Definition/Introduction
A common definition of aerospace medicine is the medicine of normal physiology in an abnormal environment. A subspecialty within occupational medicine, the challenge of aerospace medicine lies in safeguarding the health of individuals who work in a dynamic atmosphere of lowering pressure and oxygen content, coupled with increasing altitude. It is essential to comprehend the physical changes that occur as we ascend from sea level to address the medical needs of the pilot, aircrew, and aircraft passengers. Most notably, understanding the chemical gas laws that describe the characteristics of atmospheric gases, as well as those that reside within our bodies, is critical to the practice of aerospace medicine. Henry’s law is 1 of the most pertinent gas laws to aerospace medicine. Named after the English physician William Henry, this law defines the relationship between the partial pressure of gases overlying a solution and the gases’ ability to dissolve in that solution.[1]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
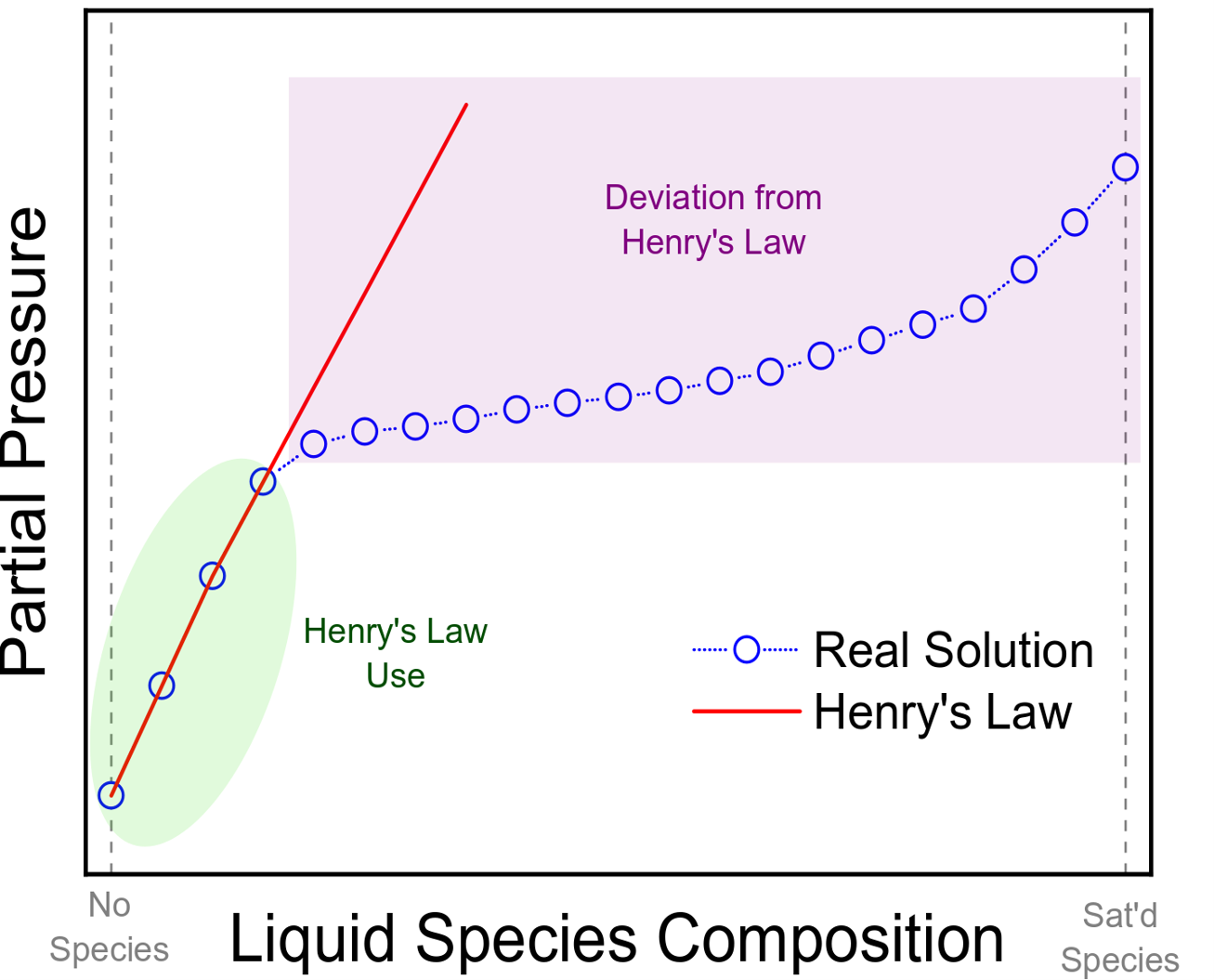
Henry's law states that when a gaseous mixture (eg, the atmosphere) is in contact with a solution, the amount of gas in that mixture that dissolves in the solution is directly proportionate to the partial pressure of that gas. The partial pressure of a gas is the amount of pressure that the gas contributes to the total pressure of that gas mixture. Per Henry's law, if the pressure of a gas over a liquid increases, the amount of gas dissolved in the liquid increases proportionally. Conversely, as the gas pressure decreases, the dissolved gas in the solution drops.
A person experiences Henry's law when they open a new soda pop bottle. Upon removing the cap, the carbon dioxide gas "atmosphere" in contact with the soda rushes out, and the gas pressure drops precipitously. In turn, less of the gas in the soda stays dissolved; the gas comes out of the solution as bubbles and foam. So long as adequate gas pressure is maintained over the liquid, the dissolved gases remain in the solution.[2]
Presented as a formula, Henry's law can be written as the following:
- P1 / A1 = P2 / A2
The left side shows the ratio of P1, the partial pressure of gas overlying a solution initially, to A1, the corresponding amount of gas dissolved in the solution at that pressure. Likewise, the right side is the ratio of the same gas at a different pressure, P2, and its corresponding amount of dissolved gas, A2, at this new pressure. Since the 2 sides are equal, a change in the size of P2 accompanies a corresponding change in A2.
For example, your blood is a solution containing multiple gases. Those gases remain in solution (ie, blood) at sea level. This is because, at sea level, air and arterial blood contain approximately the same partial pressure of gases, primarily nitrogen. As 1 rises in the atmosphere, the partial pressure decreases, and the amount of these gases held in solution (ie, blood) must decrease. This results in gas evolving in the bloodstream. As a result, the otherwise inert nitrogen supersaturates in the bloodstream or "bubbles out," like the soda pop example. While some oxygen can potentially evolve out of solution, the overwhelming majority of oxygen in the bloodstream is bound to hemoglobin, which prevents this occurrence.[3][4]
The unbound nitrogen bubbles in the vascular system result in various forms of decompression illness, a catchall term referring to both the discomfort associated with decompression sickness as well as more severe conditions like an arterial gas embolism. This problem can be partly controlled with the pressurization of the cabin. Additionally, so long as the rate of ascent is relatively slow, the risk of a supersaturation resulting in bubble formation is relatively low. It is largely because of this ascent at a controlled rate, and cabin pressurization that bubbles normally do not form during a commercial airline flight. In most commercial and military fixed-wing aircraft, pressurization is typically performed to maintain the partial pressure of a gas in the cabin to the equivalent of the pressure at 8000 feet (2438 meters). It is considered an acceptable risk to fly at elevations up to 12000 feet (3658 meters) unpressurized, though this practice is discouraged.[1]
The applications of Henry's law, however, do have their limitations. Henry's law is highly inaccurate at high concentrations of gas in the liquid phase. Low gas concentrations in a solution can be accurately captured with Henry's law.[3] See Image. Henry Law.
Clinical Significance
Henry’s law is significant to aerospace medicine in several ways. The most apparent is that it accurately describes the phenomena that result in decompression illness and affords the framework for the measures used to treat these conditions.
While Henry’s law can logically apply to both aerospace and underwater medicine, the manifestations of Henry’s law in flight do not perfectly correspond to those underwater. Specifically, manifestations such as decompression sickness directly relate to the absolute altitude achieved. While pressurization reduces the effects of decompression illness and can allow the pilot and other crew members to gradually acclimate to decreased air pressure, at higher elevations, even pressurized aircraft cannot fly safely due to the risk of supersaturation of inert gases in the bloodstream.
Current data suggests that a flyer in a pressurized cabin is less likely to experience decompression illness at an exposure equivalent of fewer than 18,000 feet (5,486 meters). In civilian and military aviation, the challenge of avoiding decompression illness tends to be higher in rotor-wing or helicopter aircraft, pilots, and crew. These aircraft have unique requirements that usually preclude cabin pressurization, and thus, they are forced to fly at lower altitudes to avoid the effects of Henry’s law on human physiology.
Several techniques are effective in preventing and treating conditions at altitude related to Henry’s law. Breathing gases used in high-altitude aircraft usually have a higher oxygen concentration than at sea level to avoid hypoxia and to help promote denitrogenation; by removing nitrogen, the hope is to avoid supersaturation effects at altitude. This approach is also the premise behind placing oxygen masks on oneself during an airline emergency with compromised cabin pressure. Cabin pressurization is the other significant preventive measure to reduce the risk of decompression.
The ideal option for resolving symptoms of bubble formation while in flight is threefold. First, drop in altitude, ideally to sea level, if possible. Second, oxygen should be used therapeutically to “blow off” nitrous gas. Finally, landing to permit evaluation by a flight surgeon is optimal to determine if further interventions are necessary. Suppose symptoms persist or significant central nervous system manifestations occur. In that case, it is essential to immediately transfer the patient to a hyperbaric chamber to pressurize the gas surrounding the patient and dissolve nitrogen gas back into solution in the bloodstream.[5]
Nursing, Allied Health, and Interprofessional Team Interventions
Nursing staff and other ancillary health team members who treat patients with diving and/or aerospace medical concerns would be well advised to have at least a basic understanding of the concepts surrounding Henry's law so they can effectively contribute to patient care and evaluation and communicate appropriately with clinicians about the physiological ramifications.
Media
(Click Image to Enlarge)
References
Ferraro G , Jadhav AJ , Barigou M . A Henry's law method for generating bulk nanobubbles. Nanoscale. 2020 Aug 7:12(29):15869-15879. doi: 10.1039/d0nr03332d. Epub 2020 Jul 22 [PubMed PMID: 32696779]
Jones MW, Brett K, Han N, Cooper JS, Wyatt HA. Hyperbaric Physics. StatPearls. 2024 Jan:(): [PubMed PMID: 28846268]
Dukes AD 3rd. Measuring the Henry's Law Constant for Carbon Dioxide and Water with UV-visible Absorption Spectroscopy. Analytical sciences : the international journal of the Japan Society for Analytical Chemistry. 2020 Aug 10:36(8):971-975. doi: 10.2116/analsci.19P477. Epub 2020 Feb 21 [PubMed PMID: 32092731]
Goldman S, Solano-Altamirano JM. An explicitly multi-component arterial gas embolus dissolves much more slowly than its one-component approximation. Mathematical biosciences. 2020 Aug:326():108393. doi: 10.1016/j.mbs.2020.108393. Epub 2020 Jun 1 [PubMed PMID: 32497622]
Van Poucke S, Hans G, Hens P. The release of dissolved gases from solution during decompression after hyperbaric treatment: effervescent tables and Henry's law. Anesthesiology. 2001 Sep:95(3):816 [PubMed PMID: 11575571]
Level 3 (low-level) evidence