Introduction
Histidine is a nutritionally essential amino acid precursor for several hormones (eg, thyrotropin-releasing hormone) and critical metabolites affecting renal function, neurotransmission, gastric secretion, and the immune system. Histidine's unique acid/base properties make it a versatile catalytic residue in many enzymes and for those proteins and enzymes that coordinate metal ions.[1]
Fundamentals
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Fundamentals
Histidine is one of the nine essential amino acids humans must obtain from their diet and is present in most protein-rich foods such as meat, fish, eggs, soy, whole grains, beans, and nuts. Histidine’s imidazole side chain is unique amongst amino acids, giving rise to its aromaticity and amphoteric properties at physiologic pH. This property makes histidine a key catalytic residue in many enzymes.[2][3] Histidine also performs important anti-inflammatory, anti-oxidant, and anti-secretory functions within the body.[4]
Issues of Concern
Appropriate dietary intake of histidine is crucial during development and throughout life. Deficiencies in histidine and genetic defects in histidine metabolism can pose problems across various body systems such as skin, kidney, blood, neuropsychiatric and immune systems. Essential histidine metabolic byproducts are histamine, urocanic acid, and muscle dipeptides such as carnosine and anserine.[4]
As a neurotransmitter, histamine modulates inflammatory response and gastric acid regulation. Urocanic acid (urocanate) is vital to epidermal barrier formation in the skin.[5] Histidine also has links to UV light absorption and immunosuppression. Finally, muscle dipeptides, like carnosine and anserine, act as homeostatic regulators protecting tissues.[6]
Cellular Level
Histidine demonstrates diverse roles in cellular function. In addition to playing a structural and catalytic role in many enzymes, histidine residues can undergo enzyme-catalyzed methylation (using S-adenosyl methionine as the methyl donor), as illustrated by the critical role of 3-methylhistidine in the ATP binding site of actin.[7] Histidine residues are also crucial to maintaining the myelin sheath as they participate in the hydroxylation of the galactosylceramide, which is responsible for the compaction of the myelin.[8]
Histamine is released by mast cells to bind histamine receptors (H1) during allergic reactions. This binding can trigger allergy symptoms like itching, prostaglandin production, smooth muscle contraction, increased vascular permeability, and tachycardia.[9] Histamine is also a paracrine element crucial in gastric acid secretion and regulation. The hormone gastrin is released by food intake to trigger histamine secretion by enterochromaffin-like (ECL) cells. Histamine then binds to H2 receptors on parietal cells, triggering a release of gastric acids by activating a proton pump.[10]
The histidine metabolite carnosine (beta-alanyl-L-histidine) also combats intramuscular acidosis by maintaining intracellular and extracellular buffering in muscle tissue pH.[11]
Molecular Level
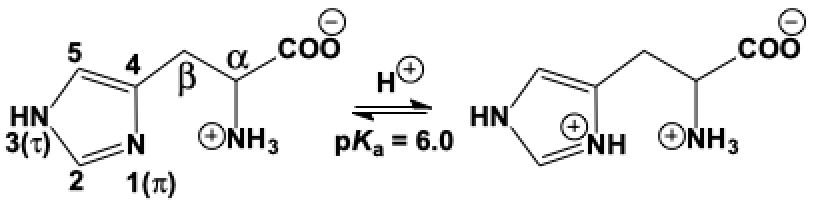
Histidine's importance to the human body derives from the properties determined by its distinctive structure. The molecule's side chain is composed of a heterocyclic imidazole ring that contains nitrogen atoms at positions 1 (pi) and 3 (tau). It is ionizable and exists in neutral and protonated forms in the body, giving histidine a pK one pH-unit below neutrality, allowing it to be both acid and base at physiologic pH. The imidazole ring of histidine is aromatic, which confers stability and makes it apolar at physiologic pH.[2]
Histidine is also a good chelator of metal ions like copper, zinc, manganese, and cobalt.[1] This ability comes from the imidazole nitrogen atoms, which can act as electron donors or acceptors in different cases. The importance of this is exemplified by the consideration of histidine-rich motifs in DNA transcription factors, which participate in the connection of proteins and nucleic acids by Zn-fingers.[12] Zinc is also found coordinated to the active-site imidazole in carbonic anhydrase, where it acts as a Lewis acid.
Mechanism
Though not synthesized in the body, histidine has an extensive metabolic pathway for breakdown and conversion to its various byproducts. The histamine biosynthesis from histidine occurs via a vitamin B-dependent decarboxylation reaction by histidine decarboxylase that occurs in multiple types of cells throughout the body, particularly in the brain and stomach. Histamine synthesis is continuous and, once made, is stored in granules, awaiting activation signals for release.[13] Another major pathway in histidine metabolism involves deamination to produce urocanic acid and ammonia, done by the histidine ammonia lyase (histidase), followed by urocanase.
Histidine is a significant catalytic residue in the enzymes of many classes of biological reactions; its efficiency at shuttling protons greatly enhances catalysis. Histidine is essential in acid-base catalysis due to its amphoteric properties. Another type of reaction catalysis histidine residues participates in are elimination-addition reactions in the body and hemolytic and redox reactions.[14]
Serine esterases, such as trypsin, chymotrypsin, acetylcholinesterase, and the various enzymes in the blood-clotting cascade provide one excellent example of histidine’s involvement as a catalytic residue. In these cases, histidine lies between a catalytic aspartate (which serves as a specific base), pulling a proton from the imidazole group, thereby making the imidazole group a better base to abstract/polarize the hydrogen ion from the active-site serine hydroxyl.
Clinical Significance
Histidinemia
Histidinemia is a metabolic disorder in which a lack of the enzyme histidase causes elevated levels of histidine and its byproducts in the blood and urine and decreased concentrations of urocanic acid in the skin and blood.[15] This condition is inherited as an autosomal recessive disorder with incidence rates similar to phenylketonuria.[16][17] Histidinemia is usually considered a metabolic variant and benign, but it can correlate with neurological deficits and speech delays in sporadic cases. While treatable with a low-histidine diet to prevent the higher levels of histidine and its metabolites, dietary management does not affect the elimination the neurological symptoms.[16]
Chronic Kidney Disease
Chronic kidney disease (CKD) patients tend to have marked changes in their urinary amino acid concentrations. CKD can correlate with low levels of histidine, which contributes to the disruption of histidine metabolism and irregularity in the concentrations of its important byproducts like histamine.[18] Research shows that low levels of plasma histidine are related to oxidative stress, protein-energy wasting, and inflammation.[19]
CKD patients have been shown to have higher levels of plasma histamine, which can damage the glomerular capillaries. This damage affects filtration ability and contributes to abnormal plasma and urinary concentrations of metabolites. Other effects of elevated histamine levels include impairment to renal and arterial endothelium and being associated with pruritus.[18][20]
Anemia
Histidine deficiencies are related to anemia, as oxidative stress plays a role in the etiology of the disease.[21] Histidine is essential for erythropoiesis and globin synthesis.[22] Histidine also works to protect cells already in circulation as its presence reduces the generation of reactive oxidative species that participate indirectly in red blood cell destruction.[21]
A histidine-deficient diet promotes anemia, particularly in CKD patients, but can also be associated with anemia in otherwise healthy subjects.[22] In particular, a lack of folate may play a role in depleting histidine levels through increased urinary output in anemic patients.[23]
Allergic Reactions
Histamine, a significant byproduct of histidine, is found in elevated levels in the tissue and plasma during allergic reactions. Histamine is released from basophils and mast cells and causes an inflammatory response by the immune system, leading to common visible allergic symptoms like itching and swelling, smooth muscle constriction, increased vascular permeability, and mucus secretion. Histamine receptors function in the characteristic mediation of allergic diseases such as urticaria, asthma, and allergic rhinitis, which are treatable with antihistamine drugs.[9]
A significant concern of histamine release is anaphylaxis, which can cause fatal complications. Though histidine contributes to anaphylaxis, it is most effectively treated not by antihistamines but with an injection of epinephrine.[24]
Tourette Syndrome
Tourette syndrome is a childhood-onset disorder caused by genetic and environmental factors. Research has shown that mutations in the histidine decarboxylase gene have high penetrance in patients with Tourette syndrome.[25] Histamine dysregulation and up-regulation of histamine H3 receptors have been found in histidine decarboxylase mutation animal models, suggesting their potential role in tic disorders and related neuropsychiatric conditions. [26][27]
Media
(Click Image to Enlarge)
References
Ingle RA. Histidine biosynthesis. The arabidopsis book. 2011:9():e0141. doi: 10.1199/tab.0141. Epub 2011 Feb 2 [PubMed PMID: 22303266]
Liao SM, Du QS, Meng JZ, Pang ZW, Huang RB. The multiple roles of histidine in protein interactions. Chemistry Central journal. 2013 Mar 1:7(1):44. doi: 10.1186/1752-153X-7-44. Epub 2013 Mar 1 [PubMed PMID: 23452343]
Schmidt AE, Sun MF, Ogawa T, Bajaj SP, Gailani D. Functional role of residue 193 (chymotrypsin numbering) in serine proteases: influence of side chain length and beta-branching on the catalytic activity of blood coagulation factor XIa. Biochemistry. 2008 Feb 5:47(5):1326-35. doi: 10.1021/bi701594j. Epub 2008 Jan 11 [PubMed PMID: 18186617]
Peterson JW, Boldogh I, Popov VL, Saini SS, Chopra AK. Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Laboratory investigation; a journal of technical methods and pathology. 1998 May:78(5):523-34 [PubMed PMID: 9605177]
Level 3 (low-level) evidenceGibbs NK, Tye J, Norval M. Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2008 Jun:7(6):655-67. doi: 10.1039/b717398a. Epub 2008 Mar 13 [PubMed PMID: 18528548]
Level 3 (low-level) evidencePeters V, Klessens CQ, Baelde HJ, Singler B, Veraar KA, Zutinic A, Drozak J, Zschocke J, Schmitt CP, de Heer E. Intrinsic carnosine metabolism in the human kidney. Amino acids. 2015 Dec:47(12):2541-50. doi: 10.1007/s00726-015-2045-7. Epub 2015 Jul 24 [PubMed PMID: 26206726]
Johnson P, Harris CI, Perry SV. 3-methylhistidine in actin and other muscle proteins. The Biochemical journal. 1967 Oct:105(1):361-70 [PubMed PMID: 6056634]
Level 3 (low-level) evidenceZhu G, Koszelak-Rosenblum M, Connelly SM, Dumont ME, Malkowski MG. The Crystal Structure of an Integral Membrane Fatty Acid α-Hydroxylase. The Journal of biological chemistry. 2015 Dec 11:290(50):29820-33. doi: 10.1074/jbc.M115.680124. Epub 2015 Oct 28 [PubMed PMID: 26515067]
White MV. The role of histamine in allergic diseases. The Journal of allergy and clinical immunology. 1990 Oct:86(4 Pt 2):599-605 [PubMed PMID: 1699987]
Schubert ML. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Current opinion in gastroenterology. 2017 Nov:33(6):430-438. doi: 10.1097/MOG.0000000000000392. Epub [PubMed PMID: 28787289]
Level 3 (low-level) evidenceLancha Junior AH, Painelli Vde S, Saunders B, Artioli GG. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports medicine (Auckland, N.Z.). 2015 Nov:45 Suppl 1():S71-81. doi: 10.1007/s40279-015-0397-5. Epub [PubMed PMID: 26553493]
Hecel A, Wątły J, Rowińska-Żyrek M, Świątek-Kozłowska J, Kozłowski H. Histidine tracts in human transcription factors: insight into metal ion coordination ability. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2018 Jan:23(1):81-90. doi: 10.1007/s00775-017-1512-x. Epub 2017 Dec 7 [PubMed PMID: 29218639]
Huang H, Li Y, Liang J, Finkelman FD. Molecular Regulation of Histamine Synthesis. Frontiers in immunology. 2018:9():1392. doi: 10.3389/fimmu.2018.01392. Epub 2018 Jun 20 [PubMed PMID: 29973935]
Holliday GL, Mitchell JB, Thornton JM. Understanding the functional roles of amino acid residues in enzyme catalysis. Journal of molecular biology. 2009 Jul 17:390(3):560-77. doi: 10.1016/j.jmb.2009.05.015. Epub 2009 May 15 [PubMed PMID: 19447117]
Level 3 (low-level) evidenceTaylor RG, Levy HL, McInnes RR. Histidase and histidinemia. Clinical and molecular considerations. Molecular biology & medicine. 1991 Feb:8(1):101-16 [PubMed PMID: 1943682]
Level 3 (low-level) evidenceVirmani K, Widhalm K. Histidinemia: a biochemical variant or a disease? Journal of the American College of Nutrition. 1993 Apr:12(2):115-24 [PubMed PMID: 8463510]
Level 3 (low-level) evidenceLam WK, Cleary MA, Wraith JE, Walter JH. Histidinaemia: a benign metabolic disorder. Archives of disease in childhood. 1996 Apr:74(4):343-6 [PubMed PMID: 8669938]
Zhang ZH, Wei F, Vaziri ND, Cheng XL, Bai X, Lin RC, Zhao YY. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Scientific reports. 2015 Sep 28:5():14472. doi: 10.1038/srep14472. Epub 2015 Sep 28 [PubMed PMID: 26412413]
Watanabe M, Suliman ME, Qureshi AR, Garcia-Lopez E, Bárány P, Heimbürger O, Stenvinkel P, Lindholm B. Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. The American journal of clinical nutrition. 2008 Jun:87(6):1860-6 [PubMed PMID: 18541578]
Gill DS, Fonseca VA, Barradas MA, Balliod R, Moorhead JF, Dandona P. Plasma histamine in patients with chronic renal failure and nephrotic syndrome. Journal of clinical pathology. 1991 Mar:44(3):243-5 [PubMed PMID: 2013627]
Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Current molecular medicine. 2008 Nov:8(7):609-19 [PubMed PMID: 18991647]
Vera-Aviles M, Vantana E, Kardinasari E, Koh NL, Latunde-Dada GO. Protective Role of Histidine Supplementation Against Oxidative Stress Damage in the Management of Anemia of Chronic Kidney Disease. Pharmaceuticals (Basel, Switzerland). 2018 Oct 21:11(4):. doi: 10.3390/ph11040111. Epub 2018 Oct 21 [PubMed PMID: 30347874]
Cooperman JM, Lopez R. The role of histidine in the anemia of folate deficiency. Experimental biology and medicine (Maywood, N.J.). 2002 Dec:227(11):998-1000 [PubMed PMID: 12486209]
Level 2 (mid-level) evidenceFineman SM. Optimal treatment of anaphylaxis: antihistamines versus epinephrine. Postgraduate medicine. 2014 Jul:126(4):73-81. doi: 10.3810/pgm.2014.07.2785. Epub [PubMed PMID: 25141245]
Xu L, Zhang C, Zhong M, Che F, Guan C, Zheng X, Liu S. Role of histidine decarboxylase gene in the pathogenesis of Tourette syndrome. Brain and behavior. 2022 Mar:12(3):e2511. doi: 10.1002/brb3.2511. Epub 2022 Feb 3 [PubMed PMID: 35114079]
Pittenger C. Histidine Decarboxylase Knockout Mice as a Model of the Pathophysiology of Tourette Syndrome and Related Conditions. Handbook of experimental pharmacology. 2017:241():189-215. doi: 10.1007/164_2016_127. Epub [PubMed PMID: 28233179]
Pittenger C. The histidine decarboxylase model of tic pathophysiology: a new focus on the histamine H(3) receptor. British journal of pharmacology. 2020 Feb:177(3):570-579. doi: 10.1111/bph.14606. Epub 2019 Mar 27 [PubMed PMID: 30714121]