Introduction
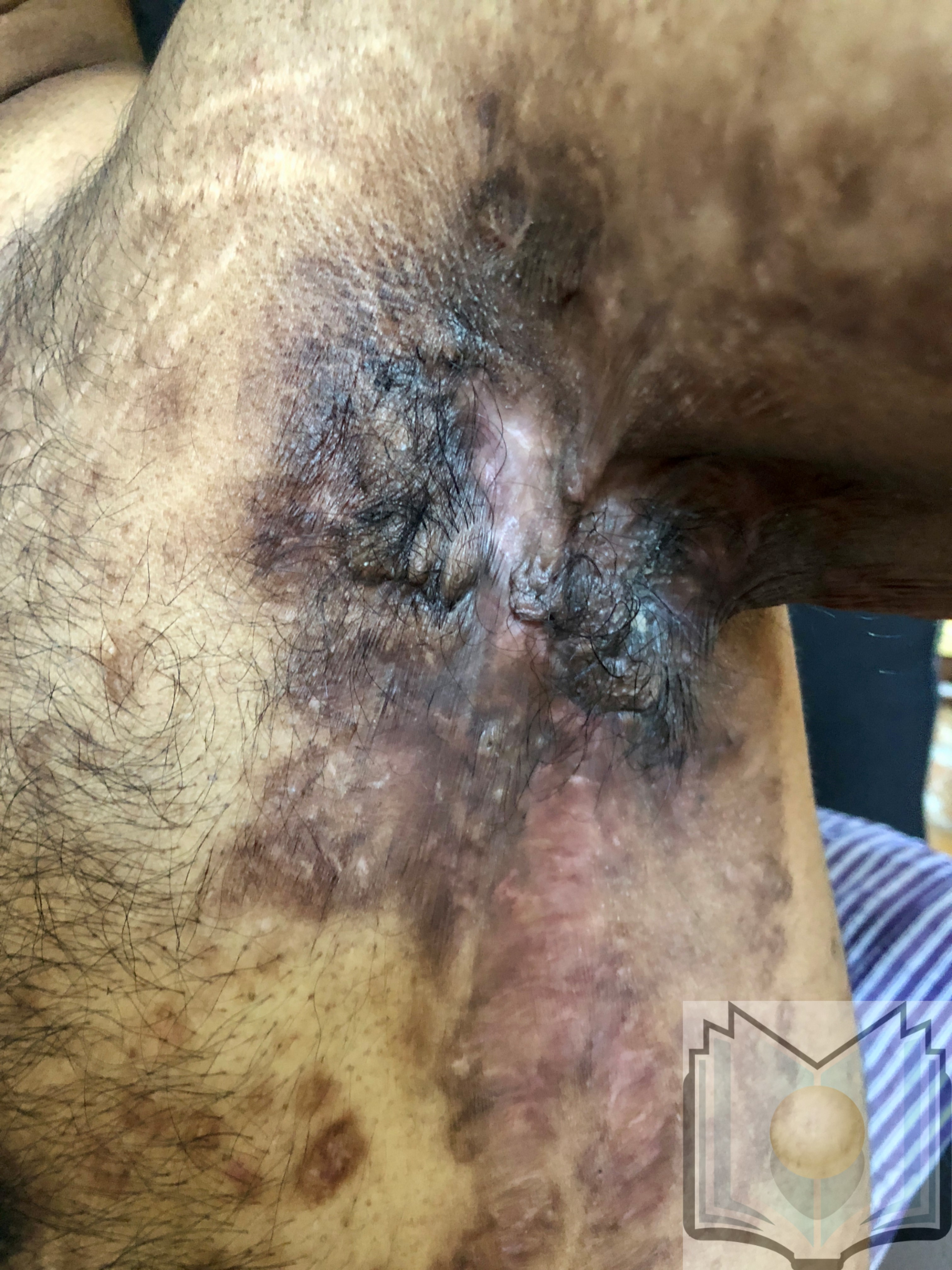
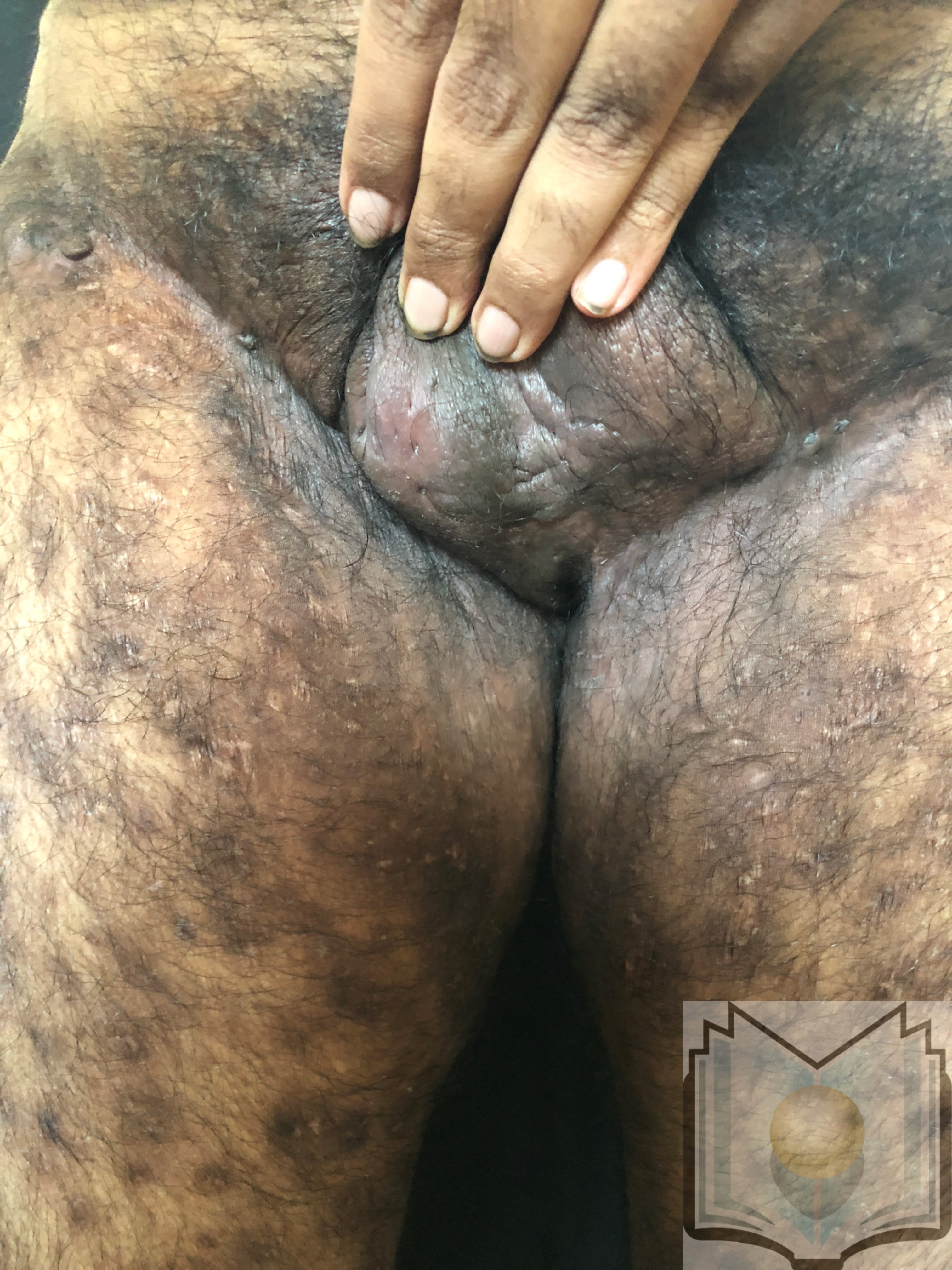
Hidradenitis suppurativa (acne inversa or Verneuil disease) is a chronic inflammatory skin condition of follicular occlusion with lesions including deep-seated nodules and abscesses, draining skin tunnels (also called sinus tracts or fistulae), and fibrotic scars.[1][2] These lesions most commonly occur in intertriginous areas, though they may present in any location with folliculopilosebaceous units. The most commonly affected areas are the axillary, groin, perianal, perineal, and inframammary regions (see Image. Hidradenitis Suppurativa, Left Axilla).[3][4]
Treatment varies based on severity and may include topical and systemic antibiotics, hormone therapy, immune modulators, and surgery.[5] This condition may have a negative psychosocial impact because of the associated pain, involvement of sensitive locations, drainage, odor, disfigurement, and scarring. Late diagnosis only worsens the patient experience.[6][7]
Skin Histology
Skin appendages include the pilosebaceous units, sweat glands, and nails, which possess distinct functions and histological features. The pilosebaceous unit is the assembly formed by the hair shaft, follicle, sebaceous gland, and arrector pili muscle. This group of skin appendages develops from the epidermis during fetal development. The hair shaft is visible on the skin's surface and is comprised of cuticle cells surrounding a cortex, with a medulla present in thicker hair. The hair follicle is essential for hair growth and is histologically divided into the infundibulum, isthmus, and inferior segment, with stem cells residing in the bulge area. Sebaceous glands are pilosebaceous unit components that produce sebum to lubricate hair. These glands have a foamy appearance microscopically due to their lipid content.
Sweat glands (sudoriferous glands) are categorized as eccrine or apocrine, which differ in their distribution and secretion mode. Eccrine glands are present in the dermis and upper hypodermis throughout the body and exhibit merocrine secretion, where the cytoplasm remains intact during secretion. Apocrine glands are found in the dermis and subcutanous fat in areas like the intertriginous zones. These glands lose the top portions of the cellular cytoplasm during secretion. Histologically, eccrine glands consist of coiled secretory segments surrounded by myoepithelial cells, while apocrine glands have less coiling and larger lumens lined by cuboidal epithelial cells.
Nails consist of the nail plate, matrix, eponychium, hyponychium, nail folds, and lunula. The matrix serves as the site of nail formation. The nail plate is composed of compact keratinocytes and lacks desquamation, contributing to its rigidity and translucency. The hyponychium lies beneath the nail plate, lacks a granular layer, and contains a rich vascular network. The eponychium serves as a pathogen barrier.[8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The development of hidradenitis suppurativa appears to have genetic, environmental, and behavioral influences.[9] Approximately 33% to 40% of individuals with hidradenitis suppurativa report an affected first-degree relative, suggesting a hereditary component with an autosomal dominant transmission pattern.[10] Researchers have identified a loss-of-function mutation of the γ-secretase complex involved in the Notch signaling pathway in a small subset of affected families.[11] Other mutations identified in hidradenitis suppurativa include DCD (encoding dermcidin), PSTPIP1 (found in hidradenitis suppurativa syndromes), SOX9, and KLF5 genes.[12][13][14]
Environmental and behavioral factors are vital in hidradenitis suppurativa's development. Individuals with this condition often have comorbidities such as obesity, leading to increased skin friction, sweat production and retention, and hormonal changes, particularly androgen excess.[15][16] Metabolic syndrome, more prevalent in obesity, is also observed in hidradenitis suppurativa cases.[17] Smoking may exacerbate the condition, with nicotine implicated in increasing follicular plugging.[18][19] As with obesity, disease progression and severity are worse in people who smoke.[20]
Hormonal influences, signified by a higher female preponderance, menstrual cycle-related symptom fluctuations, exogenous hormone intake, and pubertal and menopausal links, have been observed.[21][22] Other potential factors include medications, bacteria, and mechanical friction.[23]
Epidemiology
The estimated prevalence of hidradenitis suppurativa ranges from under 1% to 4%.[24] These numbers are likely underestimated because of underreporting and misdiagnosis. The condition's onset is commonly between puberty and 40 years, most frequently from ages 21 to 29. Women are about 3 times more affected than men.[25] Racial or ethnic predilection is currently unsupported, though the disease may be underreported, and studies have likely excluded black and Hispanic patients.[26][27]
Pathophysiology
Hidradenitis suppurativa's pathophysiologic mechanism starts with a defective hair follicle's occlusion and rupture, releasing keratin and bacteria into the surrounding dermis. This occlusion leads to keratinocyte proliferation and an immune response.[28][29] Neutrophilic and lymphocytic proliferation and heightened activity result in abscess formation and follicular destruction, with the involvement of adjacent tissues.[30] Additional contributors may include abnormal antimicrobial peptides, epidermal invaginations forming tracts, hormonal effects on follicular receptors, and complement pathways. Sebaceous glands also become deficient.[31][32]
Immunological abnormalities are evident in hidradenitis suppurativa. Inflammatory cytokines like tumor necrosis factor-α and interleukins are elevated in lesions, offering potential treatment targets.[33][34][35] While bacteria are not considered the primary cause, these organisms may trigger an inflammatory response. Patients may receive antibiotics for presumed infections, potentially leading to drug-resistant skin flora.[36] Aspirates from unruptured lesions typically yield sterile cultures. Bacterial infection and colonization may worsen hidradenitis suppurativa.
Histopathology
Hidradenitis suppurativa may be diagnosed clinically, but a skin biopsy can help confirm the diagnosis in equivocal cases. Hidradenitis suppurativa's histopathological features include:
- Follicular occlusion
- Follicular hyperkeratosis
- Infundibular follicular epithelial hyperplasia
- Epidermal psoriasiform hyperplasia
- Keratin plugging
- Perifolliculitis (predominantly lymphocytic)
- Plasmocytic infiltrate
- Pseudoepitheliomatous hyperplasia
Reports on the frequency of finding foreign body and epithelioid granulomas are conflicting, with some reporting them as frequent and others as infrequent. Eosinophils may be observed, but this finding is controversial, as studies report eosinophilic presence inconsistently.[37]
History and Physical
Hidradenitis suppurativa diagnosis is often delayed by an average of 7 years due to its resemblance to other conditions. Clinical diagnosis involves recognizing characteristic morphological features (nodules, tunnels, and scars), location (intertriginous areas), and chronicity.[38] Half of patients present prodromal symptoms such as burning, stinging, pain, pruritus, warmth, or hyperhidrosis, which may precede lesion appearance by 12 to 48 hours.[39] Triggers include menstruation, weight gain, stress, hormonal changes, excessive heat, and perspiration.[40] Individuals are usually well-appearing and afebrile at presentation unless secondary infection or advanced disease is present.
Characteristic primary hidradenitis suppurativa lesions are deep-seated nodules, typically 0.5 to 2 cm in size, persisting for days to months. The lesions are often mistaken for furuncles or boils and may have serosanguinous discharge, which can become purulent and malodorous. Unlike furuncles, these nodules are deep, recurrent, rupture-prone, and can form intercommunicating tunnels and tracts that may ulcerate or drain. Lidocaine injection or ultrasound examination can help visualize these tracts.[41] Advanced stages may feature thick fibrotic scars and plaques, causing architectural distortion and open comedones known as "tombstone" comedones.[42]
The axilla is the most common location for hidradenitis suppurativa lesions. Other common areas include the inguinal, inner thigh, perianal, perineal, inframammary, gluteal, pubic, scrotal, vulvar, and trunk regions. The scalp and retroauricular areas are less frequently involved (see Image. Hidradenitis Suppurativa, Groin).[43] Pertinent history includes onset typically from puberty to young adulthood, with recurrent lesions and intermittent improvement. A family history of a similar condition aids in diagnosis confirmation.
Once the history and physical exam are complete, the Hurley staging system may be used to classify the disease. This system categorizes lesions as follows:
- Hurley Stage I: Abscesses lack tunnels and scars.
- Hurley Stage II: Recurrent abscesses have tunnels and scars, and the lesions may be single or multiple but widely separated.
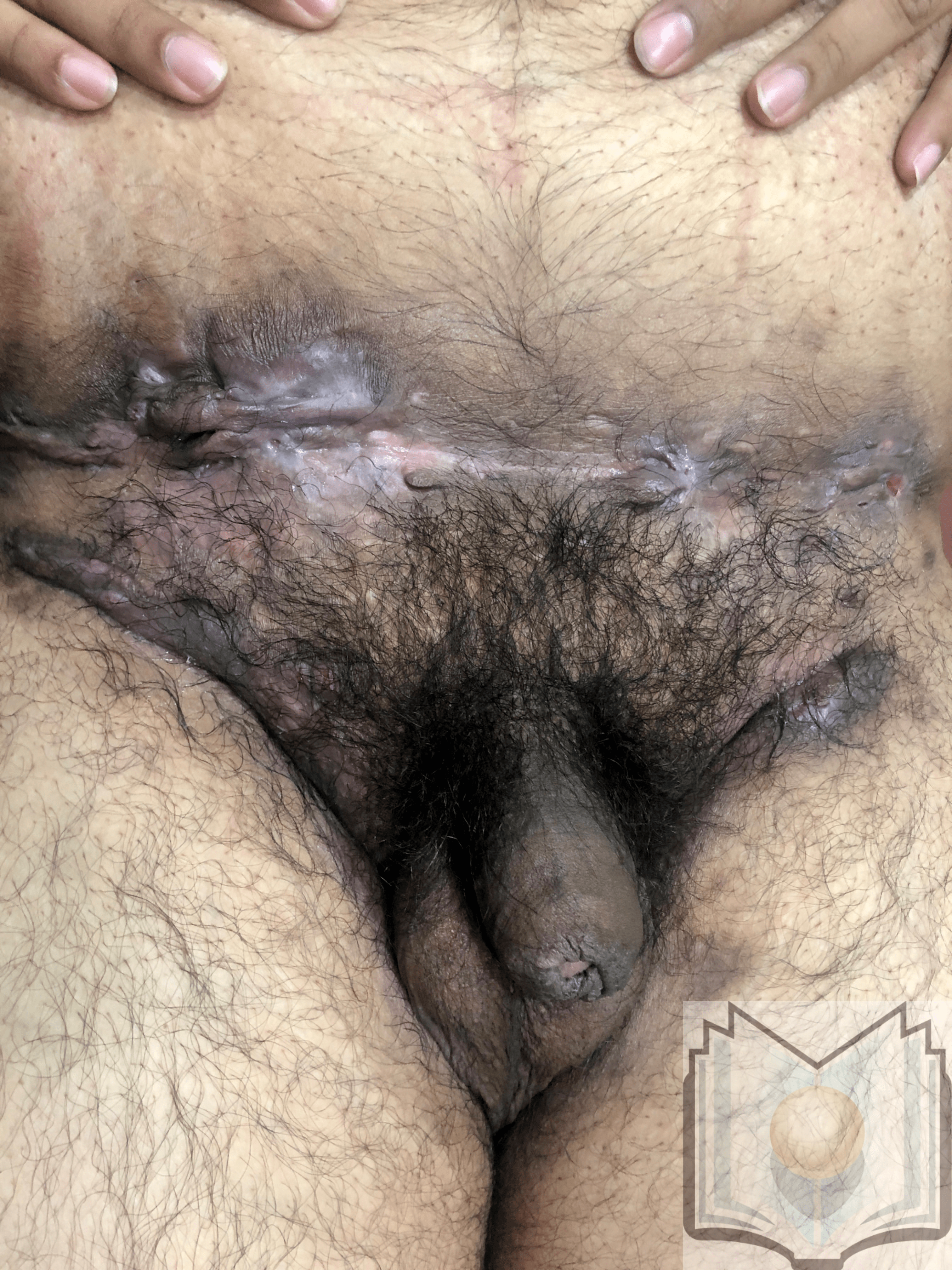
- Hurley Stage III: Lesions diffusely involve the skin, ie, multiple interconnected sinus tracts are present, and abscesses occupy large areas, leaving little to no uninvolved skin (see Image. Hidradenitis Suppurativa, Suprapubic Region). [44]
Hidradenitis suppurativa is a component of the follicular occlusion tetrad, along with acne conglobata, dissecting scalp cellulitis, and pilonidal sinus.[45] A diagnosis of hidradenitis suppurativa should prompt evaluation for possible cooccurrence of these conditions. Clinicians should also consider associations with metabolic syndrome, dyslipidemia, hypertension, diabetes mellitus, inflammatory bowel disease (especially Crohn disease), and spondyloarthropathy during assessment.[46] Syndromes like pyogenic arthritis, pyoderma gangrenosum, acne, suppurative hidradenitis (PAPASH) and pyoderma gangrenosum, acne suppurative hidradenitis (PASH), possibly related to a PSTPIP1 gene mutation, also warrant attention.[47]
Evaluation
Hidradenitis suppurativa is a clinical diagnosis. No biological or pathological diagnostic tests are required to confirm the condition. However, a biopsy may help rule out squamous cell carcinoma if the diagnosis is uncertain. Bacterial cultures are not beneficial unless a secondary infection or an alternative diagnosis is suspected. Imaging is not typically helpful, though ultrasonography may help identify skin tunneling preoperatively.[48] Severe perianal disease may warrant further imaging, preferably by magnetic resonance imaging.[49]
Guidelines recommend additional investigations to assess comorbid conditions in patients with hidradenitis suppurativa. Assessments typically involve annual physical examinations, validated screening tools, and laboratory tests. Referrals to specialists familiar with associated conditions may be necessary for further evaluation. Comorbidities in patients with hidradenitis suppurativa may include the following:
- Acne vulgaris or acne conglobata
- Dissecting cellulitis of the scalp
- Pilonidal cyst
- Pyoderma gangrenosum
- Depression
- Anxiety
- Suicidality
- Tobacco use disorder
- Substance use disorder
- Polycystic ovarian syndrome
- Obesity
- Dyslipidemia
- Diabetes mellitus
- Metabolic syndrome
- Hypertension
- Cardiovascular disease
- Inflammatory bowel disease
- SAPHO syndrome
- Behcet syndrome
- Spondyloarthritis
- Sexual dysfunction
- Down syndrome
- Thyroid disease
- Nonalcoholic fatty liver disease
- Obstructive sleep apnea
- Renal disease
- Sleep disturbances
- Alzheimer disease
- Herpes zoster
- Lymphoma
- Psoriasis vulgaris
- Pachyonychia congenita
- Dowling-Degos disease
- KID syndrome
- Stroke
- Anemia
- Cutaneous squamous cell carcinoma :[50][51]
Treatment / Management
The overall treatment goals for hidradenitis suppurativa include alleviating lesion-related symptoms (eg, pain), reducing recurrence frequency and new lesion formation, and preventing disease and comorbidity progression. Treatment approaches are largely based on expert opinion and consensus due to limited comparative studies.
Treatment response assessment involves monitoring lesion frequency, severity, extent, and morphology. Quality of life changes are also evaluated using validated tools, and progression to associated comorbidities is assessed. Various scales and scoring systems, including the International Hidradenitis Suppurativa Severity Scoring System, Sartorius score, Hidradenitis Suppurativa Physician Global Assessment, and Hidradenitis Suppurativa Core Outcome Set International Collaboration, are used to measure the condition's severity and treatment outcomes.[52][53][54](B3)
Hidradenitis suppurativa treatment should address comorbidities contributing to disease development or worsening, regardless of disease stage. Counseling and support for weight loss and smoking cessation are crucial, as obesity and smoking are associated with more severe disease progression. Minimizing skin trauma is also essential, achieved by avoiding tight clothing, harsh cleaning products, and adhesive dressings. Soft dressings with clear petroleum jelly or nonocclusive dressings can help prevent further irritation to draining lesions.[55]
Patients with hidradenitis suppurativa should be thoroughly educated about the condition's nature, wound care, and pain management. Individualized pain management plans must address potential inpatient admissions.[56][57] Hidradenitis suppurativa-related pain stems from both inflammatory and noninflammatory sources, including scarring (causing tensile pain), keloids, abscesses, open ulcerations, sinus tracts, frictional pain, lymphedema, anal fissures, and arthritis. Treatments may include topical agents like lidocaine, systemic nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants such as gabapentin or pregabalin, and serotonin-norepinephrine reuptake inhibitors, depending on disease severity and pain type. Duloxetine may be particularly beneficial for patients with comorbid depression.[58][59](B2)
A critically important but often overlooked aspect of treatment involves hidradenitis suppurativa's psychosocial aspect. Pain, drainage, odor, and involvement of sensitive areas reduce patients' quality of life.[60] Individuals with this condition may experience social isolation, employment difficulties due to missed work during flares, and heightened sexual or relationship dysfunction.[61] Assurance that this condition is not contagious or the result of poor hygiene can be helpful. Counseling and support groups are often beneficial additions to therapeutic plans.[62] Treatment for acute symptomatic lesions may include intralesional corticosteroid injection (usually triamcinolone), punch debridement, and topical resorcinol.[63][64][65] Incision and drainage is not recommended.[66](A1)
Topical antibiotics are the first-line treatment for early uncomplicated disease. Topical clindamycin has been proven the most effective and may be an appropriate adjunctive treatment to oral therapy.[67] Hurley stage I disease without skin tunneling or scarring may be treated with oral tetracycline antibiotics (eg, doxycycline), antiandrogen agents (eg, spironolactone), and metformin.[68] Tetracyclines should be discontinued and other agents retained if patient response is satisfactory after 2 to 3 months. Another tetracycline trial should be initiated if improvement occurs but is unsatisfactory. Switching to a combination of oral clindamycin and rifampin, dapsone, or acitretin may be considered if the clinical response remains unsatisfactory after 2 to 3 months on the same regimen or a 2nd tetracycline trial.[69][70](A1)
The initial treatment for Hurley stages II and III is similar to that of Hurley stage I disease. Potential treatment reactions must be considered during therapeutic planning. Patients should be educated on these medications' adverse effects before starting their regimen.
Surgical or biologic therapy should be considered if the patient poorly responds after 2 to 3 months of initial therapy, fails a 2- to 3-month second therapeutic trial, or a Hurley stage I case is treatment-refractory.[71](B2)
The preferred biologic agent for hidradenitis suppurativa is adalimumab, as it is backed by extensive research. Secukinumab, infliximab, or acitretin are alternatives. Etanercept has not shown efficacy in treating this condition.[72][73][74] The treatment must be continued if the patient demonstrates progressive improvement after 3 to 4 months of biologic therapy. Oral antiandrogen agents and metformin may be used as adjuncts.[75](A1)
Switching to a different class of biologics is advised if the response is unsatisfactory, followed by reassessment in 3 to 4 months for continued treatment or consideration of alternatives.[76][77] Surgical management and adjunctive therapies like intravenous ertapenem may be considered for severe, refractory hidradenitis suppurativa. Localized laser and pulsed light therapy may be offered as an option to disrupt the inflammatory process.[78][79][80]
Surgical management may be pursued at any stage to remove areas of active disease and limit complications, but it should be combined with medical therapy. Preoperative management should include antibiotics, anti-inflammatory agents (eg, biologics or cyclosporine), and corticosteroids (eg, prednisone or glucocorticoids).[81] Local procedures include punch debridement, deroofing, or wide excision. Punch debridement uses a 5- to 7-mm circular punch biopsy tool to evacuate an inflamed lesion. The lesion is left to heal by secondary intention with a petrolatum dressing after achieving hemostasis. Deroofing involves entering skin tunnels and sequentially removing their roofs using scissors or a carbon dioxide laser.[82]
Wide excision with reconstruction may be performed to debride a lesion until only the subcutaneous fat remains. Primary closure or a larger flap may be used for repair, depending on the anatomic location.[83] Grafting, which involves debriding the affected subepidermal skin and recycling the epidermis as a skin graft, may also be considered. Negative pressure wound therapy has also been used.[84] Petrolatum nonadherent dressings are recommended postoperatively to avoid friction. However, patients should be cautioned about the risks of bleeding, infection, or new disease when using these dressings.[85](B2)
Other therapies that may be considered include neodymium-doped yttrium aluminum garnet (Nd:YAG) laser, ustekinumab, anakinra, bimekizumab, guselkumab, canakinumab, cyclosporine, tacrolimus, apremilast, zinc, photodynamic therapy, golimumab, risankizumab, liraglutide, microwave ablation (not currently recommended), cryoinsufflation, botulinum toxin injections, and ionizing radiation.
Differential Diagnosis
The following conditions should be ruled out when diagnosing hidradenitis suppurativa:
- Follicular pyoderma (including folliculitis, furuncles, and carbuncles)
- Granuloma inguinale
- Noduloulcerative syphilis
- Tuberculous abscess
- Actinomycosis
- Lymphogranuloma venereum
- Acne vulgaris
- Epidermoid, dermoid, pilonidal, or Bartholin cysts
- Crohn disease (particularly with perianal involvement)
The following conditions should also be included in the differential diagnosis due to similar histopathology:
Prognosis
The prognosis of hidradenitis suppurativa is variable. The condition is incurable and has a chronic, relapsing course.[89] Diagnostic and treatment delays early in the disease course and comorbid conditions like smoking and obesity often worsen the prognosis.[90] Hurley stage III disease has the worst prognosis, but early recognition can help prevent complications.
Complications
Hidradenitis suppurativa has both physical and psychological complications.[91] Hidradenitis suppurativa recurrence may result in chronic pain, limb contractures, and impaired mobility from abscesses, tunnels, and scarring. Lymphatic obstruction may cause peripheral lymphedema.[92] Chronic inflammation's long-term effects include anemia, hyperproteinemia, amyloidosis, and axial and peripheral arthropathy.[93] Superimposed infections may result in systemic illness. Squamous cell carcinoma may develop, sometimes up to 30 years after diagnosis.[94]
Observational data suggests an increased risk of buccal and hepatocellular cancer.[95] Hidradenitis suppurativa's psychological impact includes depression, social isolation, decreased relationship satisfaction, sexual dysfunction, reduced work productivity, and in extreme cases, suicide.
Deterrence and Patient Education
Patient education should include reassuring individuals that hidradenitis suppurativa is not contagious or due to poor hygiene. Prompt reporting of lesions is advised to initiate timely treatment and prevent chronic progression . Lifestyle modifications, such as maintaining a healthy weight, quitting smoking, and avoiding skin trauma (eg, by avoiding tight clothing, abrasive cleansers, and adhesive bandages), should be discussed. Patients should also receive information on the risks and benefits of available treatments, with the most effective therapy for their current disease state recommended. Psychosocial education and treatment are likewise essential components of care.
Pearls and Other Issues
Hidradenitis suppurativa is a non-contagious chronic inflammatory skin disorder characterized by painful nodules, abscesses, and scarring. The condition starts to develop with hair follicle blockage, followed by rupture and inflammation. Hidradenitis suppurativa is not caused by infection or poor hygiene. Genetic and environmental elements contribute to its pathophysiology, with obesity and smoking being key factors.
Treatment options include medications (topical and oral antibiotics, intralesional and oral steroids, hormone therapy, and immune modulators) and surgery, ranging from lesion deroofing to wide excision. Hidradenitis suppurativa can cause significant psychosocial distress due to pain, drainage, odor, and involvement of sensitive or exposed areas. Early recognition and aggressive treatment offer the best prognosis, including psychosocial support.
Enhancing Healthcare Team Outcomes
Hidradenitis suppurativa is a complex condition with numerous comorbidities, requiring an interprofessional approach for effective management.[96] While this disorder is primarily a dermatologic concern, individuals may first seek care from various healthcare providers, such as family medicine, internal medicine, pediatrics, or obstetrics/gynecology. Primary care providers are crucial in early recognition and treatment initiation based on lesion characteristics. Depending on disease severity and provider training, primary care providers may manage the condition independently or refer to specialists like dermatologists, surgeons, wound care, and pain management experts.
The involvement of multiple healthcare team members, including professionals in the allied health sciences like nurses and pharmacists, may be necessary due to hidradenitis suppurativa's associated comorbidities. Nurses offer counseling, aid in history and examination, and act as liaisons between patients and clinicians. Pharmacists ensure correct dosing, check for interactions, and educate patients on medication use. Mental health providers should be part of the team, including psychiatrists, psychologists, or counselors, given the condition's significant psychosocial impact. Accurate documentation by all team members is crucial for consistent patient care. Open communication is essential for addressing any issues promptly. This interprofessional approach enhances patient outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
González-López MA. Hidradenitis suppurativa. Medicina clinica. 2024 Feb 23:162(4):182-189. doi: 10.1016/j.medcli.2023.09.018. Epub 2023 Nov 13 [PubMed PMID: 37968174]
Stancic BH, Boer J, Dolenc-Voljč M, Jemec GBE. The Role of Intra-Follicular Shear Forces in Hidradenitis Suppurativa. Skin pharmacology and physiology. 2023:36(6):302-303. doi: 10.1159/000536067. Epub 2024 Jan 4 [PubMed PMID: 38176392]
Mintoff D, Pace NP. Differences in hidradenitis suppurativa patterns of cutaneous involvement between sexes: Insights from a cross-sectional study. Human immunology. 2024 Mar:85(2):110764. doi: 10.1016/j.humimm.2024.110764. Epub 2024 Feb 6 [PubMed PMID: 38320910]
Level 2 (mid-level) evidenceScala E, Cacciapuoti S, Garzorz-Stark N, Megna M, Marasca C, Seiringer P, Volz T, Eyerich K, Fabbrocini G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells. 2021 Aug 15:10(8):. doi: 10.3390/cells10082094. Epub 2021 Aug 15 [PubMed PMID: 34440863]
Singh S, Desai K, Gillern S. Management of Pilonidal Disease and Hidradenitis Suppurativa. The Surgical clinics of North America. 2024 Jun:104(3):503-515. doi: 10.1016/j.suc.2023.11.003. Epub 2023 Dec 13 [PubMed PMID: 38677816]
Seivright J, Collier E, Grogan T, Shih T, Hogeling M, Shi VY, Hsiao JL. Pediatric hidradenitis suppurativa: epidemiology, disease presentation, and treatments. The Journal of dermatological treatment. 2022 Jun:33(4):2391-2393. doi: 10.1080/09546634.2021.1937484. Epub 2022 Mar 23 [PubMed PMID: 34057384]
Kirby J, Kim K, Zivkovic M, Wang S, Garg V, Danavar A, Li C, Chen N, Garg A. Uncovering the burden of hidradenitis suppurativa misdiagnosis and underdiagnosis: a machine learning approach. Frontiers in medical technology. 2024:6():1200400. doi: 10.3389/fmedt.2024.1200400. Epub 2024 Mar 25 [PubMed PMID: 38591045]
Yousef H, Miao JH, Alhajj M, Badri T. Histology, Skin Appendages. StatPearls. 2024 Jan:(): [PubMed PMID: 29489291]
Woodruff CM, Charlie AM, Leslie KS. Hidradenitis Suppurativa: A Guide for the Practicing Physician. Mayo Clinic proceedings. 2015 Dec:90(12):1679-93. doi: 10.1016/j.mayocp.2015.08.020. Epub [PubMed PMID: 26653298]
Balić A, Marinović B, Bukvić Mokos Z. The genetic aspects of hidradenitis suppurativa. Clinics in dermatology. 2023 Sep-Oct:41(5):551-563. doi: 10.1016/j.clindermatol.2023.08.022. Epub 2023 Aug 29 [PubMed PMID: 37652193]
Brandão LAC, Tricarico PM, Gratton R, Agrelli A, Zupin L, Abou-Saleh H, Moura R, Crovella S. Multiomics Integration in Skin Diseases with Alterations in Notch Signaling Pathway: PlatOMICs Phase 1 Deployment. International journal of molecular sciences. 2021 Feb 3:22(4):. doi: 10.3390/ijms22041523. Epub 2021 Feb 3 [PubMed PMID: 33546374]
Morales-Heil DJ, Cao L, Sweeney C, Malara A, Brown F, Milam P, Anadkat M, Kaffenberger J, Kaffenberger B, Nagele P, Kirby B, Roberson EDO. Rare missense variants in the SH3 domain of PSTPIP1 are associated with hidradenitis suppurativa. HGG advances. 2023 Apr 13:4(2):100187. doi: 10.1016/j.xhgg.2023.100187. Epub 2023 Mar 13 [PubMed PMID: 37013170]
Level 3 (low-level) evidenceTricarico PM, Gratton R, Dos Santos-Silva CA, de Moura RR, Ura B, Sommella E, Campiglia P, Del Vecchio C, Moltrasio C, Berti I, D'Adamo AP, Elsherbini AMA, Staudenmaier L, Chersi K, Boniotto M, Krismer B, Schittek B, Crovella S. A rare loss-of-function genetic mutation suggest a role of dermcidin deficiency in hidradenitis suppurativa pathogenesis. Frontiers in immunology. 2022:13():1060547. doi: 10.3389/fimmu.2022.1060547. Epub 2022 Dec 5 [PubMed PMID: 36544771]
Sun Q, Broadaway KA, Edmiston SN, Fajgenbaum K, Miller-Fleming T, Westerkam LL, Melendez-Gonzalez M, Bui H, Blum FR, Levitt B, Lin L, Hao H, Harris KM, Liu Z, Thomas NE, Cox NJ, Li Y, Mohlke KL, Sayed CJ. Genetic Variants Associated With Hidradenitis Suppurativa. JAMA dermatology. 2023 Sep 1:159(9):930-938. doi: 10.1001/jamadermatol.2023.2217. Epub [PubMed PMID: 37494057]
Chu YL, Yu S. Hidradenitis Suppurativa: An Understanding of Genetic Factors and Treatment. Biomedicines. 2024 Feb 1:12(2):. doi: 10.3390/biomedicines12020338. Epub 2024 Feb 1 [PubMed PMID: 38397941]
Level 3 (low-level) evidenceBoer J, Jemec GBE. Mechanical forces and Hidradenitis Suppurativa. Experimental dermatology. 2021 Feb:30(2):212-215. doi: 10.1111/exd.14234. Epub 2020 Nov 25 [PubMed PMID: 33155312]
Zouboulis VA, Zouboulis KC, Zouboulis CC. Hidradenitis Suppurativa and Comorbid Disorder Biomarkers, Druggable Genes, New Drugs and Drug Repurposing-A Molecular Meta-Analysis. Pharmaceutics. 2021 Dec 26:14(1):. doi: 10.3390/pharmaceutics14010044. Epub 2021 Dec 26 [PubMed PMID: 35056940]
Level 1 (high-level) evidenceRalser DJ, Basmanav FB, Tafazzoli A, Wititsuwannakul J, Delker S, Danda S, Thiele H, Wolf S, Busch M, Pulimood SA, Altmüller J, Nürnberg P, Lacombe D, Hillen U, Wenzel J, Frank J, Odermatt B, Betz RC. Mutations in γ-secretase subunit-encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. The Journal of clinical investigation. 2017 Apr 3:127(4):1485-1490. doi: 10.1172/JCI90667. Epub 2017 Mar 13 [PubMed PMID: 28287404]
Bukvić Mokos Z, Miše J, Balić A, Marinović B. Understanding the Relationship Between Smoking and Hidradenitis Suppurativa. Acta dermatovenerologica Croatica : ADC. 2020 Jul:28(1):9-13 [PubMed PMID: 32650845]
Level 3 (low-level) evidenceVossen ARJV, van Straalen KR, Swolfs EFH, van den Bosch JF, Ardon CB, van der Zee HH. Nicotine Dependency and Readiness to Quit Smoking among Patients with Hidradenitis Suppurativa. Dermatology (Basel, Switzerland). 2021:237(3):383-385. doi: 10.1159/000514028. Epub 2021 Feb 3 [PubMed PMID: 33535209]
Nowak-Liduk A, Kitala D, Ochała-Gierek G, Łabuś W, Bergler-Czop B, Pietrauszka K, Niemiec P, Szyluk K, Gierek M. Hidradenitis Suppurativa: An Interdisciplinary Problem in Dermatology, Gynecology, and Surgery-Pathogenesis, Comorbidities, and Current Treatments. Life (Basel, Switzerland). 2023 Sep 11:13(9):. doi: 10.3390/life13091895. Epub 2023 Sep 11 [PubMed PMID: 37763299]
Chu CB, Yang CC, Tsai SJ. Hidradenitis suppurativa: Disease pathophysiology and sex hormones. The Chinese journal of physiology. 2021 Nov-Dec:64(6):257-265. doi: 10.4103/cjp.cjp_67_21. Epub [PubMed PMID: 34975118]
Kisule A, Kak V, Alamelumangapuram C, Robinson C. Drug-Induced Hidradenitis Suppurativa: A Case Report. Cureus. 2023 Nov:15(11):e49637. doi: 10.7759/cureus.49637. Epub 2023 Nov 29 [PubMed PMID: 38161925]
Level 3 (low-level) evidenceJfri A, Nassim D, O'Brien E, Gulliver W, Nikolakis G, Zouboulis CC. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA dermatology. 2021 Aug 1:157(8):924-931. doi: 10.1001/jamadermatol.2021.1677. Epub [PubMed PMID: 34037678]
Level 1 (high-level) evidenceSinikumpu SP, Jokelainen J, Huilaja L. Prevalence and Characteristics of Hidradenitis Suppurativa in the Northern Finland Birth Cohort 1986 Study: A Cross-sectional Study of 2,775 Subjects. Acta dermato-venereologica. 2024 Jan 10:104():adv14732. doi: 10.2340/actadv.v104.14732. Epub 2024 Jan 10 [PubMed PMID: 38197699]
Level 2 (mid-level) evidenceMiller IM, McAndrew RJ, Hamzavi I. Prevalence, Risk Factors, and Comorbidities of Hidradenitis Suppurativa. Dermatologic clinics. 2016 Jan:34(1):7-16. doi: 10.1016/j.det.2015.08.002. Epub [PubMed PMID: 26617352]
Greif C, Gibson RS, Kimball AB, Holcomb ZE, Porter ML. Evaluating minority representation across health care settings in hidradenitis suppurativa and psoriasis. International journal of women's dermatology. 2024 Mar:10(1):e129. doi: 10.1097/JW9.0000000000000129. Epub 2024 Jan 18 [PubMed PMID: 38240009]
van Straalen KR, Prens EP, Gudjonsson JE. Insights into hidradenitis suppurativa. The Journal of allergy and clinical immunology. 2022 Apr:149(4):1150-1161. doi: 10.1016/j.jaci.2022.02.003. Epub 2022 Feb 19 [PubMed PMID: 35189127]
Brandao L, Moura R, Tricarico PM, Gratton R, Genovese G, Moltrasio C, Garcovich S, Boniotto M, Crovella S, Marzano AV. Altered keratinization and vitamin D metabolism may be key pathogenetic pathways in syndromic hidradenitis suppurativa: a novel whole exome sequencing approach. Journal of dermatological science. 2020 Jul:99(1):17-22. doi: 10.1016/j.jdermsci.2020.05.004. Epub 2020 May 22 [PubMed PMID: 32518053]
Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: An update. Journal of the American Academy of Dermatology. 2015 Nov:73(5 Suppl 1):S8-11. doi: 10.1016/j.jaad.2015.07.045. Epub [PubMed PMID: 26470623]
von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. The British journal of dermatology. 2011 Feb:164(2):367-71. doi: 10.1111/j.1365-2133.2010.10034.x. Epub 2010 Nov 23 [PubMed PMID: 20831631]
van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Experimental dermatology. 2012 Oct:21(10):735-9. doi: 10.1111/j.1600-0625.2012.01552.x. Epub 2012 Aug 7 [PubMed PMID: 22882284]
Brembach TC, Sabat R, Witte K, Schwerdtle T, Wolk K. Molecular and functional changes in neutrophilic granulocytes induced by nicotine: a systematic review and critical evaluation. Frontiers in immunology. 2023:14():1281685. doi: 10.3389/fimmu.2023.1281685. Epub 2023 Nov 23 [PubMed PMID: 38077313]
Level 1 (high-level) evidenceChu CB, Yang CC, Hsueh YY, Chen PC, Hong YK, Kuo YY, Tsai SJ. Aberrant expression of interleukin-17A in mast cells contributes to the pathogenesis of hidradenitis suppurativa. The British journal of dermatology. 2023 Nov 16:189(6):719-729. doi: 10.1093/bjd/ljad273. Epub [PubMed PMID: 37540988]
Tan IJ, Podwojniak A, Parikh A, Cohen BA. Precision Dermatology: A Review of Molecular Biomarkers and Personalized Therapies. Current issues in molecular biology. 2024 Mar 30:46(4):2975-2990. doi: 10.3390/cimb46040186. Epub 2024 Mar 30 [PubMed PMID: 38666916]
Gierek M, Ochała-Gierek G, Kitala D, Łabuś W, Bergler-Czop B. Hidradenitis suppurativa: bacteriological study in surgical treatment. Postepy dermatologii i alergologii. 2022 Dec:39(6):1101-1105. doi: 10.5114/ada.2022.119008. Epub 2022 Sep 9 [PubMed PMID: 36686013]
Smith SDB, Okoye GA, Sokumbi O. Histopathology of Hidradenitis Suppurativa: A Systematic Review. Dermatopathology (Basel, Switzerland). 2022 Jul 14:9(3):251-257. doi: 10.3390/dermatopathology9030029. Epub 2022 Jul 14 [PubMed PMID: 35892482]
Level 1 (high-level) evidenceRiva H, Hendricks A, Yoon T, Del Coro Amengual C, Maddox C. Decades Delayed in Diagnosis: Hidradenitis Suppurativa and a Review of Barriers to Care. Cureus. 2024 Mar:16(3):e56231. doi: 10.7759/cureus.56231. Epub 2024 Mar 15 [PubMed PMID: 38618324]
Ring HC, Theut Riis P, Zarchi K, Miller IM, Saunte DM, Jemec GB. Prodromal symptoms in hidradenitis suppurativa. Clinical and experimental dermatology. 2017 Apr:42(3):261-265. doi: 10.1111/ced.13025. Epub 2017 Feb 14 [PubMed PMID: 28194809]
Shams RB, Sayed CJ. Patient-reported exacerbating factors for hidradenitis suppurativa: A cross sectional study. Journal of the American Academy of Dermatology. 2024 Mar 29:():. pii: S0190-9622(24)00543-7. doi: 10.1016/j.jaad.2024.03.032. Epub 2024 Mar 29 [PubMed PMID: 38556091]
Harvey LM, Fortson JK. Hidradenitis Suppurativa at an Uncommon Site: A Review of Its Clinical Features, Diagnostic Difficulties, and Management. Cureus. 2021 Oct:13(10):e18704. doi: 10.7759/cureus.18704. Epub 2021 Oct 12 [PubMed PMID: 34790460]
Frew JW, Lowes MA, Goldfarb N, Butt M, Piguet V, O'Brien E, Ingram J, Jemec GBE, Tan J, Zouboulis C, Alavi A, Kirby JS. Global Harmonization of Morphological Definitions in Hidradenitis Suppurativa for a Proposed Glossary. JAMA dermatology. 2021 Apr 1:157(4):449-455. doi: 10.1001/jamadermatol.2020.5467. Epub [PubMed PMID: 33688910]
Miller A, Shahzeidi P, Bernhardt M. An Update on Current Clinical Management and Emerging Treatments in Hidradenitis Suppurativa. Skin therapy letter. 2024 Mar:29(2):1-6 [PubMed PMID: 38574201]
Level 3 (low-level) evidenceRamirez-GarciaLuna JL, Wang SC, Yangzom T, Piguet V, Kirby JS, Alavi A. Use of thermal imaging and a dedicated wound-imaging smartphone app as an adjunct to staging hidradenitis suppurativa. The British journal of dermatology. 2022 Apr:186(4):723-726. doi: 10.1111/bjd.20884. Epub 2022 Mar 30 [PubMed PMID: 34748648]
Treadwell S, Green M, Feschuk A, Valdebran M. Understanding patient demographics and treatment strategies observed in the follicular occlusion triad and tetrad. Archives of dermatological research. 2024 Apr 4:316(4):117. doi: 10.1007/s00403-024-02852-1. Epub 2024 Apr 4 [PubMed PMID: 38573444]
Level 3 (low-level) evidenceLi Q, Patrick MT, Sreeskandarajan S, Kang J, Kahlenberg JM, Gudjonsson JE, He Z, Tsoi LC. Large-scale epidemiological analysis of common skin diseases to identify shared and unique comorbidities and demographic factors. Frontiers in immunology. 2023:14():1309549. doi: 10.3389/fimmu.2023.1309549. Epub 2024 Jan 8 [PubMed PMID: 38259463]
Level 2 (mid-level) evidenceMaronese CA, Moltrasio C, Marzano AV. Hidradenitis Suppurativa-Related Autoinflammatory Syndromes: An Updated Review on the Clinics, Genetics, and Treatment of Pyoderma gangrenosum, Acne and Suppurative Hidradenitis (PASH), Pyogenic Arthritis, Pyoderma gangrenosum, Acne and Suppurative Hidradenitis (PAPASH), Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis (SAPHO), and Rarer Forms. Dermatologic clinics. 2024 Apr:42(2):247-265. doi: 10.1016/j.det.2023.12.004. Epub 2024 Jan 4 [PubMed PMID: 38423685]
Soto-Moreno A, Cuenca-Barrales C, Arias-Santiago S, García-Vidal JA, Medina-Mirapeix F, Molina-Leyva A. Safety and Effectiveness of Percutaneous Ultrasound-Guided Galvanic Current in Tunnels of Patients with Hidradenitis Suppurativa: A Pilot Study. Dermatology and therapy. 2024 Apr 27:():. doi: 10.1007/s13555-024-01149-5. Epub 2024 Apr 27 [PubMed PMID: 38676840]
Level 3 (low-level) evidenceNazzaro G, Calzari P, Vaienti S, Passoni E, Marzano AV. The role of imaging technologies in the diagnosis of hidradenitis suppurativa. Clinics in dermatology. 2023 Sep-Oct:41(5):611-621. doi: 10.1016/j.clindermatol.2023.08.023. Epub 2023 Aug 30 [PubMed PMID: 37652192]
Andersen R, Rostgaard K, Pedersen O, Jemec GBE, Hjalgrim H. Increased cancer incidence among patients with hidradenitis suppurativa - a Danish nationwide register study 1977-2017. Acta oncologica (Stockholm, Sweden). 2024 Apr 21:63():220-228. doi: 10.2340/1651-226X.2024.26182. Epub 2024 Apr 21 [PubMed PMID: 38647025]
Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, Alikhan A, Daveluy SD, Delorme I, Goldfarb N, Gulliver W, Hamzavi I, Jaleel T, Kimball AB, Kirby JS, Kirchhof MG, Lester J, Lev-Tov H, Lowes MA, Micheletti R, Orenstein LA, Piguet V, Sayed C, Tan J, Naik HB. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. Journal of the American Academy of Dermatology. 2022 May:86(5):1092-1101. doi: 10.1016/j.jaad.2021.01.059. Epub 2021 Jan 23 [PubMed PMID: 33493574]
Kim Y, Lee J, Kim HS, Ko HC, Kim BS, Kim MB, Shin K. Review of Scoring Systems for Hidradenitis Suppurativa. Annals of dermatology. 2024 Feb:36(1):9-17. doi: 10.5021/ad.23.090. Epub [PubMed PMID: 38325429]
Holgersen N, Nielsen VW, Rosenø NAL, Thyssen JP, Egeberg A, Nielsen SH, Ring HC, Thomsen SF. Biomarkers of systemic inflammation are associated with disease severity and metabolic syndrome in patients with hidradenitis suppurativa. JAAD international. 2024 Jun:15():170-178. doi: 10.1016/j.jdin.2024.03.002. Epub 2024 Mar 15 [PubMed PMID: 38638915]
Mastacouris N, Tannenbaum R, Strunk A, Koptyev J, Aarts P, Alhusayen R, Bechara FG, Benhadou F, Bettoli V, Brassard A, Brown D, Choon SE, Coutts P, da Silva DLF, Daveluy S, Dellavalle RP, Del Marmol V, Emtestam L, Gebauer K, George R, Giamarellos-Bourboulis EJ, Goldfarb N, Hamzavi I, Hazen PG, Horváth B, Hsiao J, Ingram JR, Jemec GBE, Kirby JS, Lowes MA, Marzano AV, Matusiak L, Naik HB, Okun MM, Oon HH, Orenstein LAV, Paek SY, Pascual JC, Fernandez-Peñas P, Resnik BI, Sayed CJ, Thorlacius L, van der Zee HH, van Straalen KR, Garg A. Outcome Measures for the Evaluation of Treatment Response in Hidradenitis Suppurativa for Clinical Practice: A HiSTORIC Consensus Statement. JAMA dermatology. 2023 Nov 1:159(11):1258-1266. doi: 10.1001/jamadermatol.2023.3282. Epub [PubMed PMID: 37755725]
Level 3 (low-level) evidenceAntia C, Alavi A, Alikhan A. Topical management and wound care approaches for hidradenitis suppurativa. Seminars in cutaneous medicine and surgery. 2017 Jun:36(2):58-61. doi: 10.12788/j.sder.2017.020. Epub [PubMed PMID: 28538745]
Schultheis M, Grabbe S, Staubach P, Hennig K, Mauch M, Burckhardt M, Langer G, Heise M, Zamsheva M, Schollenberger L, Strobel A. Drivers of disease severity and burden of hidradenitis suppurativa: a cross-sectional analysis on 553 German patients. International journal of dermatology. 2024 Feb:63(2):188-195. doi: 10.1111/ijd.16889. Epub 2023 Nov 2 [PubMed PMID: 37919257]
Level 2 (mid-level) evidenceWolinska A, Murray G, Andrawis M, Beatty P, Costa Blasco M, Doyle C, Mc Feely O, Murphy L, Tobin AM. Comparison of Social Media Content on Hidradenitis Suppurativa: A Cross-Sectional Study. Dermatology (Basel, Switzerland). 2023:239(5):840-842. doi: 10.1159/000531041. Epub 2023 May 12 [PubMed PMID: 37231820]
Level 2 (mid-level) evidenceScheinfeld N. Treatment of hidradenitis supprurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gabapentin, pegabalin, duloxetine, and venlafaxine. Dermatology online journal. 2013 Nov 15:19(11):20616 [PubMed PMID: 24314785]
Fernandez JM, Thompson AM, Borgstrom M, Orenstein LAV, Hsiao JL, Shi VY. Pain management modalities for hidradenitis suppurativa: a patient survey. The Journal of dermatological treatment. 2022 May:33(3):1742-1745. doi: 10.1080/09546634.2020.1822501. Epub 2020 Sep 20 [PubMed PMID: 32914659]
Level 3 (low-level) evidenceRichards E, Joshi A. Psychosocial effects of hidradenitis suppurativa in the literature: A systematic review. International journal of psychiatry in medicine. 2024 Apr 21:():912174241249215. doi: 10.1177/00912174241249215. Epub 2024 Apr 21 [PubMed PMID: 38644350]
Level 1 (high-level) evidenceCuenca-Barrales C, Montero-Vilchez T, Krajewski PK, Szepietowski JC, Matusiak L, Arias-Santiago S, Molina-Leyva A. Sexual Dysfunction and Quality of Life in Patients with Hidradenitis Suppurativa and Their Partners. International journal of environmental research and public health. 2022 Dec 26:20(1):. doi: 10.3390/ijerph20010389. Epub 2022 Dec 26 [PubMed PMID: 36612709]
Level 2 (mid-level) evidenceMatusiak Ł. Profound consequences of hidradenitis suppurativa: a review. The British journal of dermatology. 2020 Dec:183(6):e171-e177. doi: 10.1111/bjd.16603. Epub 2018 May 9 [PubMed PMID: 29744872]
Pascual JC, Hernández-Quiles R, Sánchez-García V, Viudez-Martínez A, Belinchón Romero I, Sivera Mascaró F. Topical and Intralesional Therapies for Hidradenitis Suppurativa: A Systematic Literature Review. Actas dermo-sifiliograficas. 2024 May:115(5):T433-T448. doi: 10.1016/j.ad.2024.02.024. Epub 2024 Feb 27 [PubMed PMID: 38423507]
Level 1 (high-level) evidenceKatoulis A, Efthymiou O, Liakou A, Pappa G, Kanelleas A, Koumaki D, Bozi E, Sgouros D. Resorcinol 10% as a Promising Therapeutic Option for Mild Hidradenitis Suppurativa: A Prospective, Randomized, Open Study. Skin appendage disorders. 2023 Dec:9(6):438-443. doi: 10.1159/000531926. Epub 2023 Aug 16 [PubMed PMID: 38058541]
Level 1 (high-level) evidenceMansour MR, Rehman RA, Daveluy S. Punch debridement (mini-deroofing): An in-office surgical option for hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2024 Jan 9:():. pii: S0190-9622(24)00054-9. doi: 10.1016/j.jaad.2023.12.050. Epub 2024 Jan 9 [PubMed PMID: 38211709]
Wang Y, Han C, Wang X. Advances in surgical treatment of hidradenitis suppurative. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences. 2023 Nov 2:52(6):795-801. doi: 10.3724/zdxbyxb-2023-0326. Epub 2023 Nov 2 [PubMed PMID: 37986703]
Level 3 (low-level) evidenceClemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. International journal of dermatology. 1983 Jun:22(5):325-8 [PubMed PMID: 6347922]
Level 1 (high-level) evidenceMasson R, Shih T, Jeong C, Shi VY, Hsiao JL. Hormonal Treatments in Hidradenitis Suppurativa: A Systematic Review. Journal of drugs in dermatology : JDD. 2023 Aug 1:22(8):785-794. doi: 10.36849/jdd.7325. Epub [PubMed PMID: 37556513]
Level 1 (high-level) evidenceBaroudi B, Bashyam AM, Feldman SR, Pichardo RO. Dapsone to Treat Moderate-to-Severe Hidradenitis Suppurativa: A Retrospective Case-Series. Journal of drugs in dermatology : JDD. 2023 Nov 1:22(11):e12-e16. doi: 10.36849/JDD.4936. Epub [PubMed PMID: 37943259]
Level 2 (mid-level) evidenceIngram JR, Bates J, Cannings-John R, Collier F, Gibbons A, Harris C, Hood K, Howells L, Howes R, Leighton P, Riaz M, Rodrigues J, Stanton H, Thomas KS, Thomas-Jones E. Treatment of Hidradenitis Suppurativa Evaluation Study: the THESEUS prospective cohort study. Health technology assessment (Winchester, England). 2023 Dec:27(30):1-107. doi: 10.3310/HWNM2189. Epub [PubMed PMID: 38149635]
Malvaso D, Chiricozzi A, Fossati B, Antonelli F, Peris K. Efficacy and safety of a combined pharmacological and surgical approach in patients affected by hidradenitis suppurativa: data from a retrospective real-world clinical study. Italian journal of dermatology and venereology. 2024 Apr:159(2):190-195. doi: 10.23736/S2784-8671.24.07620-5. Epub [PubMed PMID: 38650499]
Level 2 (mid-level) evidenceStergianou D, Kanni T, Damoulari C, Giamarellos-Bourboulis EJ. An evaluation of secukinumab for the treatment of moderate-to-severe hidradenitis suppurativa. Expert opinion on biological therapy. 2024 Apr:24(4):225-232. doi: 10.1080/14712598.2024.2343112. Epub 2024 Apr 15 [PubMed PMID: 38602836]
Level 3 (low-level) evidenceSánchez-Díaz M, Díaz-Calvillo P, Rodríguez-Pozo JÁ, Arias-Santiago S, Molina-Leyva A. Effectiveness and Safety of Acitretin for the Treatment of Hidradenitis Suppurativa, Predictors of Clinical Response: A Cohort Study. Dermatology (Basel, Switzerland). 2023:239(1):52-59. doi: 10.1159/000526019. Epub 2022 Aug 23 [PubMed PMID: 35998603]
Ring HC, Thorsen J, Kirby B, Ingram JR, Rosenø NAL, Holgersen N, Nielsen VW, Thein Aagaard DN, Maul JT, Wu JJ, Thyssen JP, Egeberg A, Thomsen SF. Long-term drug survival of adalimumab, infliximab, secukinumab and ustekinumab in hidradenitis suppurativa: a Danish nationwide cohort study. The British journal of dermatology. 2024 Apr 17:190(5):769-771. doi: 10.1093/bjd/ljae042. Epub [PubMed PMID: 38305415]
Husein-ElAhmed H, Husein-ElAhmed S. Comparative efficacy and therapeutic positioning of biologics in hidradenitis suppurativa: A systematic review with network meta-analysis of randomised trials. Indian journal of dermatology, venereology and leprology. 2024 Feb 28:():1-9. doi: 10.25259/IJDVL_665_2023. Epub 2024 Feb 28 [PubMed PMID: 38595016]
Level 1 (high-level) evidenceTchernev G. LOSS OF EFFICACY OF ADALIMUMAB IN HIDRADENITIS SUPPURATIVA: FOCUS ON ALTERNATIVES. Georgian medical news. 2023 Jul-Aug:(340-341):297-300 [PubMed PMID: 37805915]
Salvia G, Bevilacqua M, Manzo Margiotta F, Michelucci A, Granieri G, Fidanzi C, Dini V, Romanelli M, Mazzatenta C. Switch from adalimumab to brodalumab as a possible trigger factor for the onset of pyoderma gangrenosum. The Australasian journal of dermatology. 2024 Apr 10:():. doi: 10.1111/ajd.14266. Epub 2024 Apr 10 [PubMed PMID: 38597123]
Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Reviews in endocrine & metabolic disorders. 2016 Sep:17(3):343-351 [PubMed PMID: 26831295]
Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2010 Feb:36(2):208-13. doi: 10.1111/j.1524-4725.2009.01427.x. Epub 2009 Dec 21 [PubMed PMID: 20039918]
Tricarico PM, Zupin L, Ottaviani G, Rupel K, Celsi F, Genovese G, Boniotto M, Crovella S, Marzano AV. Photobiomodulation as potential novel third line tool for non-invasive treatment of hidradenitis suppurativa. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2020 Feb:155(1):88-98. doi: 10.23736/S0392-0488.19.06247-3. Epub 2019 Apr 23 [PubMed PMID: 31042851]
Taylor EM, Hamaguchi R, Kramer KM, Kimball AB, Orgill DP. Plastic Surgical Management of Hidradenitis Suppurativa. Plastic and reconstructive surgery. 2021 Mar 1:147(3):479-491. doi: 10.1097/PRS.0000000000007677. Epub [PubMed PMID: 33620946]
Clark SR, Soti V. Effectiveness of Surgical Deroofing and Carbon Dioxide Laser in Moderate-to-Severe Hidradenitis Suppurativa Patients. Cureus. 2024 Mar:16(3):e56959. doi: 10.7759/cureus.56959. Epub 2024 Mar 26 [PubMed PMID: 38545424]
La Padula S, Pensato R, Pizza C, D'Andrea F, Roccaro G, Meningaud JP, Hersant B. The Thoracodorsal Artery Perforator Flap for the Treatment of Hidradenitis Suppurativa of the Axilla: A Prospective Comparative Study. Plastic and reconstructive surgery. 2023 Nov 1:152(5):1105-1116. doi: 10.1097/PRS.0000000000010435. Epub 2023 Mar 22 [PubMed PMID: 36946904]
Level 2 (mid-level) evidenceVinnicombe Z, Singh GV, Spiers J, Pouncey AL, McEvoy H, Lancaster K. Comparison of Negative Pressure Wound Therapy with or without a Split-Thickness Skin Graft in the Surgical Management of Axillary Hidradenitis Suppurativa: A Retrospective Cohort Study. Plastic surgery (Oakville, Ont.). 2024 May:32(2):314-320. doi: 10.1177/22925503221109006. Epub 2022 Jun 27 [PubMed PMID: 38681254]
Level 2 (mid-level) evidenceGasslitter I, Häfner HM, Kofler K, Kofler L. [Postoperative wound care with custom-made wound dressings for hidradenitis suppurativa : Wound care after radical excision with secondary wound closure]. Dermatologie (Heidelberg, Germany). 2022 Dec:74(12):994-996. doi: 10.1007/s00105-023-05247-x. Epub 2023 Nov 1 [PubMed PMID: 37910227]
McKenzie SA, Ni CS, Hsiao JL. Hidradenitis Suppurativa in a Patient with Smith-Magenis Syndrome: A Case Report. Cureus. 2019 Jun 22:11(6):e4970. doi: 10.7759/cureus.4970. Epub 2019 Jun 22 [PubMed PMID: 31453042]
Level 3 (low-level) evidenceAndersen RK, Yazdanyar S, Ernst Jemec GB, Saunte DM. Concomitant Hidradenitis Suppurativa and Eruptive Xanthomas Presenting with Phimosis - The Importance of Timely Diagnosis. Acta dermatovenerologica Croatica : ADC. 2020 Dec:28(3):154-156 [PubMed PMID: 33422169]
Shamim H, Riemer C, Weenig R, Sokumbi O, Sciallis G, McEvoy M, Mischke D, Comfere N. Acneiform Presentations of Folliculotropic Mycosis Fungoides. The American Journal of dermatopathology. 2021 Feb 1:43(2):85-92. doi: 10.1097/DAD.0000000000001698. Epub [PubMed PMID: 33492839]
Lyakhovitsky A, Segal O, Galili E, Thompson CT, Tzanani I, Scope A, Baum S, Barzilai A. Diagnostic delay, comorbid hidradenitis suppurativa and the prognostic value of bacterial culture in folliculitis decalvans: A cohort study. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2023 Dec:21(12):1469-1477. doi: 10.1111/ddg.15202. Epub 2023 Oct 24 [PubMed PMID: 37875786]
Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, Jemec GB. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. The British journal of dermatology. 2014 Oct:171(4):819-24. doi: 10.1111/bjd.13090. Epub 2014 Sep 7 [PubMed PMID: 24804604]
Level 2 (mid-level) evidenceYuan JT, Naik HB. Complications of hidradenitis suppurativa. Seminars in cutaneous medicine and surgery. 2017 Jun:36(2):79-85. doi: 10.12788/j.sder.2017.022. Epub [PubMed PMID: 28538749]
Yaman A, Borman P, Eşme P, Çalışkan E. Complex decongestive therapy in hidradenitis suppurativa-related genital lymphoedema: a case report. Journal of wound care. 2024 Feb 1:33(Sup2a):xxviii-xxxi. doi: 10.12968/jowc.2024.33.Sup2a.xxviii. Epub [PubMed PMID: 38324423]
Level 3 (low-level) evidenceMac Mahon J, Murray G, McCartney Y, Crowther S, Tobin AM, Molloy K. Systemic AA amyloidosis in hidradenitis suppurativa: Should we be screening? Journal of the European Academy of Dermatology and Venereology : JEADV. 2023 May:37(5):e600-e601. doi: 10.1111/jdv.18807. Epub 2023 Jan 11 [PubMed PMID: 36463423]
Kuraitis D, Murina A. Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses. Cutis. 2024 Jan:113(1):29-34. doi: 10.12788/cutis.0914. Epub [PubMed PMID: 38478947]
Cao Y, Hong F, Conlon DM, Sidur L, Smith KM, Fang Y, Cuff CA, Kaymakcalan Z, Ruzek MC. Potential predictive biomarkers of adalimumab response in patients with hidradenitis suppurativa. The British journal of dermatology. 2021 Oct:185(4):804-814. doi: 10.1111/bjd.20097. Epub 2021 May 31 [PubMed PMID: 33811319]
Kimball AB, Sundaram M, Gauthier G, Guérin A, Pivneva I, Singh R, Ganguli A. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatology and therapy. 2018 Dec:8(4):557-569. doi: 10.1007/s13555-018-0264-z. Epub 2018 Oct 10 [PubMed PMID: 30306395]