Introduction
Immunoglobulin A vasculitis (IgAV), formerly Henoch-Schönlein purpura, involves the small vessels of the joints, kidneys, gastrointestinal tract, and skin. IgAV can also affect the central nervous system and the lungs; however, these findings are rare. It is an acute IgA-mediated disorder typically self-limited and managed with supportive care; however, serious complications, such as renal failure, can occur.
Henoch-Schönlein purpura is named after the German physician, Dr. Johann Schönlein and his student Eduard Henoch. Schönlein identified the association between joint pain and purpura, and Henoch identified gastrointestinal and renal involvement. Although Henoch-Schönlein purpura is named after Henoch and Schönlein, an English physician named William Heberden was the first to describe the disorder in the early 1800s.[1] Iga vasculitis is now the preferred term due to a tendency toward etiology-based rather than eponym-based nomenclature.
Of note, IgA vasculitis with nephritis has many overlapping features with IgA nephropathy, which is the most common glomerulonephritis in the world. The primary differences are that IgAV with nephritis is more likely to first occur in children younger than 15, while IgA nephropathy usually has an onset in patients older than 15. IgAV with nephritis is more likely to present with extrarenal symptoms; IgA nephropathy presents more often with gross hematuria. Histology in IgAV with nephritis shows more capillary staining and glomerular injury than in IgA nephropathy.
Finally, IgAV with nephritis has a 98% clinical remission; comparatively, patients with IgA nephropathy progress to end-stage renal disease within 20 years of diagnosis in 30% to 50% of cases.[2] Another interesting difference is that IgAV with nephritis presents more often during the winter "cold" season, while IgA nephropathy does not show seasonality. Much of the difference between the two disease processes results from IgAV being predominantly a disease of children and IgA nephropathy being primarily a disease of adults.[2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Environmental, genetic, and antigenic factors appear to contribute to the etiology of IgA vasculitis. Genomic studies have found an association with HLA-DQA1 and DQB1 intergenic zone, the HLA-DRB1*01:11/B1*13 loci, and DQA1*01:01/DQB1*05:01/DRB1*01:01 haplotype.[4] Many patients report a preceding infection. Upper respiratory tract infections are the most common; however, patients may also present with an antecedent gastrointestinal or pharyngeal infection.
Group A Streptococcus has been found in cultures of greater than 30% of patients with IgAV with nephritis.[5][6]
Other causative agents include.[5]
- Coxsackie virus
- Hepatitis A, hepatitis B (and hepatitis vaccines)
- Mycoplasma
- Parvovirus B19
- Infectious mononucleosis
- Subacute bacterial endocarditis
- Helicobacter pylori
- Yersinia, Shigella
- Salmonella
- Brucellosis
- Legionella
- Campylobacter
- Varicella
- Parainfluenza virus
- Influenza virus (and vaccine)
- Respiratory syncytial virus (RSV), rotavirus
- Cytomegalovirus (CMV) reactivation
- Adenoviruses
More recently, IgAV has also been found in association with COVID-19 infections.[6][7] The virus is thought to damage blood vessels directly, leading to inflammation and immune complex formation. IgAV has also been seen with COVID-19 immunizations.[8]
Epidemiology
IgAV is a rare disorder that typically affects children; however, the condition can also be seen in adults and adolescents. The majority of children are aged younger than 10. It is often more severe and likely to cause long-term renal disease in adults.[5] It is the most common vasculitis among children, affecting 10 to 20 per 100,000 per year.[9] IgAV is slightly more common among boys than girls but with about equal predilection in adults.[10]
Pathophysiology
The pathophysiology of IgA vasculitis is not fully understood; however, IgA plays a significant role. Mucous membranes of the salivary glands, lungs, and gastrointestinal tract produce IgA. Plasma B-cells produce other classes of immunoglobulins (IgG, IgE, IgM).[11] IgA1 is implicated in IgaV (rather than the IgA2 subtype) and is associated with abnormally low galactose levels.[8]
IgA-antibody immune complexes are formed in response to antigenic exposure from an infection or medication. They are then deposited in the small vessels (usually capillaries) of the skin, joints, kidneys, and gastrointestinal tract. This results in an influx of inflammatory mediators such as prostaglandins. The complement system can also be activated when C3-receptor lymphocytes bind to immune complexes and deposit in the vessel walls, contributing to the hyper-inflammatory response. If the immune complexes are deposited in the intestinal wall, they may cause gastrointestinal hemorrhage.[5] Immune complexes deposited in the skin cause palpable purpura and petechiae.[8]
Histopathology
Renal biopsy is the most definitive tool to diagnose both IgAV with nephritis and IgA nephropathy. Both are likely to show mesangial IgA deposits. IgAV with nephritis is more likely to show capillary and subendothelial IgA deposits and neutrophilic infiltration. Renal deposits of IgA-mediated immune complexes may result in mild proliferative or severe crescentic glomerulonephritis.[2][5] IgAV with nephritis is also more likely to show mixed immunocomplexes with IgA and IgG components, while biopsies from those diagnosed with IgA nephropathy tend to show only IgA complexes.
History and Physical
The classic presentation of IgA vasculitis includes palpable purpura, gastrointestinal complaints, arthralgias, and renal involvement.
Patients may present with any of the following signs or symptoms:
- Rash
- Fatigue
- Headache
- Fever
- Joint pain
- Subcutaneous edema
- Diarrhea
- Hematemesis
- Abdominal pain
- Vomiting
- Rectal bleeding
- Scrotal edema
Physical Examination
Skin
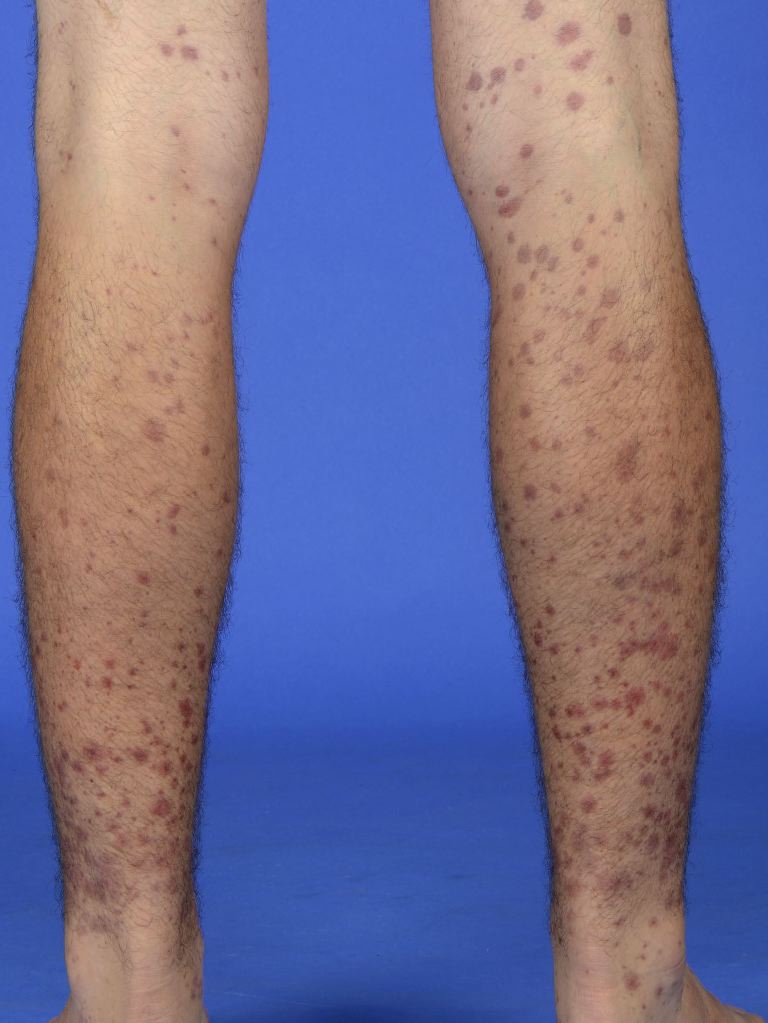
Skin involvement is present in all patients with IgAV and is usually the earliest sign.[1] The rash associated with IgA vasculitis often starts as erythematous, macular, or urticarial lesions. The rash then develops into palpable purpura and petechiae that most commonly affect the buttocks and lower extremities–particularly the extensor surfaces. Approximately one-third of patients experience the rash in the upper extremities and trunk, but the findings are primarily in dependent structures, which vary by age group and mobility.[9] The lesions can potentially become bullous or necrotic, and the bullous form has been associated with progressive, treatment-resistant renal failure [12]. The lesions change from red to purple and become rust-colored before fading. These changes occur over approximately ten days.[5]
Gastrointestinal
GI findings may occur in 10% to 40% of patients before the rashes.[9] Patients may present with nausea and vomiting, worse after meals. Potential life-threatening complications include intussusception, bowel perforation, bowel gangrene, and massive hemorrhage. Intussusception is the most common life-threatening gastrointestinal complication, affecting 3% to 4% of patients with IgAV.[1]
Renal
Renal symptoms typically occur within 1 to 3 months after rash development in 20% to 55% of children with IgAV.[9] Renal manifestations include hematuria, proteinuria, nephrotic syndrome, nephritic syndrome, and renal failure. The most common renal manifestation is microscopic hematuria.[9] Severe proteinuria may present as nephrotic syndrome, and patients with persistent proteinuria are at high risk of developing progressive glomerulonephritis. Patients may also develop ureteric obstructions. Approximately 50% of patients develop renal manifestations, with less than 1% progressing to end-stage renal failure.[13] Death from IgA vasculitis is rare; however, renal disease is the most common cause of morbidity and mortality in patients with the disorder.[5]
Joints
Approximately 15% of patients with IgA vasculitis present with arthritis as the initial symptom, and overall arthralgia or arthritis occurs in 75% of children with the disorder.[9] Patients often present with painful, swollen joints that most commonly involve the knees, ankles, hands, and feet. The arthralgias are typically transient and non-destructive.
Central Nervous System
Central nervous system involvement is rare; however, when it occurs, patients may present with headaches, dizziness, ataxia, seizures, irritability, mononeuropathy, intracranial hemorrhage, or acute motor-sensory axonal neuropathy.[9]
Evaluation
The diagnosis of IgA vasculitis is made based on the presence of petechiae (without thrombocytopenia) or palpable purpura that predominantly affects the lower limbs plus at least one of the following four characteristics:[1]
- Abdominal pain
- Arthralgia or arthritis
- Renal involvement (proteinuria, red blood cell casts, or hematuria)
- Proliferative glomerulonephritis or leukocytoclastic vasculitis with predominant deposition of IgA on histology
Clinicians should order a urinalysis with microscopy to identify hematuria, proteinuria, or red blood cell casts. If the urine dipstick is positive for protein, a 24-hour collection should be obtained to quantify the protein excretion.[1] A positive urine protein is thought to be a harbinger of IgAV recurrence [14]. Measurement of serum IgA levels is non-diagnostic as levels vary significantly, but significant elevation is suggestive of the disease. Anti-streptolysin O titers should also be sent.
Ultrasonography is often an initial imaging test to rule out hydronephrosis. Endoscopy can show purpura in the duodenum, stomach, and colon.
Treatment / Management
Unless renal involvement is present, symptomatic and supportive care are the foundations of treatment for patients with IgAV. Acetaminophen or narcotics are often preferred for pain control over NSAIDs in the setting of GI or renal involvement.[15] The presence of severe abdominal pain should support the use of prednisone or prednisolone in a tapered format.[16](B3)
Supportive and Symptomatic Care
- Rehydration with intravenous (IV) fluids
- Pain management
- Wound care for ulcerative skin lesions
Management of IgA Vasculitis Nephritis
- Angiotensin-converting enzyme inhibitors
- Corticosteroids
- Plasmapheresis or plasma exchange, with concurrent immunosuppressive therapy
- Immunosuppressants
Inhibition of the renin-angiotensin-aldosterone system has been shown to lower proteinuria levels across various pathologies. It is critical to decreasing long-term renal complications such as decreased GFR, chronic kidney disease, and progression to end-stage renal disease. These agents are most often used in adults with less spontaneous recovery than children and are more likely to have hypertension.
Early oral prednisone treatment is helpful in managing renal, joint, and gastrointestinal manifestations. Prednisone does not prevent renal disease but reduces the risk of developing persistent renal complications in children.[5] According to several randomized control trials, evidence suggests that prednisone minimizes the duration and severity of abdominal pain during the first two weeks of treatment.[15] A recent algorithm is available where oral prednisolone is given for low-level vasculitis nephritis, oral/pulse steroids for moderate, and oral/pulse steroids along with intravenous cyclophosphamide for severe forms.[4] For second-line therapy, rituximab, azathioprine, mycophenolate mofetil, along with steroids and cyclophosphamide are advocated.(B3)
Fish oil (when given in regulated medicinal form) has been proven in several trials to be more effective than placebo for IgA nephropathy through what is thought to be anti-inflammatory mechanisms and may be effective in IgAV. Dapsone is an antibiotic that inhibits bacterial folate synthesis and has been described as effective in case reports, especially against purpura. It is also thought to have an anti-inflammatory effect.[4](B3)
Intravenous immunoglobulin can also be effective in cases with rapidly progressive glomerulonephritis. Mechanisms of action include impairing autoreactive T-cells by blocking binding to antigen-presenting cells, downregulating antibody production by B-cells, and blocking the Fc-receptor-mediated immune response.[17](A1)
Differential Diagnosis
The differential diagnosis of IgAV includes the following:
- IgA nephropathy
- Acute renal failure
- Acute glomerulonephritis
- Idiopathic thrombocytopenic purpura
- Disseminated intravascular coagulation
- Thrombotic thrombocytopenic purpura
- Hemolytic uremic syndrome
- Meningococcal meningitis
- Hypersensitivity vasculitis
- Systemic lupus erythematosus
- Polyarteritis nodosa
- Bacterial endocarditis
- Inflammatory bowel disease
- Wegener granulomatosis
- Rocky Mountain spotted fever
Prognosis
IgA vasculitis is typically a self-limited illness that demonstrates an excellent prognosis in patients without renal involvement. The majority of patients fully recover in four weeks.[5] IgA vasculitis recurs in approximately one-third of patients within 4 to 6 months after the initial onset.
The long-term morbidity of vasculitis is dependent on the extent of renal involvement. Approximately 1% of patients with IgA vasculitis will develop end-stage renal disease (ESRD) and require renal transplantation.[15]
Complications
IgA vasculitis involves multiple organ systems, and the potential complications are relatively extensive. Potential complications include:
- Renal Failure
- Proteinuria
- Hematuria
- Nephrotic syndrome
- Intussusception
- Gastrointestinal bleeding
- Bowel infarction
- Bowel perforation
- CNS bleeding
- Seizures
- Neuropathy
- Pleural effusion
- Pulmonary hemorrhage
- Testicular torsion
Consultations
Patients who present with nephritic syndrome, nephrotic syndrome, hematuria, or rapidly worsening proteinuria should be urgently referred to a pediatric nephrologist.[15]
Deterrence and Patient Education
Patients should be educated that the symptoms will likely resolve within weeks but may recur. Although severe renal involvement is rare, if there is evidence of severe renal involvement, patients require aggressive treatment and management by a nephrologist.
Pearls and Other Issues
Key facts regarding IgAV are as follows:
- IgAV is primarily a disease of children, with only 10% of cases occurring in adults older than 18.
- Relapse is common in the first 4 to 6 months, but full recovery usually occurs with less than 1% mortality.
- The presentation can be similar to IgA nephropathy but with more skin, GI, and joint manifestations and fewer chronic effects.
- Histology shows more IgA deposits in the capillaries and subendothelial space and more likely co-presence of IgG than Iga nephropathy.
- Galactose-deficient-IgA1 is commonly found in IgAV, especially with renal involvement.
Enhancing Healthcare Team Outcomes
IgA vasculitis is typically a self-limiting illness; however, patients may develop life-threatening complications such as intussusception, massive gastrointestinal hemorrhage, and renal failure. An interprofessional approach is necessary to diagnose and manage the illness adequately. Patients may present with non-specific symptoms such as malaise, upper respiratory symptoms, or arthralgias before developing the characteristic rash. This may result in a delayed diagnosis. Nurse practitioners, physician assistants, and physicians may see patients during different stages of the disease; therefore, medical staff must communicate and be aware of the potential complications. A surgical team and radiologist may be required to diagnose and manage intussusception.
An essential aspect of the disease process is adequate follow-up with frequent urinalyses to screen for potential renal involvement. Patients treated with corticosteroids may require assistance from pharmacists regarding adequate therapeutic dosing and tapering. Patients with severe renal disease will need to see a nephrology team comprised of medical assistants, nurses, physicians, and rarely a transplant team.
Media
(Click Image to Enlarge)
References
Trnka P. Henoch-Schönlein purpura in children. Journal of paediatrics and child health. 2013 Dec:49(12):995-1003. doi: 10.1111/jpc.12403. Epub 2013 Oct 18 [PubMed PMID: 24134307]
Rajasekaran A, Julian BA, Rizk DV. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. The American journal of the medical sciences. 2021 Feb:361(2):176-194. doi: 10.1016/j.amjms.2020.10.003. Epub 2020 Oct 8 [PubMed PMID: 33309134]
Selvaskandan H, Shi S, Twaij S, Cheung CK, Barratt J. Monitoring Immune Responses in IgA Nephropathy: Biomarkers to Guide Management. Frontiers in immunology. 2020:11():572754. doi: 10.3389/fimmu.2020.572754. Epub 2020 Oct 6 [PubMed PMID: 33123151]
Sestan M, Jelusic M. Diagnostic and Management Strategies of IgA Vasculitis Nephritis/Henoch-Schönlein Purpura Nephritis in Pediatric Patients: Current Perspectives. Pediatric health, medicine and therapeutics. 2023:14():89-98. doi: 10.2147/PHMT.S379862. Epub 2023 Mar 7 [PubMed PMID: 36915829]
Level 3 (low-level) evidenceReamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. American family physician. 2009 Oct 1:80(7):697-704 [PubMed PMID: 19817340]
Nikolaishvili M, Pazhava A, Di Lernia V. Viral Infections May Be Associated with Henoch-Schönlein Purpura. Journal of clinical medicine. 2023 Jan 16:12(2):. doi: 10.3390/jcm12020697. Epub 2023 Jan 16 [PubMed PMID: 36675626]
Salem Y, Alam Z, Shalabi MM, Hosler GA, Acharya S. IgA Vasculitis Associated With COVID-19. Cureus. 2023 May:15(5):e38725. doi: 10.7759/cureus.38725. Epub 2023 May 8 [PubMed PMID: 37292558]
Xu L, Li Y, Wu X. IgA vasculitis update: Epidemiology, pathogenesis, and biomarkers. Frontiers in immunology. 2022:13():921864. doi: 10.3389/fimmu.2022.921864. Epub 2022 Oct 3 [PubMed PMID: 36263029]
Hetland LE, Susrud KS, Lindahl KH, Bygum A. Henoch-Schönlein Purpura: A Literature Review. Acta dermato-venereologica. 2017 Nov 15:97(10):1160-1166. doi: 10.2340/00015555-2733. Epub [PubMed PMID: 28654132]
Lei WT, Tsai PL, Chu SH, Kao YH, Lin CY, Fang LC, Shyur SD, Lin YW, Wu SI. Incidence and risk factors for recurrent Henoch-Schönlein purpura in children from a 16-year nationwide database. Pediatric rheumatology online journal. 2018 Apr 16:16(1):25. doi: 10.1186/s12969-018-0247-8. Epub 2018 Apr 16 [PubMed PMID: 29661187]
Sugino H, Sawada Y, Nakamura M. IgA Vasculitis: Etiology, Treatment, Biomarkers and Epigenetic Changes. International journal of molecular sciences. 2021 Jul 14:22(14):. doi: 10.3390/ijms22147538. Epub 2021 Jul 14 [PubMed PMID: 34299162]
Popov H, Koleva T, Stoyanov GS. Bullous Henoch-Schönlein Purpura and Associated Nephritis: A Pathological Case Report. Cureus. 2023 Feb:15(2):e35051. doi: 10.7759/cureus.35051. Epub 2023 Feb 16 [PubMed PMID: 36942172]
Level 3 (low-level) evidenceGuo D, Lam JM. Henoch-Schönlein purpura. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2016 Oct 18:188(15):E393. doi: 10.1503/cmaj.151072. Epub 2016 Jul 18 [PubMed PMID: 27431304]
Cao T, Yang HM, Huang J, Hu Y. Risk factors associated with recurrence of Henoch-Schonlein purpura: a retrospective study. Frontiers in pediatrics. 2023:11():1164099. doi: 10.3389/fped.2023.1164099. Epub 2023 Jun 12 [PubMed PMID: 37377759]
Level 2 (mid-level) evidenceBluman J, Goldman RD. Henoch-Schönlein purpura in children: limited benefit of corticosteroids. Canadian family physician Medecin de famille canadien. 2014 Nov:60(11):1007-10 [PubMed PMID: 25551129]
Bista S, Adhikari Y, Karmacharya S, Joshi S, Pandey S, Adhikari N. Henoch-Schönlein purpura with antecedent allergic diseases in a 4-year-old child: a case report. Annals of medicine and surgery (2012). 2023 Jun:85(6):3066-3069. doi: 10.1097/MS9.0000000000000782. Epub 2023 May 8 [PubMed PMID: 37363590]
Level 3 (low-level) evidenceNguyen B, Acharya C, Tangpanithandee S, Miao J, Krisanapan P, Thongprayoon C, Amir O, Mao MA, Cheungpasitporn W, Acharya PC. Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review. International journal of molecular sciences. 2023 Feb 16:24(4):. doi: 10.3390/ijms24043977. Epub 2023 Feb 16 [PubMed PMID: 36835388]
Level 1 (high-level) evidence