Introduction
The large arteries and veins directly connected with the heart are termed the great vessels, consisting of the inferior vena cava, superior vena cava, pulmonary arteries, pulmonary veins, and root of the aorta. These vessels are critical parts of the circulatory system, ensuring the delivery of deoxygenated blood from the periphery to the heart so it can be pumped into the lungs for oxygenation, returned to the left side of the heart, and then distributed back to the periphery. There are several congenital anatomical variations in the morphology of the great vessels, which can have significant deleterious effects on circulatory physiology requiring surgical management. Furthermore, thrombosis, stenosis, and aneurysm can affect the great vessels, which can be life-threatening conditions requiring prompt recognition and treatment.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
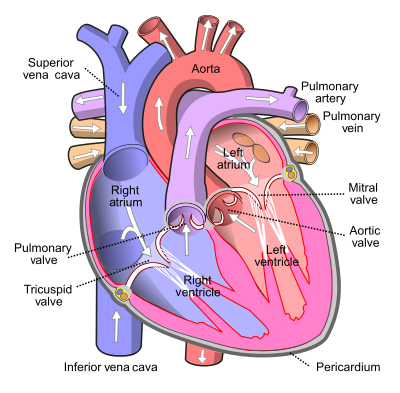
The general function of the great vessels is the supply of blood to the right atrium (inferior and superior vena cavae), supply of blood from the right ventricle to the lungs (pulmonary arteries), supply of oxygenated blood from the lungs to the left atrium (pulmonary veins), and the passage of oxygenated blood from the left ventricle to the arterial system (aorta). The structure of all vessels consists of the inner layer of endothelial cells, a middle smooth muscle layer, and an outer fibrous layer. These are termed the intima, media, and adventitia, respectively and are present in both arteries and veins, although the arterial wall is significantly thicker[1].
Vena Cavae
The vena cavae are the terminal parts of the extensive venous networks throughout the periphery returning deoxygenated blood to the heart.
The inferior vena cava carries deoxygenated blood from the tissues below the diaphragm. It passes through the diaphragm via the caval opening at the level of T11, the most superior of the diaphragmatic openings. In the thorax, it becomes invested within the pericardial sac to drain into the posteroinferior part of the right atrium.
The superior vena cava is valveless and drains the upper limbs, thorax, head, and neck. It originates from the right and left brachiocephalic veins at the level of the right first intercostal space, and enters the right atrium variably at the level of the third, fourth, or fifth intercostal space, by this point having become invested in fibrous pericardium [2] [3].
Pulmonary Arteries
The pulmonary trunk, or main pulmonary artery, is the direct continuation of the right ventricular outflow tract carrying deoxygenated blood to the lungs and is separated from it by the pulmonary valve. The pulmonary trunk is about 5cm long and has a diameter of approximately 3cm[4]. It slants posteriorly, lying directly to the left of the ascending aorta, with which it is initially invested with serous visceral pericardium before these fuse with the vessel adventitia. The trunk bifurcates at the level of T6, and the pulmonary arteries enter the lung hila to further subdivide and distribute deoxygenated blood throughout the lungs.
The right and left pulmonary arteries have slightly different relationships with their neighboring structures as they enter their respective lung hila. The left pulmonary artery lies superior to the left main bronchus and left pulmonary vein in the left lung hilum. By contrast, the longer right pulmonary artery lies anterior and inferior to the first division of the right main bronchus, which occurs just before entering the lung hilum. The right pulmonary veins still lie inferiorly within the hilum.
Pulmonary Veins
The pulmonary veins, as discussed above, lie within the inferior part of their respective lung hila[5]. The right and left lung vasculature beds return two pulmonary veins each (superior and inferior), meaning the left atrium received four pulmonary veins. The superior and inferior pulmonary veins run posterior to the superior and inferior vena cavae.
Ascending aorta and aortic arch
The ascending aorta begins at the aortic valve, approximately at the level of the third costal cartilage, as the continuation of the left ventricular outflow tract, and is usually up to 2.1cm wide [6] [7], carrying newly oxygenated blood to the body. It ascends for about 5cm before becoming the aortic arch at the sternal angle, which continues superiorly, curving slightly to the left and posteriorly over the hilum of the left lung before descending again in the posterior mediastinum where, at the level of T4, it continues as the thoracic aorta.
The apex of the aortic arch is where its three branches are given off: the brachiocephalic trunk, the left common carotid artery, and, latterly, the left subclavian artery. Just anteriorly to these near vertical branches, the left superior intercostal vein is draped obliquely across the aortic arch. As it travels horizontally from posterolateral to anteromedial, it first crosses over the left vagus nerve, then under the left phrenic nerve, before draining into the left brachiocephalic vein.
Embryology
The superior vena cava is formed mainly from the proximal right anterior cardinal veins and right common cardinal vein. The inferior vena cava forms from the fusion of the remaining cardinal veins, the subcardinal veins, and later the supracardinal veins. The separation between the subcardinal vein and the right posterior cardinal vein joins with the hepatic vein forming the inferior vena cava, which delivers deoxygenated blood from the lower limbs and abdominal viscera to the right atrium[8].
The formation of the aorta and the pulmonary trunk is dependent upon the truncus arteriosus. During development, the right and left conotruncal ridges grow in a spiral, twisted fashion forming the great vessels. This formation of the ridges is under the guidance of the combination of the mesenchymal cells that are going to create the semilunar valves (aortic and pulmonary semilunar valves) and the endocardial cells that are anteriorly and posteriorly conjoining to separate the ventricles. Eventually, from the fifth through eighth weeks, an aorticopulmonary septum will result in the separation of the Aorta and pulmonary trunk [9].
The pulmonary veins become incorporated into the left atrium through the dorsal mesocardiac and mesenchymal guidance of the pulmonary venous plexus. The sinus venosus is responsible for the formation of the crista terminalis. Some of these physiological changes take place as the hypoxic pulmonary vasoconstriction effect on the cardiac right side won't keep the fossa ovale patent, and the left atrial pressure will cause its closure alongside the degradation of the ductus arteriosus between the pulmonary trunk and aortic arch. Therefore, it results in a full separation of the four atrioventricular chambers, forming the fetal heart and great vessels[10].
Blood Supply and Lymphatics
The thoracic duct travels within the posterior mediastinum and is therefore related to the aorta here. It originates in front of the L2 vertebra as the cisterna chyli, and ascends with the aorta through the aortic hiatus of the diaphragm, where it then lies between the aorta and the azygos vein. At the level of the T5 vertebra, it continues posterior to the aortic arch before terminating as a loop arching over the subclavian artery to drain into the left internal jugular vein, jugulosubclavian angle, or left subclavian vein[11].
Nerves
Innervation of the great vessels
The innervation of vessels derives from a network of interconnected plexuses of neuronal fibers that are called the nervi vasorum, which rest upon the tunica adventitia alongside the vasa vasorum externae[1]. The primary innervation of the great vessels is the sympathetic chain, which utilizes adrenergic receptors to modulate vasoconstriction[12]. The parasympathetic system also has a partial stimulatory effect on endothelial cells, which antagonize the adrenergic receptors and result in vasodilation. Within the aortic arch, baroreceptors located between the origins of the left common carotid artery and left subclavian artery are derived from the vagus nerve and connect to the vasomotor nuclei located in the medulla oblongata[13]. These regulate responses to changes in blood pressure. Furthermore, aortic chemoreceptors scattered along the inferior aspect of the aortic arch monitor pH of the blood and respond to hypercapnia and hypoxia with increased coronary blood flow and sympathetic peripheral vasoconstriction[14]. Veins are not strongly affected by sympathetic and parasympathetic systems due to the lack of muscle within the tunica media. This lack of vasoconstriction within the venous system leads to an increased venous return via the great vessels to the right atrium[15].
Relation of the great vessels to nerves in the mediastinum
The heart and great vessels are traversed and surrounded by several important nerves that supply the area and continue inferiorly to the diaphragm and abdominal cavity. For this reason, the position of these nerves must be taken into consideration during surgical instrumentation of the great vessels to avoid iatrogenic injury[16].
The left and right phrenic nerves (C3-C5) run relatively laterally through the mediastinum[17]. The left phrenic nerve is related to the left brachiocephalic vein, passing in front of the left vagus nerve before moving laterally to pass over the anterolateral aspect of the aortic arch before descending with the pericardiophrenic artery and vein to the superior surface of the diaphragm[18]. The right phrenic nerve is shorter than its counterpart, descending posterolaterally along the surface of the right superior vena cava, right heart surface, and inferior vena cava to the superior surface of the diaphragm. Both phrenic nerves pass anteriorly to their respective lung hila, and therefore anteriorly to the pulmonary veins and arteries.
The bilateral vagus nerves (cranial nerve X) also descend within the mediastinum. They are initially related to their respective aspects of the aortic arch, having emerged between the common carotid and subclavian arteries on each side. Each gives off the important recurrent laryngeal nerve (RLN) branch, which ascends to supply the intrinsic muscles of the trachea (expect cricothyroid)[19]. The left RLN loops from the vagus nerve under the aortic arch just to the left of the ligamentum arteriosum, whereas the right RLN loops under its constituent right subclavian artery before ascending.
Surgical Considerations
There are several rare but important pathological variations of the configuration of the great vessels that may require cardiac surgery in the neonatal period or later in life as a life-saving intervention or improve quality of life. Failure of some of these procedures may ultimately lead to patients with these conditions requiring further cardiac surgery[20], or even heart transplantation[21].
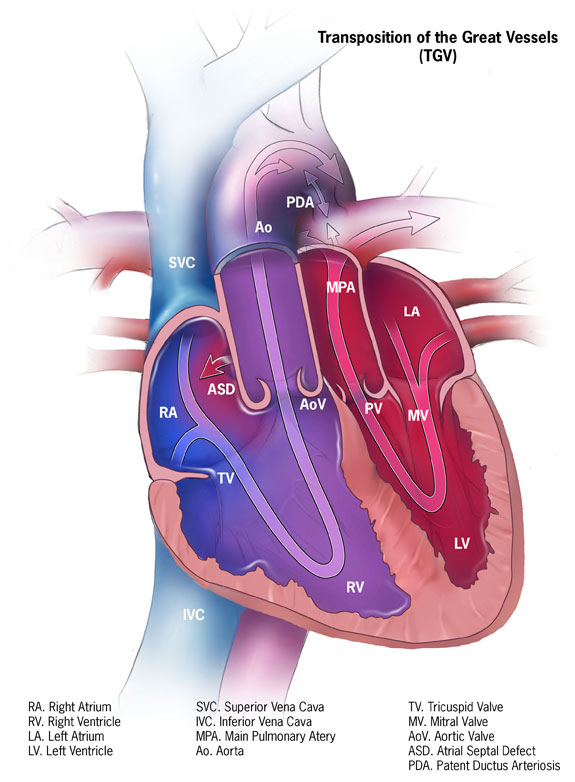
Transposition of the Great Arteries (TGA)
This condition has a prevalence of 0.2 per 1000 live births. In patients with TGA, there exists a ventriculoarterial discordance; in other words, the aorta arises from the morphological right ventricle instead of the left ventricle, and left ventricle instead gives rise to the pulmonary artery[22]. This can be surgically corrected with the arterial switch operation, in which the ventriculoarterial discordance is reversed to physiological connections between the left and right heart and the aorta and pulmonary trunk[23]. Several advances in the technique, improving on the precursor Senning and Mustard operations for this condition, as well as performing the arterial switch procedure early in the neonatal period have improved outcomes, and over 90% of patients now live to adulthood[23]. A much rarer and harder to identify condition termed congenitally corrected transposition of the great arteries (CCTGA) exists in which the right atrium connects to the left ventricle, which has outflow to the pulmonary trunk, and the left atrium connects to the right ventricle, which has outflow to the aorta. Thus, although there is, in some sense, physiological blood flow, patients progressing into adulthood without the condition being diagnosed often present with atrioventricular valve regurgitation requiring corrective surgery or even complete heart transplantation[24].
Truncus Arteriosus (TA)
TA represents approximately 3% of all congenital heart disease, presenting with a single outflow tract from the ventricles to the pulmonary, coronary, and systemic circulation[25]. The primary surgical repair involves forming a separate pathway for flow between the right ventricle and the pulmonary artery, and closure of the often present ventricular septal defect inferiorly[26] [27].
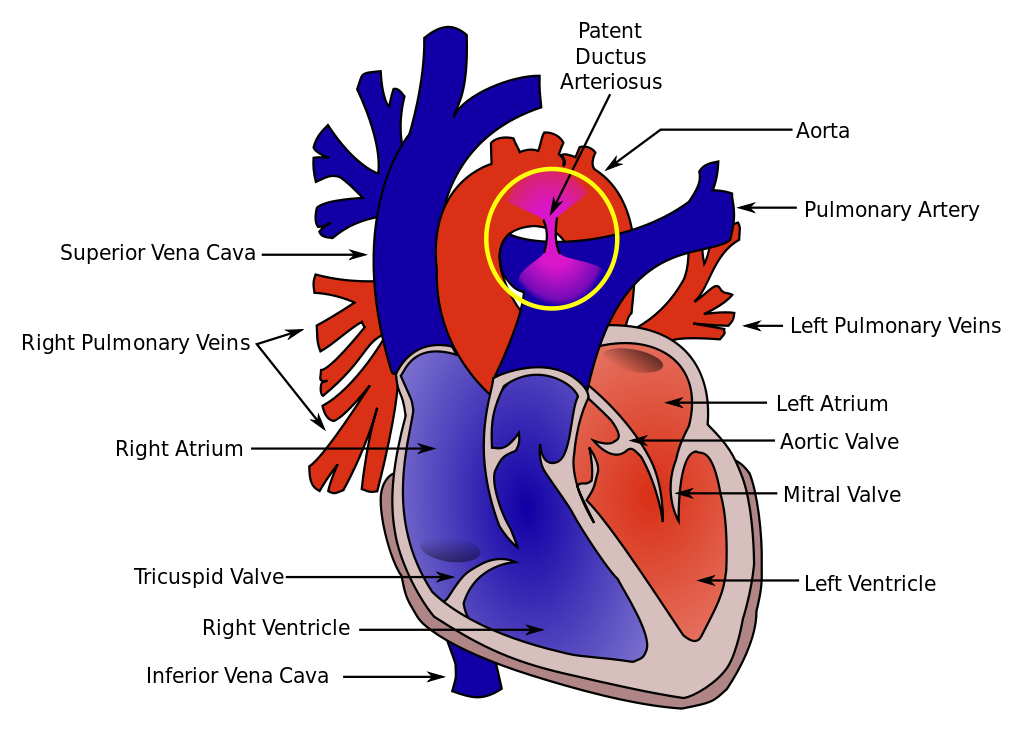
Patent Ductus Arteriosus (PDA)
A patent ductus arteriosus estimates to occur in 1 in 2000 births [28], allowing deoxygenated blood to enter the systemic circulation from the pulmonary trunk. Different strategies can be employed to treat PD, depending on symptoms and clinical picture. Pharmacological treatment with indomethacin, ibuprofen, or paracetamol can be used, all of which induce constriction of PDA through the reduction of prostaglandin synthesis[29]. Interventional management with ligations with silk, haemoclips, or excision can be performed in more severe cases, for example, with pulmonary hypertension, cyanosis, or respiratory distress syndrome[30]. This can be performed via a catheter-assisted transvenous device or surgically with thoracotomy.
Pulmonary artery sling
This rare condition involves the bifurcation of the pulmonary trunk in which the left pulmonary artery instead arises from the right pulmonary artery and, as a result, passes posterior to the trachea, forming a vascular sling[31]. This can cause associated tracheal stenosis, resulting in stridor and respiratory difficulties[32]. Surgical management with repositioning of the aberrant pulmonary artery with simultaneous tracheal repair if needed can prevent poor outcomes[33].
Clinical Significance
Congenital heart disease involving aberrations of flow from malpositioned great vessels commonly presents in the neonatal period as, although some defects may be picked up antenatal sonography, the sensitivity of prenatal testing is highly variable[34]. Thus, close examination of the newborn is essential to identifying potentially life-threatening congenital heart disease. Typical clinical manifestations include grunting, respiratory distress, tachypnoea, cough, and cyanosis[35].
Oximetry has been demonstrated to be highly specific for congenital heart disease in the newborn[36], using 95% as the threshold below which to further investigate the newborn’s cardiac health. In particular, pre- and post-ductal readings are taken, referring to the ductus arteriosus, which joins the pulmonary trunk with the aorta distal to the origin of its three branches, to short-circuit the lungs in the unborn infant. If either pre-ductal readings (at either hand) or post-ductal readings (at either foot)[37] show saturations less than 95%, or if there is a discordance in the two readings of more than 2%, further investigation is warranted with clinical examination, blood tests, chest radiography, and echocardiography to further delineate the cause[38].
Media
(Click Image to Enlarge)
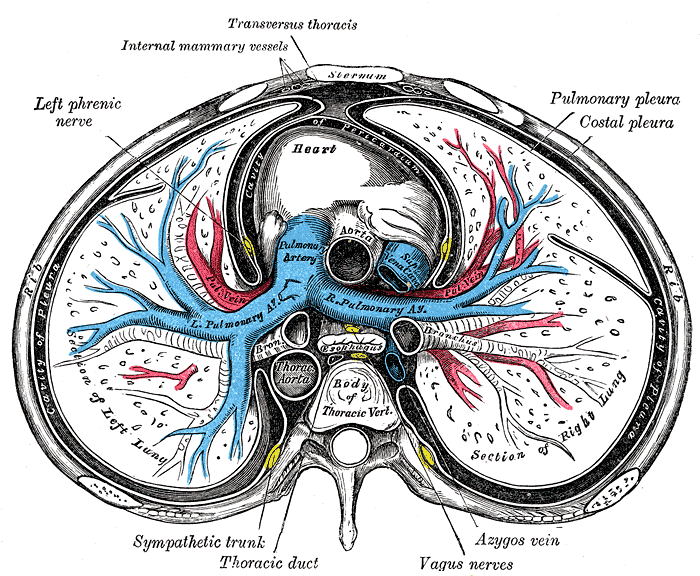
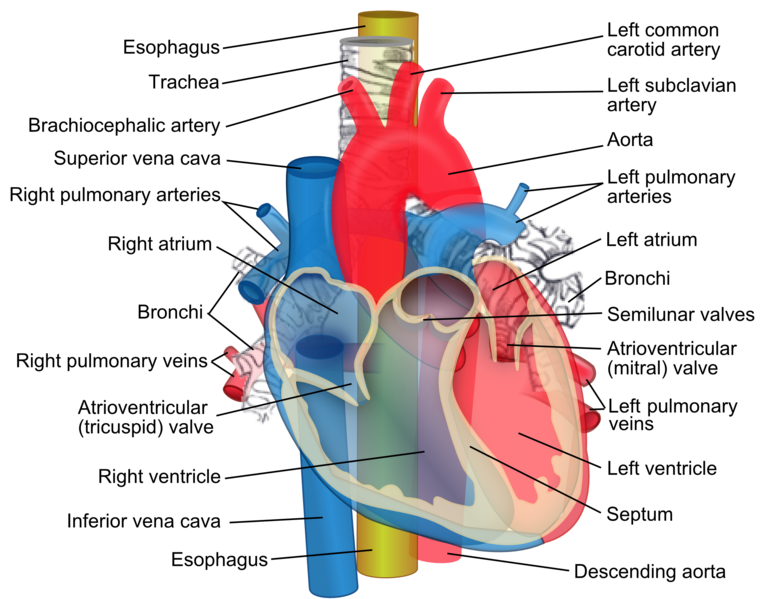
Sternum Transverse Section. This illustration shows the anatomic relationships between the right and left lungs, heart, pulmonary pleura, costal pleura, pleural cavity, ascending and thoracic aorta, pulmonary artery and its right and left branches, superior vena cava, esophagus, main bronchi, azygos vein, vagus nerves, thoracic duct, sympathetic trunk, left phrenic nerve, internal mammary vessel, transversus thoracis muscle, rib, sternum, and thoracic vertebral body.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
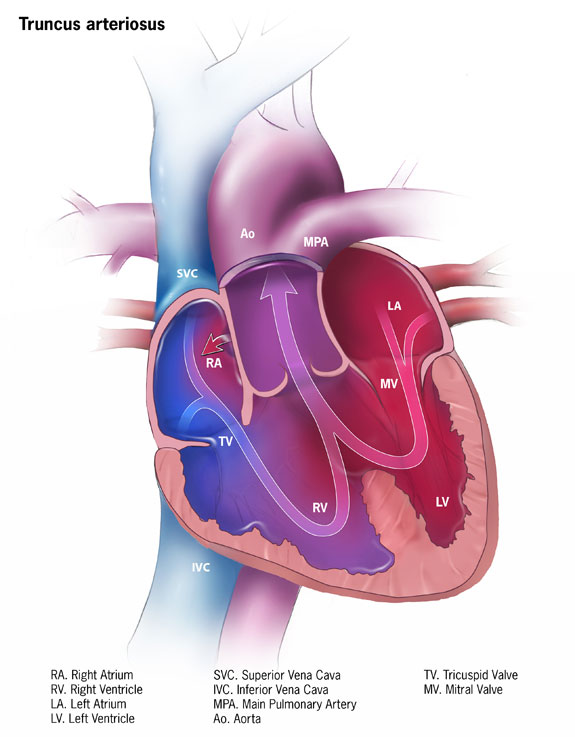
Truncus Arteriosus Pathology. The impact of truncus arteriosus on normal blood flow is illustrated as mixed oxygenated and deoxygenated blood sources.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
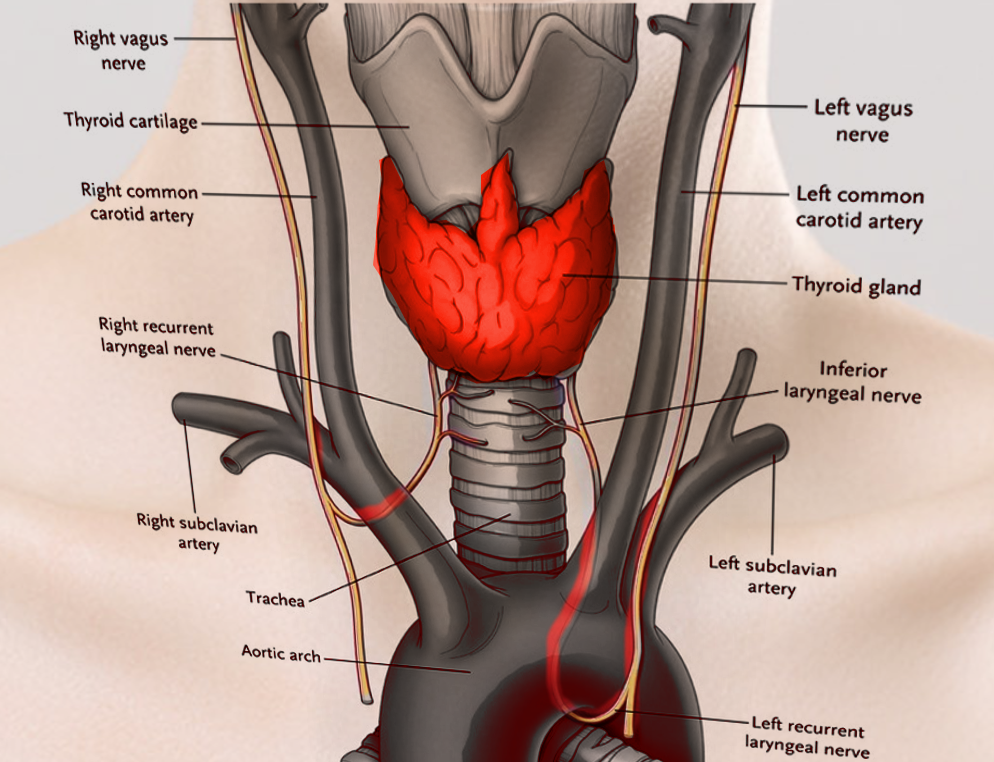
Laryngeal Nerves. This illustration shows the right and left vagus, right and left recurrent laryngeal, and inferior laryngeal nerves. Other structures included in this image are the right and left common carotid and right and left subclavian arteries, thyroid cartilage and gland, trachea, and aortic arch.
Contributed by S Bhimji, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Phillippi JA. On vasa vasorum: A history of advances in understanding the vessels of vessels. Science advances. 2022 Apr 22:8(16):eabl6364. doi: 10.1126/sciadv.abl6364. Epub 2022 Apr 20 [PubMed PMID: 35442731]
Level 3 (low-level) evidenceSanjeev S, Karpawich PP. Superior vena cava and innominate vein dimensions in growing children : an aid for interventional devices and transvenous leads. Pediatric cardiology. 2006 Jul-Aug:27(4):414-9 [PubMed PMID: 16830087]
Badshah M, Soames R, Ibrahim M, Khan MJ, Khan A. Surface anatomy of major anatomical landmarks of the neck in an adult population: A Ct Evaluation of Vertebral Level. Clinical anatomy (New York, N.Y.). 2017 Sep:30(6):781-787. doi: 10.1002/ca.22907. Epub 2017 Jun 7 [PubMed PMID: 28514499]
Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O'Donnell CJ, Hoffmann U. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circulation. Cardiovascular imaging. 2012 Jan:5(1):147-54. doi: 10.1161/CIRCIMAGING.111.968610. Epub 2011 Dec 16 [PubMed PMID: 22178898]
Level 2 (mid-level) evidenceHassani C, Saremi F. Comprehensive Cross-sectional Imaging of the Pulmonary Veins. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Nov-Dec:37(7):1928-1954. doi: 10.1148/rg.2017170050. Epub [PubMed PMID: 29131765]
Level 2 (mid-level) evidenceErbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart (British Cardiac Society). 2006 Jan:92(1):137-42 [PubMed PMID: 16365370]
Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. The American journal of cardiology. 1989 Sep 1:64(8):507-12 [PubMed PMID: 2773795]
Vuillemin M, Pexieder T. Normal stages of cardiac organogenesis in the mouse: II. Development of the internal relief of the heart. The American journal of anatomy. 1989 Feb:184(2):114-28 [PubMed PMID: 2712003]
Level 3 (low-level) evidenceDunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest. 2017 Jan:151(1):181-192. doi: 10.1016/j.chest.2016.09.001. Epub 2016 Sep 16 [PubMed PMID: 27645688]
Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science (New York, N.Y.). 1983 Jun 3:220(4601):1059-61 [PubMed PMID: 6844926]
Level 3 (low-level) evidencePhang K, Bowman M, Phillips A, Windsor J. Review of thoracic duct anatomical variations and clinical implications. Clinical anatomy (New York, N.Y.). 2014 May:27(4):637-44. doi: 10.1002/ca.22337. Epub 2013 Dec 2 [PubMed PMID: 24302465]
Level 1 (high-level) evidenceDuncan CP, Shim SS. J. Edouard Samson Address: the autonomic nerve supply of bone. An experimental study of the intraosseous adrenergic nervi vasorum in the rabbit. The Journal of bone and joint surgery. British volume. 1977 Aug:59(3):323-30 [PubMed PMID: 19482]
Level 3 (low-level) evidenceLau EO, Lo CY, Yao Y, Mak AF, Jiang L, Huang Y, Yao X. Aortic Baroreceptors Display Higher Mechanosensitivity than Carotid Baroreceptors. Frontiers in physiology. 2016:7():384. doi: 10.3389/fphys.2016.00384. Epub 2016 Aug 31 [PubMed PMID: 27630578]
Pen D, Shanks J, Barrett C, Abukar Y, Paton JFR, Ramchandra R. Aortic Body Chemoreceptors Regulate Coronary Blood Flow in Conscious Control and Hypertensive Sheep. Hypertension (Dallas, Tex. : 1979). 2022 Jun:79(6):1275-1285. doi: 10.1161/HYPERTENSIONAHA.121.18767. Epub 2022 Apr 6 [PubMed PMID: 35382553]
Berlin DA, Bakker J. Understanding venous return. Intensive care medicine. 2014 Oct:40(10):1564-6. doi: 10.1007/s00134-014-3379-4. Epub 2014 Jun 26 [PubMed PMID: 24966066]
Level 3 (low-level) evidenceWang J, Li J, Liu G, Deslauriers J. Nerves of the mediastinum. Thoracic surgery clinics. 2011 May:21(2):239-49, ix. doi: 10.1016/j.thorsurg.2011.01.006. Epub [PubMed PMID: 21477774]
Fell SC. Surgical anatomy of the diaphragm and the phrenic nerve. Chest surgery clinics of North America. 1998 May:8(2):281-94 [PubMed PMID: 9619305]
Schumpelick V, Steinau G, Schlüper I, Prescher A. Surgical embryology and anatomy of the diaphragm with surgical applications. The Surgical clinics of North America. 2000 Feb:80(1):213-39, xi [PubMed PMID: 10685150]
Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngologic clinics of North America. 2004 Feb:37(1):25-44, v [PubMed PMID: 15062685]
Prifti E, Bonacchi M, Luisi SV, Vanini V. Coronary revascularization after arterial switch operation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2002 Jan:21(1):111-3 [PubMed PMID: 11788276]
Level 3 (low-level) evidenceRoos-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, McGhie J, Bos E, Bogers AJ, Simoons ML. Decline in ventricular function and clinical condition after Mustard repair for transposition of the great arteries (a prospective study of 22-29 years). European heart journal. 2004 Jul:25(14):1264-70 [PubMed PMID: 15246646]
Warnes CA. Transposition of the great arteries. Circulation. 2006 Dec 12:114(24):2699-709 [PubMed PMID: 17159076]
Villafañe J, Lantin-Hermoso MR, Bhatt AB, Tweddell JS, Geva T, Nathan M, Elliott MJ, Vetter VL, Paridon SM, Kochilas L, Jenkins KJ, Beekman RH 3rd, Wernovsky G, Towbin JA, American College of Cardiology’s Adult Congenital and Pediatric Cardiology Council. D-transposition of the great arteries: the current era of the arterial switch operation. Journal of the American College of Cardiology. 2014 Aug 5:64(5):498-511. doi: 10.1016/j.jacc.2014.06.1150. Epub [PubMed PMID: 25082585]
Beauchesne LM, Warnes CA, Connolly HM, Ammash NM, Tajik AJ, Danielson GK. Outcome of the unoperated adult who presents with congenitally corrected transposition of the great arteries. Journal of the American College of Cardiology. 2002 Jul 17:40(2):285-90 [PubMed PMID: 12106933]
Alamri RM, Dohain AM, Arafat AA, Elmahrouk AF, Ghunaim AH, Elassal AA, Jamjoom AA, Al-Radi OO. Surgical repair for persistent truncus arteriosus in neonates and older children. Journal of cardiothoracic surgery. 2020 May 11:15(1):83. doi: 10.1186/s13019-020-01114-1. Epub 2020 May 11 [PubMed PMID: 32393289]
Yacoub MH, Hosny H, Afifi A, Nagy M, Mahgoub A, Simry W, AbouZeina MG, Doss R, El Sawy A, Shehata N, Elafifi A, Abdullah H, Romeih S. Novel concepts and early results of repairing common arterial trunk. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2022 Feb 18:61(3):562-571. doi: 10.1093/ejcts/ezab336. Epub [PubMed PMID: 34347066]
Raisky O, Ali WB, Bajolle F, Marini D, Metton O, Bonnet D, Sidi D, Vouhé PR. Common arterial trunk repair: with conduit or without? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009 Oct:36(4):675-82. doi: 10.1016/j.ejcts.2009.03.062. Epub 2009 May 22 [PubMed PMID: 19464907]
Level 2 (mid-level) evidenceDice JE, Bhatia J. Patent ductus arteriosus: an overview. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2007 Jul:12(3):138-46. doi: 10.5863/1551-6776-12.3.138. Epub [PubMed PMID: 23055849]
Level 3 (low-level) evidenceGillam-Krakauer M, Reese J. Diagnosis and Management of Patent Ductus Arteriosus. NeoReviews. 2018 Jul:19(7):e394-e402. doi: 10.1542/neo.19-7-e394. Epub [PubMed PMID: 30505242]
Tort M, Ceviz M, Sevil F, Becit N. Surgical Treatment for Patent Ductus Arteriosus: Our Experience of 12 Years. Cureus. 2021 Apr 28:13(4):e14731. doi: 10.7759/cureus.14731. Epub 2021 Apr 28 [PubMed PMID: 34079678]
Fiore AC, Brown JW, Weber TR, Turrentine MW. Surgical treatment of pulmonary artery sling and tracheal stenosis. The Annals of thoracic surgery. 2005 Jan:79(1):38-46; discussion 38-46 [PubMed PMID: 15620911]
Level 2 (mid-level) evidenceSantos N, Almeida T, Janeiro MC, Martins D. Pulmonary artery sling: a rare cause of stridor and respiratory distress. BMJ case reports. 2020 Feb 11:13(2):. doi: 10.1136/bcr-2019-233793. Epub 2020 Feb 11 [PubMed PMID: 32051151]
Level 3 (low-level) evidenceOshima Y, Yamaguchi M, Yoshimura N, Sato S, Muraji T, Nishijima E, Tsugawa C. Management of pulmonary artery sling associated with tracheal stenosis. The Annals of thoracic surgery. 2008 Oct:86(4):1334-8. doi: 10.1016/j.athoracsur.2008.04.020. Epub [PubMed PMID: 18805188]
Level 2 (mid-level) evidenceHarold JG. Cardiology patient page. Screening for critical congenital heart disease in newborns. Circulation. 2014 Aug 26:130(9):e79-81. doi: 10.1161/CIRCULATIONAHA.113.008522. Epub [PubMed PMID: 25156919]
Kishore S, Kumar M, Kumar A, Gupta A, Chandan C, Anshuman A, Prakash J, Sinha S, Kumar N. Clinical and Echocardiographic Profile of Congenital Heart Diseases in the 0-12-Year Age Group in a Tertiary Care Medical Institute in Eastern India: A Retrospective, Cross-Sectional Study. Cureus. 2022 Jun:14(6):e26114. doi: 10.7759/cureus.26114. Epub 2022 Jun 20 [PubMed PMID: 35747105]
Level 2 (mid-level) evidencePlana MN, Zamora J, Suresh G, Fernandez-Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects. The Cochrane database of systematic reviews. 2018 Mar 1:3(3):CD011912. doi: 10.1002/14651858.CD011912.pub2. Epub 2018 Mar 1 [PubMed PMID: 29494750]
Level 1 (high-level) evidenceRüegger C, Bucher HU, Mieth RA. Pulse oximetry in the newborn: is the left hand pre- or post-ductal? BMC pediatrics. 2010 May 21:10():35. doi: 10.1186/1471-2431-10-35. Epub 2010 May 21 [PubMed PMID: 20492689]
Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet (London, England). 2012 Jun 30:379(9835):2459-2464. doi: 10.1016/S0140-6736(12)60107-X. Epub 2012 May 2 [PubMed PMID: 22554860]
Level 1 (high-level) evidence