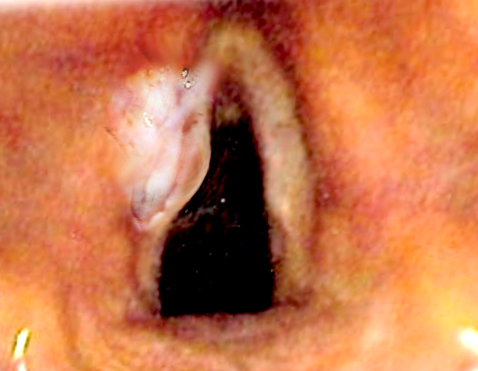
Introduction
Laryngeal cancers are one of the most common head and neck malignancies and thought to make up 1% of all cancers worldwide. Glottic cancer is defined as a malignancy arising from the true vocal cords and the anterior and posterior commissure of the larynx. Like other laryngeal cancers, smoking and alcohol abuse typically causes glottic cancer; however, tumors from this subsite have a better prognosis than other laryngeal cancers due to its reduced rate of local, nodal and distant invasion. The management of glottic cancers can vary significantly, from single modality transoral laser surgery or radiotherapy in early disease, through to chemoradiotherapy or total laryngectomy for more advanced disease. Providers, therefore, must have a thorough understanding of the anatomy and staging of glottic cancer to facilitate the most appropriate management plan for this disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
There has long been an association between the consumption of smoking and excessive alcohol consumption and the occurrence of glottic and laryngeal squamous cell carcinoma. Rates of laryngeal cancer are 15-30 times higher in smokers than non-smokers[1], with the inclusion of heavy alcohol intake having a multiplicative effect on the risk of malignancy.[2] Moreover, continued smoking following diagnosis and treatment is associated with poorer survival outcomes and a higher rate of recurrence.[3]
Other proposed risk factors include gastroesophageal reflux, low socioeconomic status, opium use, red meat, and occupational exposure, with a healthy diet thought to have a minor protective effect.[4][5][6][7][8][9] Although the role of human papillomavirus (HPV) is now well established in many oropharyngeal tumors, this is less clear in laryngeal cancers. Several meta-analyses have suggested approximately 20-30% of laryngeal cancers may be associated with HPV. However, these rates vary considerably with geographical location, and further studies will be needed to establish a true causal link between the virus and glottic cancer.[10][11]
Epidemiology
Primary laryngeal and glottic cancers are uncommon, representing approximately 1% of all male and 0.3% of female cancers worldwide. The American Cancer Society estimates the diagnosis of 12370 new cases of laryngeal carcinoma in 2020, with approximately 60% arising primarily from the glottis. Of these new cases, 9820 will occur in men, and 3750 will die from the disease with a male to female ratio of 4 to 1. Reassuringly, these figures follow a 2 to 3% year on year reduction in overall cases. Rates of laryngeal cancer vary significantly with specific patient characteristics as male, low-income, and African-American patients have shown to present with higher rates of advanced disease.[12][13] Moreover, geographical location can influence rates of disease, with England exhibiting less than half the number of cases per 100,000 compared to Scotland and Northern Ireland (2 vs. 4.2 vs. 4.3).
Pathophysiology
Squamous cell carcinoma composes over 90% of all laryngeal malignancies. It is slow-growing and can appear as an exophytic or endophytic lesion. Glottic tumors typically arise from the free margin of the anterior 1/3rd of the vocal cords. Numerous anatomical boundaries can contain the lesion within the Reinke space for a significant period. Spread may occur locally across the anterior commissure (although the anterior commissure ligament provides a resilient barrier to the invasion) or posteriorly to the arytenoid cartilages.
Cancers may eventually breach the glottis through a direct invasion of the vocal ligament and vocalis muscles, causing vocal cord fixation via involvement of the thyroarytenoid muscle. From here, tumors gain access to the paraglottic space, facilitating cranial and caudal spread. Laryngeal cartilage is relatively resistant to direct tumor invasion, although invasion of the thyroid cartilage will often occur in advanced T4a disease. While supraglottic cancers often exhibit early bilateral nodal metastasis and subglottic cancers invade paratracheal and mediastinal nodes, carcinomas of the glottis have a low rate of lymphatic spread, attributed to an inadequate submucosal lymphatic supply. Nodal metastasis tends to occur when the tumor breaches the anterior commissure or invades another laryngeal subsite, most often spreading to levels 2, 3, 4, and 6.
Lymphoma is the second most common laryngeal malignancy; however, only thought to represent less than 1% of all cancers in this region. Nevertheless, it remains an important differential diagnosis, as it rarely requires surgical intervention beyond initial biopsy or tracheostomy in acute airway obstruction. It has a good prognosis when managed with chemoradiotherapy.[14][15] Other laryngeal cancer subtypes can arise from a large variety of cells, including chondrosarcoma, neuroendocrine tumors, and salivary gland cell type cancers; however, these tumors are vanishingly rare and are described only in a limited number of case reports and case series.[16][17][18]
History and Physical
Glottic cancers often present early, as even small lesions of the glottis can result in significant hoarseness. Resultantly, current American and British guidelines advise urgent referral to an otolaryngologist for persistent hoarseness lasting 4 and 6 weeks, respectively. However, there have been suggestions for referrals as early as three weeks from the onset of hoarseness.[19][20] Patients may also present with other head and neck red flag symptoms, including dysphagia, odynophagia, and referred otalgia. Though this typically occurs in advanced disease with tumor extension beyond the glottis. In more extreme cases, patients can present late as an emergency with stridor and acute airway compromise. Looking at the patient's past medical and drug history should be done to ascertain associated risk factors and assess the patient's fitness for oncological treatment or major surgery. Quantification of etiological risk factors, in particular, smoking and alcohol consumption status, must also be performed.
Patients require a full ear, nose, and throat examination by an experienced otolaryngologist, including palpation of the neck for cervical lymphadenopathy, as well as close inspection of the oral cavity and oropharynx to exclude an oropharyngeal malignancy and assess dentition. Flexible nasal endoscopy in the outpatient clinic is essential to evaluate the glottis with particular attention paid to the involvement of one or more cords, cord mobility, and invasion to other subsites in the supraglottis and subglottis. To assess subtle vocal cord immobility, video-stroboscopic equipment is available.
Evaluation
The initial investigation of choice for glottic cancer is a CT scan of neck and thorax to accurately assess local invasion, nodal disease, and distant metastasis. However, CT scans are unlikely to be of any diagnostic benefit in early T1 cancers that do not involve the anterior commissure, as both local and nodal disease in these cancers is exceedingly uncommon.[21] MRI scanning can offer superior soft tissue definition in the neck and thus used in some centers for assessment of direct invasion of the laryngeal cartilages.[22] PET-CT may be of use when the primary site is not detectable, and a 12-week post-treatment PET-CT is the current gold standard method of assessing response to treatment.[23] While considering patients for radiotherapy, the indication of an x-ray orthopantomogram is essential to reduce the risk of highly debilitating osteoradionecrosis.[24]
A definitive diagnosis is achieved with direct laryngoscopy and biopsy under general anesthetic. Assessment with 0, 30, and 70-degree rigid endoscopes can provide the most detailed evaluation of the primary tumor and its extension to other laryngeal subsites.
Treatment / Management
Early T1-T2a Glottic Cancers
Given the low incidence of nodal metastasis and the small, slow-growing nature of the primary lesion, early T1-2 glottic cancers can be managed very successfully with single-modality treatment. Transoral laser microsurgery (TLM) and radiotherapy are the most commonly employed treatments for early glottic cancer. Although there is no direct comparison in a randomized controlled trial, a Cochrane review has noted equivalent survival outcomes and local disease control in both methods.[25] Resultantly, the use of a particular method is often determined by tumor anatomy and resectability, perceived functional outcomes, patient preference, and departmental experience.(A1)
The fundamental principles of transoral laser microsurgery are the preservation of the cricoid cartilage and of at least a single crico-arytenoid joint, failure of which can result in a narrowed airway and a non-functional larynx respectively.[26] TLM is a day case done under general anesthesia with a CO2 laser. Risks of complications are low, and it avoids the morbidity, and repeated outpatient visits seen in radiotherapy, while also reserving radiotherapy as a second-line option should the tumor recur.[27] It has been well established that a 2 mm surgical margin must be achieved to ensure complete oncological resection.[28] This may prove to be technically challenging in bulky tumors, and risk causing poor voice outcomes secondary to laryngeal scarring in anterior commissure lesions. Resultantly, radiotherapy may be preferable in T1b-T2 lesions involving the anterior commissure and patients unsuitable for an endoscopic approach (e.g., restricted c-spine movement).[27][29]
Radiotherapy regimes for glottic cancers vary between centers. Usually, they range from 50–52 Gy in 16 fractions or 53–55 Gy in 20 fractions over three to four weeks, with a higher dose per fraction resulting in improved locoregional control for both T1 and T2 lesions.[30][31] In tumors involving the anterior commissure, augmentation of radiotherapy beams is essential to provide a bolus of radiation to the anterior neck, as this is a frequent site of post-treatment recurrence.[32] Unlike early to moderately advanced supraglottic cancers, there is no routine need for bilateral nodal irradiation for early glottic cancer. Although somewhat limited due to short-term radiation toxicity side effects, radiotherapy is said to have superior voice-related outcomes when compared to TLM.[33](A1)
Partial laryngectomy is less commonly performed for primary early glottic cancers but offers a viable alternative to radiotherapy and TLM in experienced centers. Systematic reviews have demonstrated comparable oncological outcomes in both de novo and radio-recurrent cancers to radiotherapy and TLM.[34][35] However, appropriate patient selection is vital to achieving surgical clearance, as specific tumor characteristics such as subglottic or posterior commissure extension, cricoarytenoid complex fixation, or significant thyroid cartilage invasion are not appropriate candidates for partial laryngectomy.[36](A1)
Moderately Advanced T2b-T3 Glottic Cancers
At present, UK and US guidelines advise organ preservation therapy for moderately advanced glottic cancer in the form of chemoradiotherapy.[27][29] Before the early 1990s, however, the treatment of choice for moderately advanced glottic cancer was total laryngectomy. The Veterans Affairs (VALCSG) study resulted in a paradigm shift in the management of these cancers, as they found equivalent two-year survival of induction chemotherapy and radiotherapy (68%) compared to laryngectomy and radiotherapy (64%), with a lower rate of salvage laryngectomy noted in T3 patients.[37] As a result, the management of moderately advanced glottic cancers now focuses on laryngeal preservation therapies, wherein radiotherapy and chemotherapy are employed to avoid the significant lifestyle change and long term morbidity associated with a laryngectomy. The RTOG 91-11 trial further built on this, establishing that concurrent platinum-based chemotherapy (cisplatin) with radiotherapy had higher 3-year survival compared to induction chemotherapy with radiotherapy or radiotherapy alone.[38](A1)
Furthermore, a 10-year follow-up study confirmed the long term survival benefits of the concurrent chemotherapy group but did note a higher rate of non-cancer deaths in this treatment arm.[39] The addition of chemotherapy, however, can cause significant side effects with a severe impact on the patients' quality of life. This impact, combined with the reducing efficacy of chemotherapy in patients over the age of 70, means it is not routinely used in elderly patients with head and neck cancers.(A1)
Advanced T4 Glottic Cancer
Chemoradiotherapy may be used effectively in some advanced T4 cancers; however, the high rate of the thyroid cartilage and neck soft tissue invasion means many patients will be unsuitable for laryngeal preservation and will require surgery in the form of total laryngectomy. The Veterans affairs study noted T4 laryngeal tumors had a reduced response to chemotherapy and a higher rate of salvage surgery, suggesting organ preservation is not effective in advanced laryngeal cancer.[37] Additionally, epidemiological studies have shown improved survival in patients with locally advanced cancers undergoing total laryngectomy compared to chemoradiotherapy, strongly suggesting surgery is the treatment of choice for T4a glottic disease.[40][41] Total laryngectomy may also be considered in patients with significant laryngeal destruction, pre-laryngectomy tracheostomy, and non-functioning larynges.[29] T4b tumors are deemed inoperable due to the encasement of major vessels or the inability to achieve negative margins. Consideration of palliative treatment or chemoradiotherapy at this stage is feasible, and this has shown an association with a reduction of tumor progression and improved life expectancy.(A1)
Postoperative Treatment
Postoperative radiotherapy can improve locoregional outcomes in advanced head and neck cancer, and is therefore recommended in any T4 tumors, T2-3 lesions with significant nodal disease, and any patient with positive margins or extra-nodal extension.[42] The EORTC 22931 trial found that the addition of concurrent postoperative chemotherapy may further improve locoregional control and disease-free survival compared to radiotherapy alone.[42] RTOG 95-11 trial noted similar results in high-risk patients, but this stated a higher morbidity burden in the chemotherapy group. Thus postoperative chemotherapy may be used selectively in patients with a high risk of recurrence.[43](A1)
Treatment of Nodal Disease
The rate of nodal disease in glottic cancer is considerably lower than the supraglottic disease, where bilateral elective neck dissection or nodal irradiation is advised even in clinically N0 necks.[44] Nevertheless, treatment with chemoradiotherapy has an excellent complete response rate in N1-3 disease, and many patients with advanced T3/4 glottic cancer will undergo ipsilateral or bilateral level 2-5 radiotherapy, regardless of pre-treatment nodal status.[45][46](A1)
In patients undergoing total laryngectomy, elective bilateral neck dissection for staging purposes is essential, which may prevent the need for postoperative radiotherapy if no positive nodes are present. In patients who have failed to show a complete response following completion of treatment, or before chemoradiotherapy in patients with significant volume neck disease that are less likely to respond to primary oncological treatment, neck dissection is considered.[47]
Differential Diagnosis
The differential diagnosis of glottic cancer includes:
- Supraglottic, subglottic and transglottic laryngeal cancers
- Other head and neck cancers, including hypopharyngeal and upper esophageal
- Pre-malignant glottic lesions (leukoplakia, low and high-grade dysplasia)
- Vocal cord immobility
- Benign laryngeal lesions such as laryngeal papillomatosis, Reinke edema, vocal cord polyps, cysts, and nodules, granuloma, chronic laryngitis, hyperkeratosis, and functional dysphonia
Staging
The staging of glottic and other laryngeal cancers is according to the 2016 eighth edition of the AJCC TNM classification of malignant tumors. Unlike oral and oropharyngeal cancers, depth of invasion and p16 status does not have a role in staging glottic tumors. The extra-nodal extension is one of the most important predictors of clinical outcomes in head and neck cancers. To reflect this, TNM8 introduced the presence of extra-nodal extension into all laryngeal cancer staging, with its presence immediately classifying a patient as N3b regardless of nodal size, quantity, or location; to accurately reflect the negative impact this finding can have on prognosis.[48]
The TMN8 Tumour (T) staging is as follows:
- Tx: primary tumor cannot be assessed
- Tis: carcinoma in situ
- T1: tumor limited to the vocal cords (including the anterior and posterior commissure) with normal mobility
- T1a: tumor limited to one vocal cord
- T1b: tumor involving both vocal cords
- T2:
- T2a: tumor extends to supraglottis and/or subglottis with normal vocal fold mobility
- T2b: tumor extends to the supraglottis and/or subglottis, or with impaired vocal cord mobility
- T3: tumor limited to the larynx with vocal cord fixation or invasion of the paraglottic space or inner cortex of the thyroid cartilage
- T4: advanced disease
- T4a: tumor invades through the outer cortex of the thyroid cartilage and/or invades tissues beyond the larynx (trachea, cricoid cartilage, tongue muscles, strap muscles, thyroid, or esophagus)
- T4b: tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures
The TNM 8 nodal (N) staging is as follows:
- Nx: nodes unable to be assessed
- N0: no regional lymph nodes
- N1: single ipsilateral lymph node ≤3cm and no extra-nodal extension
- N2
- N2a: single ipsilateral lymph node >3cm but <6 cm in greatest dimension and no extra-nodal extension
- N2b: multiple ipsilateral lymph nodes, none >6cm in greatest dimension and no extra-nodal extension
- N2c: bilateral or contralateral lymph nodes, none >6cm in greatest dimension and extra-nodal extension
- N3
- N3a: any lymph node >6cm and no-extra-nodal extension
- N3b: any nodes with clinically overt extra-nodal extension
The TNM8 metastasis (M) staging is as follows:
- M0: no distant metastasis
- M1: distant metastasis
Prognosis
Overall, early laryngeal cancer carries a good prognosis, with 5-year survival outcomes, approximately 90% for stage 1 and 80% for stage 2 cancers when managed with single modality radiotherapy or laryngeal preserving surgery.[49] Survival outcomes are poorer for more advanced disease, with the 1990 Veterans Affairs study noting a 2-year survival of 68% and 64% for advanced disease treated with primary radiotherapy together with induction chemotherapy versus laryngectomy and postoperative radiotherapy.[37] Glottis cancer, however, carries a slightly better overall prognosis than other laryngeal sites, with 5-year survival rates of 83% for localized, 42% for advanced and 76% for all stages of the disease, compared to 61%, 30%, and 46% for supraglottic and 60%, 45%, and 52% for subglottic cancers, respectively. Unfortunately, between 1977 and 2002, the overall 5-year survival for regional and distant glottic cancer has fallen.[50]
Complications
Complications of Glottic Cancer
Compared to other laryngeal cancers, glottic cancer has a reduced propensity for regional and distant spread, but may still cause significant complications when left untreated. Dysphonia may occur through the direct invasion of the thyroarytenoid muscle and vocal ligament, progressing to dyspnoea, aspiration pneumonia, stridor, and acute airway emergencies necessitating tracheostomy in severe cases. Invasion of adjacent structures in the larynx and pharynx may also result in dysphagia and weight loss requiring nasogastric or gastrostomy feeding. The advanced local disease may encase and erode into the internal jugular vein and carotid artery, with the latter resulting in a carotid blowout and fatal hemorrhage.
Complications of Chemoradiotherapy
Despite its proven efficacy in the management of moderately advanced laryngeal cancer, chemoradiotherapy has a high rate of highly debilitating side effects and complications. Most commonly, patients will have complaints of xerostomia, mucositis, pain, and swallowing difficulty. These may manifest as late toxicity, resulting in gastrostomy support for nutritional intake in 13 to 14% of patients 1 to 2 years post-treatment.[51][52] Scarring, edema, and stenosis may also occur within the upper aerodigestive tract, necessitating recurrent endoscopic dilatation of pharyngeal strictures or tracheostomy in narrowed airways.[52] In the long term; depression, pain, dysphagia, and speech issues may have a significant effect on a patient's quality of life.[53] Further exacerbation of the rate of complications may occur due to numerous factors including advanced age and T-stage, site of primary disease, and neck dissection following oncological treatment.[51]
Complications of Surgery
Complications following transoral laser surgery are not infrequent, but are often minor and result in minimal morbidity for a patient. These can include local infection and bleeding, perforation and surgical emphysema, cutaneous fistula, dyspnoea, swallowing difficulties, and aspiration pneumonia.[54] Although uncommon, airway fires are a potentially devastating intra-operative complication of TLM, with potential sources of ignition, including the electrosurgical diathermy unit, light cords, and the CO2 laser.[55]
In more advanced cases, laryngectomy has a high rate of postoperative complications, with this rising further in salvage cases in previously irradiated patients. Pharyngocutaneous fistula remains one of the most common and challenging postoperative complications, frequently prolonging inpatient stay and delaying a return to an oral diet. Other potential complications include localized wound and lower respiratory tract infections, bleeding, damage to nerves (including the accessory, marginal mandibular and hypoglossal), and embolism.[56][57] Moreover, the long term quality of life impacts of a laryngectomy can be considerable, as patients have to re-adapt to even the most basic functions of eating, breathing, and speaking.[57]
Consultations
Management of glottic tumors requires the support of an interprofessional team. This team includes the following specialties:
- Otolaryngologists
- Maxillofacial and plastic surgeons
- Oncologists
- Radiologists
- Restorative dentists/oral surgeons
- Clinical nurse specialists
- Dieticians
- Speech and language therapists
- Palliative care
Deterrence and Patient Education
Excessive alcohol consumption and cigarette smoking are intrinsic to the development of glottic cancers, with the continuation of smoking resulting in poorer functional outcomes, reduced survival, and a higher rate of recurrence. Moreover, cessation of smoking after 1 to 4 years can result in a significant risk reduction in developing laryngeal cancer, reaching the level of never-smokers after 20 years.[58]
Enhancing Healthcare Team Outcomes
The variance in surgical and oncological management of glottic cancer across TNM stages highlights the importance of the multidisciplinary team. Invariably, there is an importance in performing an initial outpatient history and examination by an otolaryngologist. Subsequent initial investigations, including cross-sectional imaging and biopsy, radiologists, and pathologists, are essential in achieving definitive diagnosis and staging. Depending on the required treatment, otolaryngologists, and oncologists with a specialist interest in head and neck, cancer will be responsible for the provision of surgical, chemotherapy, and radiotherapy interventions. Given the myriad of complications and the often life-changing nature of these treatments, early involvement of associated specialists such as speech and language therapists, nurse specialists, dieticians and psychologists are essential in maintaining patients' quality of life and providing a patient-centered care approach.
Media
(Click Image to Enlarge)
References
Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: association by tumour type. Journal of internal medicine. 2002 Sep:252(3):206-24 [PubMed PMID: 12270001]
Level 2 (mid-level) evidenceBosetti C, Gallus S, Franceschi S, Levi F, Bertuzzi M, Negri E, Talamini R, La Vecchia C. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. British journal of cancer. 2002 Aug 27:87(5):516-8 [PubMed PMID: 12189548]
Level 2 (mid-level) evidencevan Imhoff LC, Kranenburg GG, Macco S, Nijman NL, van Overbeeke EJ, Wegner I, Grolman W, Pothen AJ. Prognostic value of continued smoking on survival and recurrence rates in patients with head and neck cancer: A systematic review. Head & neck. 2016 Apr:38 Suppl 1():E2214-20. doi: 10.1002/hed.24082. Epub 2015 Jul 30 [PubMed PMID: 25900211]
Level 1 (high-level) evidenceZhang D, Zhou J, Chen B, Zhou L, Tao L. Gastroesophageal reflux and carcinoma of larynx or pharynx: a meta-analysis. Acta oto-laryngologica. 2014 Oct:134(10):982-9. doi: 10.3109/00016489.2014.927592. Epub 2014 Aug 18 [PubMed PMID: 25131391]
Level 2 (mid-level) evidenceKhalil D, Corsten MJ, Holland M, Balram A, McDonald JT, Johnson-Obaseki S. Does Socioeconomic Status Affect Stage at Presentation for Larynx Cancer in Canada's Universal Health Care System? Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2019 Mar:160(3):488-493. doi: 10.1177/0194599818798626. Epub 2018 Sep 11 [PubMed PMID: 30200820]
Kamangar F, Shakeri R, Malekzadeh R, Islami F. Opium use: an emerging risk factor for cancer? The Lancet. Oncology. 2014 Feb:15(2):e69-77. doi: 10.1016/S1470-2045(13)70550-3. Epub [PubMed PMID: 24480557]
Level 1 (high-level) evidencePerloy A, Maasland DHE, van den Brandt PA, Kremer B, Schouten LJ. Intake of meat and fish and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Cancer causes & control : CCC. 2017 Jun:28(6):647-656. doi: 10.1007/s10552-017-0892-0. Epub 2017 Apr 5 [PubMed PMID: 28382514]
Paget-Bailly S, Cyr D, Luce D. Occupational exposures and cancer of the larynx-systematic review and meta-analysis. Journal of occupational and environmental medicine. 2012 Jan:54(1):71-84. doi: 10.1097/JOM.0b013e31823c1343. Epub [PubMed PMID: 22157731]
Level 1 (high-level) evidenceGaravello W, Lucenteforte E, Bosetti C, Talamini R, Levi F, Tavani A, Franceschi S, Negri E, La Vecchia C. Diet diversity and the risk of laryngeal cancer: a case-control study from Italy and Switzerland. Oral oncology. 2009 Jan:45(1):85-9. doi: 10.1016/j.oraloncology.2008.02.011. Epub 2008 May 19 [PubMed PMID: 18487075]
Level 2 (mid-level) evidenceGama RR, Carvalho AL, Longatto Filho A, Scorsato AP, López RV, Rautava J, Syrjänen S, Syrjänen K. Detection of human papillomavirus in laryngeal squamous cell carcinoma: Systematic review and meta-analysis. The Laryngoscope. 2016 Apr:126(4):885-93. doi: 10.1002/lary.25738. Epub 2015 Nov 6 [PubMed PMID: 26542064]
Level 1 (high-level) evidenceNdiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. The Lancet. Oncology. 2014 Nov:15(12):1319-31. doi: 10.1016/S1470-2045(14)70471-1. Epub 2014 Oct 16 [PubMed PMID: 25439690]
Level 1 (high-level) evidenceRachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the larynx in England and Wales up to 2001. British journal of cancer. 2008 Sep 23:99 Suppl 1(Suppl 1):S35-7. doi: 10.1038/sj.bjc.6604581. Epub [PubMed PMID: 18813254]
Shin JY, Truong MT. Racial disparities in laryngeal cancer treatment and outcome: A population-based analysis of 24,069 patients. The Laryngoscope. 2015 Jul:125(7):1667-74. doi: 10.1002/lary.25212. Epub 2015 Feb 18 [PubMed PMID: 25694265]
Level 2 (mid-level) evidenceHorny HP, Kaiserling E. Involvement of the larynx by hemopoietic neoplasms. An investigation of autopsy cases and review of the literature. Pathology, research and practice. 1995 Mar:191(2):130-8 [PubMed PMID: 7567682]
Level 3 (low-level) evidenceMarkou K, Goudakos J, Constantinidis J, Kostopoulos I, Vital V, Nikolaou A. Primary laryngeal lymphoma: report of 3 cases and review of the literature. Head & neck. 2010 Apr:32(4):541-9. doi: 10.1002/hed.21104. Epub [PubMed PMID: 19378323]
Level 3 (low-level) evidenceRinaldo A, Howard DJ, Ferlito A. Laryngeal chondrosarcoma: a 24-year experience at the Royal National Throat, Nose and Ear Hospital. Acta oto-laryngologica. 2000 Sep:120(6):680-8 [PubMed PMID: 11099143]
Level 3 (low-level) evidenceSoga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. Journal of experimental & clinical cancer research : CR. 2003 Dec:22(4):517-30 [PubMed PMID: 15053292]
Level 3 (low-level) evidenceDamiani JM, Damiani KK, Hauck K, Hyams VJ. Mucoepidermoid-adenosquamous carcinoma of the larynx and hypopharynx: a report of 21 cases and a review of the literature. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1981 Mar-Apr:89(2):235-43 [PubMed PMID: 6787518]
Level 3 (low-level) evidenceStachler RJ, Francis DO, Schwartz SR, Damask CC, Digoy GP, Krouse HJ, McCoy SJ, Ouellette DR, Patel RR, Reavis CCW, Smith LJ, Smith M, Strode SW, Woo P, Nnacheta LC. Clinical Practice Guideline: Hoarseness (Dysphonia) (Update). Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2018 Mar:158(1_suppl):S1-S42. doi: 10.1177/0194599817751030. Epub [PubMed PMID: 29494321]
Level 1 (high-level) evidenceTikka T, Pracy P, Paleri V. Refining the head and neck cancer referral guidelines: a two centre analysis of 4715 referrals. The British journal of oral & maxillofacial surgery. 2016 Feb:54(2):141-50. doi: 10.1016/j.bjoms.2015.09.022. Epub [PubMed PMID: 26857792]
Lewis-Jones H, Colley S, Gibson D. Imaging in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. The Journal of laryngology and otology. 2016 May:130(S2):S28-S31 [PubMed PMID: 27841111]
Becker M, Zbären P, Casselman JW, Kohler R, Dulguerov P, Becker CD. Neoplastic invasion of laryngeal cartilage: reassessment of criteria for diagnosis at MR imaging. Radiology. 2008 Nov:249(2):551-9. doi: 10.1148/radiol.2492072183. Epub [PubMed PMID: 18936314]
Level 2 (mid-level) evidenceMehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG, Nutting C, Powell N, Al-Booz H, Robinson M, Junor E, Rizwanullah M, von Zeidler SV, Wieshmann H, Hulme C, Smith AF, Hall P, Dunn J, PET-NECK Trial Management Group. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. The New England journal of medicine. 2016 Apr 14:374(15):1444-54. doi: 10.1056/NEJMoa1514493. Epub 2016 Mar 23 [PubMed PMID: 27007578]
Walker MP, Wichman B, Cheng AL, Coster J, Williams KB. Impact of Radiotherapy Dose on Dentition Breakdown in Head and Neck Cancer Patients. Practical radiation oncology. 2011:1(3):142-148 [PubMed PMID: 21857887]
Warner L, Chudasama J, Kelly CG, Loughran S, McKenzie K, Wight R, Dey P. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. The Cochrane database of systematic reviews. 2014 Dec 12:2014(12):CD002027. doi: 10.1002/14651858.CD002027.pub2. Epub 2014 Dec 12 [PubMed PMID: 25503538]
Level 1 (high-level) evidenceHartl DM, Brasnu DF. Contemporary Surgical Management of Early Glottic Cancer. Otolaryngologic clinics of North America. 2015 Aug:48(4):611-25. doi: 10.1016/j.otc.2015.04.007. Epub [PubMed PMID: 26233790]
Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA: a cancer journal for clinicians. 2017 Jan:67(1):31-50. doi: 10.3322/caac.21386. Epub 2016 Nov 29 [PubMed PMID: 27898173]
Lam KH, Lau WF, Wei WI. Tumor clearance at resection margins in total laryngectomy. A clinicopathologic study. Cancer. 1988 Jun 1:61(11):2260-72 [PubMed PMID: 3365654]
Jones TM, De M, Foran B, Harrington K, Mortimore S. Laryngeal cancer: United Kingdom National Multidisciplinary guidelines. The Journal of laryngology and otology. 2016 May:130(S2):S75-S82 [PubMed PMID: 27841116]
Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. International journal of radiation oncology, biology, physics. 2006 Jan 1:64(1):77-82 [PubMed PMID: 16169681]
Level 2 (mid-level) evidenceTrotti A 3rd, Zhang Q, Bentzen SM, Emami B, Hammond ME, Jones CU, Morrison WH, Sagar SM, Ridge JA, Fu KK, Ang KK. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). International journal of radiation oncology, biology, physics. 2014 Aug 1:89(5):958-963. doi: 10.1016/j.ijrobp.2014.04.041. Epub 2014 Jul 8 [PubMed PMID: 25035199]
Level 1 (high-level) evidenceLe QT, Fu KK, Kroll S, Ryu JK, Quivey JM, Meyler TS, Krieg RM, Phillips TL. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. International journal of radiation oncology, biology, physics. 1997 Aug 1:39(1):115-26 [PubMed PMID: 9300746]
Kinshuck AJ, Shenoy A, Jones TM. Voice outcomes for early laryngeal cancer. Current opinion in otolaryngology & head and neck surgery. 2017 Jun:25(3):211-216. doi: 10.1097/MOO.0000000000000363. Epub [PubMed PMID: 28277333]
Level 3 (low-level) evidenceThomas L, Drinnan M, Natesh B, Mehanna H, Jones T, Paleri V. Open conservation partial laryngectomy for laryngeal cancer: a systematic review of English language literature. Cancer treatment reviews. 2012 May:38(3):203-11. doi: 10.1016/j.ctrv.2011.05.010. Epub 2011 Jul 20 [PubMed PMID: 21764220]
Level 1 (high-level) evidencePaleri V, Thomas L, Basavaiah N, Drinnan M, Mehanna H, Jones T. Oncologic outcomes of open conservation laryngectomy for radiorecurrent laryngeal carcinoma: a systematic review and meta-analysis of English-language literature. Cancer. 2011 Jun 15:117(12):2668-76. doi: 10.1002/cncr.25831. Epub 2011 Feb 1 [PubMed PMID: 21287526]
Level 1 (high-level) evidenceLaccourreye H, Laccourreye O, Weinstein G, Menard M, Brasnu D. Supracricoid laryngectomy with cricohyoidoepiglottopexy: a partial laryngeal procedure for glottic carcinoma. The Annals of otology, rhinology, and laryngology. 1990 Jun:99(6 Pt 1):421-6 [PubMed PMID: 2350125]
Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, Endicott JW, McClatchey K, Henderson WG. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The New England journal of medicine. 1991 Jun 13:324(24):1685-90 [PubMed PMID: 2034244]
Level 1 (high-level) evidenceForastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. The New England journal of medicine. 2003 Nov 27:349(22):2091-8 [PubMed PMID: 14645636]
Level 1 (high-level) evidenceForastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, Thorstad W, Wagner H, Ensley JF, Cooper JS. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Mar 1:31(7):845-52. doi: 10.1200/JCO.2012.43.6097. Epub 2012 Nov 26 [PubMed PMID: 23182993]
Level 1 (high-level) evidenceGrover S, Swisher-McClure S, Mitra N, Li J, Cohen RB, Ahn PH, Lukens JN, Chalian AA, Weinstein GS, O'Malley BW Jr, Lin A. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. International journal of radiation oncology, biology, physics. 2015 Jul 1:92(3):594-601. doi: 10.1016/j.ijrobp.2015.03.004. Epub [PubMed PMID: 26068492]
Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Archives of otolaryngology--head & neck surgery. 2007 Dec:133(12):1270-6 [PubMed PMID: 18086971]
Level 2 (mid-level) evidenceBernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M, European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 2004 May 6:350(19):1945-52 [PubMed PMID: 15128894]
Level 1 (high-level) evidenceCooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK, Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2004 May 6:350(19):1937-44 [PubMed PMID: 15128893]
Level 1 (high-level) evidenceAlpert TE, Morbidini-Gaffney S, Chung CT, Bogart JA, Hahn SS, Hsu J, Kellman RM. Radiotherapy for the clinically negative neck in supraglottic laryngeal cancer. Cancer journal (Sudbury, Mass.). 2004 Nov-Dec:10(6):335-8 [PubMed PMID: 15701263]
Forest VI, Nguyen-Tan PF, Tabet JC, Olivier MJ, Larochelle D, Fortin B, Gélinas M, Soulières D, Charpentier D, Guertin L. Role of neck dissection following concurrent chemoradiation for advanced head and neck carcinoma. Head & neck. 2006 Dec:28(12):1099-105 [PubMed PMID: 16933313]
Level 2 (mid-level) evidenceCorry J, Peters L, Fisher R, Macann A, Jackson M, McClure B, Rischin D. N2-N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders-results of Trans Tasman Radiation Oncology Group Study 98.02. Head & neck. 2008 Jun:30(6):737-42. doi: 10.1002/hed.20769. Epub [PubMed PMID: 18286488]
Level 1 (high-level) evidenceHamoir M, Ferlito A, Schmitz S, Hanin FX, Thariat J, Weynand B, Machiels JP, Grégoire V, Robbins KT, Silver CE, Strojan P, Rinaldo A, Corry J, Takes RP. The role of neck dissection in the setting of chemoradiation therapy for head and neck squamous cell carcinoma with advanced neck disease. Oral oncology. 2012 Mar:48(3):203-10. doi: 10.1016/j.oraloncology.2011.10.015. Epub 2011 Nov 21 [PubMed PMID: 22104248]
Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017 Mar:67(2):122-137. doi: 10.3322/caac.21389. Epub 2017 Jan 27 [PubMed PMID: 28128848]
Stanković M, Milisavljević D, Stojanov D, Zivić M, Zivaljević S, Stanković I, Petrović S. Influential factors, complications and survival rate of primary and salvage total laryngectomy for advanced laryngeal cancer. Collegium antropologicum. 2012 Nov:36 Suppl 2():7-12 [PubMed PMID: 23397747]
Cosetti M, Yu GP, Schantz SP. Five-year survival rates and time trends of laryngeal cancer in the US population. Archives of otolaryngology--head & neck surgery. 2008 Apr:134(4):370-9. doi: 10.1001/archotol.134.4.370. Epub [PubMed PMID: 18427002]
Level 2 (mid-level) evidenceMachtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jul 20:26(21):3582-9. doi: 10.1200/JCO.2007.14.8841. Epub 2008 Jun 16 [PubMed PMID: 18559875]
O'Neill CB, O'Neill JP, Atoria CL, Baxi SS, Henman MC, Ganly I, Elkin EB. Treatment complications and survival in advanced laryngeal cancer: a population-based analysis. The Laryngoscope. 2014 Dec:124(12):2707-13. doi: 10.1002/lary.24658. Epub 2014 Oct 4 [PubMed PMID: 24577936]
Level 2 (mid-level) evidenceNguyen NP, Sallah S, Karlsson U, Antoine JE. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002 Feb 15:94(4):1131-41 [PubMed PMID: 11920484]
Level 1 (high-level) evidenceVilaseca-González I, Bernal-Sprekelsen M, Blanch-Alejandro JL, Moragas-Lluis M. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx. Head & neck. 2003 May:25(5):382-8 [PubMed PMID: 12692875]
Smith LP,Roy S, Operating room fires in otolaryngology: risk factors and prevention. American journal of otolaryngology. 2011 Mar-Apr; [PubMed PMID: 20392535]
Herranz J, Sarandeses A, Fernández MF, Barro CV, Vidal JM, Gavilán J. Complications after total laryngectomy in nonradiated laryngeal and hypopharyngeal carcinomas. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000 Jun:122(6):892-8 [PubMed PMID: 10828805]
Level 2 (mid-level) evidenceWoodard TD, Oplatek A, Petruzzelli GJ. Life after total laryngectomy: a measure of long-term survival, function, and quality of life. Archives of otolaryngology--head & neck surgery. 2007 Jun:133(6):526-32 [PubMed PMID: 17576901]
Level 2 (mid-level) evidenceMarron M, Boffetta P, Zhang ZF, Zaridze D, Wünsch-Filho V, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N, Sturgis EM, Smith E, Schwartz SM, Rudnai P, Purdue MP, Olshan AF, Eluf-Neto J, Muscat J, Morgenstern H, Menezes A, McClean M, Matos E, Mates IN, Lissowska J, Levi F, Lazarus P, La Vecchia C, Koifman S, Kelsey K, Herrero R, Hayes RB, Franceschi S, Fernandez L, Fabianova E, Daudt AW, Dal Maso L, Curado MP, Cadoni G, Chen C, Castellsague X, Boccia S, Benhamou S, Ferro G, Berthiller J, Brennan P, Møller H, Hashibe M. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. International journal of epidemiology. 2010 Feb:39(1):182-96. doi: 10.1093/ije/dyp291. Epub 2009 Oct 5 [PubMed PMID: 19805488]