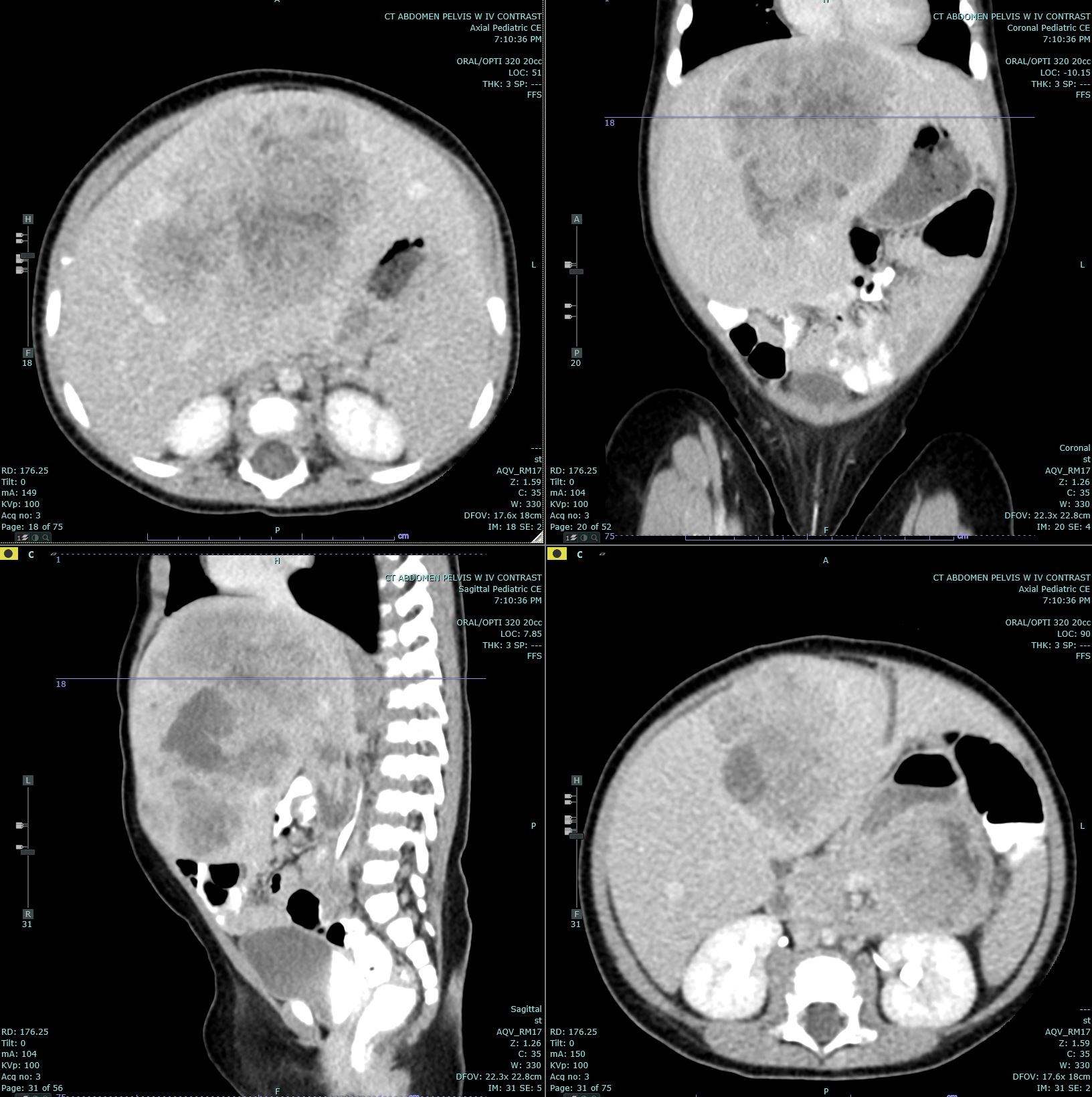
Introduction
Hepatoblastomas are the most common primary malignant liver tumor in pediatric patients, occurring mostly within the first 2 years of life.[1] The histologic types are subdivided into 2 broad categories: epithelial type and mixed type. Over the last 3 decades, the treatment has advanced with neo-adjuvant chemotherapy now the standard of care for most cases. Neo-adjuvant chemotherapy and surgical resection produce a cure rate of approximately 70%, a vast improvement over the dismal 30% cure rate in the 1970s. Prognosis is based on many factors including alpha-fetoprotein levels, age at the time of diagnosis, completeness of resection, and clinical stage of the disease.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most tumors are sporadic, but one-third of cases may be associated with Beckwith-Weidemann, familial adenomatous polyposis (FAP), Edward syndrome (trisomy 18), nephroblastoma, and Down syndrome.[3] Low birth weight infants are at higher risk of developing a hepatoblastoma and evidence has shown an association with preeclampsia and parental tobacco smoking before and during pregnancy.[3][4] Other factors thought to play a role in pathogenesis include oxygen therapy, certain medication (furosemide), radiation, plasticizers, and total parenteral nutrition (TPN).[2]
The most common genetic mutation involves the Wnt signaling pathway which results in the accumulation of beta-catenin; these mutations are present in a higher proportion of the sporadic cases.[5] By immunohistochemistry, beta-catenin usually shows a membranous staining pattern in the more differentiated fetal types and nuclear staining pattern in the less differentiated histologic types.[6] In aggressive cases, activation of TERT (human telomerase reverse transcriptase) and MYC signaling has been shown[2]
Epidemiology
Hepatoblastoma is a rare tumor comprising approximately 1% of all pediatric tumors.[7] The incidence rate is slowly increasing in North America and Europe, and there is a slight male predominance.[8]
Histopathology
Hepatoblastomas originate from primitive hepatic stem cells that give rise to the epithelial components of the liver. Classically, these tumors are divided into 2 broad categories: epithelial type (E-HB) and mixed epithelial and mesenchymal type (MEM-HB). Revision of this original classification system resulted in the pathology consensus of the pediatric hepatoblastoma classification system, which retained the subdivision of the histologic types into 2 broad categories, as described above. The E-HB includes fetal, pleomorphic, embryonal, macrotrabecular, small cell undifferentiated (SCU), cholangioblastic and mixed epithelial variants. The MEM-HB is subdivided into tumors with teratoid features and tumors without teratoid features.
The fetal subtype is further stratified into 4 categories: well-differentiated; crowded or mitotically active; pleomorphic, poorly differentiated; and anaplastic. The well-differentiated variant is characterized by a low power view demonstrating alternating light and dark areas due to variable cytoplasmic glycogen content. Assessment at higher power reveals a uniform population of hepatocytes arranged in trabeculae that are 2 to 3 cells thick. Extramedullary hematopoiesis is a typical finding, and mitotic rate is low. Description of the other variants is beyond the scope of this article.
The embryonal subtype is the most commonly encountered subtype and consists of basophilic cells with scant cytoplasm and increased mitotic rate that is arranged in nests, trabeculae, acini, pseudorosettes, or sheets. The macrotrabecular subtype is arranged in trabeculae that are more than ten cells thick. The SCU subtype consists of dyscohesive, uniform round cells arranged in sheets with increased mitotic activity. Some cases of SCU have a loss of INI1, suggesting a possible association with primary rhabdoid tumors of the liver. The cholangioblastic variant has bile ducts, typically located at the periphery of epithelial sheets.
The MEM-HB comprises 20-30% of tumors and contains a variable combination of epithelial and mesenchymal components. Most commonly, the epithelial component is fetal or embryonal, and the mesenchymal component is osteoid. Stromal derivatives include spindle cells, osteoid, skeletal muscle, and cartilage. Teratoid features include primitive endoderm, neural derivatives, melanin, squamous and glandular elements.[2][9]
History and Physical
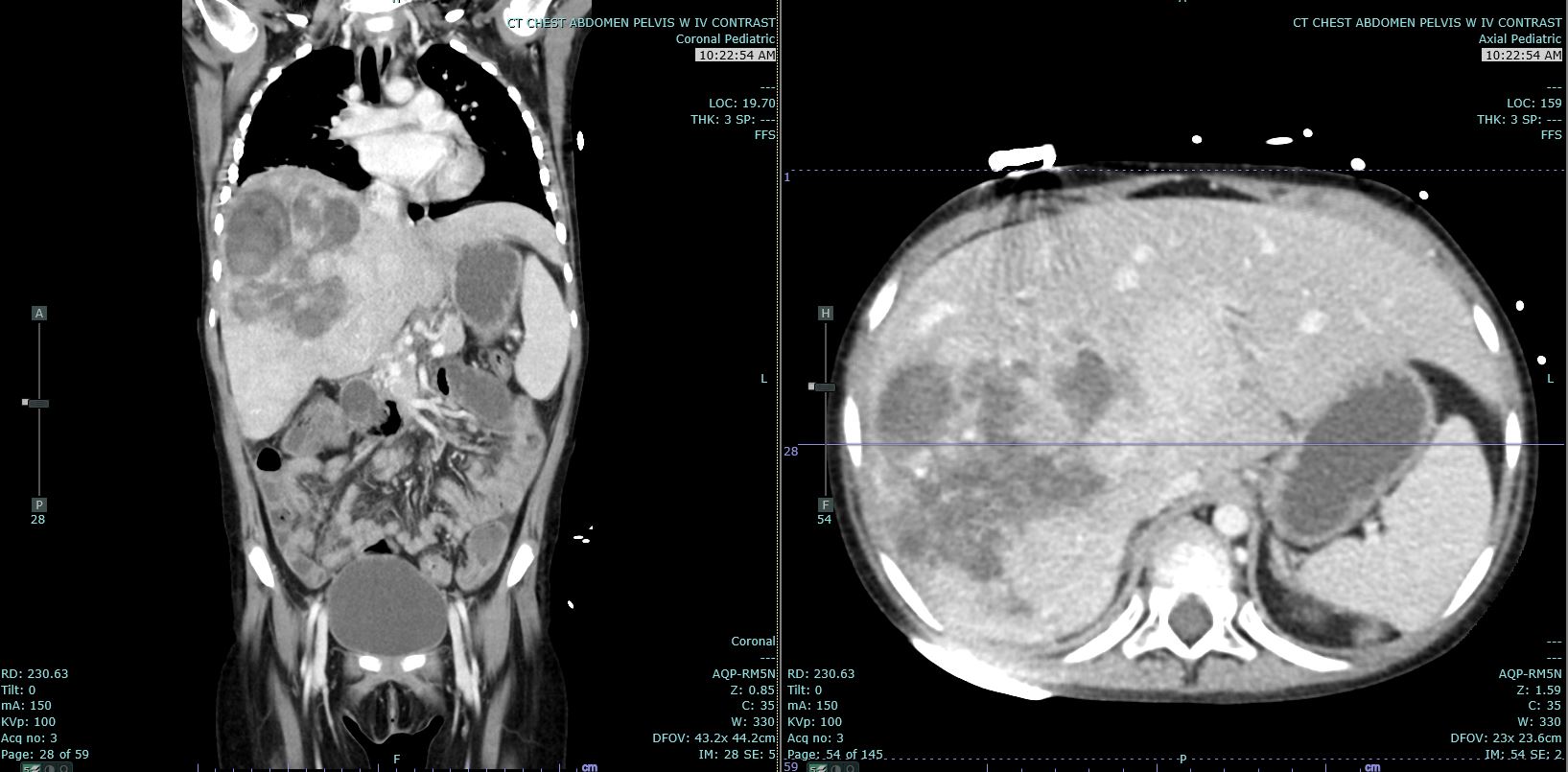
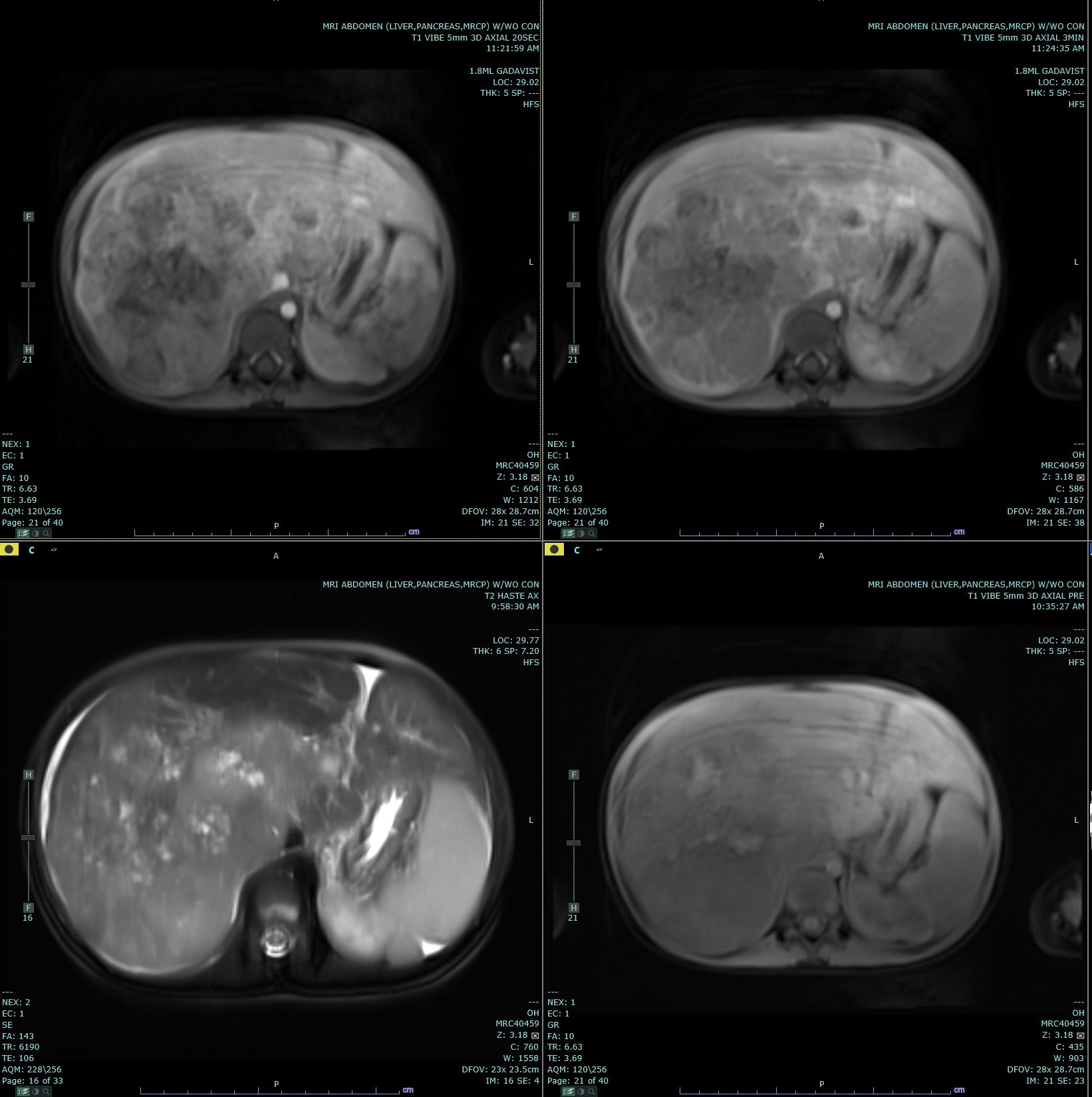
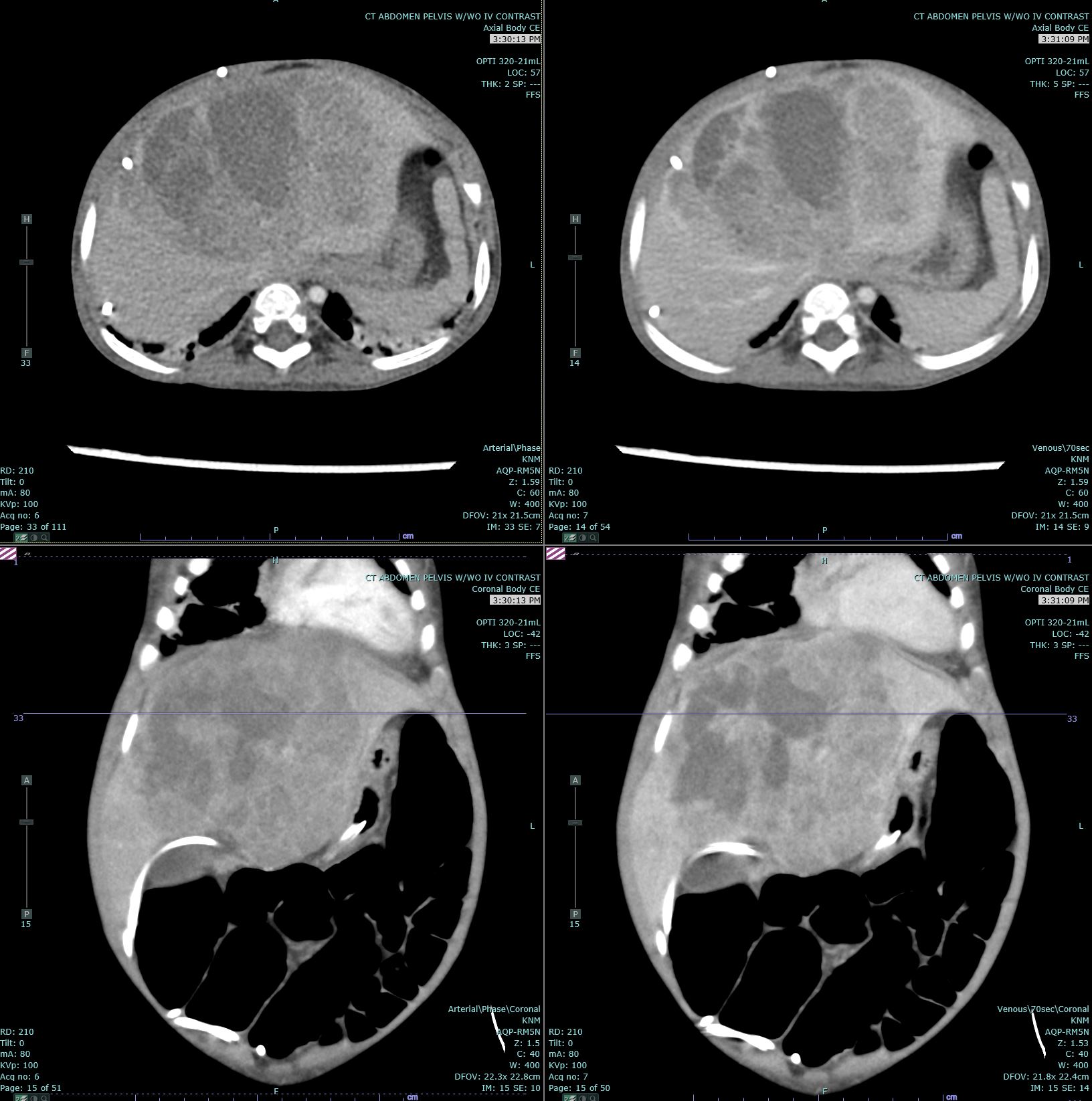
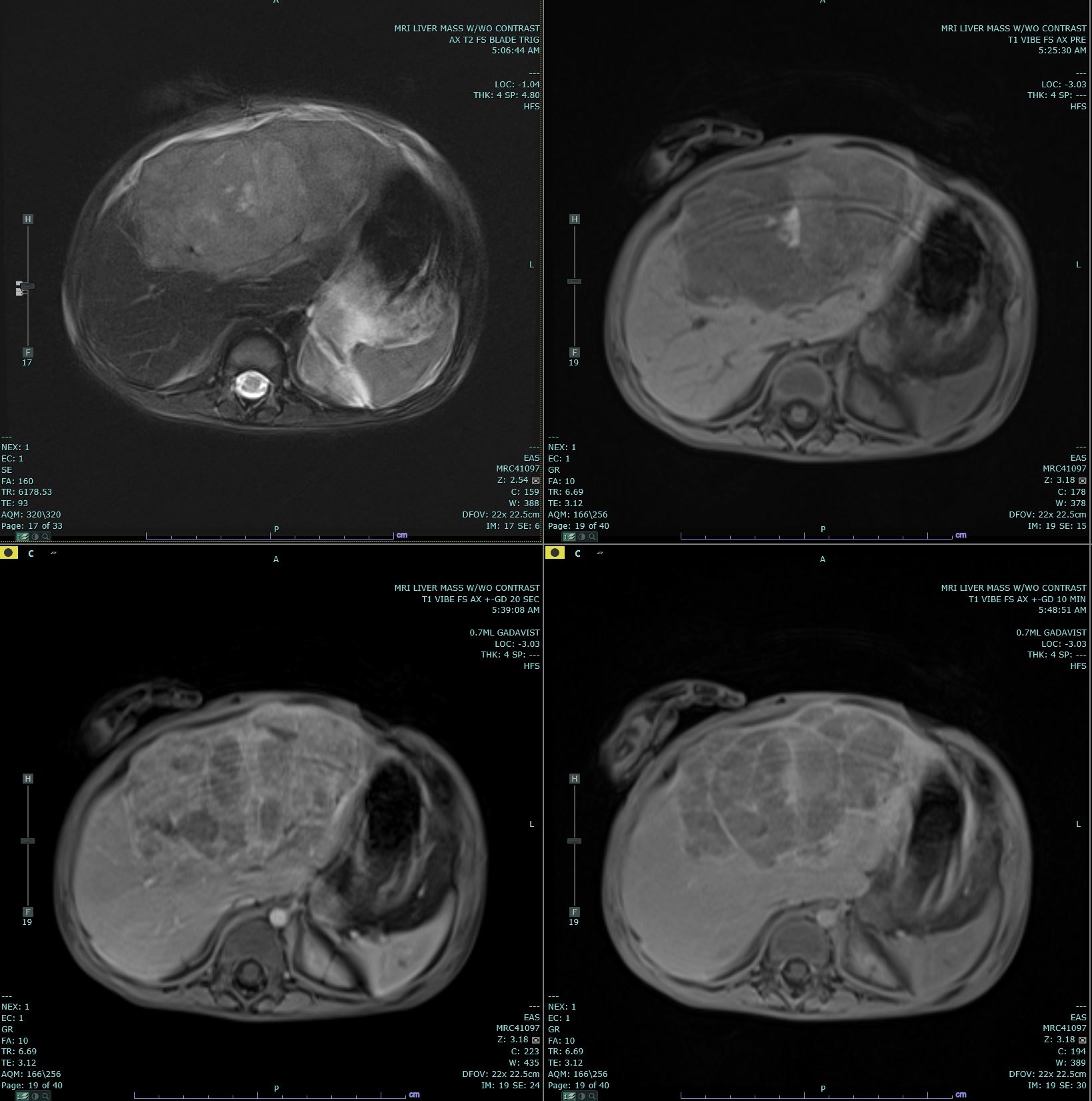
Hepatoblastomas usually present with as a single, mildly painful, rapidly enlarging abdominal mass that arises in the right lobe of the liver in 55% to 60% of cases.[10] Rapid enlargement of these tumors rarely results in tumor rupture and hemorrhage. Tumors may reach up to 25 cm in size. Most tumors are solitary; however, up to 15% of tumors are multifocal. Some cases are associated with non-specific symptoms such as weight loss, failure to thrive or anorexia.[7] Significant elevations of alpha-fetoprotein (AFP) are observed in 90% of patients, and rarely, a paraneoplastic syndrome can occur.
Evaluation
Ultrasound (US) and either computed tomography (CT) or magnetic resonance imaging (MRI) are the imaging modalities used to define the extent of tumor involvement of the liver and aid in pre-surgical planning. A chest CT can help detect lung metastasis; the lung is the most common location of metastases and up to 20% of cases present with metastases.[7] After imaging, a biopsy, alpha-fetoprotein level, liver function tests, and a hepatitis panel are performed as needed. Consent for participation in biologic studies should be requested before the biopsy is performed so the specimen can be properly allocated and handled.[9]
Treatment / Management
Surgical resection is the mainstay of treatment with resectability of the tumor determining the need for neo-adjuvant or adjuvant chemotherapy. At presentation, approximately 60% of tumors are unresectable.[11] If unresectable and chemotherapy fails to shrink the tumor to a resectable size, a liver transplant can be done and has a good long-term survival rate.[12] The benefit of radiation therapy is unclear, with some unresectable cases responding well. Alpha-fetoprotein levels are useful for tracking surgical success and whether the tumor has metastasized.[10] An increased risk of post-transplant lymphoproliferative disorder after immunosuppression for liver transplant has been suggested in some publications.[6](B3)
Differential Diagnosis
The differential diagnosis includes hepatocellular carcinoma (HCC), focal nodular hyperplasia, hepatic adenoma, lymphoma, and metastases. HCC can appear similar to the macrotrabecular subtype of hepatoblastoma, however, usually affects an adult patient population with risk factors such as metabolic disorders, liver cirrhosis, or childhood hepatitis B infection. Focal nodular hyperplasia usually affects older children and adults. Hepatic adenoma can resemble pure fetal hepatoblastomas; however, these rarely occur in persons under 5 years of age unless they have an underlying metabolic disorder.
Staging
The Children’s Hepatic Tumors International Collaboration (CHIC) constructed a staging and risk stratification system intended to standardize the assessment of this tumor across the globe. This new staging system is called the Children’s Hepatic Tumors International Collaboration – Hepatoblastoma Stratification and incorporates confirmed prognostic factors from prior risk stratification systems with new additional factors to stratify patients into 4 risk groups.
Found to be most predictive are AFP levels, patient age, Pretreatment Extent of Disease (PRETEXT) group (I, II, III, or IV), the presence of metastases, and PRETEXT annotation factor. PRETEXT group is based on the extent of the tumor in the liver. PRETEXT annotation factor is determined to be positive if at least 1 of the following 5 factors are present: involvement of the vena cava or all 3 hepatic veins, or both (V); involvement of portal bifurcation or both right and left portal veins, or both (P); extrahepatic contiguous tumor extension (E); multifocal liver tumor (F); tumor rupture at diagnosis (R). Gender, low birth weight, prematurity, and Beckwith-Wiedemann syndrome were not found to be significant. Of note, the histologic type was not included in this risk stratification system but may be incorporated at a future date. Validation of these risk groups is in progress.[2][13]
Prognosis
Prognosis is based on numerous factors including age of diagnosis, PRETEXT group, metastases, alfa fetal protein (AFP) levels, histologic subtype, completeness of resection, and clinical stage of the disease. With certainty, the well-differentiated fetal subtype is associated with a better prognosis compared with small cell undifferentiated and macrotrabecular, which are linked to unfavorable prognosis.[13] Of note, the histologic subtype is only prognostically valuable before treatment with chemotherapy. Alfa-fetoprotein is typically high upon diagnosis, but a significant drop after neo-adjuvant chemotherapy portends a better response to treatment. Younger age of diagnosis has historically been a poor prognostic factor; however, recent studies have called this into question, providing evidence that these younger patients do just as well as older children.[14] Specifically, children younger than 1 year of age have a better prognosis and children greater than 6 years of age have a worse prognosis.[2] Tumor presence at the resection margin, multifocality, and metastases have been shown to be poor prognostic factors. Beta-catenin expression has been shown to be associated with a lower period of event-free survival, while EpCAM expression has been associated with higher tumor viability and a poorer response to neo-adjuvant chemotherapy.[15][16]
Complications
- Intraperitoneal tumor rupture
- Complications related to chemotherapy
- Post-transplant complications
- Psychosocial effects of treatment and painful procedures
Enhancing Healthcare Team Outcomes
Improved clinical outcomes are achieved when hepatoblastomas are managed by an interprofessional team which may include a pediatrician, oncologist, radiologist, pediatric surgeon, hepatologist and a transplant specialist.[17] After surgical resection, these children are monitored by intensive care unit (ICU) nurses, therapists, dietitians, and an intensivist.
Media
References
Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Seminars in diagnostic pathology. 2017 Mar:34(2):192-200. doi: 10.1053/j.semdp.2016.12.015. Epub 2016 Dec 23 [PubMed PMID: 28126357]
Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Current opinion in pediatrics. 2014 Feb:26(1):19-28. doi: 10.1097/MOP.0000000000000046. Epub [PubMed PMID: 24322718]
Level 3 (low-level) evidenceFinegold MJ, Lopez-Terrada DH, Bowen J, Washington MK, Qualman SJ, College of American Pathologists. Protocol for the examination of specimens from pediatric patients with hepatoblastoma. Archives of pathology & laboratory medicine. 2007 Apr:131(4):520-9 [PubMed PMID: 17425379]
Heck JE, Meyers TJ, Lombardi C, Park AS, Cockburn M, Reynolds P, Ritz B. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer epidemiology. 2013 Aug:37(4):390-5. doi: 10.1016/j.canep.2013.03.004. Epub 2013 Apr 1 [PubMed PMID: 23558166]
Level 2 (mid-level) evidenceCuria MC, Zuckermann M, De Lellis L, Catalano T, Lattanzio R, Aceto G, Veschi S, Cama A, Otte JB, Piantelli M, Mariani-Costantini R, Cetta F, Battista P. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008 Jan:21(1):7-14 [PubMed PMID: 17962810]
Level 2 (mid-level) evidenceNg K, Rana A, Masand P, Patel K, Heczey A, Goss J, Himes R. Fatal Central Nervous System Post-Transplant Lymphoproliferative Disease in a Patient Who Underwent Liver Transplantation for Hepatoblastoma. Journal of pediatric gastroenterology and nutrition. 2018 Jan:66(1):e21-e23. doi: 10.1097/MPG.0000000000001725. Epub [PubMed PMID: 28837514]
Zhong S, Zhao Y, Fan C. Hepatoblastoma with pure fetal epithelial differentiation in a 10-year-old boy: A rare case report and review of the literature. Medicine. 2018 Jan:97(2):e9647. doi: 10.1097/MD.0000000000009647. Epub [PubMed PMID: 29480877]
Level 3 (low-level) evidencePateva IB, Egler RA, Stearns DS. Hepatoblastoma in an 11-year-old: Case report and a review of the literature. Medicine. 2017 Jan:96(2):e5858. doi: 10.1097/MD.0000000000005858. Epub [PubMed PMID: 28079820]
Level 3 (low-level) evidenceLópez-Terrada D, Alaggio R, de Dávila MT, Czauderna P, Hiyama E, Katzenstein H, Leuschner I, Malogolowkin M, Meyers R, Ranganathan S, Tanaka Y, Tomlinson G, Fabrè M, Zimmermann A, Finegold MJ, Children's Oncology Group Liver Tumor Committee. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 Mar:27(3):472-91. doi: 10.1038/modpathol.2013.80. Epub 2013 Sep 6 [PubMed PMID: 24008558]
Level 3 (low-level) evidenceHiyama E. Pediatric hepatoblastoma: diagnosis and treatment. Translational pediatrics. 2014 Oct:3(4):293-9. doi: 10.3978/j.issn.2224-4336.2014.09.01. Epub [PubMed PMID: 26835349]
Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Current opinion in pediatrics. 2014 Feb:26(1):29-36. doi: 10.1097/MOP.0000000000000042. Epub [PubMed PMID: 24362406]
Level 3 (low-level) evidenceUchida H, Sakamoto S, Sasaki K, Takeda M, Hirata Y, Fukuda A, Hishiki T, Irie R, Nakazawa A, Miyazaki O, Nosaka S, Kasahara M. Surgical treatment strategy for advanced hepatoblastoma: Resection versus transplantation. Pediatric blood & cancer. 2018 Dec:65(12):e27383. doi: 10.1002/pbc.27383. Epub 2018 Aug 7 [PubMed PMID: 30084209]
Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, Aronson DC, Malogolowkin MH, Perilongo G, von Schweinitz D, Ansari M, Lopez-Terrada D, Tanaka Y, Alaggio R, Leuschner I, Hishiki T, Schmid I, Watanabe K, Yoshimura K, Feng Y, Rinaldi E, Saraceno D, Derosa M, Czauderna P. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. The Lancet. Oncology. 2017 Jan:18(1):122-131. doi: 10.1016/S1470-2045(16)30598-8. Epub 2016 Nov 22 [PubMed PMID: 27884679]
Dall'Igna P, Brugieres L, Christin AS, Maibach R, Casanova M, Alaggio R, de Goyet JV, Zsiros J, Morland B, Czauderna P, Childs M, Aronson DC, Branchereau S, Brock P, Perilongo G. Hepatoblastoma in children aged less than six months at diagnosis: A report from the SIOPEL group. Pediatric blood & cancer. 2018 Jan:65(1):. doi: 10.1002/pbc.26791. Epub 2017 Sep 18 [PubMed PMID: 28921839]
Kiruthiga KG, Ramakrishna B, Saha S, Sen S. Histological and immunohistochemical study of hepatoblastoma: correlation with tumour behaviour and survival. Journal of gastrointestinal oncology. 2018 Apr:9(2):326-337. doi: 10.21037/jgo.2018.01.08. Epub [PubMed PMID: 29755772]
Wu JF, Chang HH, Lu MY, Jou ST, Chang KC, Ni YH, Chang MH. Prognostic roles of pathology markers immunoexpression and clinical parameters in Hepatoblastoma. Journal of biomedical science. 2017 Aug 29:24(1):62. doi: 10.1186/s12929-017-0369-1. Epub 2017 Aug 29 [PubMed PMID: 28851352]
Shanmugam N, Scott JX, Kumar V, Vij M, Ramachandran P, Narasimhan G, Reddy MS, Kota V, Munirathnam D, Kelgeri C, Sundaram K, Rela M. Multidisciplinary management of hepatoblastoma in children: Experience from a developing country. Pediatric blood & cancer. 2017 Mar:64(3):. doi: 10.1002/pbc.26249. Epub 2016 Oct 26 [PubMed PMID: 27781375]