Introduction
Hashimoto thyroiditis is an autoimmune disease that destroys thyroid follicular cells through cell- and antibody-mediated immune processes. This disease is also known as chronic autoimmune thyroiditis and chronic lymphocytic thyroiditis. Hashimoto thyroiditis is the most common cause of hypothyroidism in developed countries.[1] The pathophysiology of this disease involves the formation of antithyroid antibodies and T-cell activation that attack the thyroid tissue, causing progressive fibrosis. Together with Graves disease, this condition comes in the category of autoimmune thyroid disorders.[2] This condition was initially described by a Japanese physician, Haruto Hashimoto, in 1912 as "struma lymphomatosa" after he found enlarged thyroids having lymphocytic infiltration.[3]
Women are more commonly affected. The female-to-male ratio is at least 7 to 10:1.[4] The incidence of Hashimoto thyroiditis increases with age, with most cases found between ages 45 and 55 years. The incidence tends to be higher in countries with a lower prevalence of iodine deficiency.[2] Hashimoto thyroiditis can occur alone, or it can occur as a part of autoimmune polyglandular syndrome (APS).[5] Some individuals with Graves disease might transform into Hashimoto thyroiditis and vice versa. This could indicate a common pathogenesis for these disorders but different clinical presentations.[6][7][8][9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The underlying etiology of Hashimoto thyroiditis is still not fully understood but is thought to be a combination of genetic and environmental factors.
Genetic Factors
Hashimoto thyroiditis has been shown to have a strong familial predisposition with disease clustering in families, including some members developing Graves disease. A Danish study had shown a 50% concordance rate for Hashimoto thyroiditis and 80% for thyroid antibody positivity in monozygotic twins.
Several genes, including those encoded by the human leukocyte antigen (HLA) complex, immune regulatory genes (CD40, FoxP3, CD25, CTLA-4, and PTPN22), and thyroid-specific genes (TSH receptor and thyroglobulin), are linked to the development of Hashimoto thyroiditis.[10][11] Furthermore, Hashimoto thyroiditis is more common in individuals with Turner syndrome and Down syndrome.[12][13]
Environmental Factors
Since only 50% of the monozygotic twins in a Danish study were shown to have concordance for Hashimoto thyroiditis, environmental factors seem to have an essential role in the development of Hashimoto thyroiditis.[14]
Hygiene hypothesis
Similar to many allergic and autoimmune conditions, individuals living in a more hygienic environment with less exposure to microbial agents seem to have a higher incidence of Hashimoto thyroiditis.[15]
Iodine status
Mild iodine deficiency has been associated with a lower prevalence of Hashimoto thyroiditis, whereas chronic exposure to excess iodine intake has been associated with a higher prevalence of Hashimoto thyroiditis. This is partly because highly iodinated thyroglobulin creates a more immunogenic intrathyroid environment.[16][17][18] Significant iodine deficiency also increases thyroid autoimmunity.[19][18][16]
Selenium status
Selenoproteins are essential for thyroid function and protection.[20][21] The important thyroidal selenoproteins are the iodothyronine deiodinases, glutathione peroxidases, and thioredoxin reductases. These enzymes are involved in thyroid hormone metabolism, maintenance of cellular metabolism and regulation of redox state, and protection from oxidative damage from reactive oxygen species and hydrogen peroxide.[22][23] Deficiency in this mineral has been associated with a higher risk of Hashimoto thyroiditis. A systematic review and meta-analysis from 2024 showed that thyroid antibody levels were reduced with selenium supplementation, independent of underlying selenium deficiency and thyroid hormone replacement. Thyroid-stimulating hormone (TSH) levels also reduce with selenium supplementation, independent of thyroid hormone replacement. The certainty of this evidence was moderate.[24]
Iron status
Thyroid peroxidase is an enzyme that contains heme (iron). Hence, the presence of iron deficiency can impair thyroid activity and also increase thyroid autoimmunity.[16][25] Individuals with Hashimoto thyroiditis are at higher risk for comorbid conditions like celiac disease and autoimmune gastritis, which are often associated with iron deficiency.[26][27] Women of reproductive age are also at high risk for developing iron deficiency due to blood loss during menstruation.[28]
Vitamin D status
Vitamin D has been established as an important immunomodulator.[29] Individuals with vitamin D deficiency have higher thyroid antibody levels, possibly contributing to Hashimoto thyroiditis development and its progression.[30][31] The association of vitamin D receptor polymorphisms with thyroid autoimmunity has shown conflicting results.[32]
Role of gut microbiome
The gut microbiota regulates the immune system and contributes significantly to thyroid hormone metabolism. Hence, alterations in the gut microbiome can increase the risk of thyroid autoimmunity.[33][34][35][36] Whether the alteration in the gut microbiome occurs before or after the development of Hashimoto thyroiditis is unclear. A cross-sectional study, including euthyroid individuals, showed no robust difference in gut microbiome between individuals with or without thyroid peroxidase antibodies regarding alpha and beta diversity.[37]
Epidemiology
Hashimoto thyroiditis is the most common cause of hypothyroidism in the United States and in those areas of the world where dietary iodine intake is adequate.[38] A systematic review and meta-analysis, including studies from multiple countries, found the overall global prevalence of Hashimoto thyroiditis to be 7.5%, higher in low-middle-income areas at 11.4%.[39] Additionally, the prevalence in women was 4 times that in men.[39]
Almost 10% of the population in the United States has been shown to have positivity for thyroid antibodies. Various studies have found the prevalence of thyroid antibody positivity in the general population between 5% to 20%.[40][41][42][43][44] Thyroid peroxidase antibodies are present in 5% to 14% of pregnant females, whereas thyroglobulin antibodies are present in 3% to 18% of pregnant females.[45]
Pathophysiology
The presence of lymphocytic infiltration and fibrosis in the thyroid follicles are characteristic of Hashimoto thyroiditis. Both cellular (T-cell mediated) and humoral (B-cell mediated) immune responses have essential roles in the pathogenesis of this disorder.[46]
Role of B Cells
Individuals with Hashimoto thyroiditis have the following polyclonal [47] antibodies specific to thyroid antigens:
- Thyroid peroxidase antibody: This is the most common antibody found in Hashimoto thyroiditis. These are found in over 90% of individuals with Hashimoto thyroiditis.[48][49]
- Thyroglobulin antibody: This is present in 50% to 80% of the individuals with Hashimoto thyroiditis.[48][50]
- TSH receptor antibody (TSHR Ab): Various types of TSHR Ab have been identified. These can be stimulating, blocking, or neutral.[51][52][53] A vast majority of TSHR Ab in Hashimoto thyroiditis is the blocking type. Rarely can individuals with Hashimoto thyroiditis have TSHR-stimulating antibodies, which can also result in thyroid eye disease. This might also explain why few individuals with Hashimoto thyroiditis switch to Graves disease.[54]
Role of T Cells
CD8+ T cells are a central part of the immune dysfunction underlying the pathogenesis of Hashimoto thyroiditis. These cytotoxic cells infiltrate the thyroid tissue, causing inflammation and destruction of the follicular cells.[55]
CD4+ T cells activate other immune cells, especially macrophages and B cells, which lead to the production of autoantibodies.[46] Different subsets of CD4+ T cells, including Th1, Th2, Th17, and Tregs (regulatory T cells), have various functions, and their imbalance contributes to the pathogenesis of Hashimoto thyroiditis.[56] Th1 is upregulated in Hashimoto thyroiditis, promoting inflammation in the thyroid tissue. Tregs are critical in promoting immune tolerance and avoiding excessive immune responses. Tregs are downregulated in Hashimoto thyroiditis.[57]
Histopathology
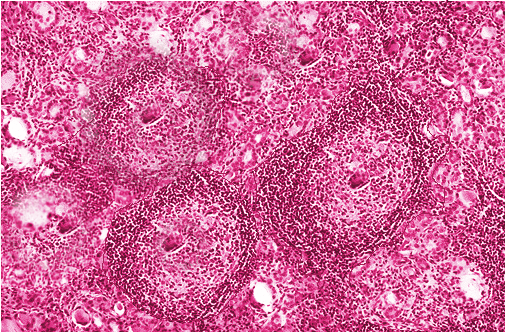
The most commonly described histopathological abnormality in Hashimoto thyroiditis is lymphocytic infiltration and destruction of follicular cells (see Image. Hashimoto Thyroiditis).[58][59] Immune cells commonly include lymphocytes and plasma cells, while others include macrophages and, occasionally, giant cells.[59] The presence of fibrotic tissue is typically noted. Lymphoid follicles containing Hurtle cells and large follicular-derived cells, also called Askanazy cells, with eosinophilic granules, are often present.[60][61]
Histopathological variants of Hashimoto thyroiditis have been described, including:
History and Physical
Individuals with Hashimoto thyroiditis have varying presentations.
Hyperthyroidism or Hashitoxicosis
A small proportion of individuals with Hashimoto thyroiditis present with hyperthyroidism. This condition is known as Hashitoxicosis. This likely occurs due to the destruction of the follicular cells, leading to leakage of prestored thyroid hormones into the bloodstream. This hyperthyroid is usually transient. This presentation is similar to subacute thyroiditis. These individuals could be at risk of developing permanent hypothyroidism.[68][69][70] A small percentage of individuals with Hashimoto thyroiditis might switch to Graves disease and present with hyperthyroidism after being hypothyroid for years before the switch.[71][72]
Euthyroid State
A majority of individuals with Hashimoto thyroiditis are euthyroid with normal thyroid function tests. About 20% to 30% of individuals with Hashimoto thyroiditis develop hypothyroidism.[73]
Subclinical Hypothyroidism
These individuals have elevated TSH, usually less than 10 mIU/L, with normal T4 and T3 levels.[74][75]
Overt Hypothyroidism
These individuals present with symptoms of hypothyroidism, a TSH of more than 10 mIU/L, or low T4 and T3 levels. Usual symptoms include fatigue, unintentional weight gain, cold intolerance, constipation, changes in menstrual cycles (typically heavy menstruation), hair loss, brittle nails, confusion, and brain fog. Individuals might notice puffiness around the eyes, ankle swelling, voice changes, muscle aches/weakness, neuropathy, and joint aches.[76][77][78]
Thyroiditis
Thyroiditis can manifest in painless and painful variants. Thyroid inflammation results in transient thyrotoxicosis, a rare manifestation of Hashimoto thyroiditis. Most cases are painless. A few may be accompanied by throat pain. These cases are resolved within a few months.[79] Rare reports on prolonged or recurrent painful thyroiditis have been published, with some individuals even needing thyroidectomy.[80][81][82]
Reproductive Effects in Women
Women may be found to have Hashimoto thyroiditis if they suffer recurrent miscarriages and/or inability to conceive. Hashimoto thyroiditis can also impair assisted conception.[83][84][85] The effects of hypothyroidism on fertility and pregnancy are not discussed in this article.
Postpartum Thyroiditis
Postpartum thyroiditis is a condition in postpartum women that usually develops around 6 months after childbirth, but it can occur anytime within a year in the postpartum phase. This condition is mostly seen in women who have underlying Hashimoto thyroiditis, most likely due to the rebound of immune response after pregnancy, which can result in an exaggerated autoimmune response destroying thyroid follicular cells. Postpartum thyroiditis is usually transient, but a few women might develop permanent hypothyroidism.[86][87]
Thyroid Nodularity
Ongoing inflammation in the thyroid gland in Hashimoto thyroiditis leads to the development of thyroid nodules. Around 20% to 30% of individuals with Hashimoto thyroiditis have thyroid nodules, and the incidence increases with age.[88] Some individuals may have compressive neck symptoms (dysphagia, dyspnea, dysphonia, chronic cough, and/or pressure) due to the development of thyromegaly with or without thyroid nodularity.
Eye Involvement
Up to 6% of individuals with Hashimoto thyroiditis can be affected by thyroid eye disease.[54] About 15% of individuals with Hashimoto thyroiditis have TSHR Ab. Most of these are the blocking type. A small proportion of individuals can have TSHR-stimulating antibodies, resulting in eye involvement.[54][88][89]
Evaluation
Laboratory Studies
The following laboratory studies may be used for diagnostic evaluation of thyroid function and identification of comorbid conditions:
- Thyroid function tests: TSH, T4, and T3 levels should be tested to assess thyroid function.
- Thyroid antibodies: Thyroid peroxidase antibody is positive in over 90% of cases, whereas thyroglobulin antibody is positive in 50% to 80% of cases. Elevated thyroid antibodies correlated with disease activity.[90] Individuals with thyroid antibody positivity have a high probability of developing overt hypothyroidism, whereas individuals with negative antibody levels are more likely to present with subclinical hypothyroidism. A positive family history is also more likely when thyroid antibodies are elevated. These individuals are also more likely to have larger thyroid gland size.[91][50] If a patient presents with thyroid eye disease, TSH receptor antibody levels should be assessed.
- Iron studies: Serum iron levels should be checked in individuals with clinical symptoms of iron deficiency and gastrointestinal symptoms due to the higher prevalence of celiac disease and atrophic gastritis in individuals with Hashimoto thyroiditis.
- Vitamin D level: Vitamin D status should be assessed due to an increased incidence of vitamin D deficiency in individuals with thyroid disorders.[92]
Imaging Studies
A thyroid ultrasound can be performed if thyroid enlargement or nodularity is noted on physical examination or if the patient has compressive symptoms.
Treatment / Management
Current clinical guidelines emphasize disease management rather than prevention or cure.
Thyroid Hormone Replacement
The mainstay of treatment for hypothyroidism that develops due to Hashimoto thyroiditis is thyroid hormone replacement. The drug of choice is titrated levothyroxine sodium administered orally, which has a half-life of 7 days and can be given daily. Levothyroxine sodium, which is best taken early in the morning on an empty stomach for optimum absorption, should not be taken with iron or calcium supplements, aluminum hydroxide, and proton pump inhibitors to avoid suboptimal absorption.
The standard dosage is 1.4 to 1.8 µg/kg per day, but this can vary from one patient to another. Individuals younger than 60 should be commenced on a standard full dose if TSH >20 mIU/L; however, lower doses should be used in patients with cardiovascular diseases and older adults. In patients older than 60, the recommended starting dose is 25 µg/day, with reevaluation in 6 to 8 weeks. In contrast, in pregnancy, the dose of thyroxine needs to be increased by 30%. In patients with short-bowel syndrome and malabsorption, increased doses of levothyroxine are required to maintain a euthyroid state.[93](A1)
Treatment in euthyroid women
Some experts will recommend initiating levothyroxine in women with TSH >2.5 with or without a history of pregnancy loss and in women using assisted reproductive measures.
Role of selenium supplementation
Recent systematic reviews and meta-analyses showed modest benefits in managing Hashimoto thyroiditis in individuals without thyroid hormone replacement, with improved thyroid function parameters and reduced thyroid antibodies.[24][94] More research is needed to establish clear benefits. Selenium supplementation of 50 to 100 µg daily could be helpful and safe, especially in areas with selenium deficiency.[95] A study including 412 individuals showed an improvement in quality of life parameters with selenium supplementation (200 µg/daily) in individuals on levothyroxine for management of hypothyroidism due to Hashimoto thyroiditis.[96](A1)
Iron deficiency treatment
Every unit increase in iron level has been demonstrated to decrease the risk of Hashimoto thyroiditis by 43% in women of reproductive age.[97](B2)
Vitamin D deficiency treatment
Correction of vitamin D deficiency may be helpful, though more large-scale studies are needed to establish clear benefits. Two small randomized controlled clinical trials have shown reduced thyroid antibody levels and improved TSH levels. Individuals with vitamin D deficiency were included in these trials. One trial based in India gave individuals in the intervention group a high dose of cholecalciferol, 60,000 IU, for 8 weeks.[98] The other trial, based in China, showed a group of individuals treated with cholecalciferol 800 IU daily compared to another group treated with cholecalciferol 800 IU daily and levothyroxine 25 to 50 µg/daily for 6 months.[99] (A1)
Furthermore, in another study in Greece, 186 vitamin D-deficient individuals were given 1200 to 400 IU of cholecalciferol daily for 4 months, with a reduction seen in thyroid peroxidase antibody levels.[100] A systematic review and meta-analysis including 862 individuals also showed the positive effect of vitamin D supplementation on thyroid function and reduction in antibody levels.[101](A1)
Dietary modifications
Limited evidence supports the benefits of an autoimmune or anti-inflammatory diet in the management of thyroid disorders. The theory behind the inflammation involves leaky gut syndrome, where an insult to the gut mucosa allows the penetrance of proteins that do not typically enter the bloodstream via transporters in the gut mucosa. Experts have theorized that a response similar to molecular mimicry occurs, and antibodies are produced against the antigens. Unfortunately, the antigen may be structurally similar to thyroid peroxidase, leading to antibody formation against this enzyme.
The concept of an autoimmune diet is based on healing the gut and decreasing the severity of the autoimmune response.[102] More research is required on this topic before becoming a component of established guidelines. A small study, which included 40 female subjects, showed positive effects on thyroid function and lower thyroid peroxidase and thyroglobulin antibody levels by following a Mediterranean and gluten-free diet.[103](A1)
Differential Diagnosis
Differential diagnoses that should be considered when evaluating Hashimoto thyroiditis:
- Euthyroid sick syndrome
- Goiter
- Graves disease (diffuse toxic goiter)
- Hypopituitarism (panhypopituitarism)
- Lithium-induced goiter
- Nontoxic goiter
- Polyglandular autoimmune syndrome type 1
- Polyglandular autoimmune syndrome type 2
- Thyroid cancer (lymphoma)
- Toxic nodular goiter
Prognosis
Many individuals with Hashimoto thyroiditis are euthyroid, but they are at a higher risk for developing overt hypothyroidism in the future. The risk of developing hypothyroidism increases by 5% every year.[104] These individuals with Hashimoto thyroiditis should have thyroid function testing done annually.
Complications
Thyroid malignancy: Whether Hashimoto thyroiditis is associated with an increased risk of papillary thyroid carcinoma remains unclear.[105] The effect of the presence of Hashimoto thyroiditis on the outcomes of papillary thyroid carcinoma is also conflicting.[106][107] Individuals with Hashimoto thyroiditis are at risk for a rare thyroid malignancy: primary thyroid lymphoma. The incidence of primary thyroid lymphoma is 0.5% to 5% of all thyroid malignancies.[108][109][110][111]
Deterrence and Patient Education
Hashimoto thyroiditis is an autoimmune disorder involving the thyroid gland that can result in thyroid dysfunction and the development of hypothyroidism. This disorder has a high familial predisposition. Individuals with Hashimoto thyroiditis are at twice the risk of developing an underactive thyroid compared to those without it.
Thyroid hormone replacement is only recommended in individuals with overt hypothyroidism. Treatment of subclinical hypothyroidism can be considered in select cases with significant symptoms of hypothyroidism affecting the quality of life of women of reproductive age, especially if they are trying to conceive. Many individuals living in iodine-replete areas might take iodine supplements for their thyroid health. Excess iodine supplementation tends to worsen Hashimoto thyroiditis. Maintenance of normal iron and vitamin D levels might improve thyroid function and reduce antibodies in Hashimoto thyroiditis. Selenium supplementation can be considered in individuals with autoimmune thyroid living in areas with selenium-deficient soil.
Pearls and Other Issues
Hashimoto thyroiditis is one of the most frequent autoimmune diseases and has been reported to be associated with gastric disorders in 10% to 40% of patients. According to research by Cellini et al, about 40% of patients with autoimmune gastritis also present with Hashimoto thyroiditis. Chronic autoimmune gastritis is characterized by the partial or complete disappearance of parietal cells, leading to impairment of hydrochloric acid and intrinsic factor production. The patients go on to develop hypochlorhydria-dependent iron-deficient anemia, which leads to pernicious anemia and severe gastric atrophy.
Thyrogastric syndrome was first described in the 1960s when thyroid autoantibodies were found in a subset of patients with pernicious anemia and atrophic gastritis. The latest guidelines have incorporated the 2 aforementioned autoimmune disorders into a syndrome now known as polyglandular autoimmune syndrome. This is characterized by 2 or more endocrine and nonendocrine disorders. The thyroid gland develops from the primitive gut; therefore, the thyroid follicular cells share similar characteristics with parietal cells of the same endodermal origin. For example, both are polarized and have apical microvilli with enzymatic activity, and both can concentrate and transport iodine across the cell membrane via the sodium/iodide symporter. Iodine not only plays an essential role in the production of thyroid hormone but is also involved in regulating gastric mucosal cell proliferation, acts as an electron donor in the presence of gastric peroxidase, and assists in removing free oxygen radicals.
Notably, due to the pharmaceutical formation of thyroxine available worldwide, there can be absorption problems in patients with gastric mucosa disorders. Most levothyroxine is obtained by salification with sodium hydroxide, making sodium levothyroxine. The absorption of T4 occurs in all areas of the small intestine and ranges from 62% to 84% of the ingested dose. Decreased gastric acid secretion can disrupt this percentage and may cause issues with reduced absorption of most pharmaceutical-grade forms of levothyroxine, except for liquid-based or soft gel formations.
Clinicians should note the association between thyroid and gastric autoimmune diseases. Iron deficiency anemia and thyroxine absorption issues should encourage further diagnostic workup.
Enhancing Healthcare Team Outcomes
Effective management of Hashimoto thyroiditis requires a collaborative, interprofessional approach to ensure patient-centered care, improve outcomes, and enhance patient safety. Physicians, including endocrinologists, primary care clinicians, and internists, play a crucial role in diagnosing and monitoring the disease, adjusting levothyroxine therapy based on individual needs, and screening for complications such as lymphoma. Advanced practitioners and nurses provide ongoing patient education, reinforcing the importance of adherence to treatment, recognizing symptoms of hormone imbalance, and scheduling regular follow-ups for thyroid function testing. Pharmacists contribute by reviewing medication interactions, counseling patients on proper levothyroxine administration, and ensuring safe and effective dosing to prevent hormone toxicity.
Interprofessional communication and care coordination are essential to optimizing team performance and patient outcomes. Clear communication among clinicians allows for timely dose adjustments, identification of potential complications, and coordination of care across specialties. Nurses and advanced practitioners serve as a bridge between patients and physicians, addressing patient concerns, managing symptoms, and facilitating referrals when needed. By fostering teamwork and prioritizing comprehensive, patient-centered care, the healthcare team can effectively manage Hashimoto thyroiditis as a lifelong condition, ensuring that patients receive the necessary support to maintain thyroid function and overall well-being.
Media
(Click Image to Enlarge)
References
McDermott MT. Hypothyroidism. Annals of internal medicine. 2020 Jul 7:173(1):ITC1-ITC16. doi: 10.7326/AITC202007070. Epub [PubMed PMID: 32628881]
Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmunity reviews. 2015 Feb:14(2):174-80. doi: 10.1016/j.autrev.2014.10.016. Epub 2014 Oct 25 [PubMed PMID: 25461470]
DAVISON TC, LETTON AH. Hashimoto's disease. The Journal of clinical endocrinology and metabolism. 1949 Oct:9(10):980-6 [PubMed PMID: 18148850]
Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto's thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid : official journal of the American Thyroid Association. 2013 Feb:23(2):142-50. doi: 10.1089/thy.2012.0554. Epub [PubMed PMID: 23151083]
Husebye ES, Anderson MS, Kämpe O. Autoimmune Polyendocrine Syndromes. The New England journal of medicine. 2018 Mar 22:378(12):1132-1141. doi: 10.1056/NEJMra1713301. Epub [PubMed PMID: 29562162]
Gonzalez-Aguilera B, Betea D, Lutteri L, Cavalier E, Geenen V, Beckers A, Valdes-Socin H. Conversion to Graves disease from Hashimoto thyroiditis: a study of 24 patients. Archives of endocrinology and metabolism. 2018:62(6):609-614. doi: 10.20945/2359-3997000000086. Epub [PubMed PMID: 30624501]
Ahmad E, Hafeez K, Arshad MF, Isuga J, Vrettos A. Hypothyroidism conversion to hyperthyroidism: it's never too late. Endocrinology, diabetes & metabolism case reports. 2018:2018():. doi: 10.1530/EDM-18-0047. Epub 2018 Aug 3 [PubMed PMID: 30083349]
Level 3 (low-level) evidenceDaramjav N, Takagi J, Iwayama H, Uchino K, Inukai D, Otake K, Ogawa T, Takami A. Autoimmune Thyroiditis Shifting from Hashimoto's Thyroiditis to Graves' Disease. Medicina (Kaunas, Lithuania). 2023 Apr 13:59(4):. doi: 10.3390/medicina59040757. Epub 2023 Apr 13 [PubMed PMID: 37109715]
McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid : official journal of the American Thyroid Association. 2013 Jan:23(1):14-24. doi: 10.1089/thy.2012.0374. Epub [PubMed PMID: 23025526]
Zaletel K, Gaberšček S. Hashimoto's Thyroiditis: From Genes to the Disease. Current genomics. 2011 Dec:12(8):576-88. doi: 10.2174/138920211798120763. Epub [PubMed PMID: 22654557]
Qiu K, Li K, Zeng T, Liao Y, Min J, Zhang N, Peng M, Kong W, Chen LL. Integrative Analyses of Genes Associated with Hashimoto's Thyroiditis. Journal of immunology research. 2021:2021():8263829. doi: 10.1155/2021/8263829. Epub 2021 Aug 28 [PubMed PMID: 34493981]
Level 2 (mid-level) evidenceAversa T, Messina MF, Mazzanti L, Salerno M, Mussa A, Faienza MF, Scarano E, De Luca F, Wasniewska M. The association with Turner syndrome significantly affects the course of Hashimoto's thyroiditis in children, irrespective of karyotype. Endocrine. 2015 Dec:50(3):777-82. doi: 10.1007/s12020-014-0513-6. Epub 2014 Dec 27 [PubMed PMID: 25542186]
Amr NH. Thyroid Disorders in Subjects with Down Syndrome: An Update. Acta bio-medica : Atenei Parmensis. 2018 Mar 27:89(1):132-139. doi: 10.23750/abm.v89i1.7120. Epub 2018 Mar 27 [PubMed PMID: 29633736]
Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. The Journal of clinical endocrinology and metabolism. 2000 Feb:85(2):536-9 [PubMed PMID: 10690851]
Wiersinga WM. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinology and metabolism (Seoul, Korea). 2016 Jun:31(2):213-22. doi: 10.3803/EnM.2016.31.2.213. Epub 2016 May 13 [PubMed PMID: 27184015]
Hu S, Rayman MP. Multiple Nutritional Factors and the Risk of Hashimoto's Thyroiditis. Thyroid : official journal of the American Thyroid Association. 2017 May:27(5):597-610. doi: 10.1089/thy.2016.0635. Epub 2017 Apr 6 [PubMed PMID: 28290237]
Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, Gillett MJ, Gilbert R, Tanner M, Stuckey BG. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. The Journal of clinical endocrinology and metabolism. 2006 Jul:91(7):2624-30 [PubMed PMID: 16670161]
Level 1 (high-level) evidenceTeng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. Effect of iodine intake on thyroid diseases in China. The New England journal of medicine. 2006 Jun 29:354(26):2783-93 [PubMed PMID: 16807415]
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carlé A. Iodine intake as a determinant of thyroid disorders in populations. Best practice & research. Clinical endocrinology & metabolism. 2010 Feb:24(1):13-27. doi: 10.1016/j.beem.2009.08.013. Epub [PubMed PMID: 20172467]
Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nature reviews. Endocrinology. 2011 Oct 18:8(3):160-71. doi: 10.1038/nrendo.2011.174. Epub 2011 Oct 18 [PubMed PMID: 22009156]
Duntas LH. Selenium and the thyroid: a close-knit connection. The Journal of clinical endocrinology and metabolism. 2010 Dec:95(12):5180-8. doi: 10.1210/jc.2010-0191. Epub 2010 Sep 1 [PubMed PMID: 20810577]
Köhrle J, Jakob F, Contempré B, Dumont JE. Selenium, the thyroid, and the endocrine system. Endocrine reviews. 2005 Dec:26(7):944-84 [PubMed PMID: 16174820]
Wang F, Li C, Li S, Cui L, Zhao J, Liao L. Selenium and thyroid diseases. Frontiers in endocrinology. 2023:14():1133000. doi: 10.3389/fendo.2023.1133000. Epub 2023 Mar 24 [PubMed PMID: 37033262]
Huwiler VV, Maissen-Abgottspon S, Stanga Z, Mühlebach S, Trepp R, Bally L, Bano A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid : official journal of the American Thyroid Association. 2024 Mar:34(3):295-313. doi: 10.1089/thy.2023.0556. Epub 2024 Feb 16 [PubMed PMID: 38243784]
Level 1 (high-level) evidenceGarofalo V, Condorelli RA, Cannarella R, Aversa A, Calogero AE, La Vignera S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients. 2023 Nov 15:15(22):. doi: 10.3390/nu15224790. Epub 2023 Nov 15 [PubMed PMID: 38004184]
Level 1 (high-level) evidenceCellini M, Santaguida MG, Virili C, Capriello S, Brusca N, Gargano L, Centanni M. Hashimoto's Thyroiditis and Autoimmune Gastritis. Frontiers in endocrinology. 2017:8():92. doi: 10.3389/fendo.2017.00092. Epub 2017 Apr 26 [PubMed PMID: 28491051]
Roy A, Laszkowska M, Sundström J, Lebwohl B, Green PH, Kämpe O, Ludvigsson JF. Prevalence of Celiac Disease in Patients with Autoimmune Thyroid Disease: A Meta-Analysis. Thyroid : official journal of the American Thyroid Association. 2016 Jul:26(7):880-90. doi: 10.1089/thy.2016.0108. Epub [PubMed PMID: 27256300]
Level 1 (high-level) evidenceFernandez-Jimenez MC, Moreno G, Wright I, Shih PC, Vaquero MP, Remacha AF. Iron Deficiency in Menstruating Adult Women: Much More than Anemia. Women's health reports (New Rochelle, N.Y.). 2020:1(1):26-35. doi: 10.1089/whr.2019.0011. Epub 2020 Jan 29 [PubMed PMID: 33786470]
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Current opinion in pharmacology. 2010 Aug:10(4):482-96. doi: 10.1016/j.coph.2010.04.001. Epub 2010 Apr 27 [PubMed PMID: 20427238]
Level 3 (low-level) evidenceGiovinazzo S, Vicchio TM, Certo R, Alibrandi A, Palmieri O, Campennì A, Cannavò S, Trimarchi F, Ruggeri RM. Vitamin D receptor gene polymorphisms/haplotypes and serum 25(OH)D(3) levels in Hashimoto's thyroiditis. Endocrine. 2017 Feb:55(2):599-606. doi: 10.1007/s12020-016-0942-5. Epub 2016 Apr 4 [PubMed PMID: 27043843]
Bozkurt NC, Karbek B, Ucan B, Sahin M, Cakal E, Ozbek M, Delibasi T. The association between severity of vitamin D deficiency and Hashimoto's thyroiditis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013 May-Jun:19(3):479-84. doi: 10.4158/EP12376.OR. Epub [PubMed PMID: 23337162]
Vieira IH, Rodrigues D, Paiva I. Vitamin D and Autoimmune Thyroid Disease-Cause, Consequence, or a Vicious Cycle? Nutrients. 2020 Sep 11:12(9):. doi: 10.3390/nu12092791. Epub 2020 Sep 11 [PubMed PMID: 32933065]
Ludgate ME, Masetti G, Soares P. The relationship between the gut microbiota and thyroid disorders. Nature reviews. Endocrinology. 2024 Sep:20(9):511-525. doi: 10.1038/s41574-024-01003-w. Epub 2024 Jun 21 [PubMed PMID: 38906998]
Gong B, Wang C, Meng F, Wang H, Song B, Yang Y, Shan Z. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Frontiers in endocrinology. 2021:12():774362. doi: 10.3389/fendo.2021.774362. Epub 2021 Nov 17 [PubMed PMID: 34867823]
Level 1 (high-level) evidenceChen J, Wang Y, Yao H, Li Y, Song H. Uncovering a Causal Connection between Gut Microbiota and Six Thyroid Diseases: A Two-Sample Mendelian Randomization Study. Biology. 2024 Sep 11:13(9):. doi: 10.3390/biology13090714. Epub 2024 Sep 11 [PubMed PMID: 39336141]
Yan K, Sun X, Fan C, Wang X, Yu H. Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives. International journal of molecular sciences. 2024 Oct 10:25(20):. doi: 10.3390/ijms252010918. Epub 2024 Oct 10 [PubMed PMID: 39456701]
Level 3 (low-level) evidenceFenneman AC, Boulund U, Collard D, Galenkamp H, Zwinderman AH, van den Born BH, van der Spek AH, Fliers E, Rampanelli E, Blaser MJ, Nieuwdorp M. Comparative Analysis of Taxonomic and Functional Gut Microbiota Profiles in Relation to Seroconversion of Thyroid Peroxidase Antibodies in Euthyroid Participants. Thyroid : official journal of the American Thyroid Association. 2024 Jan:34(1):101-111. doi: 10.1089/thy.2023.0346. Epub 2024 Jan 3 [PubMed PMID: 38010921]
Level 2 (mid-level) evidenceJonklaas J Optimal Thyroid Hormone Replacement. Endocrine reviews. 2022 Mar 9 [PubMed PMID: 34543420]
Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto's thyroiditis in adults: A systematic review and meta-analysis. Frontiers in public health. 2022:10():1020709. doi: 10.3389/fpubh.2022.1020709. Epub 2022 Oct 13 [PubMed PMID: 36311599]
Level 1 (high-level) evidenceAmouzegar A, Gharibzadeh S, Kazemian E, Mehran L, Tohidi M, Azizi F. The Prevalence, Incidence and Natural Course of Positive Antithyroperoxidase Antibodies in a Population-Based Study: Tehran Thyroid Study. PloS one. 2017:12(1):e0169283. doi: 10.1371/journal.pone.0169283. Epub 2017 Jan 4 [PubMed PMID: 28052092]
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism. 2002 Feb:87(2):489-99 [PubMed PMID: 11836274]
Level 3 (low-level) evidencePedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clinical endocrinology. 2003 Jan:58(1):36-42 [PubMed PMID: 12519410]
Level 3 (low-level) evidenceVanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical endocrinology. 1995 Jul:43(1):55-68 [PubMed PMID: 7641412]
Level 3 (low-level) evidenceTaylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nature reviews. Endocrinology. 2018 May:14(5):301-316. doi: 10.1038/nrendo.2018.18. Epub 2018 Mar 23 [PubMed PMID: 29569622]
De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. The lancet. Diabetes & endocrinology. 2018 Jul:6(7):575-586. doi: 10.1016/S2213-8587(17)30402-3. Epub 2017 Dec 12 [PubMed PMID: 29246752]
Rydzewska M, Jaromin M, Pasierowska IE, Stożek K, Bossowski A. Role of the T and B lymphocytes in pathogenesis of autoimmune thyroid diseases. Thyroid research. 2018:11():2. doi: 10.1186/s13044-018-0046-9. Epub 2018 Feb 13 [PubMed PMID: 29449887]
McLachlan SM, Feldt-Rasmussen U, Young ET, Middleton SL, Dlichert-Toft M, Siersboek-Nielsen K, Date J, Carr D, Clark F, Rees Smith B. IgG subclass distribution of thyroid autoantibodies: a 'fingerprint' of an individual's response to thyroglobulin and thyroid microsomal antigen. Clinical endocrinology. 1987 Mar:26(3):335-46 [PubMed PMID: 3652478]
Li J, Huang Q, Sun S, Zhou K, Wang X, Pan K, Zhang Y, Wang Y, Han Q, Si C, Li S, Fan S, Li D. Thyroid antibodies in Hashimoto's thyroiditis patients are positively associated with inflammation and multiple symptoms. Scientific reports. 2024 Nov 13:14(1):27902. doi: 10.1038/s41598-024-78938-7. Epub 2024 Nov 13 [PubMed PMID: 39537841]
Mehanathan PB, Erusan RR, Shantaraman K, Kannan SM. Antithyroid Peroxidase Antibodies in Multinodular Hashimoto's Thyroiditis Indicate a Variant Etiology. Journal of thyroid research. 2019:2019():4892329. doi: 10.1155/2019/4892329. Epub 2019 Jul 21 [PubMed PMID: 31428301]
Iwamoto Y, Kimura T, Itoh T, Mori S, Sasaki T, Sugisaki T, Nakao E, Ohnishi M, Kusano T, Takenouchi H, Iwamoto H, Sanada J, Fushimi Y, Katakura Y, Tatsumi F, Shimoda M, Nakanishi S, Mune T, Kaku K, Kaneto H. Structural and functional differences in auto-antibody positive compared to auto-antibody negative hypothyroid patients with chronic thyroiditis. Scientific reports. 2023 Sep 20:13(1):15542. doi: 10.1038/s41598-023-42765-z. Epub 2023 Sep 20 [PubMed PMID: 37731007]
Diana T, Krause J, Olivo PD, König J, Kanitz M, Decallonne B, Kahaly GJ. Prevalence and clinical relevance of thyroid stimulating hormone receptor-blocking antibodies in autoimmune thyroid disease. Clinical and experimental immunology. 2017 Sep:189(3):304-309. doi: 10.1111/cei.12980. Epub 2017 May 16 [PubMed PMID: 28439882]
Napolitano G, Bucci I, Di Dalmazi G, Giuliani C. Non-Conventional Clinical Uses of TSH Receptor Antibodies: The Case of Chronic Autoimmune Thyroiditis. Frontiers in endocrinology. 2021:12():769084. doi: 10.3389/fendo.2021.769084. Epub 2021 Nov 5 [PubMed PMID: 34803929]
Level 3 (low-level) evidenceTamaki H, Amino N, Kimura M, Hidaka Y, Takeoka K, Miyai K. Low prevalence of thyrotropin receptor antibody in primary hypothyroidism in Japan. The Journal of clinical endocrinology and metabolism. 1990 Nov:71(5):1382-6 [PubMed PMID: 1977757]
Kahaly GJ, Diana T, Glang J, Kanitz M, Pitz S, König J. Thyroid Stimulating Antibodies Are Highly Prevalent in Hashimoto's Thyroiditis and Associated Orbitopathy. The Journal of clinical endocrinology and metabolism. 2016 May:101(5):1998-2004. doi: 10.1210/jc.2016-1220. Epub 2016 Mar 10 [PubMed PMID: 26964732]
Ehlers M, Thiel A, Bernecker C, Porwol D, Papewalis C, Willenberg HS, Schinner S, Hautzel H, Scherbaum WA, Schott M. Evidence of a combined cytotoxic thyroglobulin and thyroperoxidase epitope-specific cellular immunity in Hashimoto's thyroiditis. The Journal of clinical endocrinology and metabolism. 2012 Apr:97(4):1347-54. doi: 10.1210/jc.2011-2178. Epub 2012 Jan 18 [PubMed PMID: 22259066]
Wrońska K, Hałasa M, Szczuko M. The Role of the Immune System in the Course of Hashimoto's Thyroiditis: The Current State of Knowledge. International journal of molecular sciences. 2024 Jun 23:25(13):. doi: 10.3390/ijms25136883. Epub 2024 Jun 23 [PubMed PMID: 38999993]
Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in Hashimoto's thyroiditis: what do we know so far? Journal of immunology research. 2015:2015():979167. doi: 10.1155/2015/979167. Epub 2015 Apr 27 [PubMed PMID: 26000316]
Chandanwale SS, Nair R, Gambhir A, Kaur S, Pandey A, Shetty A, Naragude P. Cytomorphological Spectrum of Thyroiditis: A Review of 110 Cases. Journal of thyroid research. 2018:2018():5246516. doi: 10.1155/2018/5246516. Epub 2018 Mar 1 [PubMed PMID: 29686830]
Level 3 (low-level) evidenceAvramidou E, Gkantaras A, Dermitzakis I, Sapalidis K, Manthou ME, Theotokis P. Histological Alterations in Hashimoto's Disease: A Case-Series Ultrastructural Study. Medicines (Basel, Switzerland). 2023 Sep 2:10(9):. doi: 10.3390/medicines10090051. Epub 2023 Sep 2 [PubMed PMID: 37755241]
Level 3 (low-level) evidenceWong KS, Angell TE, Barletta JA, Krane JF. Hürthle cell lesions of the thyroid: Progress made and challenges remaining. Cancer cytopathology. 2021 May:129(5):347-362. doi: 10.1002/cncy.22375. Epub 2020 Oct 27 [PubMed PMID: 33108684]
Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Archives of pathology & laboratory medicine. 2008 Aug:132(8):1241-50 [PubMed PMID: 18684023]
Iannaci G, Luise R, Sapere P, Coluccino V, Ronchi A, Faggiano A, Marotta V, Colao A, Spiezia S. Fibrous Variant of Hashimoto's Thyroiditis as a Diagnostic Pitfall in Thyroid Pathology. Case reports in endocrinology. 2013:2013():308908. doi: 10.1155/2013/308908. Epub 2013 Dec 5 [PubMed PMID: 24381770]
Level 3 (low-level) evidenceFalhammar H, Juhlin CC, Barner C, Catrina SB, Karefylakis C, Calissendorff J. Riedel's thyroiditis: clinical presentation, treatment and outcomes. Endocrine. 2018 Apr:60(1):185-192. doi: 10.1007/s12020-018-1526-3. Epub 2018 Jan 29 [PubMed PMID: 29380231]
Zala A, Berhane T, Juhlin CC, Calissendorff J, Falhammar H. Riedel Thyroiditis. The Journal of clinical endocrinology and metabolism. 2020 Sep 1:105(9):. pii: dgaa468. doi: 10.1210/clinem/dgaa468. Epub [PubMed PMID: 32687163]
Li Y, Zhou G, Ozaki T, Nishihara E, Matsuzuka F, Bai Y, Liu Z, Taniguchi E, Miyauchi A, Kakudo K. Distinct histopathological features of Hashimoto's thyroiditis with respect to IgG4-related disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012 Aug:25(8):1086-97. doi: 10.1038/modpathol.2012.68. Epub 2012 May 4 [PubMed PMID: 22555173]
Jin M, Kim B, Jang A, Jeon MJ, Choi YJ, Lee YM, Song DE, Kim WG. Immunoglobulin G4-Related Thyroid Disease: A Single-Center Experience and Literature Review. Endocrinology and metabolism (Seoul, Korea). 2022 Apr:37(2):312-322. doi: 10.3803/EnM.2021.1318. Epub 2022 Apr 25 [PubMed PMID: 35504602]
Han X, Zhang P, Li J, Liu Z, Lu H, Luo X, Pan B, Lian X, Zeng X, Zhang W, Zeng X. Clinical features and treatment efficacy for IgG4-related thyroiditis. Orphanet journal of rare diseases. 2021 Jul 21:16(1):324. doi: 10.1186/s13023-021-01942-x. Epub 2021 Jul 21 [PubMed PMID: 34289855]
Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Polish archives of internal medicine. 2022 Mar 30:132(3):. pii: 16222. doi: 10.20452/pamw.16222. Epub 2022 Mar 3 [PubMed PMID: 35243857]
Shahbaz A, Aziz K, Umair M, Sachmechi I. Prolonged Duration of Hashitoxicosis in a Patient with Hashimoto's Thyroiditis: A Case Report and Review of Literature. Cureus. 2018 Jun 14:10(6):e2804. doi: 10.7759/cureus.2804. Epub 2018 Jun 14 [PubMed PMID: 30123726]
Level 3 (low-level) evidenceCaturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmunity reviews. 2014 Apr-May:13(4-5):391-7. doi: 10.1016/j.autrev.2014.01.007. Epub 2014 Jan 13 [PubMed PMID: 24434360]
Level 3 (low-level) evidenceArshad I, Zahra T, Vargas-Jerez J. New-Onset Graves' Disease in the Background of Hashimoto's Thyroiditis: Spectrums of the Same Disease With Changing Autoantibodies. Cureus. 2022 Aug:14(8):e28296. doi: 10.7759/cureus.28296. Epub 2022 Aug 23 [PubMed PMID: 36158389]
Jadhav R, Alberawi M, Gupta K. Graves' disease: A rare fate of Hashimoto's thyroiditis. World journal of nuclear medicine. 2021 Jan-Mar:20(1):102-104. doi: 10.4103/wjnm.WJNM_34_20. Epub 2020 Oct 23 [PubMed PMID: 33850498]
Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, Churilov LP, Ferrari SM, Antonelli A. Hashimotos' thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best practice & research. Clinical endocrinology & metabolism. 2019 Dec:33(6):101367. doi: 10.1016/j.beem.2019.101367. Epub 2019 Nov 26 [PubMed PMID: 31812326]
Biondi B, Cappola AR, Cooper DS. Subclinical Hypothyroidism: A Review. JAMA. 2019 Jul 9:322(2):153-160. doi: 10.1001/jama.2019.9052. Epub [PubMed PMID: 31287527]
Gosi SKY, Kaur J, Garla VV. Subclinical Hypothyroidism. StatPearls. 2025 Jan:(): [PubMed PMID: 30725655]
Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. The Medical clinics of North America. 2012 Mar:96(2):203-21. doi: 10.1016/j.mcna.2012.01.005. Epub 2012 Feb 14 [PubMed PMID: 22443971]
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid : official journal of the American Thyroid Association. 2014 Dec:24(12):1670-751. doi: 10.1089/thy.2014.0028. Epub [PubMed PMID: 25266247]
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012 Nov-Dec:18(6):988-1028 [PubMed PMID: 23246686]
Level 1 (high-level) evidenceRalli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, de Vincentiis M, Greco A. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmunity reviews. 2020 Oct:19(10):102649. doi: 10.1016/j.autrev.2020.102649. Epub 2020 Aug 15 [PubMed PMID: 32805423]
Peng CC, Munir KM, Song L, Papadimitriou JC, Pennant MA. RECURRENT PAINFUL HASHIMOTO THYROIDITIS SUCCESSFULLY TREATED BY THYROIDECTOMY. AACE clinical case reports. 2020 Jan-Feb:6(1):e9-e13. doi: 10.4158/ACCR-2019-0184. Epub 2020 Sep 26 [PubMed PMID: 32984515]
Level 3 (low-level) evidencePeng CC,Huai-En Chang R,Pennant M,Huang HK,Munir KM, A Literature Review of Painful Hashimoto Thyroiditis: 70 Published Cases in the Past 70 Years. Journal of the Endocrine Society. 2020 Feb 1 [PubMed PMID: 32047869]
Level 3 (low-level) evidenceKon YC, DeGroot LJ. Painful Hashimoto's thyroiditis as an indication for thyroidectomy: clinical characteristics and outcome in seven patients. The Journal of clinical endocrinology and metabolism. 2003 Jun:88(6):2667-72 [PubMed PMID: 12788871]
Li N, Lu Y, Si P, Li Z, Qin Y, Jiao X. The Impact of Moderately High Preconception Thyrotropin Levels on Ovarian Reserve Among Euthyroid Infertile Women Undergoing Assisted Reproductive Technology. Thyroid : official journal of the American Thyroid Association. 2022 Jul:32(7):841-848. doi: 10.1089/thy.2021.0534. Epub 2022 Apr 22 [PubMed PMID: 35317605]
Level 2 (mid-level) evidenceUnuane D, Velkeniers B. Impact of thyroid disease on fertility and assisted conception. Best practice & research. Clinical endocrinology & metabolism. 2020 Jul:34(4):101378. doi: 10.1016/j.beem.2020.101378. Epub 2020 Jan 30 [PubMed PMID: 32037280]
Brown EDL, Obeng-Gyasi B, Hall JE, Shekhar S. The Thyroid Hormone Axis and Female Reproduction. International journal of molecular sciences. 2023 Jun 6:24(12):. doi: 10.3390/ijms24129815. Epub 2023 Jun 6 [PubMed PMID: 37372963]
Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocrine reviews. 2001 Oct:22(5):605-30 [PubMed PMID: 11588143]
Nguyen CT, Mestman JH. Postpartum Thyroiditis. Clinical obstetrics and gynecology. 2019 Jun:62(2):359-364. doi: 10.1097/GRF.0000000000000430. Epub [PubMed PMID: 30844908]
Jain D, Mor S, Aggarwal HK, Chhabra P, Jain P. Thyroid Association Ophthalmopathy in Hashimoto's Thyroiditis: a Case Report. Maedica. 2017 Jan:12(1):65-67 [PubMed PMID: 28878841]
Level 3 (low-level) evidenceSawicka-Gutaj N, Ziółkowska P, Wojciechowska K, Shawkat S, Czarnywojtek A, Warchoł W, Sowiński J, Szczepanek-Parulska E, Ruchała M. Eye symptoms in patients with benign thyroid diseases. Scientific reports. 2021 Sep 21:11(1):18706. doi: 10.1038/s41598-021-98232-0. Epub 2021 Sep 21 [PubMed PMID: 34548580]
Williams DE, Le SN, Godlewska M, Hoke DE, Buckle AM. Thyroid Peroxidase as an Autoantigen in Hashimoto's Disease: Structure, Function, and Antigenicity. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2018 Dec:50(12):908-921. doi: 10.1055/a-0717-5514. Epub 2018 Oct 25 [PubMed PMID: 30360003]
Rotondi M, de Martinis L, Coperchini F, Pignatti P, Pirali B, Ghilotti S, Fonte R, Magri F, Chiovato L. Serum negative autoimmune thyroiditis displays a milder clinical picture compared with classic Hashimoto's thyroiditis. European journal of endocrinology. 2014 Jul:171(1):31-6. doi: 10.1530/EJE-14-0147. Epub 2014 Apr 17 [PubMed PMID: 24743395]
Mikosch P, Aistleitner A, Oehrlein M, Trifina-Mikosch E. Hashimoto's thyroiditis and coexisting disorders in correlation with HLA status-an overview. Wiener medizinische Wochenschrift (1946). 2023 Feb:173(1-2):41-53. doi: 10.1007/s10354-021-00879-x. Epub 2021 Sep 15 [PubMed PMID: 34524590]
Level 3 (low-level) evidenceGarber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid : official journal of the American Thyroid Association. 2012 Dec:22(12):1200-35. doi: 10.1089/thy.2012.0205. Epub 2012 Nov 6 [PubMed PMID: 22954017]
Level 1 (high-level) evidenceZuo Y, Li Y, Gu X, Lei Z. The correlation between selenium levels and autoimmune thyroid disease: a systematic review and meta-analysis. Annals of palliative medicine. 2021 Apr:10(4):4398-4408. doi: 10.21037/apm-21-449. Epub 2021 Apr 16 [PubMed PMID: 33894732]
Level 1 (high-level) evidenceKryczyk-Kozioł J, Zagrodzki P, Prochownik E, Błażewska-Gruszczyk A, Słowiaczek M, Sun Q, Schomburg L, Ochab E, Bartyzel M. Positive effects of selenium supplementation in women with newly diagnosed Hashimoto's thyroiditis in an area with low selenium status. International journal of clinical practice. 2021 Sep:75(9):e14484. doi: 10.1111/ijcp.14484. Epub 2021 Jun 30 [PubMed PMID: 34107151]
Larsen C, Winther KH, Cramon PK, Rasmussen ÅK, Feldt-Rasmusssen U, Knudsen NJ, Bjorner JB, Schomburg L, Demircan K, Chillon TS, Gram J, Hansen SG, Brandt F, Nygaard B, Watt T, Hegedus L, Bonnema SJ. Selenium supplementation and placebo are equally effective in improving quality of life in patients with hypothyroidism. European thyroid journal. 2024 Jan 1:13(1):. pii: ETJ-23-0175. doi: 10.1530/ETJ-23-0175. Epub 2024 Jan 1 [PubMed PMID: 38215286]
Level 2 (mid-level) evidenceZhang L, Li Y, Yang L, Luo Z, Wu Z, Wang J, Qin S, Ren F, Hu T. Inverse association between serum iron levels and Hashimoto's thyroiditis in United States females of reproductive age: analysis of the NHANES 2007-2012. Frontiers in nutrition. 2024:11():1410538. doi: 10.3389/fnut.2024.1410538. Epub 2024 Oct 1 [PubMed PMID: 39416653]
Level 2 (mid-level) evidenceBhakat B, Pal J, Das S, Charaborty SK, SircarMedical NR, Kolkata, RGKar, NorthBengal, Siliguri. A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis. The Journal of the Association of Physicians of India. 2023 Jan:71(1):1 [PubMed PMID: 37116030]
Jiang X, Huang Y, Li Y, Xia Y, Liu L, Lin F, Shi Y. Therapeutic effect of vitamin D in Hashimoto's thyroiditis: a prospective, randomized and controlled clinical trial in China. American journal of translational research. 2023:15(10):6234-6241 [PubMed PMID: 37969187]
Level 1 (high-level) evidenceMazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA. Is vitamin D related to pathogenesis and treatment of Hashimoto's thyroiditis? Hellenic journal of nuclear medicine. 2015 Sep-Dec:18(3):222-7 [PubMed PMID: 26637501]
Tang J, Shan S, Li F, Yun P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto's thyroiditis: A systematic review and meta-analysis. Medicine. 2023 Dec 29:102(52):e36759. doi: 10.1097/MD.0000000000036759. Epub [PubMed PMID: 38206745]
Level 1 (high-level) evidenceOsowiecka K, Myszkowska-Ryciak J. The Influence of Nutritional Intervention in the Treatment of Hashimoto's Thyroiditis-A Systematic Review. Nutrients. 2023 Feb 20:15(4):. doi: 10.3390/nu15041041. Epub 2023 Feb 20 [PubMed PMID: 36839399]
Level 1 (high-level) evidenceÜlker MT, Çolak GA, Baş M, Erdem MG. Evaluation of the effect of gluten-free diet and Mediterranean diet on autoimmune system in patients with Hashimoto's thyroiditis. Food science & nutrition. 2024 Feb:12(2):1180-1188. doi: 10.1002/fsn3.3833. Epub 2023 Nov 20 [PubMed PMID: 38370054]
Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. The Journal of clinical endocrinology and metabolism. 2002 Jul:87(7):3221-6 [PubMed PMID: 12107228]
Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. European journal of endocrinology. 2013 Mar:168(3):343-9. doi: 10.1530/EJE-12-0903. Epub 2013 Feb 15 [PubMed PMID: 23211578]
Level 1 (high-level) evidenceXu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, Liu S, Xu Z, Liu J. Prevalence of Hashimoto Thyroiditis in Adults With Papillary Thyroid Cancer and Its Association With Cancer Recurrence and Outcomes. JAMA network open. 2021 Jul 1:4(7):e2118526. doi: 10.1001/jamanetworkopen.2021.18526. Epub 2021 Jul 1 [PubMed PMID: 34313737]
Level 2 (mid-level) evidenceHussein O, Abdelwahab K, Hamdy O, Awny S, Megahed NA, Hafez MT, Elalfi AF, Abdelaziz M, Gaballah K, Abdelkhalek M. Thyroid cancer associated with Hashimoto thyroiditis: similarities and differences in an endemic area. Journal of the Egyptian National Cancer Institute. 2020 Jan 17:32(1):7. doi: 10.1186/s43046-020-0017-9. Epub 2020 Jan 17 [PubMed PMID: 32372240]
Dündar HZ, Sarkut P, Kırdak T, Korun N. Primary thyroid lymphoma. Ulusal cerrahi dergisi. 2016:32(1):75-7. doi: 10.5152/UCD.2015.2935. Epub 2015 Jul 10 [PubMed PMID: 26985163]
Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. The Journal of clinical endocrinology and metabolism. 2013 Aug:98(8):3131-8. doi: 10.1210/jc.2013-1428. Epub 2013 May 28 [PubMed PMID: 23714679]
Walsh S, Lowery AJ, Evoy D, McDermott EW, Prichard RS. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. The oncologist. 2013:18(9):994-1003. doi: 10.1634/theoncologist.2013-0036. Epub 2013 Jul 23 [PubMed PMID: 23881987]
Level 3 (low-level) evidenceLee JS, Shin SJ, Yun HJ, Kim SM, Chang H, Lee YS, Chang HS. Primary thyroid lymphoma: A single-center experience. Frontiers in endocrinology. 2023:14():1064050. doi: 10.3389/fendo.2023.1064050. Epub 2023 Feb 9 [PubMed PMID: 36843586]