Introduction
The pilosebaceous unit is the hair follicle's structural unit, comprised of the hair follicle and its associated sebaceous gland and arrector pili muscle (see Image. The Common Integument, Section of Skin). The hair follicle originates from the surface of the epidermis. Follicles that produce terminal hairs extend into the deep dermis and, sometimes, even the subcutis. Meanwhile, follicles producing vellus hairs extend only to the upper reticular dermis. Three essential segments of hair follicles are found on the head: the infundibulum, isthmus, and lower follicle. The lower follicle is also known as the inferior segment and includes the bulb.[1][2]
Sebaceous holocrine glands are associated with hair follicles, especially in some skin regions, such as the face. These glands open onto hair follicles, except in areas like the lips, where they empty directly onto the mucosal surface due to the absence of hair follicles. When stimulated by hormones such as androgens, sebaceous glands secrete a lipid-rich sebum that protects the hair and provides the skin with a hydrophobic protective barrier.[3]
The arrector pili muscle is a bundle of smooth muscle fibers that connects to the bulge area of the external root sheath of the hair follicle and extends obliquely to its superior attachment point just below the epidermis (papillary dermis). Sympathetic stimulation causes these muscles to contract in cold climates, slightly raising the skin and making the hair stand erect, an occurrence commonly referred to as "goose-bumps."[4][5]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
The 3 essential hair follicle segments found on the head are the infundibulum, isthmus, and inferior segment. The infundibulum is the follicle's upper portion, beginning at the epidermal surface and extending to the sebaceous duct opening. The isthmus is the area between the sebaceous duct opening and the bulge. The bulge is marked by the arrector pili muscle insertion and contains several epidermal stem cells that are part of the outer root sheath. These stem cells can be stained for the markers cytokeratin 19, cytokeratin 15, and CD200 (OX-2 membrane glycoprotein).
The inferior segment extends from the bulge to the base of the follicle. This segment includes the bulb, which contains the follicular matrix surrounding the sides and top of the dermal papilla. The dermal papilla contains capillaries and interacts with the matrix, which has the highest mitotic rate of any organ. The matrix is where matrix keratinocytes proliferate to form the hair shaft. Mixed among the matrix cells, melanocytes provide the hair shaft with color.
The hair shaft consists of an inner core—the medulla—surrounded by the cortex, which makes up the bulk of the hair (see Image. Hair Follicle Transverse Section). Moving outward, a single layer of cells makes up the shaft cuticle, a structure encased in 3 layers that form the inner (internal) root sheath. The inner sheath shapes the hair shaft as it grows upward from the matrix. The inner sheath keratinizes from the outside and eventually disintegrates mid-follicle around the isthmus level. The outer (external) root sheath encases the entirety of the hair shaft and undergoes trichilemmal keratinization around the isthmus level (see Image. Cross Section, Layers of the Skin).[6][7]
Hair production and growth
Hair follicles undergo a cyclical process comprising growth (anagen), regression (catagen), rest (telogen), and shedding (exogen). This cycle is essential for continuous hair renewal throughout an individual's life.
The anagen (proliferation) phase occurs when the hair follicle grows a new shaft. The length of this phase can vary. The growth phase can last 2 to 6 years for scalp hair and only a few months for eyebrows and eyelashes. The anagen phase is the only phase during which the hair follicle's inferior segment is present. The dermal papilla signals to the multipotent epithelial stem cells in the bulge at the beginning of anagen. Once stimulated, the inferior segment grows downward, forming a bulb around the dermal papilla. The dermal papilla then signals the matrix cells in the bulb to proliferate, differentiate, and grow upward, creating a new hair.
The catagen (transition or regression) phase lasts only a few weeks and is the shortest hair growth phase. Cell division in the matrix ceases during this phase. The inferior segment also regresses and eventually disappears, and the dermal papilla moves up to contact the bulge. This process forms a club hair with a white, hard node at the end.
In the telogen (resting) phase, the hair is fully formed but not actively growing. Club hairs, essentially dead, remain on the scalp for about 100 days.
The exogen (shedding) phase is sometimes considered a telogen sub-phase. Old hair is shed and makes way for new hair growth during exogen.[8][9]
All hairs begin in the anagen average at birth. The human scalp contains over 100,000 hairs. Most hairs (85% to 95%) are in the anagen phase at any given time. These hairs can grow approximately 1 cm per month. Meanwhile, hair loss is continuous, with people losing about 100 hairs daily on average. The number of hairs lost can double with shampooing.[10] Stem cells in the hair follicle bulge region are activated to regenerate the lower part of the hair follicle during the anagen phase, leading to new hair growth.[11]
Function
The hair follicle is essential for producing and sustaining hair growth. This structure also contributes to various skin functions and responses.
Sensory and protective functions
Hair follicles have nerve endings, making them sensitive to touch and vibrations. Hair follicle sensations help people detect external stimuli and respond appropriately. Hair also provides a physical barrier that helps protect the skin from environmental damage, UV radiation, and mechanical injury.[12]
Thermoregulation
Hair helps maintain body temperature by providing an insulating layer, which is particularly important in mammals with dense fur, as fur traps heat and reduces heat loss in cold environments. Hair aids in dissipating heat through mechanisms like sweating, influencing the efficiency of these cooling processes.[13]
Wound healing and skin regeneration
Hair follicles are crucial in wound healing and skin regeneration. Stem cells from the hair follicle bulge can migrate to the epidermis to aid in tissue repair. Additionally, hair follicles release various cytokines and growth factors that promote cellular proliferation and tissue repair during wound healing.
Endocrine function
Hair follicles respond to various hormones, influencing hair growth, density, and distribution. For instance, androgens play a significant role in the development of male pattern baldness.[14] Hair follicles secrete sebum, an oily substance produced by sebaceous glands associated with the follicles. Sebum helps lubricate and waterproof the hair and skin.[15]
Immune response
The hair follicle, particularly the bulge region, is an immune-privileged site. The hair follicle can thus suppress immune responses to protect the stem cells within it, preventing autoimmunity and chronic inflammation.[16]
Embryology
Hair follicle embryogenesis is characterized by intricate interactions and communications between the epidermis and dermis. This developmental process begins around day 14 of embryogenesis with the formation of the dermal papilla. An epidermal thickening, the placode, initially forms. The cells within the placode send signals that lead to the formation of the dermal condensate beneath it. The interaction between the placode and the dermal condensate stimulates the proliferation of epithelial placode cells and their downward extension. The placode eventually envelops the dermal condensate, now termed the dermal papilla, forming the hair bulb.
The dermal papilla, composed of mesenchymal cells, plays a crucial role in regulating the growth and development of the hair follicle both during embryogenesis and postnatal life. The appearance of the dermal papilla signals adjacent epithelial cells, known as matrix cells, to proliferate and differentiate. Dermal papilla cells migrate upward during maturation, contributing to the formation of the inner root sheath and the hair shaft layers. Surrounding the inner root sheath is the outer root sheath, which is continuous with the interfollicular epidermal cells. The bulge, a specific region within the outer root sheath, houses epithelial stem cells crucial for hair follicle regeneration.[17]
Sebocytes, which form sebaceous glands, originate from superficial hair follicle cells. Sebaceous gland development occurs between the 13th and 16th weeks of gestation, stemming from the superficial bulge of the emerging hair follicle. Several signaling pathways and factors, including Sonic Hedgehog, Wnt, heparan sulfate proteins, and C-myc, are implicated in sebocyte development, regulation, and proliferation.[18][19]
Lanugo hairs grow in utero once hair follicles develop. Lanugo hairs are thin and short. These hairs eventually shed by about 36 to 40 weeks gestation and are replaced by vellus hairs, which cover most body areas. Meanwhile, thicker, courser terminal hairs can be found on the head in areas such as the scalp, eyebrows, and eyelashes. Beard hairs arise once puberty is reached, with androgen hormones transforming the vellus hairs in the beard area into terminal hairs. New hair follicles do not form after birth, but new hair can grow from the existing follicles. Follicle size can still be altered after birth.[20]
Blood Supply and Lymphatics
Blood supply to the hair follicles is primarily provided by a network of capillaries derived from dermal blood vessels. These capillaries deliver essential nutrients and oxygen to the rapidly dividing cells in the hair bulb and follicle, ensuring the metabolic demands of active hair growth are met. The dermal papilla, located at the base of the hair follicle, is rich in capillaries that supply blood to the follicle. This vascular network is crucial for providing the necessary growth factors and nutrients to the hair follicle cells.[21]
Angiogenesis, the formation of new blood vessels, is stimulated during the anagen phase of the hair cycle to support the follicle's increased metabolic activity. This process is vital for sustaining prolonged hair growth.[22] Various growth factors, such as vascular endothelial growth factor, modulate the blood supply to hair follicles, ensuring adequate blood flow throughout the hair cycle.
The lymphatic vasculature within the dermis also drains the hair follicles. The lymph vessels participate in the skin's immune response.
Nerves
Two distinct neural networks innervate the hair and hair follicles, comprising sensory and autonomic nerve fibers. Sensory C fibers and sympathetic fibers form a neural network around the hair follicle neck. This network is critical in transmitting sensory information and modulating autonomic responses. These fibers also innervate the proximal arrector pili muscles.[23] A second neural network comprising longitudinal Aδ fibers and circular C fibers encircles the midsection of the hair follicle. These fibers are involved in the mechanosensory and pain responses of the hair follicle.[24]
Muscles
The arrector pili muscles are small smooth muscles located in the skin's dermis, attached to hair follicles. These muscles are crucial in the body's thermoregulatory and sensory responses. Arrector pili muscle contraction causes the hair to stand up, a reaction known as piloerection or "goosebumps." This action is primarily a response to cold or emotional stimuli, aiding in heat retention by trapping a layer of air beneath the erect hairs.[21] The arrector pili muscles originate near the sebaceous glands and insert onto the hair follicle at an angle. The contraction of these muscles also assists in sebum secretion from the sebaceous glands, which helps lubricate and protect both the hair and the skin.[21]
Clinical Significance
Hair growth rate and hair density decrease with age. Hair loss, known as alopecia, is a common occurrence. The condition can be due to factors like drugs, diet, hormone imbalances, altered mitotic activity, and growth cycle abnormalities. A thorough history, physical exam, hair pull test, daily hair counts, part width, hair shaft clip tests, hair growth windows, hair pluck, and trichograms can all be used to diagnose hair disease. Scalp biopsies, hormone studies, and a potassium hydroxide examination for fungi may also need to be performed in certain cases. Hair disease must be correctly diagnosed, as the treatment for hair loss depends on the diagnosis.
Male-pattern baldness, known as androgenetic alopecia, is a common condition where hair loss occurs as men age. Anagen cycles shorten progressively with increased dihydrotestosterone binding to the follicle of hair receptors. The hair follicles shrink and gradually produce shorter, thinner, more vellus-like hairs. Possible treatments may include slowing the conversion of testosterone to dihydrotestosterone in men with this condition.[25][26]
Media
(Click Image to Enlarge)
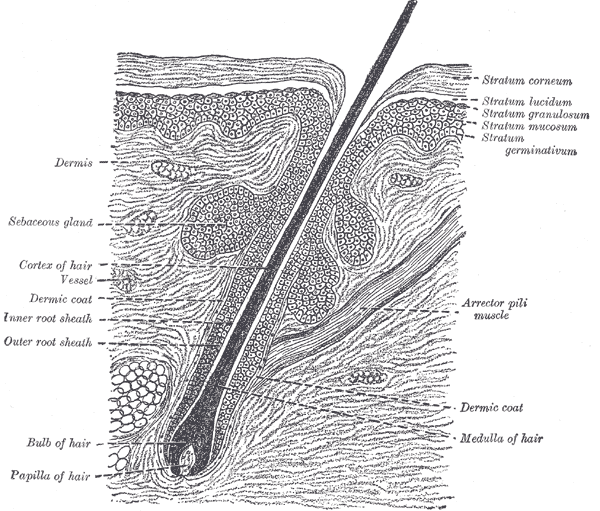
The Common Integument, Section of the Skin. A section of the skin showing the epidermis, dermis, hair shaft and follicle, arrector pili muscles, and sebaceous glands.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
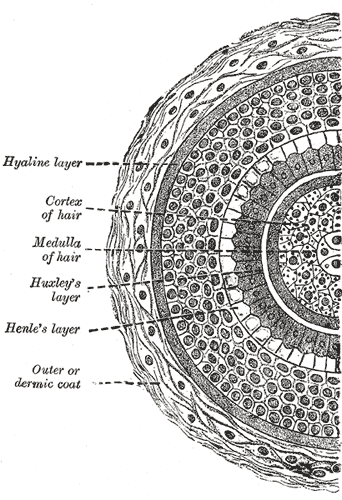
Transverse Section of a Hair Follicle. This transverse hair follicle section shows the hyaline layer, hair cortex and medulla, Huxley and Henle layers, and outer or dermic coat.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
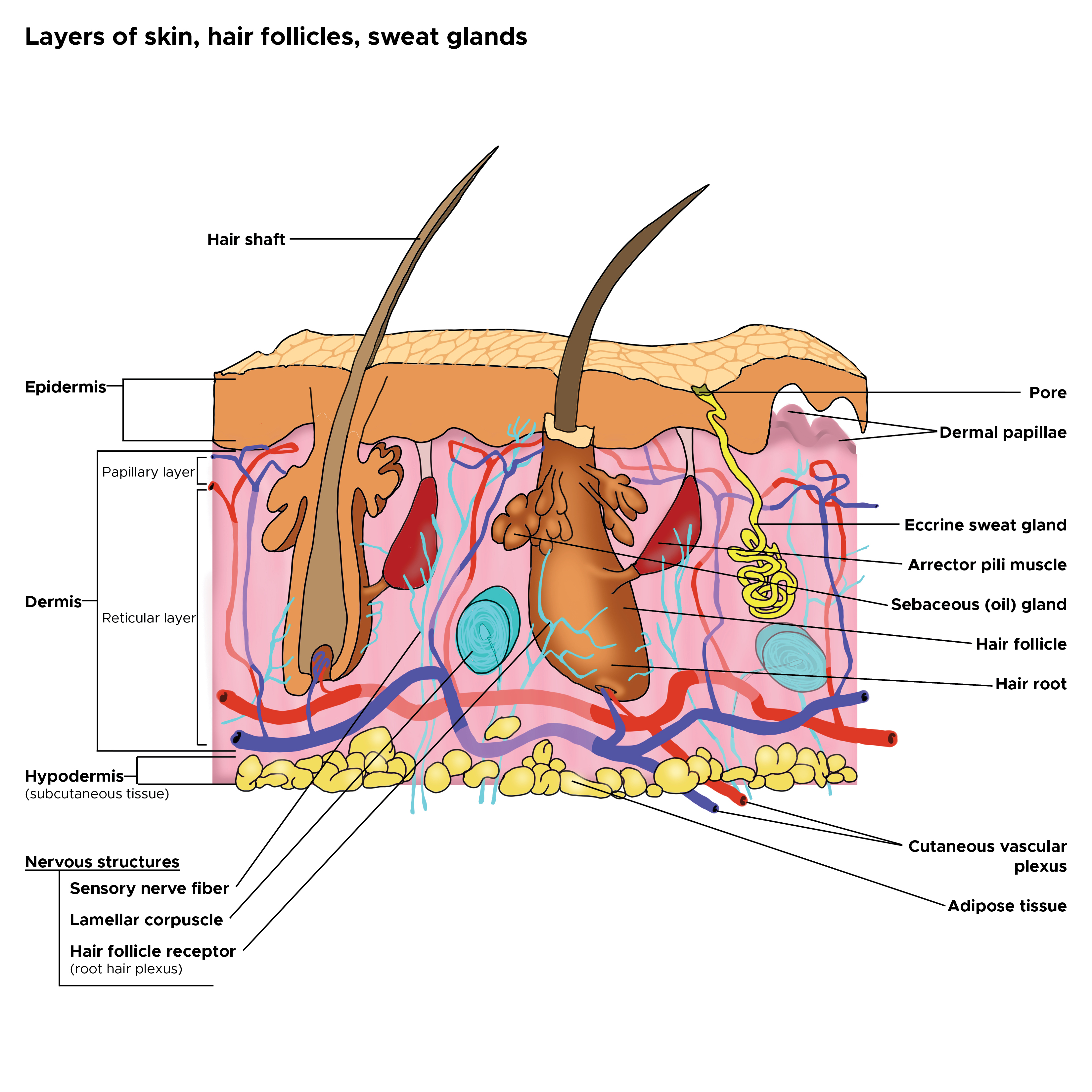
Cross Section, Layers of the Skin. This is a cross-section view of the hair follicles, hair roots and shafts, sweat glands, pores, epidermis, dermis, and hypodermis. The papillary and reticular layers are also included. The eccrine sweat gland is located in the arrector pili muscles, and the sebaceous oil glands are located in the reticular layer.
Contributed by C Rowe
References
Rahmani W, Abbasi S, Hagner A, Raharjo E, Kumar R, Hotta A, Magness S, Metzger D, Biernaskie J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Developmental cell. 2014 Dec 8:31(5):543-58. doi: 10.1016/j.devcel.2014.10.022. Epub 2014 Nov 26 [PubMed PMID: 25465495]
Paniagua Gonzalez LM, Tschen JA, Cohen PR. Ectopic Sebaceous Glands in the Hair Follicle Matrix: Case Reports and Literature Review of this Embryogenic Anomaly. Cureus. 2018 Nov 17:10(11):e3605. doi: 10.7759/cureus.3605. Epub 2018 Nov 17 [PubMed PMID: 30680266]
Level 3 (low-level) evidenceWortsman X, Carreño L, Ferreira-Wortsman C, Poniachik R, Pizarro K, Morales C, Calderon P, Castro A. Ultrasound Characteristics of the Hair Follicles and Tracts, Sebaceous Glands, Montgomery Glands, Apocrine Glands, and Arrector Pili Muscles. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2019 Aug:38(8):1995-2004. doi: 10.1002/jum.14888. Epub 2018 Dec 20 [PubMed PMID: 30570163]
Poblet E, Jiménez F, Ortega F. The contribution of the arrector pili muscle and sebaceous glands to the follicular unit structure. Journal of the American Academy of Dermatology. 2004 Aug:51(2):217-22 [PubMed PMID: 15280840]
Poblet E, Ortega F, Jiménez F. The arrector pili muscle and the follicular unit of the scalp: a microscopic anatomy study. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2002 Sep:28(9):800-3 [PubMed PMID: 12269872]
Glover JD, Wells KL, Matthäus F, Painter KJ, Ho W, Riddell J, Johansson JA, Ford MJ, Jahoda CAB, Klika V, Mort RL, Headon DJ. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS biology. 2017 Jul:15(7):e2002117. doi: 10.1371/journal.pbio.2002117. Epub 2017 Jul 11 [PubMed PMID: 28700594]
Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. The British journal of dermatology. 2006 Apr:154(4):609-18 [PubMed PMID: 16536801]
Level 3 (low-level) evidenceChen R, Miao Y, Hu Z. Dynamic Nestin expression during hair follicle maturation and the normal hair cycle. Molecular medicine reports. 2019 Jan:19(1):549-554. doi: 10.3892/mmr.2018.9691. Epub 2018 Nov 26 [PubMed PMID: 30483790]
Dobreva A, Paus R, Cogan NG. Analysing the dynamics of a model for alopecia areata as an autoimmune disorder of hair follicle cycling. Mathematical medicine and biology : a journal of the IMA. 2018 Sep 11:35(3):387-407. doi: 10.1093/imammb/dqx009. Epub [PubMed PMID: 28992198]
Stenn KS, Paus R. Controls of hair follicle cycling. Physiological reviews. 2001 Jan:81(1):449-494 [PubMed PMID: 11152763]
Zhang B, Chen T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nature reviews. Molecular cell biology. 2024 Feb:25(2):87-100. doi: 10.1038/s41580-023-00662-3. Epub 2023 Oct 30 [PubMed PMID: 37903969]
Panteleyev AA. Functional anatomy of the hair follicle: The Secondary Hair Germ. Experimental dermatology. 2018 Jul:27(7):701-720. doi: 10.1111/exd.13666. Epub [PubMed PMID: 29672929]
Welle MM, Wiener DJ. The Hair Follicle: A Comparative Review of Canine Hair Follicle Anatomy and Physiology. Toxicologic pathology. 2016 Jun:44(4):564-74. doi: 10.1177/0192623316631843. Epub 2016 Mar 21 [PubMed PMID: 27000375]
Level 2 (mid-level) evidenceCeruti JM, Leirós GJ, Balañá ME. Androgens and androgen receptor action in skin and hair follicles. Molecular and cellular endocrinology. 2018 Apr 15:465():122-133. doi: 10.1016/j.mce.2017.09.009. Epub 2017 Sep 12 [PubMed PMID: 28912032]
Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, Meczekalski B. Hormonal Effects on Hair Follicles. International journal of molecular sciences. 2020 Jul 28:21(15):. doi: 10.3390/ijms21155342. Epub 2020 Jul 28 [PubMed PMID: 32731328]
Contreras-Jurado C, Lorz C, García-Serrano L, Paramio JM, Aranda A. Thyroid hormone signaling controls hair follicle stem cell function. Molecular biology of the cell. 2015 Apr 1:26(7):1263-72. doi: 10.1091/mbc.E14-07-1251. Epub 2015 Feb 5 [PubMed PMID: 25657324]
Peña-Jimenez D, Fontenete S, Megias D, Fustero-Torre C, Graña-Castro O, Castellana D, Loewe R, Perez-Moreno M. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. The EMBO journal. 2019 Oct 1:38(19):e101688. doi: 10.15252/embj.2019101688. Epub 2019 Sep 2 [PubMed PMID: 31475747]
Park S. Hair Follicle Morphogenesis During Embryogenesis, Neogenesis, and Organogenesis. Frontiers in cell and developmental biology. 2022:10():933370. doi: 10.3389/fcell.2022.933370. Epub 2022 Jul 22 [PubMed PMID: 35938157]
Ji S, Zhu Z, Sun X, Fu X. Functional hair follicle regeneration: an updated review. Signal transduction and targeted therapy. 2021 Feb 17:6(1):66. doi: 10.1038/s41392-020-00441-y. Epub 2021 Feb 17 [PubMed PMID: 33594043]
Grubbs H, Nassereddin A, Morrison M. Embryology, Hair. StatPearls. 2024 Jan:(): [PubMed PMID: 30521215]
Murphrey MB, Agarwal S, Zito PM. Anatomy, Hair. StatPearls. 2025 Jan:(): [PubMed PMID: 30020684]
Li KN, Jain P, He CH, Eun FC, Kang S, Tumbar T. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. eLife. 2019 Jul 25:8():. doi: 10.7554/eLife.45977. Epub 2019 Jul 25 [PubMed PMID: 31343406]
Peng J, Chen H, Zhang B. Nerve-stem cell crosstalk in skin regeneration and diseases. Trends in molecular medicine. 2022 Jul:28(7):583-595. doi: 10.1016/j.molmed.2022.04.005. Epub 2022 May 17 [PubMed PMID: 35595610]
Shwartz Y, Gonzalez-Celeiro M, Chen CL, Pasolli HA, Sheu SH, Fan SM, Shamsi F, Assaad S, Lin ET, Zhang B, Tsai PC, He M, Tseng YH, Lin SJ, Hsu YC. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell. 2020 Aug 6:182(3):578-593.e19. doi: 10.1016/j.cell.2020.06.031. Epub 2020 Jul 16 [PubMed PMID: 32679029]
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, Sansone A, Lombardo F. Androgenetic alopecia: a review. Endocrine. 2017 Jul:57(1):9-17. doi: 10.1007/s12020-017-1280-y. Epub 2017 Mar 28 [PubMed PMID: 28349362]
Piraccini BM, Alessandrini A. Androgenetic alopecia. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2014 Feb:149(1):15-24 [PubMed PMID: 24566563]