Introduction
The hepatitis A virus (HAV) is a common infectious etiology of acute hepatitis worldwide. HAV is most commonly transmitted through the fecal-oral route via exposure to contaminated food, water, or close physical contact with an infectious person. According to the World Health Organization, infection rates in high-resource countries are low. However, high-risk groups include injected drug users, men who have sex with men, people traveling to endemic areas, and isolated communities.
HAV does not cause chronic liver disease, unlike hepatitis B or C.[1] Acute HAV infection usually presents as a self-limited illness; the development of fulminant hepatitis is rare. Typical symptoms of acute infection include nausea, vomiting, abdominal pain, fatigue, malaise, poor appetite, and fever; management is with supportive care. Alternate clinical patterns include cholestatic, prolonged, and relapsing disease.
Vaccination against HAV is recommended for children 12 months or older and adults with the risk of exposure, including travelers to endemic countries, men who have sex with men, people who use drugs (injected and non injected), potential occupational exposure, and chronic liver disease.[2][3][4][5] Globally, the rates of HAV have decreased due to improvements in public healthcare policies, sanitation, and education, but infection rates of other hepatitis viruses appear to be increasing.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
HAV is one of the most common causes of acute hepatitis infection worldwide. The discovery of a virus-like antigen in 1973 via immune electron microscopy marked a pivotal advancement in comprehending acute infectious hepatitis, culminating in identifying HAV.[6] Initially categorized as enterovirus type 72, HAV is now classified as the sole member of the Hepatovirus genus within Picornaviridae.[7] Despite sharing structural similarities with insect-infecting picornaviruses and having evolutionary roots in bats, rodents, and shrews, HAV exhibits distinctive features in its life cycle and hepatotropism.[8]
This nonenveloped, icosahedral virus, measuring 27 to 32 nm, is transmitted fecal-orally and displays notable differences in its physical form when excreted versus circulating in the blood. Genomic analysis reveals 6 genotypes, 3 infecting humans and 3 found in primates. Genotype distribution varies geographically, with human infections predominantly involving genotype 1. The HAV genome, a 7.5 kb positive-sense single-stranded ribonucleic acid, contains an internal ribosome entry site, modified capsid proteins, and specific replication elements.
Hepatitis A Risk Factors
Factors that increase an individual's risk of HAV infection include:
- Close personal contact with infected persons (eg, household members, caretakers, sexual partners)
- Close contact with international adoptees from high endemicity regions
- Occupational exposure (eg, laboratory workers and primate handlers)
- Injection and non injection drug use
- High-population group settings (eg, homeless shelters, syringe service programs, and correctional facilities)
- International travel to regions with high or intermediate HAV endemicity
Additionally, risk factors for severe HAV disease include:
- Immunocompromised persons (eg, human immunodeficiency virus, chronic renal failure, transplant recipients, and those on immunosuppressive therapy),
- Chronic liver disease (eg, hepatitis B or C infection, cirrhosis, fatty liver disease, and elevated liver enzymes)
- Adults older than 40
- Pregnancy
Epidemiology
Improved sanitation has resulted in a shift in the age group that acquires hepatitis A, which has led to a decline in the incidence of new cases in recent years. The United States has a low endemicity; however, Mexico has a high prevalence of individuals with anti-HAV antibodies, indicating a previous infection. The frequency of acute hepatitis is higher in countries that are adjacent to Mexico. The reported hepatitis A incidence has declined by 90% to as low as 1.2 cases per 100,000 population, with the most significant reductions seen in children and countries where routine vaccination started in 1999.[9]
Over the last 4 decades, the average age of hepatitis A-infected individuals has increased, and those in high-risk populations account for most cases of HAV infection. The classification of HAV endemicity is based on the age-specific seroprevalence of anti-HAV immunoglobulin G (IgG). Age-associated seroprevalence surveys of anti-HAV IgG are essential for elucidating historical infection rates and population susceptibility. However, incidence studies relying on case reports are often faced with data incompleteness and bias.
Geographical Incidence and Transmission Patterns
Individuals in high-endemicity regions such as sub-Saharan Africa and certain parts of South Asia exhibit 90% or more anti-HAV IgG positivity by age 10. These areas experience low disease rates, with most infections occurring in early childhood (ie, younger than 5). Transmission is predominantly interpersonal, facilitated by inadequate sanitation.
Patients in medium-endemicity areas, including Asia, Latin America, Eastern Europe, and the Middle East, demonstrate 50% or more seroprevalence by age 15 but less than 90% by age 10. These regions face high disease rates, affecting late childhood and young adulthood. Transmission occurs via person-to-person contact and through contaminated food and water sources during outbreaks.
Economically advanced regions, including Western Europe, Australia, New Zealand, Canada, the United States, Japan, and Singapore, are low endemicity areas with 50% or more seroprevalence by age 30 but less than 50% by age 15. These areas experience low disease rates, with infections primarily occurring in susceptible individuals. Transmission modes include interpersonal contact, food and water-related outbreaks, and travel-associated infections. Individuals in very low-endemicity regions (eg, Northern Europe) exhibit less than 50% seroprevalence by age 30. These areas experience low disease rates, with infections predominantly affecting susceptible individuals, similar to low-endemicity regions (CDC. Hepatitis A, https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/hepatitis-a).
Acute Liver Failure Incidence and Hepatitis A Infection
In regions with low endemicity and developed settings, HAV contributes to less than 2% of acute liver failure (ALF) cases.[10] However, in medium and high endemicity regions, the incidence is highly variable, ranging from around 2% in some adult studies to approximately 30% in certain series.[11][12] Recent observational studies from India indicate a concerning trend of symptomatic HAV infections in adolescents and adults, accompanied by an increased incidence of ALF and higher mortality rates among adolescents.
Age-Dependent Variations of Hepatitis A Infection
The clinical manifestations and outcomes of HAV infection exhibit significant age-dependent variations. In adults, symptomatic acute hepatitis A occurs in more than 70% of cases, with 3% to 20% experiencing relapsing or prolonged hepatitis.[13] ALF is rare, with less than 1% of adult cases, but when it does occur, approximately 30% may require liver transplantation. In contrast, children demonstrate a markedly different clinical profile, with fewer than 30% exhibiting symptomatic acute hepatitis A.
Adults and children share a high spontaneous recovery rate exceeding 99%, underscoring the self-limiting nature of HAV infection. However, children generally experience a more benign course, rarely progressing to ALF or developing prolonged or relapsing hepatitis. Despite these age-related differences, the overall prognosis remains favorable for both groups with appropriate supportive care. Most patients recover without significant medical intervention, although severe outcomes such as ALF and the need for liver transplantation are slightly more prevalent in adults.
These age-dependent clinical patterns highlight the generally milder course of HAV infection in pediatric populations and emphasize the importance of age as a factor in predicting disease progression and outcomes. The epidemiological impact of HAV is intricately linked to the average age of infection, with high-endemicity regions experiencing early, often asymptomatic infections in children. As incidence decreases and the average age of infection rises, more severe symptoms and increased healthcare costs are observed.
Pathophysiology
Following oral transmission, the virus enters the bloodstream from the gastrointestinal tract and is carried to the basolateral membrane of the hepatocyte via the portal circulation.[7] HAV exhibits 2 infectious forms: naked, nonenveloped virions (nHAV) and quasi-enveloped virions (eHAV). The naked virions, characterized by a highly conserved antigenic structure, are shed in feces, while eHAV, a distinctive feature of HAV, is secreted from infected cells and found in blood. The dual nature of HAV virions plays a crucial role in its pathogenesis and transmission, highlighting the virus's adaptation to different host environments.
HAV infection of hepatocytes occurs via the HAV cellular receptor-1, also known as the T-cell immunoglobulin mucin domain-1 receptor, in the space of Disse. Results from recent studies suggest alternative entry pathways.[14] A complex interplay of innate and adaptive immune mechanisms characterizes the host response to HAV infection. HAV employs strategies to evade innate immunity, wherein the viral proteases target key signaling molecules and thereby suppress the type I interferon response. However, innate immunity is not completely evaded as the plasmacytoid dendritic cells produce significant type I interferons in response to HAV-infected cells or eHAV.[15]
The adaptive immune response, particularly T-cell–mediated immunity, appears crucial in viral clearance, potentially causing hepatocellular injury. While human studies have identified virus-specific cytotoxic CD8 T cells, results from recent research in chimpanzee models suggest a more prominent role for CD4 helper T cells in infection resolution.[16][17] The dynamics of regulatory T cells during acute infection have also been implicated in the modulation of liver injury. Diagnosing acute hepatitis A primarily relies on detecting serum IgM antibodies to HAV or observing seroconversion in symptomatic individuals. Humans and primates exhibit delayed B-cell response due to the sequestration of viral antigens. A rapid, dominant, neutralizing IgG response targets conserved viral capsid protein epitopes, providing lifelong protection postinfection. Passive immunization or postexposure vaccination within 2 weeks can prevent liver disease, likely through post-endocytic neutralization of eHAV. Multifunctional virus-specific CD4 T cells correlate with declining intrahepatic viral ribonucleic acid, indicating their primary role in controlling infection and interferon-stimulated genes.[18]
Following replication in the liver, HAV is excreted in bile and released into the stool. The virus concentration is highest in the stool during the 2 weeks before the onset of jaundice, at which point the individual is most infectious (see Image. Hepatitis A Replication Cycle).[19] Most people are no longer contagious 1 week after jaundice appears, at which time stool shedding and viremia are decreased.
Histopathology
Liver biopsy is rarely performed in uncomplicated acute hepatitis A infections. When a biopsy is performed, histopathological examination reveals inflammation and necrosis in the periportal regions with varied levels of apoptosis.[20][21] While cholestasis accompanied by an increased presence of plasma cells in the portal and periportal areas can be more indicative of hepatitis A, definitive diagnosis necessitates laboratory confirmation. In cases of severe hepatitis, necrotic bridges may form between portal areas.
History and Physical
Hepatitis A Clinical Features
The following clinical presentations are frequently associated with HAV infection:
- Asymptomatic infection
- Symptomatic infection with jaundice, dark urine, and clay-colored stool
- Cholestatic hepatitis with prolonged alkaline phosphatase and bilirubin elevation and pruritus
- Relapsing infection
- Fulminant hepatitis [15]
HAV infection usually presents with an acute onset, similar to other hepatotropic virus infections, following an average incubation period of approximately 28 days (15-50 days).[22] The clinical course is characterized by initial prodromal symptoms, including malaise, vomiting, anorexia, fever, and right upper quadrant abdominal pain, followed by a more severe symptomatic phase. This phase, typically manifesting 1 week after the initial symptoms, may include fever, malaise, anorexia, nausea, abdominal discomfort, and jaundice, persisting for a median duration of 8 weeks.[23]
The most prevalent symptoms include jaundice, observed in 40% to 80% of cases, and dark urine, reported by 68% to 94% of patients. Fatigue affects 52% to 91% of individuals, while loss of appetite or anorexia is noted in 42% to 90% of cases during the prodromal phase. Abdominal pain or discomfort is experienced by a third during the various stages. Half of the patients report cholestatic symptoms, including acholic stools. The severity and manifestation of symptoms demonstrate a notable age-dependent pattern, with children younger than 6 years often presenting asymptomatically or with mild symptoms. In contrast, older children and adults exhibit more pronounced symptomatology, including jaundice, in more than 70% of cases. Peak infectivity occurs 2 weeks before and at least 1 week following symptom onset. The acute illness typically resolves within 2 months, with a median duration of 2 weeks.
Physical examination reveals icterus and tender hepatomegaly. Acute HAV is associated with self-limiting pleural effusion, usually mild and undetected on examination. In the cholestatic phase, scratch marks and shiny nails can be observed. Extrahepatic manifestations are rarely associated with splenomegaly, rash, lymphadenopathy, and arthritis. A high index of suspicion must be kept for identifying hepatic encephalopathy and signs of coagulopathy, though it is a rare complication.
Cholestatic hepatitis presentations
Marked by prolonged jaundice, cholestatic symptoms, including pruritus and acholic stools, with the labs showing elevated serum bilirubin and alkaline phosphatase levels, occur in less than 5% of patients. Cholestatic hepatitis typically resolves spontaneously without sequelae. Rarely, patients with intractable pruritus might require specific therapy.
Relapsing infection presentations
Although HAV does not progress to a chronic stage or result in chronic viral shedding, cases of prolonged illness with recurrent symptoms or ALF have been documented and affect up to 10% of patients, with episodes of relapse lasting up to 12 months. These protracted cases may involve delayed viral clearance, persistent symptoms, elevated liver enzymes, and severe cholestasis, with some instances of relapsing hepatitis and detectable HAV ribonucleic acid.
Up to 10% of patients experience a relapse of symptoms within 6 months after the acute illness. Clinical relapse generally lasts less than 3 weeks, though biochemical relapse can persist for up to 12 months. The cause of relapsing hepatitis remains unknown, and no predisposing factors have been identified. Clinical relapse typically follows apparent recovery, with near normalization of serum aminotransferases, followed by biochemical and sometimes clinical relapse.
Manifestations during relapse are often milder than the initial episode, but serum aminotransferases can exceed 1000 IU/dL, and serum anti-HAV IgM antibodies persist throughout the disease. HAV can be recovered from stool during relapses, indicating continued infectivity. During relapses, extrahepatic manifestations such as arthritis, vasculitis, nephritis, and cryoglobulinemia have been reported. Autoimmune hepatitis may be triggered by HAV infection in susceptible individuals, characterized by hyperglobulinemia, circulating autoantibodies, and inflammatory liver changes on histology, which might be another cause for this pattern.
Acute liver failure and fulminant hepatitis presentations
Individuals with ALF and fulminant hepatitis frequently present with coagulopathy and altered sensorium after the onset of jaundice. The risk of ALF and mortality increases with age and preexisting liver conditions, with an overall case-fatality ratio of approximately 0.3%. Superinfection with HAV in patients with chronic hepatitis C can lead to severe outcomes, including ALF, and may trigger autoimmune mechanisms exacerbating liver damage. Coinfection with other viruses (eg, hepatitis G) has been observed, although the role of HAV in severe cases remains unclear.
Hepatitis A and Pregnancy
Acute HAV infection is associated with increased risks of preterm labor and gestational complications.[24] Extrahepatic manifestations, occurring in 10% to 15% of cases, include rash, arthralgias, leukocytoclastic vasculitis, arthritis, glomerulonephritis, cryoglobulinemia, optic neuritis, transverse myelitis, toxic epidermal necrolysis, myocarditis, thrombocytopenia, aplastic anemia, and red cell aplasia.[25]
Evaluation
In patients presenting with the syndrome of acute hepatitis with characteristic clinical features as mentioned above, an HAV infection is suspected, and laboratory tests and exclusion of other conditions are necessary for the diagnosis. Biochemical parameters include a characteristic pattern in the liver function tests observed across various acute viral hepatitis presentations. Elevated bilirubin, serum aminotransferases, and alkaline phosphatase (ALP) are noted. The chronology of this derangement and the pattern of aminotransferase elevation are essential. Among the transaminases, the alanine aminotransferase level (ALT) is generally higher than in cases of other viral hepatitis and can rise 10- to 100-fold. Typically, the bilirubin level starts to increase later. Blood work usually reveals mild lymphocytosis and normal prothrombin time. If the prothrombin time becomes deranged, severe liver damage, including the risk for encephalopathy, should be suspected.
Shortly after the ALT levels increase, HAV particles can be detected in the stool, though stool is not routinely tested. Anti-HAV IgM starts to rise after this, and the clinical symptoms appear shortly after the initial excretion of particles. When differential diagnoses are excluded, the presence of anti-HAV IgM and characteristic clinical features confirms the diagnosis of hepatitis A. Anti-HAV IgM usually remains positive for 4 to 6 months. Additional testing can include reverse transcriptase-polymerase chain reaction to detect viral ribonucleic acid, which can show false negative results in an acute infection. Anti-HAV IgG emerges soon after infection and remains present for the person’s lifetime.
An abdominal ultrasound can help exclude other causes, including differentiating between extrahepatic and intrahepatic cholestasis and evaluating fever with abdominal pain. However, findings are frequently nonspecific (eg, gallbladder wall and periportal edema). Pleural effusion and perihepatic fluid are seen on rare occasions while visualizing the liver.
Acute liver failure should be suspected in cases with deranged prolonged prothrombin time and monitoring for minimal hepatic encephalopathy. Close monitoring of prothrombin time and liver function tests are mandated. Signs of raised intracranial pressures (ICT) should be carefully monitored, as bradycardia, hypertension, and altered breathing patterns can indicate raised ICT. ICT can be monitored by transcranial Doppler, optic nerve sheath diameter, and reverse jugular monitoring. Moreover, a noncontrast computed tomography (CT) of the brain can demonstrate changes secondary to increased ICT. Deranged kidney function tests are a poor prognostic marker. Clinicians should also exclude sepsis. Evaluation and management are like any other case of ALF.
In the cholestatic variant of hepatitis A infection, ALP and gamma-glutamyl transferase are raised along with cholestatic symptoms, typical of intrahepatic cholestasis. Pruritus can be worrisome; testing of individuals with pruritus will typically have findings that show elevated bile acid levels. Usually, cholestasis does not persist long enough to cause a severe deficiency of fat-soluble vitamins.
Treatment / Management
Supportive Management
No specific treatment is needed for most patients with acute, uncomplicated HAV infection beyond supportive care. In individuals with fulminant hepatitis within regions where universal immunization is not possible, antiviral treatment would be helpful; this type of therapy is currently not available for HAV. Cholestatic hepatitis A is also self-limiting; if pruritus is severe, antipruritic measures, including cholestyramine, rifampicin, or ondansetron, may be used. Refractory pruritus, though a self-limiting condition, can rarely require plasma exchange.
Severe HAV infections with ALF should be managed like other ALF cases. The Kings College criteria give a lower priority to ALF due to acute viral hepatitis as these are less severe. Despite the implementation of priority scoring systems like the Model for End-Stage Liver Disease (MELD) score or King’s College criteria for liver transplantation referrals, it remains challenging to determine which patients with hepatitis A-associated ALF will need a transplant due to the lack of reliable biomarkers. However, Kings College criteria may not apply to high endemicity regions with higher prevalence where dynamic prognostication models have been developed.[26]
Patients exhibiting worsening hepatic synthetic function require special considerations. Hepatic synthetic dysfunction is indicated by coagulopathy with prolonged international normalized ratio, increasing creatinine, serum albumin levels below 3%, rising blood ammonia levels, and clinical signs of hepatic encephalopathy, necessitating prompt transfer to an intensive care unit, ideally at a liver transplant center. Patients should be classified using the United Network for Organ Sharing liver failure medical urgency classification (eg, MELD 1 or PELD 2) or by assigning a status (eg, 1A or 1B) for liver allocation based on country-specific guidelines and the availability of access to a transplant center. Extrahepatic complications are managed accordingly.
In rare cases of severe relapse and cholestatic symptoms, patients have been given a short course of corticosteroids based on anecdotal evidence and limited case reports, but randomized controlled trials are lacking.[27] Corticosteroids are also used for pure red cell aplasia caused by hepatitis A and have been tried in children with fulminant liver failure from hepatitis A.[28][29][30][31][32][33][34][35] N-acetyl cysteine was not found to be useful.[36](B2)
Differential Diagnosis
Differential diagnoses that should also be considered include:
- Other acute hepatitis viral infections (eg, hepatitis E virus and hepatitis B virus)
- Non-hepatotropic viral infection (eg, dengue virus, cytomegalovirus infection, herpes simplex virus infection, Ebstein-Barr virus)
- Parasitic and zoonotic infectious diseases (eg, leptospiral and rickettsial infections, Q fever, malaria, and tropical fever syndromes)
- Drug-induced liver injury
- Autoimmune hepatitis
- Ischemic hepatitis
- Alcohol-associated hepatitis
- Wilson disease
- Acute Budd-Chiari syndrome
Prognosis
The outcomes for most patients with HAV are excellent. After an infection, long-term immunity is common, and unlike other viral hepatitis infections, recurrence of symptoms is rare. Death is rare but may occur in older individuals or those with underlying liver disease. In the United States, approximately 100 deaths from HAV occur every year. Liver transplant is rarely undertaken in children with fulminant disease. The most important prognostic factor is age; the older the individual, the more likely an adverse reaction or event may occur. Long-term sequelae are very rare.
Complications
Complications of HAV include:
- Acute liver failure and fulminant hepatitis
- Acute liver injury
- Prolonged cholestasis
- Acute-on-chronic liver failure in patients with preexisting hepatitis C virus infection or metabolic dysfunction associated steatotic liver disease
- Severe complications associated with HAV infection including pericarditis, renal failure, thrombocytopenia, acute pancreatitis, aplastic anemia, autoimmune hemolytic anemia, Guillain-Barré syndrome, vasculitis, and arthritis
Deterrence and Patient Education
Patient Education
Patient education efforts are tailored to specific at-risk populations, including persons who use injection and non-injection drugs, men who have sex with men, and individuals with chronic liver diseases, to address their unique vulnerabilities. Healthcare professionals should receive specialized training to enhance their ability to recognize HAV symptoms, comprehend transmission dynamics, and effectively educate patients. Travel-related education focuses on pre-travel vaccination and safe practices in high-endemicity areas. Management of the close contacts of patients with HAV involves education on postexposure prophylaxis and hygiene measures to prevent further transmission. During outbreaks, emphasis is placed on early identification, implementation of preventive measures, and coordinated vaccination campaigns.
Prevention Strategies
The cornerstone of preventing HAV transmission involves rigorous hygiene practices, emphasizing food and water safety, and advocating for widespread vaccination, particularly for high-risk groups and travelers to endemic regions. Preventing HAV infection requires public health measures such as improving hygiene, sanitation, socioeconomic conditions, immunoglobulin prophylaxis, and vaccination.[37]
Data on HAV's persistence on surfaces, soil, water, food, and inactivation methods are limited. HAV can remain infectious on some foods for days and on frozen berries, feces, and soil for months. On surfaces, HAV can survive for months, influenced by temperature and humidity, and on hands for hours; however, thorough handwashing can remove the virus. HAV resists low pH, mild heat, pasteurization, and freezing. Personal hygiene and environmental sanitation are crucial to preventing HAV transmission through fecal contamination or personal contact. Monitoring HAV ribonucleic acid in sewage helps assess transmission risks.
Universal pediatric vaccination is the most effective control measure in intermediate-risk regions. Before the advent of HAV vaccines, short-term pre-exposure and postexposure prophylaxis involved injecting intramuscular human immunoglobulin. Effective HAV vaccines have reduced immunoglobulin use due to their high cost, limited availability, and shorter protection duration compared to vaccines. Currently, the only indication of immunoglobulin is probably in individuals with hypersensitivity to vaccine components.
In the United States, vaccination against hepatitis A is available as inactivated, single-antigen vaccines (HAVRIX and VAQTA) or in combination with hepatitis B (TWINRIX). The Centers for Disease Control and Prevention recommends vaccination for children 12 months or older, travelers to endemic countries, men who have sex with men, persons with human immunodeficiency virus infection, persons who use injection and noninjection drugs, and individuals with occupational risk exposure or chronic liver disease. Standard adult dosing recommends administration of 2 doses of the vaccine 6 to 12 months apart. These vaccines are highly efficacious, with seroconversion rates approaching 100%. Live-attenuated HAV vaccines, licensed in China since 1992, have shown high efficacy and long-lasting immunity after a single dose. China's extensive infant vaccination program has significantly increased coverage and reduced hepatitis A incidence. Vaccination has proven effective in large-scale hepatitis A prevention, supporting its role in public health strategies.[38]
Enhancing Healthcare Team Outcomes
Effective management of hepatitis A infection, especially in individuals at higher risk for adverse outcomes, requires a coordinated effort from an interprofessional healthcare team. Physicians, advanced clinicians, nurses, pharmacists, and other healthcare professionals must collaborate to deliver patient-centered care. Specialists and infectious disease experts are responsible for diagnosing and overseeing the treatment plan, while primary care clinicians ensure that patients receive appropriate supportive care.
Pharmacists, advanced clinicians, and physicians are crucial in educating patients, particularly travelers to endemic areas, about the hepatitis A vaccine and preventive measures like proper hygiene and safe food practices. Nurses, especially those in infectious disease, must also counsel patients on necessary precautions to prevent the spread of the virus, such as adhering to strict enteric precautions and avoiding certain high-risk behaviors. Clear, interprofessional communication and care coordination are essential to enhance patient outcomes, safety, and overall team performance, ensuring that each professional's expertise contributes to comprehensive and effective care for patients with hepatitis A.
Media
(Click Image to Enlarge)
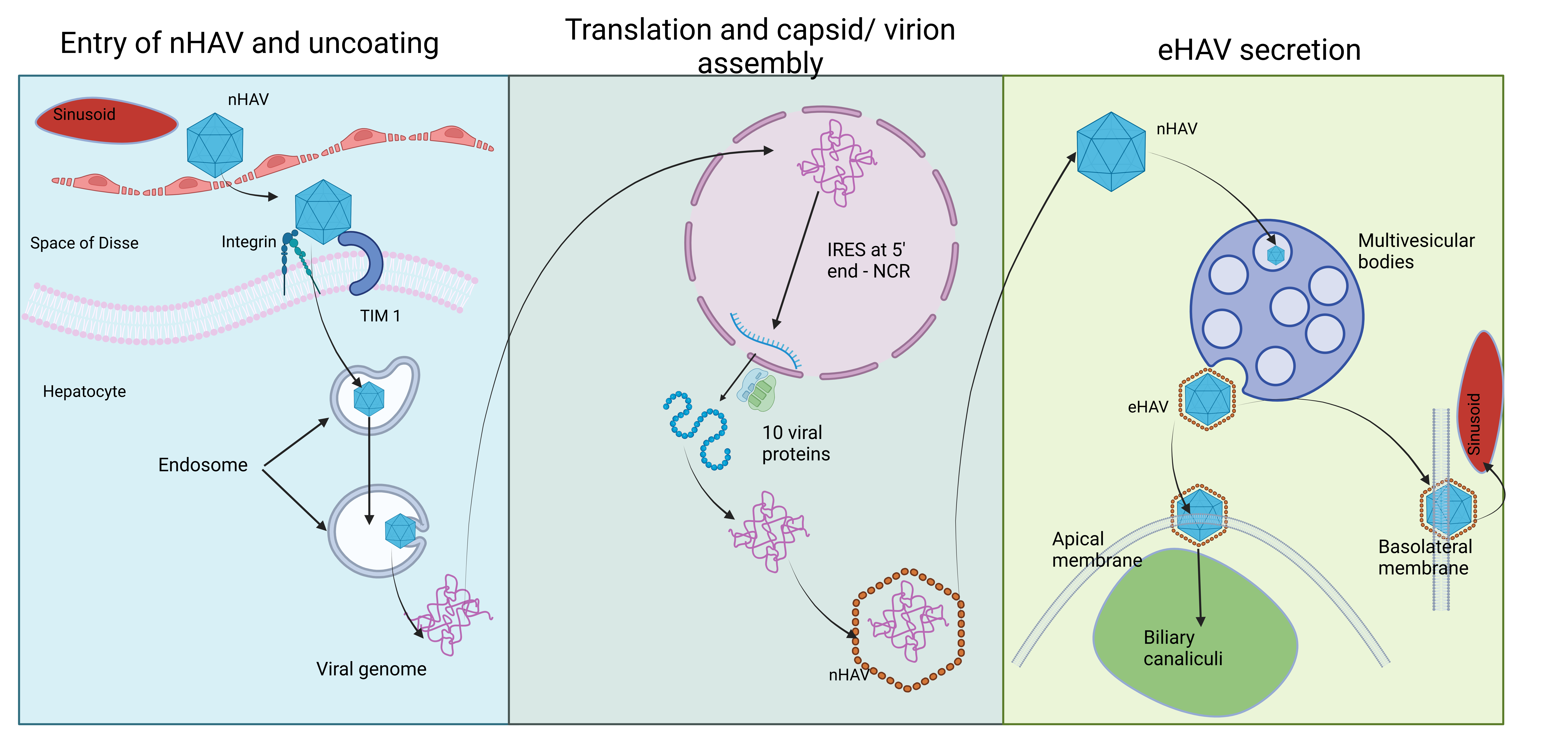
Hepatitis A Replication Cycle. The HAV replication cycle begins with virus attachment to hepatocytes via integrins and TIM1 receptors, followed by endosomal entry. In early endosomes, the virus dissociates from receptors, while in more acidic late endosomes capsid changes trigger RNA release into the cytoplasm. IRES-mediated translation produces a viral polyprotein, which undergoes proteolytic processing. Genome replication occurs on membrane structures, followed by virion assembly. New particles are released via multivesicular bodies (MVBs) as quasi-enveloped HAV (eHAV). Basolaterally released eHAV enters the bloodstream, while apically released eHAV enters the biliary tract, where bile salts remove the quasi-envelope, producing naked HAV (nHAV) for fecal shedding and transmission. This cycle encompasses key steps from attachment to transmission, highlighting HAV's unique quasi-enveloped state and bile salt-mediated envelope removal.
Contributed by V Girish, MD
References
Odenwald MA, Paul S. Viral hepatitis: Past, present, and future. World journal of gastroenterology. 2022 Apr 14:28(14):1405-1429. doi: 10.3748/wjg.v28.i14.1405. Epub [PubMed PMID: 35582678]
Alberts CJ, Boyd A, Bruisten SM, Heijman T, Hogewoning A, Rooijen MV, Siedenburg E, Sonder GJB. Hepatitis A incidence, seroprevalence, and vaccination decision among MSM in Amsterdam, the Netherlands. Vaccine. 2019 May 9:37(21):2849-2856. doi: 10.1016/j.vaccine.2019.03.048. Epub 2019 Apr 13 [PubMed PMID: 30992222]
Johnson KD, Lu X, Zhang D. Adherence to hepatitis A and hepatitis B multi-dose vaccination schedules among adults in the United Kingdom: a retrospective cohort study. BMC public health. 2019 Apr 15:19(1):404. doi: 10.1186/s12889-019-6693-5. Epub 2019 Apr 15 [PubMed PMID: 30987613]
Level 2 (mid-level) evidenceBrennan J, Moore K, Sizemore L, Mathieson SA, Wester C, Dunn JR, Schaffner W, Jones TF. Notes from the Field: Acute Hepatitis A Virus Infection Among Previously Vaccinated Persons with HIV Infection - Tennessee, 2018. MMWR. Morbidity and mortality weekly report. 2019 Apr 12:68(14):328-329. doi: 10.15585/mmwr.mm6814a3. Epub 2019 Apr 12 [PubMed PMID: 30973852]
Wilson E, Hofmeister MG, McBee S, Briscoe J, Thomasson E, Olaisen RH, Augustine R, Duncan E, Bamrah Morris S, Haddy L. Notes from the Field: Hepatitis A Outbreak Associated with Drug Use and Homelessness - West Virginia, 2018. MMWR. Morbidity and mortality weekly report. 2019 Apr 12:68(14):330-331. doi: 10.15585/mmwr.mm6814a4. Epub 2019 Apr 12 [PubMed PMID: 30973849]
Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science (New York, N.Y.). 1973 Dec 7:182(4116):1026-8 [PubMed PMID: 4356028]
. Hepatitis A virus infection. Nature reviews. Disease primers. 2023 Sep 28:9(1):52. doi: 10.1038/s41572-023-00467-w. Epub 2023 Sep 28 [PubMed PMID: 37770501]
Sander AL, Corman VM, Lukashev AN, Drexler JF. Evolutionary Origins of Enteric Hepatitis Viruses. Cold Spring Harbor perspectives in medicine. 2018 Dec 3:8(12):. doi: 10.1101/cshperspect.a031690. Epub 2018 Dec 3 [PubMed PMID: 29610146]
Level 3 (low-level) evidenceKlevens RM, Miller JT, Iqbal K, Thomas A, Rizzo EM, Hanson H, Sweet K, Phan Q, Cronquist A, Khudyakov Y, Xia GL, Spradling P. The evolving epidemiology of hepatitis a in the United States: incidence and molecular epidemiology from population-based surveillance, 2005-2007. Archives of internal medicine. 2010 Nov 8:170(20):1811-8. doi: 10.1001/archinternmed.2010.401. Epub [PubMed PMID: 21059974]
Level 2 (mid-level) evidenceTaylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ, US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology (Baltimore, Md.). 2006 Dec:44(6):1589-97 [PubMed PMID: 17133489]
Shalimar, Kedia S, Gunjan D, Sonika U, Mahapatra SJ, Nayak B, Kaur H, Acharya SK. Acute Liver Failure Due to Hepatitis E Virus Infection Is Associated with Better Survival than Other Etiologies in Indian Patients. Digestive diseases and sciences. 2017 Apr:62(4):1058-1066. doi: 10.1007/s10620-017-4461-x. Epub 2017 Jan 27 [PubMed PMID: 28130708]
Alam S, Khanna R, Sood V, Lal BB, Rawat D. Profile and outcome of first 109 cases of paediatric acute liver failure at a specialized paediatric liver unit in India. Liver international : official journal of the International Association for the Study of the Liver. 2017 Oct:37(10):1508-1514. doi: 10.1111/liv.13370. Epub 2017 Mar 3 [PubMed PMID: 28111909]
Level 3 (low-level) evidenceShin EC, Jeong SH. Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A. Cold Spring Harbor perspectives in medicine. 2018 Sep 4:8(9):. doi: 10.1101/cshperspect.a031708. Epub 2018 Sep 4 [PubMed PMID: 29440324]
Level 3 (low-level) evidenceDas A, Rivera-Serrano EE, Yin X, Walker CM, Feng Z, Lemon SM. Cell entry and release of quasi-enveloped human hepatitis viruses. Nature reviews. Microbiology. 2023 Sep:21(9):573-589. doi: 10.1038/s41579-023-00889-z. Epub 2023 Apr 25 [PubMed PMID: 37185947]
Lemon SM, Ott JJ, Van Damme P, Shouval D. Type A viral hepatitis: A summary and update on the molecular virology, epidemiology, pathogenesis and prevention. Journal of hepatology. 2017 Sep 5:():. pii: S0168-8278(17)32278-X. doi: 10.1016/j.jhep.2017.08.034. Epub 2017 Sep 5 [PubMed PMID: 28887164]
Vallbracht A, Fleischer B, Busch FW. Hepatitis A: hepatotropism and influence on myelopoiesis. Intervirology. 1993:35(1-4):133-9 [PubMed PMID: 8407240]
Lanford RE, Walker CM, Lemon SM. The Chimpanzee Model of Viral Hepatitis: Advances in Understanding the Immune Response and Treatment of Viral Hepatitis. ILAR journal. 2017 Dec 1:58(2):172-189. doi: 10.1093/ilar/ilx028. Epub [PubMed PMID: 29045731]
Level 3 (low-level) evidenceMisumi I, Mitchell JE, Lund MM, Cullen JM, Lemon SM, Whitmire JK. T cells protect against hepatitis A virus infection and limit infection-induced liver injury. Journal of hepatology. 2021 Dec:75(6):1323-1334. doi: 10.1016/j.jhep.2021.07.019. Epub 2021 Jul 29 [PubMed PMID: 34331968]
Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology. 2010:53(1):15-9. doi: 10.1159/000252779. Epub 2010 Jan 5 [PubMed PMID: 20068336]
Okuno T, Sano A, Deguchi T, Katsuma Y, Ogasawara T, Okanoue T, Takino T. Pathology of acute hepatitis A in humans. Comparison with acute hepatitis B. American journal of clinical pathology. 1984 Feb:81(2):162-9 [PubMed PMID: 6695857]
Usuda D, Kaneoka Y, Ono R, Kato M, Sugawara Y, Shimizu R, Inami T, Nakajima E, Tsuge S, Sakurai R, Kawai K, Matsubara S, Tanaka R, Suzuki M, Shimozawa S, Hotchi Y, Osugi I, Katou R, Ito S, Mishima K, Kondo A, Mizuno K, Takami H, Komatsu T, Nomura T, Sugita M. Current perspectives of viral hepatitis. World journal of gastroenterology. 2024 May 14:30(18):2402-2417. doi: 10.3748/wjg.v30.i18.2402. Epub [PubMed PMID: 38764770]
Level 3 (low-level) evidenceLemon SM. Hepatitis A: Current view of an ancient disease. Journal of hepatology. 2022 Jul:77(1):243-244. doi: 10.1016/j.jhep.2021.09.028. Epub 2022 May 2 [PubMed PMID: 35513903]
Abutaleb A, Kottilil S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterology clinics of North America. 2020 Jun:49(2):191-199. doi: 10.1016/j.gtc.2020.01.002. Epub 2020 Mar 29 [PubMed PMID: 32389358]
Elinav E, Ben-Dov IZ, Shapira Y, Daudi N, Adler R, Shouval D, Ackerman Z. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology. 2006 Apr:130(4):1129-34 [PubMed PMID: 16618407]
Terrault NA, Levy MT, Cheung KW, Jourdain G. Viral hepatitis and pregnancy. Nature reviews. Gastroenterology & hepatology. 2021 Feb:18(2):117-130. doi: 10.1038/s41575-020-00361-w. Epub 2020 Oct 12 [PubMed PMID: 33046891]
Shalimar, Sonika U, Kedia S, Mahapatra SJ, Nayak B, Yadav DP, Gunjan D, Thakur B, Kaur H, Acharya SK. Comparison of Dynamic Changes Among Various Prognostic Scores in Viral Hepatitis-Related Acute Liver Failure. Annals of hepatology. 2018 May-June:17(3):403-412. doi: 10.5604/01.3001.0011.7384. Epub 2018 Apr 9 [PubMed PMID: 29735790]
Yoon EL, Yim HJ, Kim SY, Kim JH, Lee JH, Lee YS, Lee HJ, Jung SW, Lee SW, Choi JH. Clinical courses after administration of oral corticosteroids in patients with severely cholestatic acute hepatitis A; three cases. The Korean journal of hepatology. 2010 Sep:16(3):329-33. doi: 10.3350/kjhep.2010.16.3.329. Epub [PubMed PMID: 20924218]
Level 3 (low-level) evidenceZakaria HM, Salem TA, El-Araby HA, Salama RM, Elbadry DY, Sira AM, Ali MA, Salem ME, Abd-Alaaty BM, Goda SS, Eltaras SM, Khalil FO, Abou-Zeinah SS, Sira MM. Steroid therapy in children with fulminant hepatitis A. Journal of viral hepatitis. 2018 Jul:25(7):853-859. doi: 10.1111/jvh.12873. Epub 2018 Feb 21 [PubMed PMID: 29397017]
Darchis I, Colombel JF, Cortot A, Morel L, Devienne A, Bauters F, Paris JC. Pure red cell aplasia during the course of virus A hepatitis. Gastroenterologie clinique et biologique. 1990:14(12):1027 [PubMed PMID: 2127029]
Tan EM, Marcelin JR, Virk A. Pre-travel counseling for immunocompromised travelers: A 12-year single-center retrospective review. Infection, disease & health. 2019 Feb:24(1):13-22. doi: 10.1016/j.idh.2018.09.083. Epub 2018 Oct 26 [PubMed PMID: 30541695]
Level 2 (mid-level) evidenceNelson NP, Link-Gelles R, Hofmeister MG, Romero JR, Moore KL, Ward JW, Schillie SF. Update: Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Postexposure Prophylaxis and for Preexposure Prophylaxis for International Travel. MMWR. Morbidity and mortality weekly report. 2018 Nov 2:67(43):1216-1220. doi: 10.15585/mmwr.mm6743a5. Epub 2018 Nov 2 [PubMed PMID: 30383742]
Gervasi G, Biticchi M, Zaratti L, Franco E. [Epidemics of Hepatitis A and opportunities for vaccination: a focus on the category of men who practice sex with men (MSM)]. Igiene e sanita pubblica. 2018 May-Jun:74(3):295-304 [PubMed PMID: 30235469]
Waszczuk K, Waszczuk E, Szenborn L. Can we better protect patients with inflammatory bowel disease against infections - patient attitude and personal immunization knowledge. Acta gastro-enterologica Belgica. 2018 Apr-Jun:81(2):257-261 [PubMed PMID: 30024696]
O'Leary ST, Kimberlin DW. Update From the Advisory Committee on Immunization Practices. Journal of the Pediatric Infectious Diseases Society. 2018 Aug 17:7(3):181-187. doi: 10.1093/jpids/piy050. Epub [PubMed PMID: 29961833]
Singh V, Crosby RA, Gratzer B, Gorbach PM, Markowitz LE, Meites E. Disclosure of Sexual Behavior Is Significantly Associated With Receiving a Panel of Health Care Services Recommended for Men Who Have Sex With Men. Sexually transmitted diseases. 2018 Dec:45(12):803-807. doi: 10.1097/OLQ.0000000000000886. Epub [PubMed PMID: 29944645]
Gunduz H, Karabay O, Tamer A, Ozaras R, Mert A, Tabak OF. N-acetyl cysteine therapy in acute viral hepatitis. World journal of gastroenterology. 2003 Dec:9(12):2698-700 [PubMed PMID: 14669316]
Dunn R, Wetten A, McPherson S, Donnelly MC. Viral hepatitis in 2021: The challenges remaining and how we should tackle them. World journal of gastroenterology. 2022 Jan 7:28(1):76-95. doi: 10.3748/wjg.v28.i1.76. Epub [PubMed PMID: 35125820]
Migueres M, Lhomme S, Izopet J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses. 2021 Sep 22:13(10):. doi: 10.3390/v13101900. Epub 2021 Sep 22 [PubMed PMID: 34696330]