Introduction
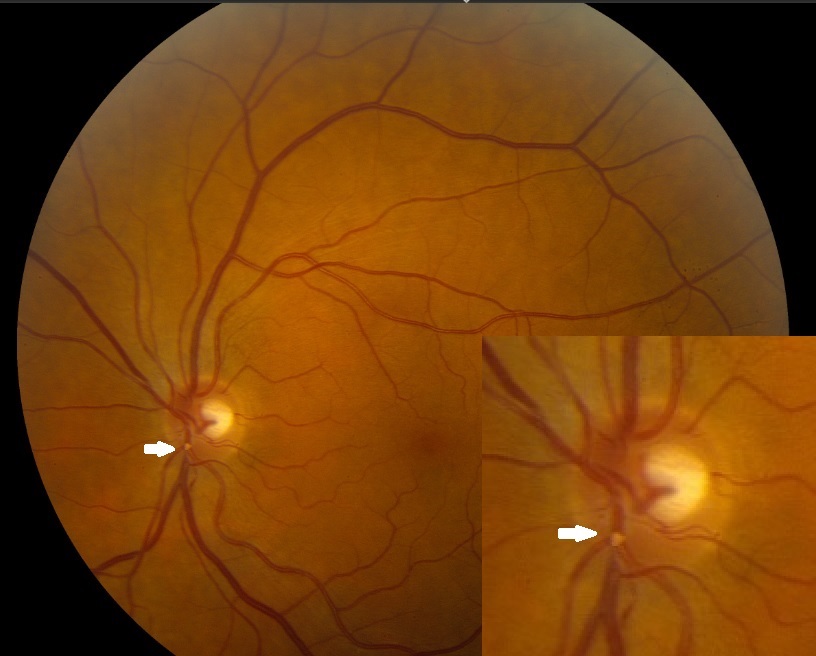
Stroke is the third most common cause of death in the United States. Eighty percent of all strokes are due to vessel occlusion secondary to atherothrombosis or embolus. Hollenhorst plaque (HP) was discovered in 1961 by Dr. Robert Hollenhorst. He defined them as tiny emboli caused by cholesterol plaques and found in the retina's small blood vessels. The appearance of these emboli indicates that they are yellow, refractile, and typically located at an arterial bifurcation.[1][2] Hollenhorst plague is the most common cause accounting for the ocular ischemic syndrome (OIS) following retinal artery occlusion (RAO).[3]
History
In 1927, T. Harrison Butler first mentioned a bright retinal embolus involving the inferior temporal arteriole. Hollenhorst, Witmer, and Schmid described this lesion in 1958.[4] Hollenhorst postulated that the cholesterol ester is the fundamental component of the lesion, and he proposed a temporal relationship, foreseeing the risk of subsequent cerebral ischemic events on the side ipsilateral to symptomatic carotid disease. He also confirmed that this plague was mobile upon manual ocular pressure.[4] In 1961, Hollenhorst detailed the characteristics of these vivid, orange-appearing plaques in his paper titled "Significance of Bright Plaques in Retinal Arterioles." These plaques were identified in 11% of cohorts with carotid disease and 4% with vertebrobasilar diseases. The plaques rarely obstruct arterioles, instead frequently migrating to distal vessels and ultimately dissipating through fragmentation.[4] Hollenhorst and Jack Whisnant reproduced retinal plaques mirroring those observed in humans through the injection of cholesterol crystals and human atheromatous material into the carotids of experimental animals. This was validated in 1963 from an autopsy of a patient who had similar appearing retinal plaques and who had died following carotid endarterectomy (CEA).[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
HPs tend to originate from carotid arteries or the aorta. This finding is consistent with carotid disease originating from atherosclerotic lesions. Upon discovering an HP, eye care professionals initially assumed that it originated from the stenosed, ipsilateral internal carotid artery (ICA). The direct anatomical route between the ICA and the central retinal artery (CRA) supports this assumption. The ophthalmic artery is the first branch of the ICA, which then leads into the CRA.[5][6]
Epidemiology
The Blue Mountains Eye study stated that the prevalence of retinal emboli is 1.4% in the general population older than 49 years.[7] The prevalence increases with age. Retinal emboli are significantly more prevalent in men than in women. HPs make up 80% of retinal emboli. An estimated 10% of carotid emboli reach the retinal arteries.[8]
In the Beaver Dam Eye Study, comprising almost 5000 patients, the prevalence of HPs was 1.3%, and the 5-year incidence was 0.9%.[9] In a study of 130 consecutive patients with a diagnosis of HP alongside central or branch RAO, the mean age of the patients was 68 ± 16 years, and the incidence of symptomatic patients was 61%.[10]
The incidence of asymptomatic retinal emboli is 1.4%. Cholesterol emboli are more prevalent in men than women (2.2% vs 0.8%).[11][12] Eighty percent of all retinal emboli are of the cholesterol type.[4][11] Significant links with hypertension (odds ratio [OR], -2.2), vascular disease (OR, -2.4), past vascular surgery (OR, -3.5), and current smoking history (OR, -2.2) or any smoking history (OR, -2.6) have been observed.[11]
Pathophysiology
Ulcerated plaques just distal to the bifurcation of the common carotid artery into its external and internal branches may be a source of retinal emboli that can be asymptomatic or produce transient monocular blindness.[13][14] Cholesterol plaques are seen in the retina owing to their relatively small size and decreased velocity while traveling through the internal carotid system.
History and Physical
HP is a marker of past embolic events but is a poor predictor of future events. These plaques may or may not cause an RAO. The discovery of asymptomatic emboli has a greater concern for a patient’s systemic health than visual health. Most HPs are discovered incidentally during funduscopy. They often dislodge and are not noted on subsequent fundus examinations.
An HP is a clinical sign, commonly a contributing factor in diagnosing RAO. The plaque must completely obstruct the vessel for an HP to cause an RAO. RAOs can occur in the central retinal artery (CRAO) or one of its branches (BRAO). HP is a possible cause of a CRAO/BRAO and should not be considered synonymous.
As noted in the differential diagnosis, other types of emboli can occlude the relevant vessels. There are also nonembolic causes of RAO, including nocturnal arterial hypotension and transient vasospasm. HP, like other types of retinal emboli, may not stay in 1 place. The plaque may dislodge and move to a smaller diameter vessel before it gets lodged again, or the plaque may dissolve completely. The presence of an HP is a confirming diagnosis; however, the absence of a plaque does not rule out the possibility of embolic occlusion.
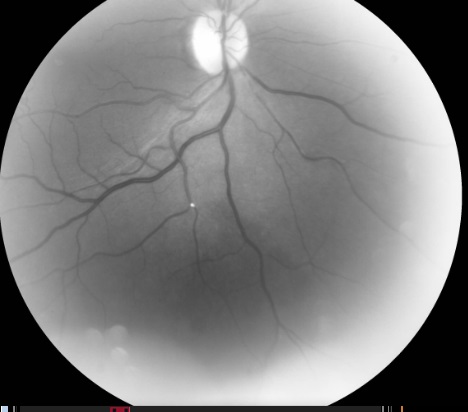
If an RAO occurs, the most common symptom is sudden, painless vision loss. The fundus will display typical ischemic signs, such as retinal whitening around the occluded vessel (see Images. Hollenhorst Plaques). The macular area will remain “cherry-red” due to its secondary outer retinal blood supply. If the plaque only partially occludes the vessel, blood can still flow through the lumen, and no damage occurs. A single eye can undergo more than 1 transient RAO.
Evaluation
Diagnostic tools which may be employed in the evaluation of HPs include the following:
Auscultation of the ipsilateral carotid for the presence of a bruit.
Blood pressure measurement to rule out hypertension or hypotension.
Electrocardiogram (ECG) for atrial fibrillation (AF).
Carotid duplex study- A significant stenosis is characterized by a diameter reduction of 80% to 99%, a peak systolic velocity of >125cm/sec, and an end-diastolic velocity of>140cm/sec alongside extensive spectral broadening. Carotid bifurcation stenosis was <30% in 68% of the patients, between 30% and 60% in 22% of the patients, and >60% in only 8% of the patients.[10] Significant carotid internal artery stenosis was detected in 7% to 20% of patients with asymptomatic retinal emboli and 20% to 25% of patients with BRAOs.[15]
Echocardiography (ECHO) study for determining the cardio-embolic source.
CT or MRI 4-vessel carotid angiography to evaluate for stenosis, dissection, and/or dysplasia. CT angiography has a sensitivity of 85% and a specificity of 93%.[16]
Blood test for ruling out any hypercoagulopathy.
Fluorescein angiography shows delayed or absent fill in the retinal artery (most specific sign), prolonged arteriovenous transit time (most sensitive sign), and capillary nonperfusion (loss of endothelial cells, pericytes, and lumen obliteration). Staining of retinal vessels (endothelial cell damage due to chronic ischemia), macular edema, and hyperfluorescence within the optic disk in OIS are also observed, caused by leakage from the disk and capillaries.[3][17] Indocyanine green angiography shows prolonged arm-to-choroid and intrachoroidal circulation times and slow filling of the watershed zones.[3]
Visual-evoked potentials (VEP), electroretinography (ERG), and ophthalmo-tonometry are seldom used.[3] Electroretinography shows reduced amplitudes of both the a- and b-waves.[18]
Optical coherence tomography (OCT) shows thick, hyperreflective inner retinal layers with blocked outer retina reflectivity. Eventually, the retina is thinned out and appears atrophic. This also demonstrates a reduction in the foveal avascular zone and improved vessel density in all retinal layers after carotid artery stenting for OIS.[19]
B-scan ultrasonography or orbital computed tomography (CT) scan to rule out compressive lesions.
Treatment / Management
Immediate ocular massage may help dislodge the emboli.[17] Irreversible retinal damage occurs within 240 minutes of central RAO.
Surgical embolus removal was first attempted in 1990 by Peyman and Gremillion. This was achieved in 87.5% with reperfusion and improved visual acuity in 4 of 6 patients in 1 study.[17]
The treatment decision for carotid stenosis is primarily based on the degree of stenosis and associated symptomatology.
For patients with <50% stenosis - antiplatelet therapy is advocated in the form of:
- Cyclooxygenase-2 (COX-2) inhibitor- Aspirin
- Adenosine diphosphate (ADP) receptor inhibitor- Clopidogrel and
- ADP reuptake inhibitor- Dipyridamole
This minimizes the risk of a 5-year stroke rate by almost 50%. Subgroup analysis in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial revealed that atorvastatin reduced the risk of both cerebrovascular and cardiovascular adverse events.[20]
For patients with >70% stenosis - are considered for surgical interventions in the form of either CEA or carotid angioplasty and stenting (CAS). Both are safe and effective, with increased odds of myocardial infarction following CEA and an increased risk of stroke following CAS. They also have a comparable risk for fatal or disabling stroke.[21] Evidence is poor for purely ocular transient ischemic attacks (TIAs).(A1)
North American Symptomatic Carotid Endarterectomy Trial (NASCET) showed that surgical intervention was markedly effective with cohorts with 70% to 99% stenosis.[22] This significantly reduced the 2-year risk of ipsilateral stroke rate compared to medical management alone (9% vs 26%) among symptomatic cohorts presenting with transient monocular visual loss, transient ischemic attack, or nondisabling strokes. Comparable findings were observed among symptomatic patients within the European Carotid Surgical Trial.[23] A meta-analysis comprising 5223 patients with asymptomatic moderate to severe stenosis and low perioperative risks favored CEA (relative risk, 0.69). CAS is also comparable to endarterectomy in terms of risk of procedural stroke (2.9 vs 1.7%) and survival (87.1% vs 89.4%). The 5-year stroke-free survival was similar (93.1% vs 94.7%).[24](A1)
For patients with 50% to 69% stenosis - there was a considerable drop in the benefits following surgery.
The Asymptomatic Carotid Atherosclerosis Study and Asymptomatic Carotid Surgery Trial showed a risk reduction of 53% and 46% among cohorts with 60% stenosis with CEA.[25] Guidelines recommend CEA when the risk of perioperative stroke, myocardial infarction, or mortality is considerably low.(A1)
No ocular treatment is necessary unless an HP completely obstructs a vessel, causing an RAO. All patients with retinal emboli should be referred to the patient’s primary care provider for a bilateral carotid duplex study. The management considers the following:
- Patient education on HA as primarily an underlying etiology
- Lifestyle modifications and combating risk variables (diabetes, hypertension, hyperlipidemia, sedentary lifestyles, obesity, cigarette smoking) and
- Aspirin for arteriosclerosis
Differential Diagnosis
Types of Retinal Emboli:
- Calcific emboli- appear whitish, involve the central retinal artery, do not dissolve, and originate from cardiac valves or aorta calcification.
- Fibrinoplatelet emboli- appear dull white within the retinal arteriole and arise from carotid thrombus.
- Talc emboli- observed in patients with an intravenous drug and/or cocaine addiction, are relatively small and appear parafoveally.[26]
- Metastatic tumor cells
- Septic emboli- following bacterial endocarditis
Prognosis
The atheromatous disease of the ICA can be associated with HPs and is usually an indicator of potential stroke. Carotid stenosis increases stroke risk by 1.18 times for every 10% increase in stenosis. This risk of stroke rises <1% per year for a vessel that is <80% stenosed. On the contrary, a vessel >90% stenosed raises the stroke risk by 4.8% per year.
Almost 75% of HPs are asymptomatic. Asymptomatic HP, however, is a poor predictor of future embolic events.[12] However, 25% of these cohorts have carotid stenosis of >40%.[4] Symptomatic patients were more likely to harbor carotid stenosis >69% when compared with asymptomatic patients (25% vs 9.2%).[12]
Patients with retinal cholesterol plaques had a higher incidence of stroke compared to their healthy counterparts (8.5% vs 0.8%).[12] Cholesterol emboli have a 15% risk of mortality at 1 year, 29% at 3 years, and 54% at 7 years.[12] Hollenhorst observed that survival reduced by almost 15% and 40% at 1 and 8 years, respectively, in patients with retinal cholesterol embolism.[4] These patients have increased odds of stroke-related deaths (hazard ratio, 2.61).[9] The cumulative mortality was 56% with retinal emboli and 30% without retinal emboli.[4] The risk of nonfatal myocardial infarction or vascular death was 7.7% (4.9% among controls).[30]
Asymptomatic retinal cholesterol embolism is a risk variable for cerebral infarction.[30] In a meta-analysis of 1343 patients with asymptomatic cholesterol emboli, 17.8% had a history of either cerebrovascular accident (CVA) or TIAs at presentation. Twelve percent of these patients evolved to stroke, TIAs, or death during their follow-up.[31] The evidence suggests that these patients warrant referral for medical optimization of pertinent cardiovascular risk factors.
Currently, no recommendation exists to support CEA in patients with HPs or retinal emboli alone.[31] In a study with 159 patients with CRAO, 284 with BRAO, and 85 with HP, mortality and cerebrovascular events were statistically significantly higher in only the CRAO and BRAO groups.
Complications
Complications of HPs include the following:
- Central retinal artery occlusion (CRAO)
- Branch retinal artery occlusion (BRAO)
- Ischemic strokes [32]
Deterrence and Patient Education
Deterrence and patient education play pivotal roles in addressing the implications of HPs and promoting vascular health. Clinicians engaging in deterrence strategies focus on identifying and managing risk factors associated with plaque formation, such as hypertension and atherosclerosis. By emphasizing lifestyle modifications, such as maintaining a heart-healthy diet, regular exercise, and smoking cessation, healthcare providers contribute to preventing HPs and related vascular issues.
Concurrently, patient education is a cornerstone in empowering individuals with knowledge about the significance of these cholesterol emboli, potential risks, and available preventive measures. Educating patients on recognizing early symptoms, the importance of regular eye examinations, and adherence to prescribed medications fosters proactive involvement in their own healthcare, facilitating early detection and intervention to mitigate the impact of HPs on both ocular and systemic health. Together, deterrence and patient education create a foundation for informed decision-making and collaborative efforts in preserving overall vascular well-being.
Pearls and Other Issues
HPs are yellow and refractile, typically located at the carotid artery bifurcation. They tend to originate from carotid arteries or the aorta secondary to atherosclerotic lesions. The salient features include the following:
- HPs are considered to be the most common form of emboli.
- HPs are a common finding in the aging population.
- Approximately 75% of HPs seen in ophthalmic practice are asymptomatic.
Enhancing Healthcare Team Outcomes
Even though there are several other causes of HPs, the most problematic diagnosis is atherosclerosis of the ICA. Often, the presence of an HP indicates an impending stroke, especially in older individuals. Once an HP has been diagnosed, the management generally involves a neurologist, ophthalmologist, cardiologist, vascular surgeon, interventional radiologist, nurse, and pharmacist, functioning as a cohesive interprofessional team.
Healthcare professionals managing patients with HPs need refined clinical skills for accurate diagnosis, including proficiency in ophthalmic examinations and the interpretation of retinal findings. Advanced practitioners, such as nurse practitioners and physician assistants, may play a crucial role in gathering comprehensive patient histories and conducting initial assessments.
The patient should undergo a duplex ultrasound of the neck to determine the presence of atherosclerotic disease at the carotid bifurcation. If the lesion is ulcerated and has >70% stenosis, the patient should be referred to a vascular surgeon or an interventional radiologist for either CEA or CAS. The patient should be encouraged to lower his blood pressure and cholesterol, discontinue smoking, and take aspirin while awaiting surgery. The nurse should educate the patient on possible stroke symptoms and when to return to the emergency room. When patients with carotid artery atherosclerosis are managed with elective surgery or stenting, the morbidity and mortality rates are <3%.[6][10] This type of interprofessional collaboration will foster improved results.
For patients with symptomatic HP, risk factors and cost-utility analyses are justified.[9] Despite asymptomatic emboli, a medical referral is beneficial. HP with concurrent venous stasis retinopathy confers more conclusive evidence for the same.[33] Positive predictive values of carotid stenosis for the ocular signs/symptoms of amaurosis fugax (18.2%), HPs (20.0%), and venous stasis retinopathy (20.0%) have been observed. Patients with carotid duplex showing ulcerated atheromatous plagues causing >70% stenosis should be referred for either CEA or CAS.
Developing a cohesive strategy for managing patients with HPs involves aligning diagnostic and treatment approaches among the healthcare team. Physicians, in collaboration with pharmacists, can strategize effective medication regimens for managing underlying vascular conditions contributing to plaque formation. Advanced care practitioners may contribute to the development and implementation of patient care plans, ensuring continuity and consistency.
Effective communication among healthcare professionals is essential for delivering patient-centered care. Team members must share relevant information, discuss treatment plans, and collaborate on interventions. Clear and open communication ensures that all team members are aligned in their understanding of the patient's condition and goals of care. A coordinated approach ensures that patients with HPs receive holistic and timely care across different healthcare settings.
A multidisciplinary approach involving physicians, advanced care practitioners, nurses, pharmacists, and other health professionals is crucial for enhancing patient-centered care, improving outcomes, ensuring patient safety, and optimizing team performance in managing patients with HPs. Effective communication and coordinated interprofessional team efforts contribute to a comprehensive, patient-focused care strategy.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Majstruk L, Giocanti-Aurégan A. [Multimodal imaging of a Hollenhorst plaque]. Journal francais d'ophtalmologie. 2016 Sep:39(7):655-6. doi: 10.1016/j.jfo.2016.07.004. Epub 2016 Aug 27 [PubMed PMID: 27575573]
Coca MN, Palau AE, Morgan ML, Lee AG. Embolic anterior ischemic optic neuropathy associated with a Hollenhorst plaque. JAMA ophthalmology. 2015 Feb:133(2):e143264. doi: 10.1001/jamaophthalmol.2014.3264. Epub 2015 Feb 12 [PubMed PMID: 25674893]
Level 3 (low-level) evidenceTerelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome - a systematic review. Medical science monitor : international medical journal of experimental and clinical research. 2012 Aug:18(8):RA138-144 [PubMed PMID: 22847215]
Level 3 (low-level) evidenceGraff-Radford J, Boes CJ, Brown RD Jr. History of Hollenhorst plaques. Stroke. 2015 Apr:46(4):e82-4. doi: 10.1161/STROKEAHA.114.007771. Epub 2015 Jan 15 [PubMed PMID: 25593136]
Lawlor M. Bakri et al.: is carotid ultrasound necessary in the evaluation of the asymptomatic Hollenhorst plaque? (Ophthalmology 2013;120:2747-8). Ophthalmology. 2014 Sep:121(9):e49-50. doi: 10.1016/j.ophtha.2014.04.032. Epub 2014 Jun 3 [PubMed PMID: 24907057]
Level 3 (low-level) evidenceBakri SJ, Luqman A, Pathik B, Chandrasekaran K. Is carotid ultrasound necessary in the evaluation of the asymptomatic Hollenhorst plaque? Ophthalmology. 2013 Dec:120(12):2747-2748.e1. doi: 10.1016/j.ophtha.2013.09.005. Epub [PubMed PMID: 24246827]
Level 2 (mid-level) evidenceJoachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The Incidence and Progression of Age-Related Macular Degeneration over 15 Years: The Blue Mountains Eye Study. Ophthalmology. 2015 Dec:122(12):2482-9. doi: 10.1016/j.ophtha.2015.08.002. Epub 2015 Sep 14 [PubMed PMID: 26383995]
Tokoyoda T, Tsujimoto I, Sugiura Y, Sezaki R. A hollenhorst plaque in cholesterol crystal embolism. Internal medicine (Tokyo, Japan). 2012:51(2):223 [PubMed PMID: 22246495]
Level 3 (low-level) evidenceKlein R, Klein BE, Jensen SC, Moss SE, Meuer SM. Retinal emboli and stroke: the Beaver Dam Eye Study. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Aug:117(8):1063-8 [PubMed PMID: 10448750]
Dunlap AB, Kosmorsky GS, Kashyap VS. The fate of patients with retinal artery occlusion and Hollenhorst plaque. Journal of vascular surgery. 2007 Dec:46(6):1125-9 [PubMed PMID: 17950567]
Level 2 (mid-level) evidenceMitchell P, Wang JJ, Li W, Leeder SR, Smith W. Prevalence of asymptomatic retinal emboli in an Australian urban community. Stroke. 1997 Jan:28(1):63-6 [PubMed PMID: 8996490]
Bakri SJ, Luqman A, Pathik B, Chandrasekaran K. Is carotid ultrasound necessary in the clinical evaluation of the asymptomatic Hollenhorst plaque? (An American Ophthalmological Society thesis). Transactions of the American Ophthalmological Society. 2013 Sep:111():17-23 [PubMed PMID: 24072943]
Level 2 (mid-level) evidenceSaric M, Kronzon I. Cholesterol embolization syndrome. Current opinion in cardiology. 2011 Nov:26(6):472-9. doi: 10.1097/HCO.0b013e32834b7fdd. Epub [PubMed PMID: 21993354]
Level 3 (low-level) evidenceWolintz RJ. Carotid endarterectomy for ophthalmic manifestations: is it ever indicated? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2005 Dec:25(4):299-302 [PubMed PMID: 16340498]
Kassotis A, Sharma T. Migrating Emboli in Branch Retinal Artery Occlusion. The New England journal of medicine. 2023 Sep 21:389(12):e24. doi: 10.1056/NEJMicm2303399. Epub 2023 Sep 16 [PubMed PMID: 37721379]
Filatov V, Tom D, Alexandrakis G, Skolik SA, Klassen H, Liggett PE. Branch retinal artery occlusion associated with directional coronary atherectomy after percutaneous transluminal coronary angioplasty. American journal of ophthalmology. 1995 Sep:120(3):391-3 [PubMed PMID: 7661214]
García-Arumí J, Martinez-Castillo V, Boixadera A, Fonollosa A, Corcostegui B. Surgical embolus removal in retinal artery occlusion. The British journal of ophthalmology. 2006 Oct:90(10):1252-5 [PubMed PMID: 16854826]
Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. International ophthalmology. 1988 Feb:11(4):239-51 [PubMed PMID: 3182177]
Saito K, Akiyama H, Mukai R. ALTERATION OF OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN A PATIENT WITH OCULAR ISCHEMIC SYNDROME. Retinal cases & brief reports. 2021 Sep 1:15(5):588-592. doi: 10.1097/ICB.0000000000000857. Epub [PubMed PMID: 30730456]
Level 3 (low-level) evidenceHuisa BN, Stemer AB, Zivin JA. Atorvastatin in stroke: a review of SPARCL and subgroup analysis. Vascular health and risk management. 2010 Apr 15:6():229-36 [PubMed PMID: 20407630]
Halliday A, Bulbulia R, Bonati LH, Chester J, Cradduck-Bamford A, Peto R, Pan H, ACST-2 Collaborative Group. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet (London, England). 2021 Sep 18:398(10305):1065-1073. doi: 10.1016/S0140-6736(21)01910-3. Epub 2021 Aug 29 [PubMed PMID: 34469763]
Level 1 (high-level) evidenceFerguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ. The North American Symptomatic Carotid Endarterectomy Trial : surgical results in 1415 patients. Stroke. 1999 Sep:30(9):1751-8 [PubMed PMID: 10471419]
Level 1 (high-level) evidence. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet (London, England). 1998 May 9:351(9113):1379-87 [PubMed PMID: 9593407]
Level 1 (high-level) evidenceRosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W, ACT I Investigators. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. The New England journal of medicine. 2016 Mar 17:374(11):1011-20. doi: 10.1056/NEJMoa1515706. Epub 2016 Feb 17 [PubMed PMID: 26886419]
Level 1 (high-level) evidence. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995 May 10:273(18):1421-8 [PubMed PMID: 7723155]
Level 1 (high-level) evidenceLuong PM, Tsui E, Batra NN, Zegans ME. Endogenous endophthalmitis and other ocular manifestations of injection drug use. Current opinion in ophthalmology. 2019 Nov:30(6):506-512. doi: 10.1097/ICU.0000000000000606. Epub [PubMed PMID: 31589187]
Level 3 (low-level) evidenceLópez-Herrero F, Muñoz-Morales A, Rueda-Rueda T, Sánchez-Vicente JL. Bilateral macular structural analysis after fat embolism syndrome. Archivos de la Sociedad Espanola de Oftalmologia. 2019 Dec:94(12):e93-e94. doi: 10.1016/j.oftal.2019.05.011. Epub 2019 Jun 3 [PubMed PMID: 31171386]
Young BK, Florine Magdelijns P, Chervenak JL, Chan M. Amniotic fluid embolism: a reappraisal. Journal of perinatal medicine. 2024 Feb 26:52(2):126-135. doi: 10.1515/jpm-2023-0365. Epub 2023 Dec 13 [PubMed PMID: 38082418]
Bradley LM, McDonald AG, Lantz PE. Fatal systemic (paradoxical) air embolism diagnosed by postmortem funduscopy. Journal of forensic sciences. 2021 Sep:66(5):2029-2034. doi: 10.1111/1556-4029.14781. Epub 2021 Jun 15 [PubMed PMID: 34132391]
Bruno A, Jones WL, Austin JK, Carter S, Qualls C. Vascular outcome in men with asymptomatic retinal cholesterol emboli. A cohort study. Annals of internal medicine. 1995 Feb 15:122(4):249-53 [PubMed PMID: 7825759]
Level 2 (mid-level) evidenceGhoneim BM, Westby D, Elsharkawi M, Said M, Walsh SR. Systematic review of the relationship between Hollenhorst plaques and cerebrovascular events. Vascular. 2024 Aug:32(4):784-791. doi: 10.1177/17085381231163339. Epub 2023 Mar 13 [PubMed PMID: 36914563]
Level 1 (high-level) evidenceRoskal-Wałek J, Wałek P, Biskup M, Odrobina D, Mackiewicz J, Głuszek S, Wożakowska-Kapłon B. Central and Branch Retinal Artery Occlusion-Do They Harbor the Same Risk of Further Ischemic Events? Journal of clinical medicine. 2021 Jul 13:10(14):. doi: 10.3390/jcm10143093. Epub 2021 Jul 13 [PubMed PMID: 34300257]
McCullough HK, Reinert CG, Hynan LS, Albiston CL, Inman MH, Boyd PI, Welborn MB 3rd, Clagett GP, Modrall JG. Ocular findings as predictors of carotid artery occlusive disease: is carotid imaging justified? Journal of vascular surgery. 2004 Aug:40(2):279-86 [PubMed PMID: 15297821]