Introduction
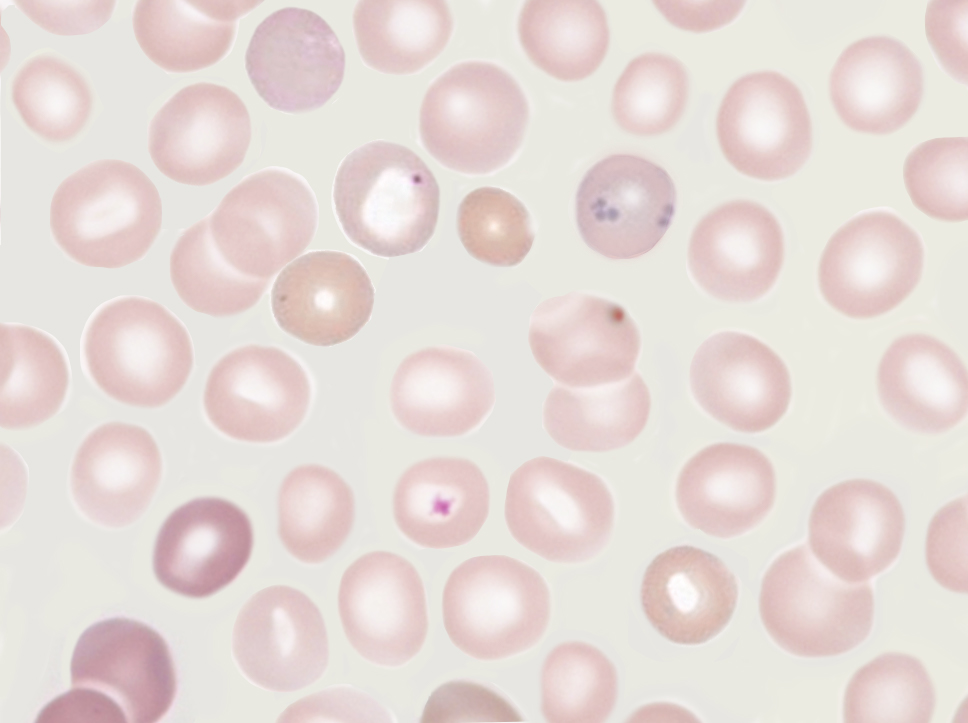
Howell-Jolly bodies are nuclear remnants found within red blood cells, named after the pioneering work of William Howell and Justin Jolly in the late 19th and early 20th centuries. These bodies are typically extruded during the final stages of erythropoiesis in the bone marrow but can persist in the peripheral blood under certain pathological conditions, particularly when the spleen is absent or functionally impaired. Howell-Jolly bodies may persist in patients with spleen impairment because one of the spleen's functions is to filter deranged blood cells and remove the intracellular inclusions left by the erythrocyte precursors (see Image. Howell-Jolly Body). Identifying and studying Howell-Jolly bodies have provided significant insights into both normal and abnormal erythropoiesis and are crucial in diagnosing various hematological disorders.[1]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Howell-Jolly bodies appear in peripheral blood smears in patients with absent or deficient spleen function. Moreover, Howell-Jolly bodies are pathognomonic for splenic dysfunction but can also be found in several conditions, including:
- Splenectomy
- Sepsis
- Severe hemolytic anemia
- Megaloblastic anemia
- Congenital disorders
- Congenital asplenia
- Functional asplenia and sickle cell hemoglobinopathies
- Alcoholism
- Lupus and other autoimmune disorders
- Bone marrow disorders, such as myelodysplastic syndromes or bone marrow infiltration by malignancies, disrupt normal erythropoiesis
- Posttransplantation of bone marrow
- Severe postpartum hemorrhage
- Chemotherapy and radiation therapy
- Hereditary spherocytosis
Conditions less commonly associated with Howell-Jolly bodies include:
- Gastrointestinal diseases, such as celiac and inflammatory bowel diseases
- Neoplastic disorders
- Splenic vascular thrombosis
- Amyloidosis, elderly
- Postmethyldopa treatment
- High-dose corticosteroid treatment [2][3][4][5][6][7][8]
Detection and the number of Howell-Jolly bodies do not reflect an accurate assessment of spleen function; however, Howell-Jolly bodies often present concurrently with spleen dysfunction.[9] Other erythrocyte inclusions that should be distinguished from Howell-Jolly bodies include:
- Basophilic stippling
- Pappenheimer bodies
- Heinz bodies
- Howell-Jolly body–like inclusions in neutrophils
- Howell-Jolly body–like inclusions in COVID-19 (Please refer to the Clinical Significance section for more information)
Structure
Howell-Jolly bodies are DNA-containing inclusions found in red blood cells after erythrocyte maturation. The exact composition of the DNA remains unknown. However, studies show that these inclusions are of centromeric origin. Smaller Howell-Jolly bodies contain nuclear material from 1 to 2 centromeres, whereas larger Howell-Jolly bodies have nuclear fragments from up to 8 centromeres. The most frequently observed fragments are centromeres from chromosomes 1, 5, 7, 8, and 18. The average size of Howell-Jolly bodies is 0.73 µm.[10]
Function
Howell-Jolly bodies are not functional structures but rather cellular remnants that serve as diagnostic markers, as their presence in the peripheral blood indicates certain pathological conditions primarily related to spleen function. Howell-Jolly bodies are observed in patients with asplenia or splenic dysfunctions and in conditions with ineffective erythropoiesis, such as megaloblastic anemia. In this condition, due to vitamin B12 or folate deficiency, the maturation of red blood cells in the bone marrow is impaired, releasing immature red blood cells with nuclear remnants into the bloodstream. Howell-Jolly bodies may also indicate a stressed or recovering bone marrow. During periods of high erythropoietic demand, such as hemolytic crisis or following chemotherapy, immature red blood cells with Howell-Jolly bodies may be released into the circulation before the spleen can remove these remnants.[5][11]
Tissue Preparation
Detecting Howell-Jolly bodies in red blood cells is a critical diagnostic procedure for assessing splenic function and identifying underlying hematological disorders. Proper tissue preparation and staining techniques are essential for accurately identifying these nuclear remnants.
A peripheral blood sample is typically collected using standard venipuncture techniques, with an anticoagulant, such as ethylenediaminetetraacetic acid, to prevent clotting. A small drop of the blood sample is placed on a clean glass microscope slide to prepare the smear. Another slide spreads the drop by placing it at a 30° to 45° angle and smoothly spreads the blood across the slide to create a thin smear. The smear is then allowed to air dry completely, as rapid drying helps preserve cell morphology.
Once dried, the smear is fixed by immersing the slide in methanol for a few minutes, which preserves the cells and prevents further degradation. Howell-Jolly bodies are best visualized using Romanowsky-type stains, including Wright, Giemsa, or a combination of Wright-Giemsa stains. For the Wright-Giemsa stain procedure, the fixed slide is dipped into a jar of Wright-Giemsa stain for about 1 to 3 min, then gently rinsed with distilled water or buffer solution to remove excess stain, and air dried before microscopic examination. Alternative staining methods include the Feulgen reaction, a DNA-specific stain involving hydrolyzing the slide in hydrochloric acid and then staining with Schiff's reagent, and the May-Grünwald stain, another Romanowsky-type stain used similarly to Wright-Giemsa.
The stained blood smear is examined under a light microscope at high magnification, usually with a 100× oil immersion magnification. Howell-Jolly bodies appear as small, round, dark-purple inclusions within the red blood cells, typically singular and located eccentrically within the cell. The findings are documented by taking photomicrographs of representative fields and recording the number of Howell-Jolly bodies per a specified number of red blood cells to quantify their presence. To ensure the staining and detection process is functioning correctly, include slides with known Howell-Jolly bodies as positive controls and use slides without Howell-Jolly bodies as negative controls to confirm the specificity of the staining. If the initial results are ambiguous, prepare and stain additional smears to confirm the presence of Howell-Jolly bodies.[5]
Histochemistry and Cytochemistry
No specific histochemical markers for detecting Howell-Jolly bodies have been established. However, recent advances have been made in detecting and quantifying erythrocyte chromosomal fragments using flow cytometry. Sample preparation uses an RNase/antibody solution and anti-CD71 fluorescein isothiocyanate to detect young reticulocytes. However, this process is not standard in medical practice.[12]
Microscopy, Light
Howell-Jolly bodies appear as small, round, or oval, strongly basophilic (dark purple) inclusions within red blood cells when stained with Romanowsky-type stains, such as Wright or Giemsa. These inclusions are typically singular, located eccentrically within the red blood cell, and vary in diameter. Using the Wright-Giemsa stain, Howell-Jolly bodies appear dark purple due to their high affinity for basic dyes, which bind to the DNA material within the inclusions. This contrast makes Howell-Jolly bodies distinct from other cytoplasmic components. Howell-Jolly bodies are generally found at the periphery of the red blood cell, although they can sometimes be located more centrally. These bodies can be confused with overlapping platelets but do not have the halo that typically overlies platelets.[13]
Microscopy, Electron
Although performing electron microscopy is not routine for examining peripheral blood smears, electron microscopy provides unparalleled resolution and detail in studying cellular structures, making it an invaluable tool for detecting Howell-Jolly bodies in red blood cells. The high resolution of electron microscopy, up to the nanometer scale, allows for detailed visualization of Howell-Jolly bodies within erythrocytes. This level of detail can differentiate between Howell-Jolly bodies and other cellular inclusions or artifacts. In addition, electron microscopy can provide detailed images of the subcellular organization of HJBs, including chromatin arrangement within the nuclear remnants.
Electron microscopy also distinguishes Howell-Jolly bodies from other erythrocyte inclusions, including basophilic stippling, pappenheimer bodies, and Heinz bodies, which is crucial for accurate diagnosis. Advanced electron microscopy techniques, such as scanning electron and transmission electron microscopy, offer three-dimensional reconstructions of erythrocytes, providing a comprehensive understanding of Howell-Jolly bodies' spatial relationships and morphology. Furthermore, electron microscopy can be used for quantitative analysis, such as measuring the size and frequency of Howell-Jolly bodies in a given sample, providing valuable data for clinical assessments. This quantitative capability enhances its utility in evaluating splenic function and related hematological disorders.[14][15][16]
Pathophysiology
Erythrocytes undergo many vital changes and growth to become functional cells for oxygen transportation. Erythropoiesis, the production and maturation of red blood cells, is the process by which new red blood cells are produced. Erythropoiesis is initiated by hematopoietic stem cells in the bone marrow and involves the following stages of maturation:
- Proerythroblast: The earliest recognizable precursor in the erythroid lineage is a large cell with a round nucleus and prominent nucleoli.
- Basophilic erythroblast: The cytoplasm begins to become basophilic due to ribosomal RNA.
- Polychromatic erythroblast: Hemoglobin synthesis starts, and the cell's cytoplasm becomes grayish-blue due to the mix of ribosomes and hemoglobin.
- Orthochromatic erythroblast (normoblast): The nucleus becomes smaller and more condensed, eventually extruding from the cell.
- Reticulocyte: The cell loses its nucleus and enters the bloodstream. The reticulocyte still contains some residual RNA and organelles, which are gradually lost as it matures into a fully functional erythrocyte within 1 to 2 days.
The spleen plays a crucial role in maintaining the quality of circulating erythrocytes by removing defective cells and nuclear remnants through a process known as pitting. In pitting, the red pulp of the spleen contains macrophages that remove inclusions, such as Howell-Jolly bodies, from erythrocytes without destroying the cells themselves. This process ensures that only mature, defect-free erythrocytes remain in circulation.
Howell-Jolly bodies are remnants of nuclear material that are typically removed from erythrocytes during their maturation process in the bone marrow. These remnants appear as small, round, dark-staining inclusions within red blood cells on a peripheral blood smear. In healthy individuals, the spleen effectively removes these nuclear remnants through filtration. The macrophages in the spleen's red pulp identify and extract Howell-Jolly bodies from the erythrocytes, ensuring the removal of defective or immature cells from circulation.[1][17][18]
Clinical Significance
Hyposplenism is associated with several conditions, most commonly including splenectomy; sepsis; congenital disorders, such as congenital asplenia, Ivemark syndrome, Stormorken syndrome, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy [APECED] syndrome, cyanotic heart disease, and prematurity; sickle hemoglobinopathies; alcoholism; lupus; and posttransplantation of bone marrow. Other less common causes are gastrointestinal diseases, such as celiac disease, ulcerative colitis, and Crohn disease; other autoimmune disorders; neoplastic disorders; splenic vascular thrombosis; amyloidosis in older individuals; methyldopa treatment; and high-dose corticosteroid treatment.[3] Splenic dysfunction leaves patients susceptible to infections by encapsulated bacterial organisms, such as Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis, because splenic macrophages are responsible for removing encapsulated bacteria. Therefore, these patients should receive prophylactic vaccines, such as pneumococcal and meningococcal.
Historically, nuclear imaging modalities such as spleen scintigraphy have been employed to assess splenic function, but these methods are invasive and time-consuming. Other techniques evaluate the immunological function of the spleen by correlating its volume with spleen-dependent functional B-cell subsets. Indicators such as pitted erythrocytes or Howell-Jolly bodies are useful for scoring splenic function through imaging or spectroscopic approaches. Although Howell-Jolly bodies are sensitive markers for asplenia, they are less effective for detecting mild forms of hyposplenism. Pitted erythrocytes are characterized by depressions on their membranes due to a specific membrane defect, impairing vacuole formation and leading to their pitted appearance.
Although sensitive, detecting pitted erythrocytes requires specialized Nomarski optics-equipped microscopes. Conversely, although less sensitive, detecting Howell-Jolly bodies is feasible with conventional wide-field microscopes, making it a widely used method. Flow cytometry–based techniques for detecting Howell-Jolly bodies–like micronuclei allow measuring large-cell numbers quickly but may face challenges such as insufficient specificity and potential false positives or negatives.
Transferrin-positive reticulocytes are also used to assess splenic function as they represent the youngest erythrocytes in blood, keeping the same stage for typically 2 to 5 h in healthy adults. These reticulocytes have fewer splenic passages, and the frequency of Howell-Jolly bodies in transferrin-positive reticulocytes is much higher compared to mature erythrocytes.[19][20] In addition, current methods for measuring spleen function do not sufficiently address the risk of pneumococcal infection, which is the primary concern associated with spleen impairment in children with sickle cell disease. A complementary method for spleen assessment is necessary, likely involving immunological assays of immunoglobulin M memory B cells and other B-cell subsets, along with anti-pneumococcal antibody titers. Until specific and reliable immunological markers are available and validated, it is suggested that maintaining anti-pneumococcal prophylaxis in children with sickle cell disease until at least 5 years is reasonable, even if Howell-Jolly body counts are normal.[21]
Howell-Jolly bodies are pathognomonic for splenic dysfunction. They are one of many types of inclusions found in circulating erythrocytes, resembling Heinz bodies present in patients with glucose-6-phosphate dehydrogenase deficiency or other hemolytic anemias. Howell-Jolly bodies are also confused with basophilic stippling, a process most attributed to lead toxicity but can occur in conditions including thalassemia, sickle cell disease, vitamin B12 or folate deficiency, and myelodysplastic syndrome. Recent studies have found that Howell-Jolly body–like inclusions in neutrophils and other important immune cells could also be a potential feature to help differentiate between COVID-19 and bacterial pneumonia.[22] Howell-Jolly body–like inclusions occur in immunocompromised states, such as HIV, posttransplant immunosuppression, and chemotherapy.[23] Howell-Jolly bodies must be distinguished from pappenheimer bodies. Pappenheimer bodies are similar to red blood cell inclusions in asplenic patients and are basophilic-staining granules composed of iron compounds, such as ferritin aggregates. Pappenheimer bodies are most common in sideroblastic anemia, myelodysplastic syndrome, and sickle cell disease.[24] Howell-Jolly bodies can interfere with accurately calculating reticulocyte counts, which is essential for evaluating bone marrow erythropoiesis and diagnosing hemolytic anemias.[25]
Media
(Click Image to Enlarge)
References
Sears DA, Udden MM. Howell-Jolly bodies: a brief historical review. The American journal of the medical sciences. 2012 May:343(5):407-9. doi: 10.1097/MAJ.0b013e31823020d1. Epub [PubMed PMID: 21946828]
Level 3 (low-level) evidenceOng SY, Ng HJ. Howell-Jolly bodies in systemic amyloidosis. International journal of hematology. 2018 Aug:108(2):119-120. doi: 10.1007/s12185-018-2473-8. Epub 2018 May 22 [PubMed PMID: 29790005]
William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology (Amsterdam, Netherlands). 2007 Feb:12(1):1-13 [PubMed PMID: 17364987]
Johns J, Goel AK, Jondhale S, Kumar Venkatesan D, Ravina M, Shah S, Syal S. Splenic Dysfunction in Children With Sickle Cell Disease: A Single Centre Experience From Central India. Indian pediatrics. 2024 Jun 20:():. pii: S097475591600654. Epub 2024 Jun 20 [PubMed PMID: 38910365]
Wagener MG, Marahrens H, Ganter M. Anaemia in South American camelids - an overview of clinical and laboratory diagnostics. Veterinary research communications. 2024 Apr:48(2):633-647. doi: 10.1007/s11259-023-10274-z. Epub 2023 Dec 5 [PubMed PMID: 38049672]
Level 3 (low-level) evidenceNarvestad-Bøttger H, Winther-Larsen A, Haugbølle Bjerre J, Dziegiel MH, Hansen AT, Hasle H. Extreme Reticulocytosis After Splenectomy in a Patient With Hemoglobin Mizuho. Journal of pediatric hematology/oncology. 2024 Jan 1:46(1):e111-e114. doi: 10.1097/MPH.0000000000002790. Epub 2023 Nov 24 [PubMed PMID: 38011049]
Butel-Simoes GI, Jones P, Wood EM, Spelman D, Woolley IJ, Ojaimi S. Congenital asplenia study: clinical and laboratory characterisation of adults with congenital asplenia. Annals of hematology. 2022 Jul:101(7):1421-1434. doi: 10.1007/s00277-022-04765-3. Epub 2022 Apr 22 [PubMed PMID: 35451619]
Scott-Charlton A, Reynolds G. A case of systemic lupus erythematosus associated auto-splenectomy presenting as invasive pneumococcal sepsis. Modern rheumatology case reports. 2020 Jul:4(2):233-236. doi: 10.1080/24725625.2020.1751407. Epub 2020 May 7 [PubMed PMID: 33087009]
Level 3 (low-level) evidencede Porto AP, Lammers AJ, Bennink RJ, ten Berge IJ, Speelman P, Hoekstra JB. Assessment of splenic function. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010 Dec:29(12):1465-73. doi: 10.1007/s10096-010-1049-1. Epub 2010 Sep 19 [PubMed PMID: 20853172]
Felka T, Lemke J, Lemke C, Michel S, Liehr T, Claussen U. DNA degradation during maturation of erythrocytes - molecular cytogenetic characterization of Howell-Jolly bodies. Cytogenetic and genome research. 2007:119(1-2):2-8 [PubMed PMID: 18160774]
Yan X, Kong J, Wang J, Wang C, Shen H. Severe megaloblastic anemia in a patient with advanced lung adenocarcinoma during treatment with erlotinib: a case report and literature review. BMC pulmonary medicine. 2024 Mar 6:24(1):121. doi: 10.1186/s12890-024-02935-9. Epub 2024 Mar 6 [PubMed PMID: 38448889]
Level 3 (low-level) evidenceHarrod VL, Howard TA, Zimmerman SA, Dertinger SD, Ware RE. Quantitative analysis of Howell-Jolly bodies in children with sickle cell disease. Experimental hematology. 2007 Feb:35(2):179-83 [PubMed PMID: 17258066]
Level 2 (mid-level) evidenceMathew H, Dittus C, Malek A, Negroiu A. Howell-Jolly bodies on peripheral smear leading to the diagnosis of congenital hyposplenism in a patient with septic shock. Clinical case reports. 2015 Aug:3(8):714-7. doi: 10.1002/ccr3.323. Epub 2015 Jul 4 [PubMed PMID: 26331020]
Level 3 (low-level) evidenceHilberg RW, Ringle RD, Balcerzak SP. Howell-Jolly bodies. Intracellular or extracellular? Archives of internal medicine. 1973 Feb:131(2):236-7 [PubMed PMID: 4682982]
JENSEN WN, MORENO GD, BESSIS MC. AN ELECTRON MICROSCOPIC DESCRIPTION OF BASOPHILIC STIPPLING IN RED CELLS. Blood. 1965 Jun:25():933-43 [PubMed PMID: 14294770]
Level 3 (low-level) evidenceRIFKIND RA, DANON D. HEINZ BODY ANEMIA--AN ULTRASTRUCTURAL STUDY. I. HEINZ BODY FORMATION. Blood. 1965 Jun:25():885-96 [PubMed PMID: 14294766]
Kashimura M. The human spleen as the center of the blood defense system. International journal of hematology. 2020 Aug:112(2):147-158. doi: 10.1007/s12185-020-02912-y. Epub 2020 Jun 16 [PubMed PMID: 32557229]
Nardo-Marino A, Braunstein TH, Petersen J, Brewin JN, Mottelson MN, Williams TN, Kurtzhals JAL, Rees DC, Glenthøj A. Automating Pitted Red Blood Cell Counts Using Deep Neural Network Analysis: A New Method for Measuring Splenic Function in Sickle Cell Anaemia. Frontiers in physiology. 2022:13():859906. doi: 10.3389/fphys.2022.859906. Epub 2022 Apr 5 [PubMed PMID: 35480040]
Angay O, Friedrich M, Pinnecker J, Hintzsche H, Stopper H, Hempel K, Heinze KG. Image-based modeling and scoring of Howell-Jolly Bodies in human erythrocytes. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2018 Mar:93(3):305-313. doi: 10.1002/cyto.a.23123. Epub 2017 May 24 [PubMed PMID: 28544333]
Araújo NC, Orlando MMC, Neves MB, Rioja SS, de Lucena SBG, Mandarim-de-Lacerda CA. Howell-Jolly bodies and liver-spleen scanning for assessment of splenic filtrative function yields discordant results in renal transplant recipients. Medicine. 2017 Dec:96(51):e9242. doi: 10.1097/MD.0000000000009242. Epub [PubMed PMID: 29390481]
Pourdieu C, El Hoss S, Le Roux E, Pages J, Koehl B, Missud F, Holvoet L, Ithier G, Benkerrou M, Haouari Z, Da Costa L, El Nemer W, Laurance S, Aronovicz YC, Le Van Kim C, Fenneteau O, Lainey E, Brousse V. Relevance of Howell-Jolly body counts for measuring spleen function in sickle cell disease. American journal of hematology. 2023 May:98(5):E110-E112. doi: 10.1002/ajh.26879. Epub 2023 Feb 27 [PubMed PMID: 36794434]
Oehadian A, Huang I, Kartikasari A, Alisjahbana B, Prihatni D. Howell-Jolly Body-Like Inclusions in Coronavirus Disease 2019 (COVID-19): Possible Novel Findings. Journal of blood medicine. 2023:14():233-238. doi: 10.2147/JBM.S399596. Epub 2023 Mar 29 [PubMed PMID: 37016662]
Sharma P, Nampoothiri RV, Sharma P, Prakash G, Malhotra P, Varma N. Howell-Jolly Body-Like Inclusions in Neutrophils and Monocytes of a Transplant Recipient. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2018 Apr:34(2):381-382. doi: 10.1007/s12288-017-0865-1. Epub 2017 Aug 17 [PubMed PMID: 29622896]
Sears DA, Udden MM. Pappenheimer bodies: a brief historical review. American journal of hematology. 2004 Apr:75(4):249-50 [PubMed PMID: 15054821]
van Berkel M, Besselaar E, Kuijper P, Scharnhorst V. Instrument-dependent interference of Howell-Jolly bodies in reticulocyte enumeration. Clinical chemistry and laboratory medicine. 2013 Jun:51(6):e137-9. doi: 10.1515/cclm-2012-0790. Epub [PubMed PMID: 23314550]
Level 3 (low-level) evidence