lowered by more than 20% to 25% of the baseline preop value or clinic value. It is understood that young, healthy patients, such as those with baseline BP of 110/65, can tolerate lower values than the recommendations above for patients with HTN or cardiovascular disease (CVD). Still, even in healthy patients, a prolonged period of hypotension may increase morbidity and mortality.
These recommended BP ranges may not be applicable in patients undergoing neurosurgery, in whom appropriate BP targets may be influenced by baseline BP, neurological status, intracranial pressure, and surgical procedure. Specifically, patients with intracerebral hemorrhage may require higher intraoperative BP than suggested here, to maintain cerebral blood flow in the face of increased intracranial pressure. BP guidelines for neurosurgery are another topic and have been reviewed elsewhere.
Even patients with baseline BP of 150/90 may demonstrate significant fluctuations in BP during the perioperative period. If the planned procedure is intracranial, intrathoracic, or major abdominal, insertion of an arterial line for beat-to-beat measurement of BP may be advisable. For less invasive procedures, an arterial line may not be necessary but, as the risk/benefit ratio of an arterial line is low, any significant fluctuations in BP should result in arterial line placement. If perioperative BP fluctuations are significant, hospitalization for 24 to 48 hours postoperatively may be necessary to begin an antihypertensive regimen to control BP prior to hospital discharge.
In critically ill patients in whom invasive blood pressure monitoring is not immediately available, non-invasive BP should be repeated every one to two minutes. Still, in patients who are severely hypertensive, hypotensive, receiving drugs that rapidly change blood pressure, or are undergoing surgical procedures that may result in significant fluctuations in BP or bleeding, invasive measurement of BP via an arterial catheter should be initiated as soon as possible. The radial artery is the most common location where arterial catheters are inserted, but other possible sites include the femoral, axillary, brachial, and dorsal pedis arteries. In neonates, the umbilical artery may be the easiest artery to cannulate.
Differential Diagnosis
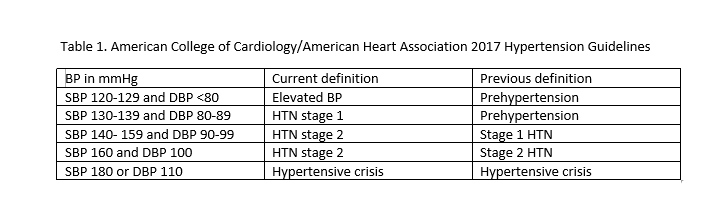
Guidelines published in 2017 have lowered the values at which HTN is defined so that SBP of 130 or DBP of 80 is now labeled HTN (see table 1).[17]
The guidelines also discuss the differentiation between white coat HTN and masked HTN, and the use of ambulatory BP monitoring to make this distinction.[32]
Prognosis
HTN is the most common risk factor for the development of CVD and mortality. Since HTN impacts >60% of people over the age of 60 in the developed world, most studies have focused on outcomes in older patients. Still, even HTN in late adolescence is associated with increased cardiovascular causes of death in middle age, as compared with people with lower BP.[33] In other words, HTN is no longer a disease of the elderly. As in older persons, a sedentary lifestyle and obesity are contributing factors to HTN in late adolescence.[33]
The continuous relationship between increasing BP and increased morbidity and mortality has been demonstrated at all ages. Furthermore, the treatment of elevated SBP has been shown to significantly reduce total mortality, CVD, coronary heart disease-related mortality, and stroke.[34][35] Although SBP appears to be a better predictor of events than DBP after age 50, high DBP is associated with increased CVD risk and is more commonly elevated in patients < 50 years of age, compared with older patients. The strong association between elevated DBP and morbidity and mortality was reported in two studies, one in veterans whose DBP ranged between 115 and 129 mm Hg, and one in veterans with DBP 90 to 114 mm Hg.[36][37] DBP tends to decline from starting at midlife due to arterial stiffening. Consequently, SBP assumes even greater importance as a risk factor starting at midlife.[38]
Complications
Complications of HTN impact the vasculature, heart, brain, kidneys, and eyes. Specific complications include atherosclerotic vascular disease, CAD, cerebrovascular disease, renal disease including ESRD, and retinal hemorrhages.
Congestive Heart Failure
HTN is the most common cause of heart failure.[39] Marked systolic HTN is associated with a frequent occurrence of APE. Most literature explains APE as a consequence of ischemia decreasing systolic function. This results in secretion of norepinephrine, release of renin-angiotensin due to decreased renal perfusion, and sometimes activation of the autonomic nervous system. The fact that marked HTN results in APE in patients who have been revascularized, as well as in patients who have not been revascularized, suggests that HTN alone may be the etiology of APE in some patients.[40] Indeed, these authors reported not only that the reoccurrence of APE is similar in patients with normal and decreased LVEF, but also in patients with and without CAD.[40] Furthermore, in patients who were revascularized, CHF was not less common than in patients with CAD who were not revascularized. Since BP is often severely elevated during APE, in at least some patients, HTN itself may be the cause of APE.[13] In fact, in a study of patients with APE and severe HTN due to renovascular disease, surgical correction of the renal vasculature resulted in a dramatic decrease in the incidence of APE, even in patients in whom BP did not normalize.[41] DD may also result in APE, but the clinical picture may be less severe due to preserved systolic function. Maintenance of both BP and heart rate in an appropriate range will minimize the risk of perioperative CHF. The judicious administration of crystalloids, colloids, and blood products is also important in this regard. Central venous and pulmonary artery pressures may be useful for trending, but no specific values ensure appropriate volume status, as both these measurements are influenced by cardiac pathologies, such as LVH, decreased compliance, and valvular heart disease. When optimization of volume status is judged essential, either TTE or TEE, as appropriate for the location of the surgical incision, are advised. If TTE/TEE are unavailable, esophageal Doppler can be used to measure cardiac output and stroke volume. Still, it does not provide additional information beyond what can be obtained with TTE/TEE.
Stroke
Risk factors for perioperative stroke are the same as those for non-operative stroke, that is, HTN, diabetes, older age, kidney disease, coronary artery disease, CHF, and AFIB. In noncardiac surgery, the reported incidence of overt stroke is 0.7%.[42] This relatively low incidence is not surprising since the maintenance of BP in an appropriate range is a primary task of the anesthesiologist. The risk of stroke is higher in the carotid artery and cardiac surgery, which is explained by embolic phenomena.
A recent study reported covert stroke in 10% of patients undergoing non-surgery and that covert stroke was associated with cognitive decline, delirium, and subsequent stroke or transient ischemic attack within one year. [43] Cognitive decline was detected in 42% of subjects with a covert perioperative stroke vs. in 29% of subjects who did not have a covert perioperative stroke. The latter suggests that other etiologies may be contributing to cognitive decline. Of note, this study did not have a control group of patients who did not undergo surgery. This study did not measure intraoperative physiologic parameters, nor document anesthetic techniques, so it cannot be known whether anesthetic management could decrease the risk of covert stroke. Additional work to confirm these findings will be welcomed.
Many elderly patients are receiving anticoagulants and/or platelet inhibitors, which are held pre-operatively to minimize the risk of bleeding. Both thrombosis and bleeding are causes of stroke, but there are no specific guidelines when to withhold and when to re-institute these agents in order to minimize the risk of stroke. At present, these decisions should be at the recommendation of the physician who prescribed these agents, as the decision to withhold might impact the occurrence of complications they are intended to prevent. Depending on the indication for the anticoagulant and/or antiplatelet agent, these physicians might include a cardiologist, neurologist, cardiac surgeon, vascular surgeon, or primary care provider.
Acute Kidney Injury
The literature contains numerous definitions of kidney injury. In 2004, the RIFLE classification was proposed to distinguish between different levels of kidney injury and to facilitate comparison of studies of AKI.[44] It has been shown useful to predict outcomes. One limitation of the RIFLE criteria is the requirement to know baseline serum creatinine (sCr), a value that is sometimes unavailable. Additional limitations of the RIFLE criteria have been described.[44] The Acute Kidney Injury Network (AKIN) classification was first reported in 2007. It is a modification of the RIFLE criteria. The advantages of the AKIN classification are that it can be used when baseline sCr is unknown and has been shown to increase the identification of patients with AKI as compared with RIFLE.[44] A weakness of both RIFLE and AKIN is their use of sCr, which may take several days to rise after renal insult. In contrast, urinary and serum biomarkers now exist that rise one to three days before sCr.[45]
Acute kidney injury (AKI) and renal replacement therapy are serious complications after surgery. Both increase morbidity and mortality. Shortened lifespan has been documented after AKI with permanently increased sCr. Furthermore, shortened lifespan has also been reported in patients with AKI whose serum creatinine returns to baseline.[30] Therefore, the anesthesiologist should take precautions to prevent the occurrence of perioperative AKI. These include maintaining the MAP at least 60 mm Hg in adults, hydrating patients before contrast injection, and care with the use of nephrotoxic drugs. Since biomarkers can detect AKI one to three days before sCr rises, it can be expected that their adoption into clinical practice will provide new insights into causes of AKI and new preventive measures that could be instituted.
Deterrence and Patient Education
It sometimes occurs that HTN is first detected during the perioperative period. The anesthesia provider must discuss such an occurrence with the surgeon and/or the primary care provider. Complications of untreated HTN can be devastating, so the opportunity to refer patients for antihypertensive treatment could be life-changing.
Pearls and Other Issues
It sometimes occurs that patients with no history of HTN, in whom echocardiography is performed for other reasons, manifest LVH and/or changes consistent with diastolic dysfunction DD, suggesting HTN has been present, but undiagnosed, for some time. Also, patients in whom severe BP gyrations occur under anesthesia, but SBP does not rise above 130 mm Hg may reflect patients with HTN who are hypovolemic.
It sometimes occurs that a patient is brought to the operating room for lower extremity surgery for gangrene, with SBP of 180 and/or DBP of 110. As such, patients may develop sepsis, or may already be septic, surgery is emergent. In such cases, a saddle block via spinal anesthesia is often performed. If spinal is chosen, we recommend it be performed prior to the administration of any antihypertensive agents. These patients may be hypovolemic, especially if they are concurrently hyperglycemic, and, in such patients, even a saddle block may lower BP dramatically. We emphasize the importance of a large-bore IV, and that antihypertensive treatment only be considered, in small doses, after the hemodynamic impact of the spinal has stabilized. Sometimes, the spinal will bring BP to normal levels, in which case antihypertensive treatment should be initiated slowly when HTN reappears as hemodynamic effects of the spinal regress.
If surgery is performed in the sitting position, as is sometimes done during neurosurgery and shoulder surgery, it should be appreciated that non-invasive BP measured at the level of the brachial artery does not reflect BP perfusing the brain. Therefore, cuff BP goals should be maintained higher than normal. If an arterial line is inserted, the transducer should be placed and zeroed at the level of the ear. Placement of the arterial line transducer at the level of the operating room table, and management of BP to that arterial line, has resulted in brain injury in a patient undergoing neurosurgery in the sitting position.
Not all adrenal tumors are pheochromocytomas. Most are non-functioning tumors, some are metastases, and some are aldosterone-producing adenomas. Others may be due to Cushing disease or cancer. Urinary metanephrine and normetanephrine levels may be used to differentiate pheochromocytomas from other lesions. The classic triad of headache, HTN, and palpitations is present in less than a quarter of patients. Not all patients with pheochromocytoma are hypertensive, and 10% to 15% are asymptomatic. Pheochromocytoma is just one of the possible causes of secondary HTN. Still, it is the most important to be aware of in the perioperative period as the administration of anesthesia to patients with unknown/untreated pheochromocytoma has been reported to result in perioperative mortality as high as 50%, due to severe HTN or arrhythmias. Preoperative preparation includes alpha blockade, followed by the addition of CCB or beta-blockers as needed to control BP. Several measurements of SBP should be less than 160 mm Hg before surgery. As alpha blockade takes effect, patients are advised to increase liquid and salt intake orally. Beta-blockade should never be initiated before alpha-blockade in patients with pheochromocytoma, as the unopposed alpha effect can result in severe HTN. Historically, preoperative preparation was performed over one to two weeks. Some recent reports indicate just one week of preoperative preparation is as safe. However, these are retrospective reports, not prospective randomized studies. It should also be appreciated that current improved outcomes may be due to improved anesthesia and intensive care techniques. In the absence of preoperative alpha blockade and fluid supplementation, hypotension after tumor removal is more common than HTN.
Enhancing Healthcare Team Outcomes
The perioperative period is unique in that multiple healthcare providers contribute to the care of the patient. Communication between these providers will ensure patient safety preoperatively. Referral to existing or new providers if perioperative BP values are concerning is emphasized. This will ensure that an antihypertensive regimen is initiated, and follow up visits are scheduled to optimize the antihypertensive regimen.
Media
(Click Image to Enlarge)
References
Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011 Apr 26:123(16):1737-44. doi: 10.1161/CIRCULATIONAHA.110.005645. Epub [PubMed PMID: 21518989]
Level 2 (mid-level) evidenceChow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S, PURE (Prospective Urban Rural Epidemiology) Study investigators. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013 Sep 4:310(9):959-68. doi: 10.1001/jama.2013.184182. Epub [PubMed PMID: 24002282]
Level 2 (mid-level) evidenceHall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circulation research. 2015 Mar 13:116(6):991-1006. doi: 10.1161/CIRCRESAHA.116.305697. Epub [PubMed PMID: 25767285]
Level 3 (low-level) evidenceLiu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd-Jones DM. Can Antihypertensive Treatment Restore the Risk of Cardiovascular Disease to Ideal Levels?: The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association. 2015 Sep 21:4(9):e002275. doi: 10.1161/JAHA.115.002275. Epub 2015 Sep 21 [PubMed PMID: 26391135]
Mueller NT, Noya-Alarcon O, Contreras M, Appel LJ, Dominguez-Bello MG. Association of Age With Blood Pressure Across the Lifespan in Isolated Yanomami and Yekwana Villages. JAMA cardiology. 2018 Dec 1:3(12):1247-1249. doi: 10.1001/jamacardio.2018.3676. Epub [PubMed PMID: 30427998]
Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997 Jul 1:96(1):308-15 [PubMed PMID: 9236450]
Level 2 (mid-level) evidenceWriting Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):e38-360. doi: 10.1161/CIR.0000000000000350. Epub 2015 Dec 16 [PubMed PMID: 26673558]
Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodríguez-Artalejo F, Stegmayr B, Thamm M, Tuomilehto J, Vanuzzo D, Vescio F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003 May 14:289(18):2363-9 [PubMed PMID: 12746359]
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet (London, England). 2005 Jan 15-21:365(9455):217-23 [PubMed PMID: 15652604]
Aziz F, Tk LA, Enweluzo C, Dutta S, Zaeem M. Diastolic heart failure: a concise review. Journal of clinical medicine research. 2013 Oct:5(5):327-34. doi: 10.4021/jocmr1532w. Epub 2013 Aug 5 [PubMed PMID: 23986796]
Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002 Nov 6:288(17):2144-50 [PubMed PMID: 12413374]
Bhuiyan T, Maurer MS. Heart Failure with Preserved Ejection Fraction: Persistent Diagnosis, Therapeutic Enigma. Current cardiovascular risk reports. 2011 Oct:5(5):440-449 [PubMed PMID: 22081782]
Zampaglione B, Pascale C, Marchisio M, Cavallo-Perin P. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension (Dallas, Tex. : 1979). 1996 Jan:27(1):144-7 [PubMed PMID: 8591878]
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005 Feb 8:111(5):697-716 [PubMed PMID: 15699287]
Level 3 (low-level) evidenceBartels K, Esper SA, Thiele RH. Blood Pressure Monitoring for the Anesthesiologist: A Practical Review. Anesthesia and analgesia. 2016 Jun:122(6):1866-79. doi: 10.1213/ANE.0000000000001340. Epub [PubMed PMID: 27195632]
Andrès E, Gass R, Charloux A, Brandt C, Hentzler A. Respiratory sound analysis in the era of evidence-based medicine and the world of medicine 2.0. Journal of medicine and life. 2018 Apr-Jun:11(2):89-106 [PubMed PMID: 30140315]
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex. : 1979). 2018 Jun:71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. Epub 2017 Nov 13 [PubMed PMID: 29133354]
Level 3 (low-level) evidenceVetter TR. Perioperative Surgical Home Models. Anesthesiology clinics. 2018 Dec:36(4):677-687. doi: 10.1016/j.anclin.2018.07.015. Epub 2018 Oct 12 [PubMed PMID: 30390787]
Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. The New England journal of medicine. 2005 Jul 28:353(4):349-61 [PubMed PMID: 16049209]
Level 2 (mid-level) evidenceRoshanov PS, Rochwerg B, Patel A, Salehian O, Duceppe E, Belley-Côté EP, Guyatt GH, Sessler DI, Le Manach Y, Borges FK, Tandon V, Worster A, Thompson A, Koshy M, Devereaux B, Spencer FA, Sanders RD, Sloan EN, Morley EE, Paul J, Raymer KE, Punthakee Z, Devereaux PJ. Withholding versus Continuing Angiotensin-converting Enzyme Inhibitors or Angiotensin II Receptor Blockers before Noncardiac Surgery: An Analysis of the Vascular events In noncardiac Surgery patIents cOhort evaluatioN Prospective Cohort. Anesthesiology. 2017 Jan:126(1):16-27 [PubMed PMID: 27775997]
Hollmann C, Fernandes NL, Biccard BM. A Systematic Review of Outcomes Associated With Withholding or Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Before Noncardiac Surgery. Anesthesia and analgesia. 2018 Sep:127(3):678-687. doi: 10.1213/ANE.0000000000002837. Epub [PubMed PMID: 29381513]
Level 1 (high-level) evidenceWax DB, Porter SB, Lin HM, Hossain S, Reich DL. Association of preanesthesia hypertension with adverse outcomes. Journal of cardiothoracic and vascular anesthesia. 2010 Dec:24(6):927-30. doi: 10.1053/j.jvca.2010.06.022. Epub [PubMed PMID: 20817562]
Level 2 (mid-level) evidencePrys-Roberts C, Meloche R, Foëx P. Studies of anaesthesia in relation to hypertension. I. Cardiovascular responses of treated and untreated patients. British journal of anaesthesia. 1971 Feb:43(2):122-37 [PubMed PMID: 5550843]
Aronson S, Stafford-Smith M, Phillips-Bute B, Shaw A, Gaca J, Newman M, Cardiothoracic Anesthesiology Research Endeavors. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology. 2010 Aug:113(2):305-12. doi: 10.1097/ALN.0b013e3181e07ee9. Epub [PubMed PMID: 20571360]
Level 2 (mid-level) evidenceVidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG, CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet (London, England). 2016 Oct 29:388(10056):2142-2152. doi: 10.1016/S0140-6736(16)31326-5. Epub 2016 Aug 30 [PubMed PMID: 27590221]
Sandesara PB, O'Neal WT, Kelli HM, Topel M, Samman-Tahhan A, Sperling LS. Diastolic Blood Pressure and Adverse Outcomes in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial. Journal of the American Heart Association. 2018 Feb 23:7(5):. doi: 10.1161/JAHA.117.007475. Epub 2018 Feb 23 [PubMed PMID: 29475874]
Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Annals of internal medicine. 2006 Jun 20:144(12):884-93 [PubMed PMID: 16785477]
Level 1 (high-level) evidenceMcEvoy JW, Chen Y, Nambi V, Ballantyne CM, Sharrett AR, Appel LJ, Post WS, Blumenthal RS, Matsushita K, Selvin E. High-Sensitivity Cardiac Troponin T and Risk of Hypertension. Circulation. 2015 Sep 1:132(9):825-33. doi: 10.1161/CIRCULATIONAHA.114.014364. Epub 2015 Jul 7 [PubMed PMID: 26152706]
Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013 Sep:119(3):507-15. doi: 10.1097/ALN.0b013e3182a10e26. Epub [PubMed PMID: 23835589]
Level 2 (mid-level) evidenceBihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Annals of surgery. 2009 May:249(5):851-8. doi: 10.1097/SLA.0b013e3181a40a0b. Epub [PubMed PMID: 19387314]
Level 2 (mid-level) evidencevan Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011 Jul 19:124(3):289-96. doi: 10.1161/CIRCULATIONAHA.110.011130. Epub 2011 Jun 27 [PubMed PMID: 21709059]
Level 2 (mid-level) evidencePickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. The New England journal of medicine. 2006 Jun 1:354(22):2368-74 [PubMed PMID: 16738273]
Sundström J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ (Clinical research ed.). 2011 Feb 22:342():d643. doi: 10.1136/bmj.d643. Epub 2011 Feb 22 [PubMed PMID: 21343202]
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015 Nov 26:373(22):2103-16. doi: 10.1056/NEJMoa1511939. Epub 2015 Nov 9 [PubMed PMID: 26551272]
Level 1 (high-level) evidenceSamuelsson OG, Wilhelmsen LW, Svärdsudd KF, Pennert KM, Wedel H, Berglund GL. Mortality and morbidity in relation to systolic blood pressure in two populations with different management of hypertension: The Study of Men Born in 1913 and the Multifactorial Primary Prevention Trial. Journal of hypertension. 1987 Feb:5(1):57-66 [PubMed PMID: 3584964]
. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970 Aug 17:213(7):1143-52 [PubMed PMID: 4914579]
Level 1 (high-level) evidence. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967 Dec 11:202(11):1028-34 [PubMed PMID: 4862069]
Level 1 (high-level) evidenceFranklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999 Jul 27:100(4):354-60 [PubMed PMID: 10421594]
Level 2 (mid-level) evidenceLevy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996 May 22-29:275(20):1557-62 [PubMed PMID: 8622246]
Level 2 (mid-level) evidenceKramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. American heart journal. 2000 Sep:140(3):451-5 [PubMed PMID: 10966547]
Weatherford DA, Freeman MB, Regester RF, Serrell PF, Stevens SL, Goldman MH. Surgical management of flash pulmonary edema secondary to renovascular hypertension. American journal of surgery. 1997 Aug:174(2):160-3 [PubMed PMID: 9293835]
Level 2 (mid-level) evidencePOISE Study Group, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Málaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet (London, England). 2008 May 31:371(9627):1839-47. doi: 10.1016/S0140-6736(08)60601-7. Epub 2008 May 12 [PubMed PMID: 18479744]
Level 1 (high-level) evidenceNeuroVISION Investigators. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet (London, England). 2019 Sep 21:394(10203):1022-1029. doi: 10.1016/S0140-6736(19)31795-7. Epub 2019 Aug 15 [PubMed PMID: 31422895]
Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clinical kidney journal. 2013 Feb:6(1):8-14 [PubMed PMID: 27818745]
Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria. Journal of intensive care medicine. 2007 Jul-Aug:22(4):187-93 [PubMed PMID: 17712054]