Introduction
A broad spectrum of hand infections is commonly encountered by hand surgeons, primary care physicians, and emergency medicine practitioners.[1] Acute hand infections vary significantly with regard to their location and etiology, but when misdiagnosed or untreated, they may result in significant morbidity or mortality to patients.[2] Hand infections may broadly be characterized as being superficial or deep, dependent upon whether they involve the skin and subcutaneous soft tissues, or whether they involve the deeper structures, respectively. Differentiation between superficial and deep infections of the hand has general implications on the requisite treatment modality and setting. Given the anatomical complexity of the hand and its delicate function, however, further characterization of the specific structures involved is necessary to establish the diagnosis and treatment fully.
Superficial infections are more common and may include the skin, nail fold, fingertip pulp, or subcutaneous tissues.[3] Superficial infections are more likely to resolve with conservative management alone than deeper infections.[3] Deep infections may involve the tendons, and their sheaths, bone, joint, or deep spaces of the hand and frequently require aspects of surgical management in addition to antimicrobial therapy to achieve resolution.[2] Regardless of the respective diagnosis, prompt evaluation and treatment are paramount to resolve the infection and optimize outcomes for patients. This article reviews the broad spectrum of infectious conditions affecting the hand and details their basic evaluation and general principles of treatment with the goal of improving recognition of pathology and understanding the acuity of treatment necessary.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Infections of the hand may arise in a myriad of ways, but most frequently occur due to direct inoculation and contiguous spread to adjacent structures after a traumatic injury.[1] In many cases, an infection may develop due to delayed or inadequate treatment after minor trauma.[4] Infections may also spread hematogenously, though this occurs more commonly in children, and individuals who are immunocompromised or who engage in intravenous drug use.[1][5]
Numerous bacterial and fungal pathogens have been isolated from infections of the hand. Staphylococcus aureus is cited as the overall most common microorganism causing infections of the hand, followed by Streptococcus species.[3] Methicillin-resistant Staphylococcus aureus is increasingly being identified, especially in urban communities, with some studies suggesting it may account for around 50% of hand infections in this setting.[6][7] Several other classical associations with particular microorganisms exist depending upon the nature of the injury. For example, Eikenella corrodens and Pasteurella multocida are identified frequently in human and animal bites, respectively.[8][9] Group A, B-hemolytic Streptococcus, is cited as being the most common, single organism isolated in cases of necrotizing soft tissue infections.[10] Chronic paronychia, which may arise in patients working in wet environments (i.e., dishwashers, bartenders, ‘thumb suckers’), is commonly determined to be caused by Candida albicans.[11] This is particularly true when a case of chronic paronychia is found to be unresponsive to oral antibiotic therapy.[11]
Atypical infections with nontuberculous mycobacterial species, such as Mycobacterium marinum or Vibrio spp may cause soft tissue infections of the hand after injury occurring in aquatic environments.[12] Other mycobacterial species, such as Mycobacterium avium, can cause infection in both immunocompromised and immunocompetent patients. Frequently these infections are insidious, and diagnosis is delayed.[13] Knowledge of such associations may be helpful in determining empiric antimicrobial therapy.
Epidemiology
Superficial hand infections are commonly encountered by hand surgeons, primary care physicians, and emergency medicine practitioners. As many superficial infections are managed conservatively in an outpatient setting, determination of the exact incidence of various infections is challenging. One study estimated 70% to 85% of hand infections are located within the skin, nail fold, fingertip pulp, or subcutaneous tissues.[14] Deep infections are less common, but frequently require inpatient admission for intravenous antibiotics and surgical management. Some studies estimate that 25 to 50 admissions at major hospital centers occur annually due to deep infections of the hand.[14] Other series’ demonstrate over 100 admissions may occur at an individual hospital due to hand infections over a year.[15]
Certain populations of patients have been determined to be at greater risk for hand infections. Manual laborers are more predisposed to superficial trauma, which can lead to the development of infection.[3] Intravenous drug users, diabetics, and immunocompromised patients are other subpopulations identified as being at greater risk.[16][17] Compared to a healthy patient population, diabetic patients are at greater risk to present with deeper infections, have infections caused by more atypical organisms, and to require multiple surgical procedures to treat an infection.[18][19] Patients with transplants with infections of the hand are more likely to undergo digital amputation and require longer hospitalizations than a non-transplant population.[19][20] Biological disease-modifying antirheumatic drugs (DMARDs) are also particularly cited as increasing risk of infection in patients with autoimmune conditions.[19] Elderly patients may also be at higher risk due to an increased burden of comorbidity, as well as having a weakened skin barrier and more poor vascular perfusion in the distal extremities.[21]
History and Physical
Acquiring a detailed history and physical exam is necessary to determine the correct diagnosis and necessary treatment. It is important to determine the patient’s age, handedness, and occupation. Knowledge of occupation may provide the physician insight into risk factors for certain exposures, and give a better understanding of the patient’s individualized needs when considering treatment and rehabilitation strategies. A full understanding of the patient’s comorbidities and past medical history should be obtained as certain medications, and medical conditions may place the patient in an immunocompromised state. A targeted review of systems should allow the physician to evaluate the patient for signs of hematogenous spread or systemic illness. Patients should also be queried regarding previous injuries or surgeries to their hands.If known by the patient, it is important to establish the time of onset and nature of the inoculation event. Understanding the chronicity and the setting of inoculation will help to guide empiric antibiotic coverage. This is especially important in the cases of penetrating trauma or bite wounds, as specific microorganisms have classical associations for particular types and settings of injury.[2] Knowledge of previous treatment of the present condition should be elicited, especially in the referral setting, as a failure of conservative management may suggest the presence of more insidious pathology and prompt greater consideration toward operative intervention or initiation of parenteral antibiotics.
A complete investigation into the patient’s presenting symptoms should always be undertaken to create and narrow differential diagnosis. Location and characterization of pain, erythema, effusion, and discharge may help to determine the particular pathology present. Patients should also be assessed for signs of nerve compression. In all cases, a full inspection of the hand and upper extremity should be performed to examine for erythema or other discoloration, effusion, deformity or malalignment, and lacerations or open wounds. Erythema should be outlined with a marking pen to help determine the progression of the infectious process, which may help to differentiate superficial cellulitis from a necrotizing soft tissue infection.[2] Location of tenderness or effusion can also provide insight regarding anatomic involvement. Various patterns characteristically arise depending upon the pathology present. Erythema, swelling, and effusion of the lateral or proximal nail fold indicate paronychia or eponychia, respectively.[11] Erythema, effusion, and pain in the pulp of the fingertip may represent the presence of a felon.[16] A herpetic whitlow is oftentimes signified by digital pain and erythema in the setting of clustered vesicles that coalesce together over time.[16] Palpation of fluctuance in the setting of localized erythema and effusion suggests the presence of an abscess.[1]
The existence of an abscess may also be suggested by the failure of apparent cellulitis to resolve upon initiation of antibiotic treatment. The location of the abscess should be noted if one is present, as they may occur superficially, in the webspace between digits, or the midpalmar, thenar, or hypothenar spaces.[1] Noting the presence of crepitation may suggest an infection with a gas-forming microorganism, and should raise suspicion for gas gangrene or a necrotizing soft tissue infection.[1] Presence of some or all of Kanavel’s cardinal signs (flexor posturing and fusiform swelling of the digit, tenderness to palpation along the flexor tendon sheath, and pain upon passive digit extension) may indicate the presence of flexor tenosynovitis.[22][23] Septic arthritis may be signified by pain, erythema, and effusion of any of the joints of the hand or wrist, with significant pain upon passive or active range of motion.[1]
A patient with osteomyelitis oftentimes has risk factors predisposing them to this condition, and on a physical exam may demonstrate localized erythema, bony pain, and edema. In some cases of osteomyelitis, a draining sinus tract will also be present.[1] Finally, in all cases, a complete neurovascular exam should be performed and documented. The presence of infectious processes within certain enclosed spaces, such as the carpal tunnel, can result in symptoms of nerve compression.
Evaluation
The remainder of the objective patient assessment should include an examination of a patient’s vital signs to evaluate for fever or other abnormalities suggesting a systemic illness. Laboratory evaluation should include assessment of the white blood cell count. Other inflammatory markers, such as the erythrocyte sedimentation rate (ESR) and C-reactive protein test (CRP) can be collected to assess the systemic inflammatory response. It certain instances, it may be helpful to evaluate the CRP serially as a marker of the acute phase reaction, in order to monitor the patient’s response to therapy.[4] Depending upon the setting, blood, and local fluid or tissue cultures can also be acquired to identify the responsible organism and evaluate for antibiotic susceptibility.[1] Cultures should ideally be taken prior to the initiation of antibiotic therapy to maximize yield and should be sent routinely if an associated debridement is performed.
If they are determined to be clinically indicated, standard radiographs or other advanced imaging modalities may be used for diagnosis or therapy. Radiographs can be assessed for osteolysis or periosteal reaction if osteomyelitis is suspected.[1] Radiographs may also show the presence of radiolucent foreign bodies, which can serve as a nidus for infection. Ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI) may be used to identify and localize abscesses. Gas tracking along the facial planes on advanced imaging can also signify the presence of gas gangrene or necrotizing fasciitis.[1]
Treatment / Management
Although the treatment of hand infections is specific to the particular pathology, several basic tenets of management exist. Conservative management of infections includes splint immobilization, soaks, and elevation in addition to adequate antimicrobial coverage. Broad-spectrum antimicrobial coverage should be started while awaiting objective culture data, and with consideration to preliminary gram stain results and epidemiologic clues.[1] Empiric antibiotic selection should include coverage against Gram-positive, Gram-negative, and anaerobic organisms. Given the frequency of MRSA in hand-infections, vancomycin (1 g every 12 hours) is commonly selected for Gram-positive coverage.[6][22] Piperacillin/tazobactam (3.375 g every 6 hours) is commonly added to cover Gram-negative rods and anaerobes, given the risk of polymicrobial infection.[1][6][22] Cat bites are usually treated with amoxicillin-clavulanic acid. As culture data results, the antibiotic spectrum should be narrowed appropriately to cover the specific organisms that are identified and their respective susceptibilities.[1][2][3] In cases where the skin barrier is penetrated, tetanus prophylaxis should be given dependent upon the patient’s immunization status.[16] Despite being able to manage many superficial infections on an outpatient basis, a local incision and drainage with subsequent wound care may need to be performed at the bedside in the clinic or the emergency department. Early evaluation for surgical management should occur in situations where the infection doesn’t respond to conservative management, or in cases involving the deeper structures of the hand.(B3)
The typical treatment for chronic paronychia is the avoidance of predisposing factors and the application of topical antifungals and steroids. In certain cases, additional oral antibiotics may be included in the management of chronic paronychia to cover for potential secondary bacterial infections.[24] However, paronychia or eponychia that fail to respond to conservative measures may require incision and drainage with partial or complete nail removal, and with the possible release of the eponychial fold.[11] A felon may require incision and drainage with care taken to release all septae.[1][16] Initially localized, but untreated felons can spread to involve adjacent joints, flexor tendons, tendon sheaths, and bone.[1][21] Human or animal bites may also progress to require surgical irrigation and debridement if conservative measures fail.[1][2] Physicians must maintain a high index of suspicion for the presence of a “fight bite” in patients presenting with lacerations over the dorsal metacarpophalangeal joint. These injuries, frequently sustained when a closed fist makes contact with a tooth in an altercation, require prompt treatment with incision and drainage, especially if joint or tendon sheath is involved to avoid the development of infection in these structures.[5] Abscesses require incision and drainage, which are performed at the bedside or in an operating room depending upon their anatomic location.[2](B3)
Deeper infections more frequently require intravenous antibiotic treatment and prompt surgical management. In certain cases of flexor tenosynovitis, a trial of intravenous antibiotics under close inpatient observation may be attempted, provided the patient presents within 24 hours of symptom onset.[22] Typically, however, the treatment of choice for flexor tenosynovitis is incision and drainage of the flexor tendon sheath.[22] Incision and drainage of the flexor tendon sheath may be accomplished by passing an angiocatheter through a small proximal or distal incision on the digit. Depending on the presentation, however, a full open exposure may be required.[22] If untreated, thumb or index finger flexor tenosynovitis may spread to the deep thenar space or radial bursa, and the more ulnar digits may spread to the deep midpalmar space or ulnar bursa.[22][25] The development of a “horseshoe abscess” is based upon similar connections, where infection communicates between the thumb and small finger flexor tendon sheaths in a potential space located between the pronator quadratus and flexor digitorum profundus tendons (i.e., Parona space).[22][25] (B3)
Given their location and deep anatomic connections, infections of the flexor tendons, web spaces, and deep spaces of the hand require incision and drainage to eradicate the infection and prevent further contiguous spread.[25] Similarly, necrotizing soft tissue infections and gas gangrene require emergent, extensive surgical debridement, and broad-spectrum, intravenous antimicrobial coverage to prevent rapid systemic decompensation.[1][2] Osteomyelitis generally requires long-term intravenous antibiotic treatment, and in some cases, requires surgical debridement. Septic arthritis requires urgent irrigation and debridement in all cases to prevent inflammatory-mediated destruction of cartilage and the onset of arthritis.[1]
Differential Diagnosis
While the presence of infection can usually be determined based on the history and physical exam in conjunction with laboratory analysis, several non-infectious inflammatory conditions can mimic the appearance of different infectious conditions. Osteoarthritis exacerbations, crystalline arthritis, or inflammatory arthropathies may appear similar to septic arthritis but can be differentiated based upon synovial fluid analysis, gram stain, and culture.[1] Pyoderma gangrenosum, a dermatologic condition often associated with a concomitant systemic inflammatory disorder, can appear similar to a localized skin and soft tissue infection.[5] Retained foreign bodies or metastases are other conditions that can generate a local inflammatory response, which can appear similar to an infection.[2]
Prognosis
The prognoses of hand infections differ significantly depending upon the pathology present and the acuity with which it is recognized and treated by the physician. Provided the infection is promptly recognized, and appropriate antimicrobial coverage is initiated early, superficial infections typically resolve with good functional outcome. In cases of infections involving deep structures, the prognosis depends upon the chronicity of infection, the respective structures involved, and the adequacy of surgical and antimicrobial treatment. With deeper infections, postoperative care, and structured hand therapy are paramount to minimize tendon adhesions and digital stiffness.[2] Utilization of proper splinting techniques, which maintain the length of capsular ligaments and leave uninvolved joints free whenever possible, is also important to preserve function.[1][2] Referral to dedicated hand therapy for supervised mobilization and edema control may be necessary or helpful for patients to achieve good outcomes.
Complications
Prompt evaluation and treatment are paramount to resolving the infection and optimizing outcomes for patients. If unrecognized or inadequately treated, infections may progress to involve contiguous structures, resulting in greater morbidity to patients. Functional limitations and neurovascular compromise may arise as a result of mismanagement. Other complications include stiffness, tendon rupture, joint destruction, osteomyelitis, nerve compression, and wound complications requiring amputation or flap coverage.[2][3][5][14] Untreated or mistreated infections can also progress to cause systemic illness, sepsis, and even death.[1][2]
Deterrence and Patient Education
Given the risk for complications, patients should be educated regarding the signs and symptoms of hand infections and be encouraged to acquire an evaluation from a physician in the setting of any concerning findings. Prompt recognition and treatment of infections can prevent the progression of a more minor infection into one that requires surgical management. If appropriate, counseling for appropriate wound care, soaking, and dressings should be given once they are initiated.
Enhancing Healthcare Team Outcomes
While some simple, superficial patterns of hand infections may be managed by primary care or emergency physicians in an outpatient setting, it is essential to recognize that certain pathology may require an interdisciplinary approach. There should exist a low threshold for the primary care or emergency medicine physician to provide referral or consultation to a hand surgeon for further management. Furthermore, depending upon the etiology of the infection and microorganism susceptibilities, consultation with an infectious disease specialist may help determine the optimal antimicrobial regimen. In cases of immunocompromised patients or in dealing with drug-resistant organisms, an interprofessional approach may be necessary to ensure appropriate patient care.
Media
(Click Image to Enlarge)
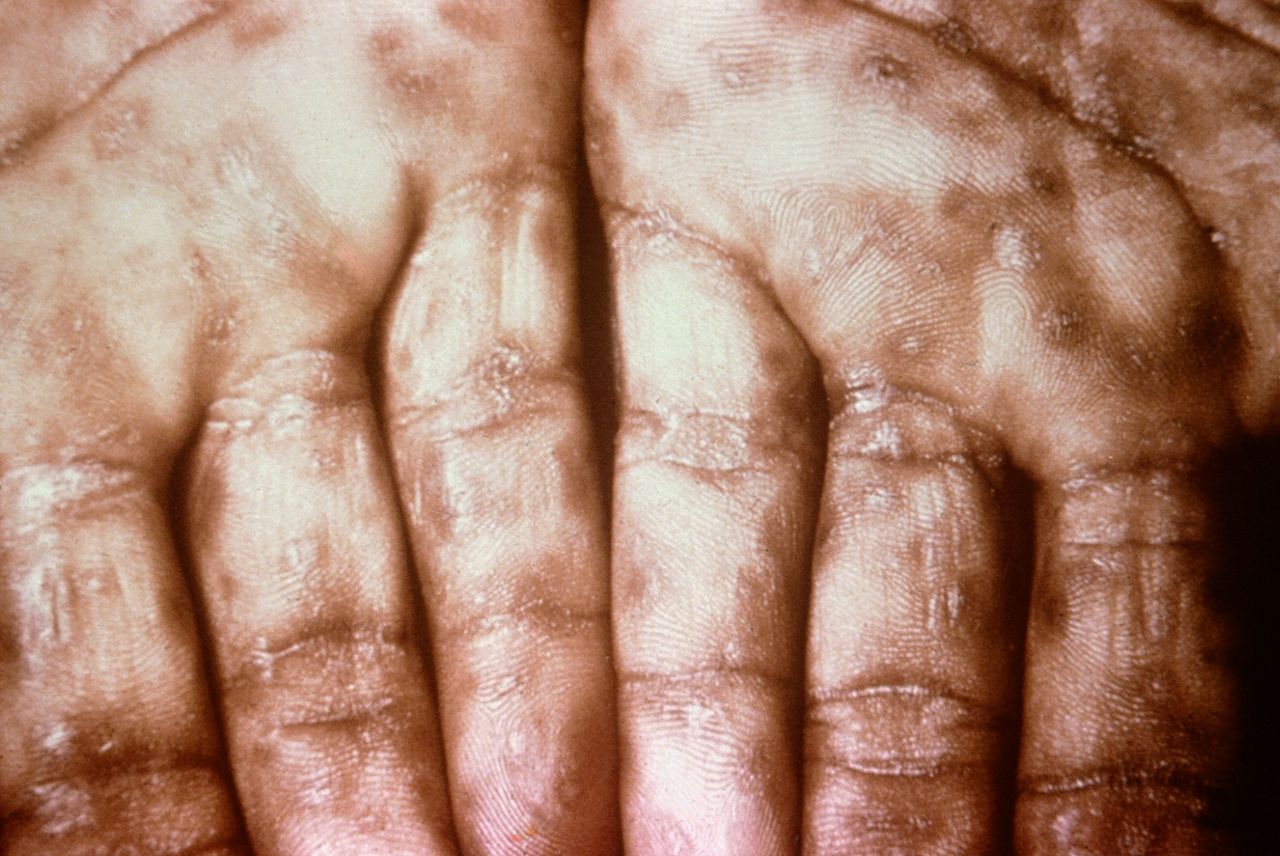
Keratotic Lesions on the Palms. This photograph shows a close-up view of keratotic lesions on the palms of a patient’s hands due to a secondary syphilitic infection. Syphilis is a complex sexually transmitted disease (STD) caused by the bacterium Treponema pallidum. It has often been called “the great imitator” because many signs and symptoms are indistinguishable from those of other diseases.
Robert Sumpter; Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
References
Koshy JC, Bell B. Hand Infections. The Journal of hand surgery. 2019 Jan:44(1):46-54. doi: 10.1016/j.jhsa.2018.05.027. Epub 2018 Jul 14 [PubMed PMID: 30017648]
Teo WZW, Chung KC. Hand Infections. Clinics in plastic surgery. 2019 Jul:46(3):371-381. doi: 10.1016/j.cps.2019.03.003. Epub [PubMed PMID: 31103082]
Rerucha CM, Ewing JT, Oppenlander KE, Cowan WC. Acute Hand Infections. American family physician. 2019 Feb 15:99(4):228-236 [PubMed PMID: 30763047]
Houshian S, Seyedipour S, Wedderkopp N. Epidemiology of bacterial hand infections. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2006 Jul:10(4):315-9 [PubMed PMID: 16483816]
Level 3 (low-level) evidenceMcDonald LS, Bavaro MF, Hofmeister EP, Kroonen LT. Hand infections. The Journal of hand surgery. 2011 Aug:36(8):1403-12. doi: 10.1016/j.jhsa.2011.05.035. Epub [PubMed PMID: 21816297]
Kistler JM, Thoder JJ, Ilyas AM. MRSA Incidence and Antibiotic Trends in Urban Hand Infections: A 10-Year Longitudinal Study. Hand (New York, N.Y.). 2019 Jul:14(4):449-454. doi: 10.1177/1558944717750921. Epub 2018 Jan 11 [PubMed PMID: 29322874]
Fowler JR, Ilyas AM. Epidemiology of adult acute hand infections at an urban medical center. The Journal of hand surgery. 2013 Jun:38(6):1189-93. doi: 10.1016/j.jhsa.2013.03.013. Epub 2013 May 3 [PubMed PMID: 23647640]
Schmidt DR, Heckman JD. Eikenella corrodens in human bite infections of the hand. The Journal of trauma. 1983 Jun:23(6):478-82 [PubMed PMID: 6345799]
Level 2 (mid-level) evidenceArons MS, Fernando L, Polayes IM. Pasteurella multocida--the major cause of hand infections following domestic animal bites. The Journal of hand surgery. 1982 Jan:7(1):47-52 [PubMed PMID: 7061808]
Level 3 (low-level) evidenceDeLullo JA, Lubahn JD, Loeffler OP, Dominy DD. Necrotizing soft tissue infections of the upper extremity: a case series. Journal of surgical orthopaedic advances. 2013 Fall:22(3):198-203 [PubMed PMID: 24063795]
Level 3 (low-level) evidenceShafritz AB, Coppage JM. Acute and chronic paronychia of the hand. The Journal of the American Academy of Orthopaedic Surgeons. 2014 Mar:22(3):165-74. doi: 10.5435/JAAOS-22-03-165. Epub [PubMed PMID: 24603826]
Diaz JH, Lopez FA. Skin, soft tissue and systemic bacterial infections following aquatic injuries and exposures. The American journal of the medical sciences. 2015 Mar:349(3):269-75. doi: 10.1097/MAJ.0000000000000366. Epub [PubMed PMID: 25374398]
Al-Qattan MM, Helmi AA. Chronic hand infections. The Journal of hand surgery. 2014 Aug:39(8):1636-45. doi: 10.1016/j.jhsa.2014.04.003. Epub [PubMed PMID: 25070033]
Brown DM, Young VL. Hand infections. Southern medical journal. 1993 Jan:86(1):56-66 [PubMed PMID: 8420019]
Türker T, Capdarest-Arest N, Bertoch ST, Bakken EC, Hoover SE, Zou J. Hand infections: a retrospective analysis. PeerJ. 2014:2():e513. doi: 10.7717/peerj.513. Epub 2014 Sep 2 [PubMed PMID: 25210653]
Level 2 (mid-level) evidenceClark DC. Common acute hand infections. American family physician. 2003 Dec 1:68(11):2167-76 [PubMed PMID: 14677662]
Chan E, Bagg M. Atypical Hand Infections. The Orthopedic clinics of North America. 2017 Apr:48(2):229-240. doi: 10.1016/j.ocl.2016.12.013. Epub 2017 Feb 1 [PubMed PMID: 28336045]
Sharma K, Pan D, Friedman J, Yu JL, Mull A, Moore AM. Quantifying the Effect of Diabetes on Surgical Hand and Forearm Infections. The Journal of hand surgery. 2018 Feb:43(2):105-114. doi: 10.1016/j.jhsa.2017.11.003. Epub 2017 Dec 12 [PubMed PMID: 29241843]
Schmidt G, Piponov H, Chuang D, Gonzalez M. Hand Infections in the Immunocompromised Patient: An Update. The Journal of hand surgery. 2019 Feb:44(2):144-149. doi: 10.1016/j.jhsa.2018.07.001. Epub 2018 Aug 23 [PubMed PMID: 30145028]
Francel TJ, Marshall KA, Savage RC. Hand infections in the diabetic and the diabetic renal transplant recipient. Annals of plastic surgery. 1990 Apr:24(4):304-9 [PubMed PMID: 2353778]
Flevas DA, Syngouna S, Fandridis E, Tsiodras S, Mavrogenis AF. Infections of the hand: an overview. EFORT open reviews. 2019 May:4(5):183-193. doi: 10.1302/2058-5241.4.180082. Epub 2019 May 10 [PubMed PMID: 31191986]
Level 3 (low-level) evidenceDraeger RW, Bynum DK Jr. Flexor tendon sheath infections of the hand. The Journal of the American Academy of Orthopaedic Surgeons. 2012 Jun:20(6):373-82. doi: 10.5435/JAAOS-20-06-373. Epub [PubMed PMID: 22661567]
Level 3 (low-level) evidenceKennedy CD, Huang JI, Hanel DP. In Brief: Kanavel's Signs and Pyogenic Flexor Tenosynovitis. Clinical orthopaedics and related research. 2016 Jan:474(1):280-4. doi: 10.1007/s11999-015-4367-x. Epub 2015 May 29 [PubMed PMID: 26022113]
Hochman LG. Paronychia: more than just an abscess. International journal of dermatology. 1995 Jun:34(6):385-6 [PubMed PMID: 7657434]
Rigopoulos N, Dailiana ZH, Varitimidis S, Malizos KN. Closed-space hand infections: diagnostic and treatment considerations. Orthopedic reviews. 2012 May 9:4(2):e19. doi: 10.4081/or.2012.e19. Epub 2012 Jun 13 [PubMed PMID: 22802987]