Introduction
Echinococcosis is a zoonotic larval infection that infects humans globally. The parasite Echinococcus causes the disease. According to the World Health Organization (WHO), the global burden of controlling the disease exceeds three billion US dollars annually.[1]
There are several identified species of Echinococcosis, four of which are of concern in humans:
- Echinococcus granulosus: infections cause cystic echinococcosis, also called hydatidosis.
- Echinococcus multilocularis: infections cause alveolar echinococcosis.
- Echinococcus vogeli: infections cause polycystic echinococcosis.
- Echinococcus oligarthrus: infections cause polycystic echinococcosis.
This article will discuss and summarize the essential aspects of Echinococcus granulosus infections.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Hydatidosis results from a larval infection of the tapeworm (cestode) Echinococcus granulosus. The disease characteristically demonstrates the growth of hydatid cysts (metacestode) in the internal organs of intermediate hosts, including humans. The definitive hosts of the cestode are carnivores such as dogs. Humans and other hosts ingest eggs or gravid proglottids that are excreted in the definitive host's feces, causing the infection.[2]
Epidemiology
Due to the nature of the pathogen, hydatidosis is more predominant in communities that keep dogs for guarding and herding cattle [3]. Geographically, the disease has a worldwide distribution with higher prevalence in the Mediterranean, Russia, China, North and East Africa, Australia, and South America.[2]
Pathophysiology
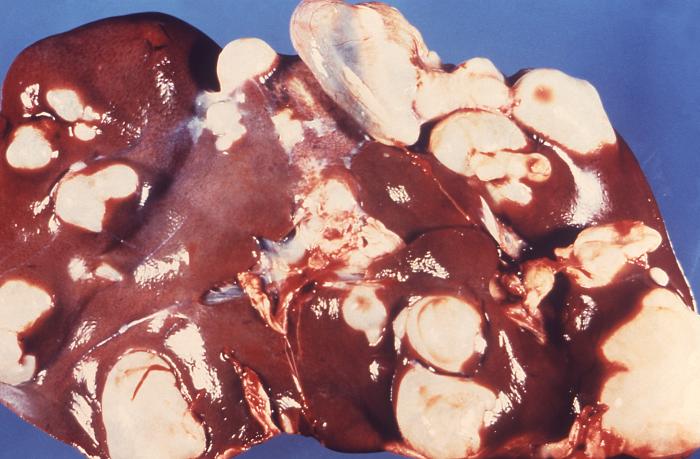
As humans ingest the eggs of the tapeworm, an oncosphere larva releases from the egg, which can penetrate the lamina propria of the intestine. Once it penetrates, it gets passively transported via blood or lymphatics to the liver, lungs, or other internal organs, where it develops into hydatid cysts (metacestode larvae). These cysts have an inner germinal layer and an outer laminated layer surrounded by a fibrous capsule derived from the host. Smaller "daughter" cysts bud from the inner cellular layer. In humans, cysts grow slowly and can be up to multiple liters in volume and contain thousands of protoscolices. Over time, septations and daughter cysts disrupt the typical unilocular pattern of echinococcal cysts.[2][4]
Histopathology
Under the microscope, the hydatid cysts are recognizable by three distinct characteristics[5]:
1. Thick, acellular, laminated layer with characteristic acidophilic staining
2. Cellular germinal layer
3. Brood capsules or protoscolices
A host produced granulomatous reaction can be seen surrounding the cyst
History and Physical
Infected hosts may go on for months or years without showing any signs or symptoms of infection; some patients have persistent cysts for years without noticing, and others cysts may rupture spontaneously or due to trauma and disappear entirely.
If a cyst continues growing, patients may exhibit symptoms gradually due to the cyst exerting pressure on surrounding tissue. Sudden signs and symptoms are more likely due to rupture rather than the growth of the cyst.
Rupture of the cyst can induce an IgE-mediated hypersensitivity reaction in patients, presenting as hives, mucous membrane swelling, and flushing. The reaction can be life-threatening.[6]
Ruptured or leaking cysts can release protoscolices into the peritoneum, causing secondary hydatidosis.[7]
The nature of signs and symptoms varies depending on the site of the cyst, which is most common in the liver (greater than 65% in pastoral strains) and lungs (25%) but can affect other sites, including bones, spleen, central nervous system, and the heart. Most primary infections consist of one cyst, but 20 to 40% of patients display multiple organ involvement.[8]
While infections can be acquired in childhood, most causes of hydatidosis present later in life due to the slow growing nature of cysts. However, cysts in the brain or eye can cause symptoms early on and thus mostly present in childhood.
Common findings in liver hydatidosis include abdominal pain, decreased appetite, hepatomegaly, a palpable mass, and abdominal distention.
Common findings in lung hydatidosis include chronic cough, chest pain, and shortness of breath.
During the history, it's important to look for risk factors of echinococcosis, especially contact with canines and cattle.
Evaluation
Diagnosis of echinococcosis starts from a thorough history, including a history of exposure or immigration from endemic areas, followed by a combination of serology and imaging.
Liver function tests are unreliable in detecting the severity of disease and are abnormal in only about 40% of patients. Alkaline phosphatase is the one typically elevated, while AST, ALT, and bilirubin levels typically remain within the normal range. The complete blood count may show eosinophilia.[7]
Serological testing for Echinococcus antibodies is a common method for diagnosis. ELISA tests are usually used to screen. A confirmatory immunoblot assay for echinococcal antigens is carried out following a positive result. However, a significant number of echinococcosis patients do not elicit an immune response.[9] The strength and detectability of the immune response depend on various factors, including cyst viability and cyst wall intactness.
Imaging is extremely useful in detecting and monitoring hydatidosis cases, especially those that are seronegative. Ultrasonography is the preferred modality for liver and intraabdominal hydatidosis, but its accuracy remains operator-dependent. It remains the modality of choice for screening due to its accessibility and portability. It's also helpful in post-treatment monitoring of the disease.
For hepatic echinococcosis, the World Health Organization Informal Working Group (WHO-IWGE) developed an ultrasound classification to stage the disease[10]:
- CE 1: unilocular fluid collection/simple cyst with double line sign.
- CE 2: multivesicular/multiseptated cyst. "honeycomb" or "rosette-like."
- CE 3A: a fluid collection with a detached membrane (water lily sign).
- CE 3B: the presence of daughter cysts in a solid matrix.
- CE 4: cysts with a heterogeneous hypoechoic/hyperechoic matrix without daughter cysts.
- CE 5: solid cystic wall.
Stages CE1 and CE2 indicate active disease; stage CE3 indicates a transitional stage where the cyst has suffered a compromise, whereas CE4 & CE5 indicate inactive disease.
Radiography can be used to detect calcifications in up to 30% of cases. The calcifications are typically ring-like and can progress throughout all stages of the disease.[11]
CT imaging is highly sensitive and serves a vital role in cases where ultrasonography is difficult (e.g., obese patients). It's also crucial in the perioperative period, as it's excellent at detecting complications, including cyst rupture, underlying infection, and biliary or vascular involvement.[12]
Other modalities used for diagnosis include ultrasound-guided fine needle aspiration (in seronegative cases with inconclusive imaging) and endoscopic retrograde cholangiopancreatography (both diagnostic and therapeutic for cases affecting the biliary tree).[13]
Treatment / Management
Management options range from expectant therapy to invasive surgical intervention; the choice depends on a variety of factors, including the patient's clinical picture and the size of the cyst (ultrasound-guided staging system is the method used in liver cysts).
Treatment of Hepatic Cysts[13] (Strength of recommendations B, quality of evidence III)(B3)
- The "Watch and wait" approach can be an option in cases in which the cysts are uncomplicated (stages CE4 and CE5), given that long-term follow-up with ultrasonography can be ensured.
- Medical treatment with benzimidazoles (BMZ) is indicated for CE1 and CE3a cysts smaller than 5 cm in the liver and lung, patients with cysts in two or more organs, and inoperable patients. BMZs are also used following surgery or percutaneous procedures to prevent recurrence. Albendazole (ABZ) is currently the drug of choice at a dose of 10 to 15 mg/kg/day. Mebendazole (MBZ) at a dose of 40 to 50 mg/kg/day is another therapeutic option. Medical treatment with BMZ is contraindicated in cysts vulnerable to rupture and early pregnancy. Duration of treatment is based on the clinical situation and can range from 1 to 6 months.
- Surgery is the modality of choice for complicated cysts. It is necessary for the removal of large CE2-CE3b cysts, superficial cysts that may rupture with trauma or spontaneously, infected cysts, cysts with biliary tree communication, and cysts exerting pressure on adjacent organs.
- PAIR (puncture, aspiration, injection, re-aspiration) procedure is an ultrasound-guided, minimally invasive modality used in hepatic and other abdominal cysts. Indications include inoperable patients, relapsing cases after surgery, and failure to respond to medical treatment. It provides the best results in CE1 and CE3a cysts over 5 cm and is used in combination with medical therapy (BMZ). It is contraindicated in for CE2, CE3b, CE4, and CE5 cysts. Physicians performing this procedure must be equipped to deal with the potential anaphylactic shock. Other percutaneous treatments can be used but are usually reserved for CE2 and CE3b cysts.
Post-treatment monitoring using leukocyte count, aminotransferases, and ultrasound should be done every 3 to 6 months initially, then once yearly once the patient is stable.[13](B3)
Differential Diagnosis
Hydatidosis can mimic a large number of conditions, depending on the site of the cyst.
Liver hydatidosis differential diagnoses include:
- Liver abscess
- Liver cysts
- Budd-Chiari syndrome
- Biliary colic
- Biliary cirrhosis
- Tuberculosis
- Primary hepatic carcinoma
A detailed history and physical exam, alongside proper workup including serology and imaging, is key in making the diagnosis and ruling out diseases that may mimic hydatidosis.
Prognosis
The prognosis is considered generally good with appropriate treatment. However, cysts that develop in difficult sites for surgery, such as the heart and spine, have a poorer prognosis. Recurrence at the site of the cyst or in other sites occurs in some cases.[14][15][16][17]
Complications
Rupture of the cyst, whether spontaneous or traumatic, can cause site-specific complications. Rupture of liver cysts into the biliary tree can result in biliary obstruction, superinfection of the cyst, and secondary peritonitis, while rupture of the lung cysts into the bronchial tree can result in pneumonitis, pneumothorax, pleural effusion, and secondary pleuritis.[2][3]
Deterrence and Patient Education
Education regarding the disease and its mode of transmission is especially crucial in endemic areas. People who work with cattle and dogs should receive education on the transmissibility of the disease and proper ways of dealing with sheep viscera.
Mass screening using ultrasound has been used to monitor prevalence in endemic areas, which helps guide a public health discussion on ways to limit the disease in these areas.[18]
Enhancing Healthcare Team Outcomes
The diagnosis of hydatidosis can pose some difficulty to the nurse practitioner, internist, or primary physician, especially in areas where the disease is not endemic. However, being mindful of the possibility alongside picking up clues from the history should help in guiding the workup and, eventually, the diagnosis.
Management options should be evaluated based on a patient-to-patient basis. Infectious disease specialists, surgeons, anesthetists, and potentially interventional radiologists could all weigh in on deciding what could lead to the best outcome for the patient. These physicians, as part of a broader interprofessional team approach, including nurses and pharmacists, can provide the best potential result. This interprofessional discussion is emphasized in complicated cases or in atypical sites for hydatidosis where the literature is less informative on outcomes. [Level 5]
Media
(Click Image to Enlarge)
References
Pourseif MM, Moghaddam G, Saeedi N, Barzegari A, Dehghani J, Omidi Y. Current status and future prospective of vaccine development against Echinococcus granulosus. Biologicals : journal of the International Association of Biological Standardization. 2018 Jan:51():1-11. doi: 10.1016/j.biologicals.2017.10.003. Epub [PubMed PMID: 29100669]
McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet (London, England). 2003 Oct 18:362(9392):1295-304 [PubMed PMID: 14575976]
Moro P, Schantz PM. Echinococcosis: a review. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2009 Mar:13(2):125-33. doi: 10.1016/j.ijid.2008.03.037. Epub 2008 Oct 19 [PubMed PMID: 18938096]
Level 3 (low-level) evidenceWen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: Advances in the 21st Century. Clinical microbiology reviews. 2019 Mar 20:32(2):. doi: 10.1128/CMR.00075-18. Epub 2019 Feb 13 [PubMed PMID: 30760475]
Level 3 (low-level) evidenceLi T, Ito A, Nakaya K, Qiu J, Nakao M, Zhen R, Xiao N, Chen X, Giraudoux P, Craig PS. Species identification of human echinococcosis using histopathology and genotyping in northwestern China. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008 Jun:102(6):585-90. doi: 10.1016/j.trstmh.2008.02.019. Epub 2008 Apr 8 [PubMed PMID: 18396303]
Level 3 (low-level) evidenceLanger JC, Rose DB, Keystone JS, Taylor BR, Langer B. Diagnosis and management of hydatid disease of the liver. A 15-year North American experience. Annals of surgery. 1984 Apr:199(4):412-7 [PubMed PMID: 6712316]
Symeonidis N, Pavlidis T, Baltatzis M, Ballas K, Psarras K, Marakis G, Sakantamis A. Complicated liver echinococcosis: 30 years of experience from an endemic area. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2013:102(3):171-7. doi: 10.1177/1457496913491877. Epub [PubMed PMID: 23963031]
Level 2 (mid-level) evidenceKammerer WS, Schantz PM. Echinococcal disease. Infectious disease clinics of North America. 1993 Sep:7(3):605-18 [PubMed PMID: 8254162]
Zhang W, Wen H, Li J, Lin R, McManus DP. Immunology and immunodiagnosis of cystic echinococcosis: an update. Clinical & developmental immunology. 2012:2012():101895. doi: 10.1155/2012/101895. Epub 2011 Dec 25 [PubMed PMID: 22235225]
Level 3 (low-level) evidenceWHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta tropica. 2003 Feb:85(2):253-61 [PubMed PMID: 12606104]
Level 3 (low-level) evidenceBeggs I. The radiology of hydatid disease. AJR. American journal of roentgenology. 1985 Sep:145(3):639-48 [PubMed PMID: 3895873]
Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS neglected tropical diseases. 2012:6(10):e1880. doi: 10.1371/journal.pntd.0001880. Epub 2012 Oct 25 [PubMed PMID: 23145199]
Level 2 (mid-level) evidenceBrunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta tropica. 2010 Apr:114(1):1-16. doi: 10.1016/j.actatropica.2009.11.001. Epub 2009 Nov 30 [PubMed PMID: 19931502]
Level 3 (low-level) evidenceMansfield BS, Pieton K, Pather S. Spinal Cystic Echinococcosis. The American journal of tropical medicine and hygiene. 2019 Jan:100(1):9-10. doi: 10.4269/ajtmh.18-0588. Epub [PubMed PMID: 30652663]
Sayek I, Tirnaksiz MB, Dogan R. Cystic hydatid disease: current trends in diagnosis and management. Surgery today. 2004:34(12):987-96 [PubMed PMID: 15580379]
Level 3 (low-level) evidenceDhar P, Chaudhary A, Desai R, Agarwal A, Sachdev A. Current trends in the diagnosis and management of cystic hydatid disease of the liver. The Journal of communicable diseases. 1996 Dec:28(4):221-30 [PubMed PMID: 9057445]
Level 3 (low-level) evidenceBouraoui H, Trimeche B, Mahdhaoui A, Majdoub A, Zaaraoui J, Hajri Ernez S, Gouider J, Ammar H. Echinococcosis of the heart: clinical and echocardiographic features in 12 patients. Acta cardiologica. 2005 Feb:60(1):39-41 [PubMed PMID: 15779850]
Level 2 (mid-level) evidenceMacpherson CN, Bartholomot B, Frider B. Application of ultrasound in diagnosis, treatment, epidemiology, public health and control of Echinococcus granulosus and E. multilocularis. Parasitology. 2003:127 Suppl():S21-35 [PubMed PMID: 15027603]
Level 3 (low-level) evidence