Introduction
Glaucoma is a complex eye condition characterized by elevated intraocular pressure (IOP) that may progress to vision loss over time. Glaucoma is the second leading cause of permanent blindness in the United States and occurs most often in older adults.[1] Glaucoma can be categorized into either primary or secondary types and further into open-angle or closed-angle variants within each type of glaucoma. Adult glaucoma includes primary open-angle glaucoma (POAG) and angle-closure glaucoma, as well as secondary open and angle-closure glaucoma,[2][3] with a specific focus on the most prevalent type, POAG.[4][5]
Glaucoma is an acquired loss of retinal ganglion cells and axons within the optic nerve or optic neuropathy that results in a characteristic optic nerve head appearance and a corresponding progressive loss of vision.[6] This unique pattern of peripheral vision loss serves as a distinguishing feature from other types of vision impairment.[7]
Patients with POAG are often asymptomatic until significant optic nerve damage occurs unless early signs of glaucoma are identified during routine eye examinations.[8] On the other hand, acute angle-closure glaucoma can develop suddenly and lead to a rapid decline in vision, accompanied by symptoms such as corneal edema, eye pain, headache, nausea, and emesis.[9][10] Secondary glaucoma often arises due to a previous eye injury or underlying medical conditions, resulting in elevated IOP and subsequent optic neuropathy. This category encompasses various subtypes, including congenital, pigmentary, neovascular, exfoliative, traumatic, and uveitic glaucoma.[11] Normal or low-tension type of glaucoma presents as an optic neuropathy with glaucomatous visual loss despite normal or unremarkable IOP readings.[12]
Although congenital, infantile, and developmental glaucoma, along with a juvenile variant of POAG, primarily affect younger individuals, most types of glaucoma are commonly diagnosed in individuals aged 40 and older. While IOP is often associated with glaucoma, a direct causal relationship has not been definitively established. Researchers are investigating genetic and environmental factors contributing to glaucoma development. Evidence from studies involving monozygotic twin pairs, who exhibit a higher concordance rate compared to dizygotic pairs, suggests that environmental factors also have a significant role in the disease's development.[13]
Although the available treatments cannot cure existing optic nerve damage or reverse visual field loss, they can help control the disease progression through medication, laser treatment, or incisional glaucoma surgeries to prevent further vision loss. All therapeutic interventions are focused on lowering IOP and minimizing the impact of this vision-threatening condition. This approach aims to prevent the onset of glaucoma in patients with risk factors and to manage the condition effectively to limit its progression in affected individuals.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The exact etiology of glaucoma is unknown, but a clear correlation with elevated eye pressure exists in most cases. High IOP is the primary risk factor for developing glaucoma and for the progression of the disease, and it is also the sole factor that current treatments can effectively address.[14]
Primary Open-Angle Glaucoma
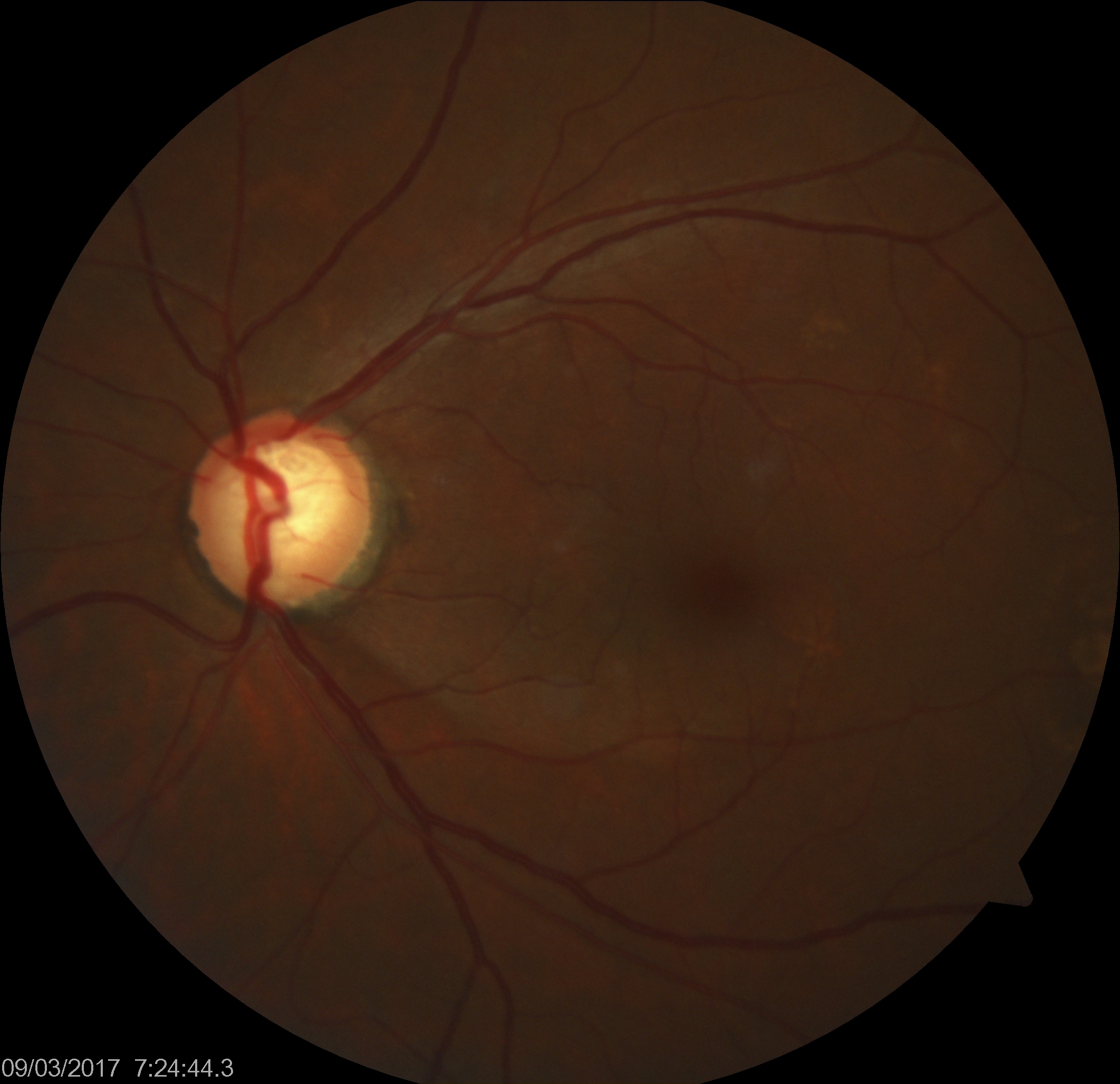
POAG typically manifests as slow, painless damage to the optic nerve due to an ineffective drainage system in the eye. In glaucoma, the resistance to drainage of aqueous humor most commonly starts at the inner wall of Schlemm canal at the juxtacanalicular trabecular meshwork. This decreased outflow facility or increased resistance to aqueous outflow results in a gradual rise in IOP, leading to characteristic damage patterns in the visual field and the optic nerve ganglion cell nerve fiber layer.[15] Recent studies have highlighted that elevated IOP can also reduce blood flow to the optic nerve fibers, resulting in subtle ischemic damage (see Image. Glaucomatous Optic Nerve Head Showing Inferotemporal Retinal Nerve Fiber Layer Defect).[16]
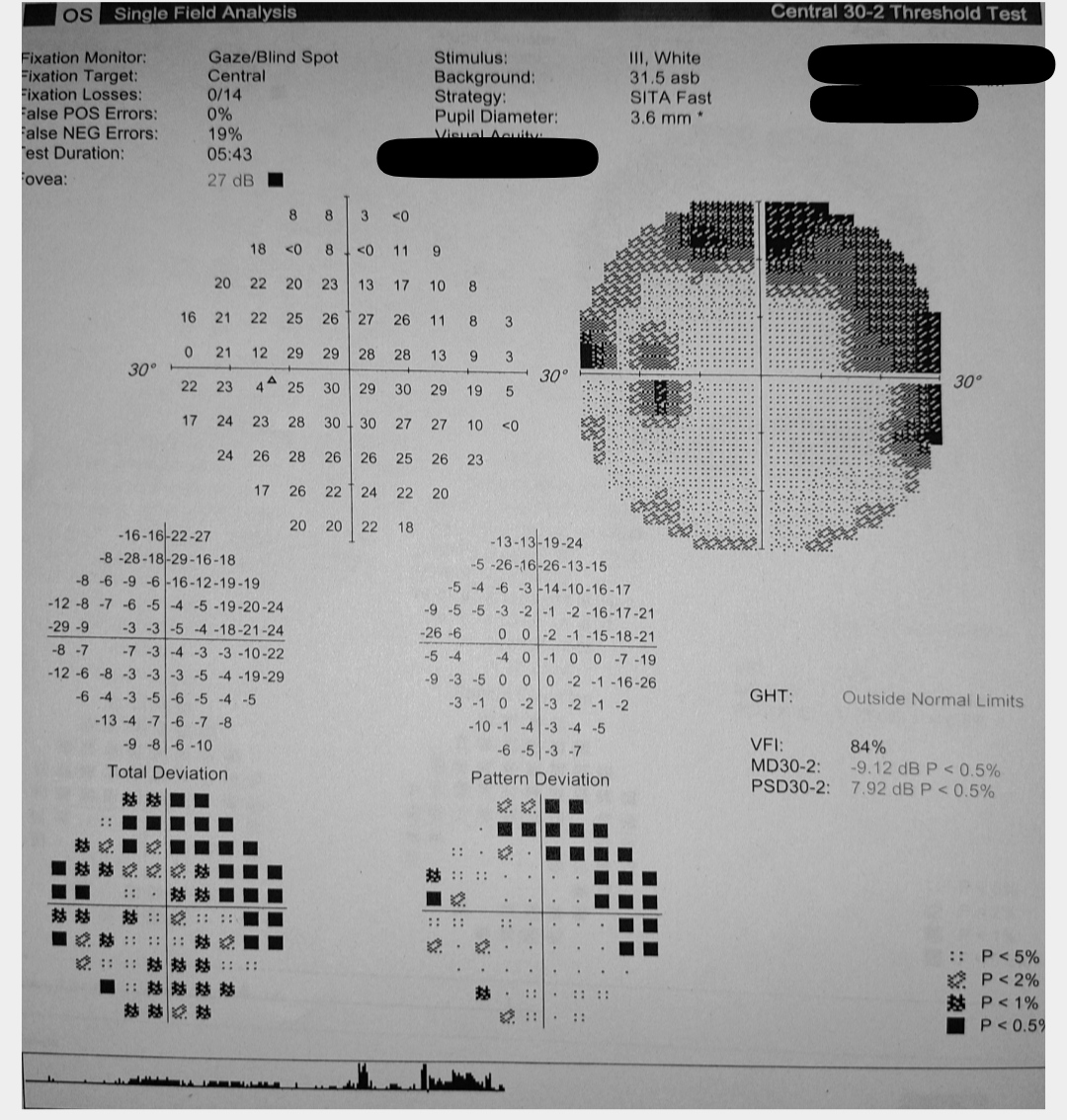
POAG patients often have elevated IOP readings correlating with characteristic optic nerve damage and visual field defect patterns (see Image. Glaucoma Visual Field Changes in the Left Eye). As the disease progresses, a slow loss of peripheral vision in one or typically both eyes eventually leads to loss of central vision. Because of this loss pattern, affected persons do not notice a change in their vision until their loss is advanced and affects the central vision, in which case damage is permanent and irreversible.[7]
Glaucoma can manifest at different ages, with the age of onset often characterizing its presentation. Although POAG is typically associated with adulthood, it can also affect younger individuals and children, suggesting a significant genetic component. Primary congenital glaucoma is diagnosed in newborns aged up to 1 month, often suspected when there is eye enlargement at birth.[17] Infantile glaucoma affects individuals between the ages of 1 and 36 months,[18] while juvenile glaucoma is used to indicate individuals diagnosed with glaucoma between the ages of 3 and 40.[19] Juvenile open-angle glaucoma shares similarities with POAG in terms of IOP leading to optic nerve damage, but it occurs in a younger age group with higher IOP levels and potentially more severe visual field defects.[20]
Low-Tension or Normal-Tension Glaucoma
This type of glaucoma resembles POAG in terms of characteristic optic disc cupping and peripheral visual-field loss findings.[16] However, what sets it apart is that IOP readings are consistently normal, typically measuring less than 21 mmHg.[12] Theories suggest that patients with this type of glaucoma may have an optic nerve that is abnormally sensitive to pressure or may experience intermittent ischemic changes due to atherosclerosis or vascular insufficiency. These patients often exhibit a higher prevalence of migraines, Raynaud phenomenon, autoimmune diseases, ischemic vascular diseases, and coagulopathies. This observation may suggest the involvement of a vascular autoregulatory defect in the pathogenesis of the disease.[21][22][23][24] In addition, these patients tend to have a greater frequency of nerve fiber layer hemorrhages and a neuroretinal rim that is thinner inferiorly and inferotemporal than those with POAG. Visual field defects in this type of glaucoma are typically more focal, deeper, and closer to fixation rather than following the classic arcuate scotoma pattern seen in open-angle glaucoma.[25]
Angle-Closure Glaucoma
Angle-closure glaucoma is classified based on ocular anatomy and can manifest as a medical emergency in the acute setting or as a chronic condition.[26] In the acute form, this type of glaucoma occurs when the eye's drainage system is abruptly blocked due to the closure of the angle formed between the cornea and the iris.[27] Typically, this blockage arises from age-related lens thickening, leading to a gradual increase in a relative pupillary block that pushes the iris anteriorly. This anteriorly displaced iris, coupled with a natural anatomical variation such as a smaller angle seen in hypermetropia or specific ethnic groups, predisposes easier blockage of the outflow tract.[28]
A pupillary block is considered the underlying cause in the majority of cases.[29] When sudden pupil dilation occurs due to certain stimuli, darkness, or drugs, the iris is thick enough in its contracted state or anteriorly displaced by pupillary block to block fluid drainage via the trabecular meshwork. The pressure rapidly increases within the eye. This rapid change in IOP can cause central and/or peripheral vision loss within a few days of onset without intervention and very high IOP. A significantly elevated IOP and acute angle closure can lead to complications such as retinal vascular occlusion, ischemic optic neuropathy, or glaucomatous optic nerve damage. However, it is important to note that only about 10% of glaucoma cases fall into the acute angle-closure type category.[30]
Angle-closure glaucoma can also occur as a secondary condition due to various causes. Examples include lens subluxation in Marfan syndrome,[31] lens dislocation,[32] and lens-induced glaucoma.[33] The displacement of the lens into the pupil or anterior chamber can lead to an acute pupillary block.[34] Plateau iris configuration can also cause an acute pupillary block and chronic angle closure, attributed to elongated or anteriorly positioned ciliary processes pushing the iris edges forward.[35][36] In iridocorneal endothelial syndrome, irregular corneal endothelium migration onto the trabecular meshwork and peripheral iris can lead to high peripheral anterior synechiae, closing the angle and hindering outflow.[37][38]
Neovascularization can cause angle closure in neovascular glaucoma by forming a fibrovascular membrane that flattens and displaces the iris anteriorly. This process, along with new vessel formation in the iris and angle in rubeosis iridis, can lead to total synechial closure of the angle.[39] The most common etiologies of neovascular glaucoma are central retinal or branch retinal vein occlusion, proliferative diabetic retinopathy, and ocular ischemic syndrome.[40] Angle-closure glaucoma can also occur post-ophthalmic surgery due to factors such as ciliary body edema, scleral buckle placement, fibrin deposition, gas, or silicone oil used in retinal surgery.[41] Additionally, sulfa drugs such as topiramate can induce angle closure by causing ciliochoroidal effusion, which compresses the lens-iris diaphragm and anteriorly displaces it, resulting in angle closure.[42]
Secondary Open-Angle Glaucoma
Secondary open-angle glaucoma can result from various factors such as eye injury, eye disease, and occasionally eye surgery. These conditions can elevate IOP, leading to optic nerve damage and functional defects similar to those seen in primary open-angle glaucoma. One notable mechanism of secondary open-angle glaucoma arises from laser surgery, which can trigger pigment release, inflammatory cell accumulation, debris deposition, and mechanical deformation. These factors can collectively obstruct the trabecular meshwork, contributing to elevated IOP levels (see Image. Hypermature Morgagnian Cataract).
Pseudoexfoliation Glaucoma
Pseudoexfoliation syndrome (PEX) is a type of glaucoma characterized by the presence of flaky material within the anterior chamber of the eye that collects in the angle. This material can clog the trabecular meshwork, leading to increased resistance to aqueous outflow and elevated IOP.[43] PEX syndrome is a systemic disorder affecting the extracellular matrix, with ocular manifestations such as white deposits on the pupil's margin and the anterior capsule of the lens, which can occur in one or both eyes.[44] This secondary form of open-angle glaucoma poses additional risks during cataract surgery, including zonular dialysis, capsular bag rupture, and vitreous loss. These risks are attributed to eyes with unstable lens zonules and poor pupillary dilation associated with PEX syndrome.[45]
Individuals with pigment dispersion syndrome (PDS) are at an increased risk of elevated IOP.[46] Ocular manifestations of PDS share similarities with PEX syndrome, except that in PDS, the debris consists of pigment granules from the posterior iris surface. These granules can detach and obstruct the trabecular meshwork, especially in myopic or near-sighted eyes, due to contact with the peripheral lens capsule and zonules.[47] Features of PDS include anterior chamber pigment dispersion, spoke-like iris defects on trans-illumination, central corneal endothelial deposits (known as the Krukenberg spindle), and increased pigmentation in the iridocorneal angle.[48] Some individuals may progress to pigment dispersion glaucoma or pigmentary glaucoma characterized by elevated IOP levels, glaucomatous optic neuropathy, and/or visual field defects.[49]
Steroid-Induced Glaucoma
Steroid-induced glaucoma can occur in susceptible individuals undergoing cortisone therapy due to glucocorticoid's effects. Glucocorticoids can upregulate receptors on cells within the trabecular meshwork, leading to increased outflow resistance. Additionally, the accumulation of glycosaminoglycans in the meshwork pores contributes to this resistance.[50] Steroids can also suppress phagocytic activity, which decreases debris deposition removal from the meshwork and stimulates the expression of extracellular matrix proteins.[51]
Another form of secondary glaucoma is a carotid-cavernous fistula, characterized by an abnormal connection between the cavernous sinus and the carotid artery.[52] This condition causes arterial flow and venous engorgement, leading to elevated episcleral venous pressure. The consequent dilation of retinal veins and swelling of the optic disc can cause concurrent damage to optic nerve fibers.[53]
Secondary glaucoma can also result from posttraumatic or postoperative conditions, elevated episcleral venous pressure, and tumor-related factors.[54] Ellingson syndrome, also known as uveitis-glaucoma-hyphema syndrome, is a complication that can cause elevated IOP following the implantation of an intraocular lens. This elevation in IOP can occur either immediately after surgery or manifest years later as a complication.[55]
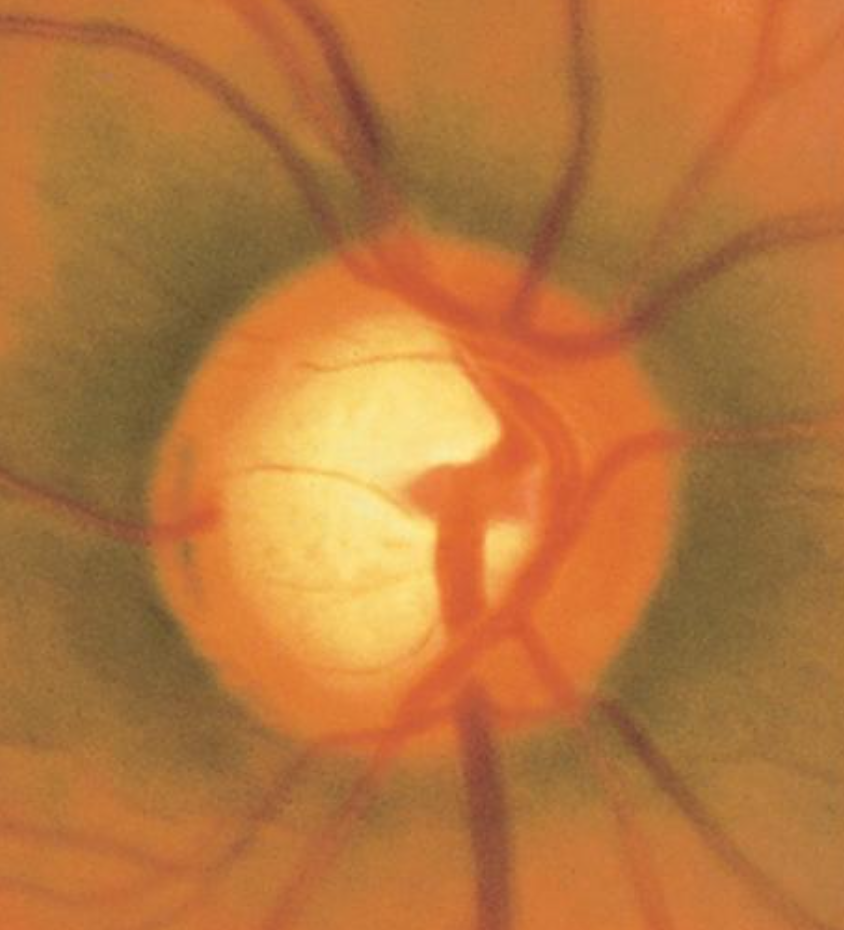
Glaucomatocyclitic crisis, also known as Posner-Schlossman syndrome, presents with recurrent acute episodes of elevated IOP that typically resolve spontaneously without treatment. However, repeated episodes have been associated with glaucomatous damage to the optic nerve over time (see Image. Advanced Glaucomatous Damage to the Optic Nerve).[56][57][58]
Epidemiology
Glaucoma is a condition marked by the gradual loss of peripheral vision and irreversible damage to the optic nerve and retinal ganglion cells, making it a significant public health concern. Its multifactorial etiology involves anatomical, genetic, vascular, and immune factors. Glaucoma is a fundamental public health concern, considering that, after cataracts, it is the second cause of irreversible blindness. Currently affecting over 60 million individuals worldwide, this number is expected to surpass 110 million by 2040.[59] POAG is the most prevalent type, affecting approximately 2% to 4% of individuals aged 40 and older and around 10% of those aged 75 and older.[60]
The African population exhibits the highest prevalence of open-angle glaucoma. Individuals of African descent face up to a 15-fold increased risk of blindness from open-angle glaucoma compared to other population groups.[7] On the other hand, the Inuit population has the highest prevalence of angle-closure glaucoma. Women are more commonly affected than men in this group, along with individuals of Asian descent, who generally have a shallower anterior chamber, contributing to the higher rates of angle closure.[61] The normal-tension type of glaucoma is most prevalent in Japanese populations.
Age represents a significant risk factor in the progressive loss of retinal ganglion cells across all types of glaucoma. Additionally, other risk factors for developing glaucoma include a family history of the condition in a primary relative (mother, father, brother, sister, or children) and medical conditions such as diabetes, high blood pressure, and heart disease. Eye trauma, anatomical differences such as thinner corneas, a history of retinal detachment, eye tumors or inflammation, and prolonged use of corticosteroids are also associated with an increased risk of glaucoma.[62]
Pathophysiology
The optic nerve carries over 1 million nerve fibers that transmit visual signals from the photoreceptors in the outer retina to the visual processing areas of the occipital lobe. Damage to the retinal nerve fiber layer occurs in various types of glaucoma. Aqueous humor, the fluid in the anterior chamber of the eye, is crucial in maintaining intraocular pressure and nourishing ocular tissues. Aqueous humor is produced by the non-pigmented epithelial cells of the ciliary body processes and follows a circadian production pattern.[63] Aqueous humor drains continuously through the pupil, then via the trabecular meshwork anterior to the scleral spur and iris insertion, into Schlemm canal, and finally into the episcleral venous system, larger orbital venous system, and systemic venous circulation. The trabecular meshwork consists of multiple layers of connective tissue and the endothelium of Schlemm canal, forming the primary drainage pathway for aqueous humor.[64]
The conventional outflow pathway regulates fluid drainage from the eye in a pressure-dependent manner, acting as a one-way valve for aqueous humor drainage. In contrast, the uveoscleral outflow pathway allows pressure-independent passage of aqueous humor through the ciliary muscle and iris root into the supraciliary and suprachoroidal space.[65] This pathway is believed to experience reduced outflow with age. Over time, there is a decline in aqueous humor drainage through the trabecular meshwork, while the production of aqueous humor by the ciliary body decreases slightly. This imbalance between outflow and aqueous production leads to elevated average IOP and larger diurnal fluctuations in IOP, which are common features in patients with glaucoma.[66]
Prolonged elevation of IOP leads to the death and atrophy of nerve fibers, resulting in a "cupped" or curved appearance of the optic nerve head observed during fundoscopy. [67] The normal range of IOP is approximately 16±3 mmHg, with values typically falling between 12 and 21 mmHg.[68] However, IOP fluctuates throughout the day due to various factors including heart rate, respiration, exercise, hydration status, systemic medications, time of day, alcohol intake, patient posture, and use of topical medications.
Elevated pressure readings during screening, surpassing 21 mmHg, indicate pressures beyond normal physiological levels and raise concerns about potential future damage to the optic nerve due to glaucoma. However, it is challenging to determine if patients experience transient spikes in pressure during the day, leading to damage that remains undetected during screening. Consequently, elevated screening pressure serves only as a risk factor for developing glaucoma and is not sufficient for a glaucoma diagnosis. Continuous monitoring of IOP throughout the day (diurnal pressure monitoring) may aid in identifying individuals at risk in this patient population.[69]
Patients diagnosed with normal-tension glaucoma often exhibit systemic vascular conditions such as the Raynaud phenomenon, migraines, sleep apnea, carotid artery disease, and significant nocturnal blood pressure fluctuations.[70][71] In acute angle-closure glaucoma, the trabecular meshwork drainage pathway becomes obstructed due to the iris being pushed forward from pressure, such as an anteriorly displaced lens, or by fibrous tissue pulling the iris forward. The most common cause is a pupillary block, where the iris dilates to a mid-position, bows anteriorly upon contact with the lens, and obstructs the trabecular meshwork, thereby impeding aqueous outflow. Secondary glaucoma, as mentioned earlier, can arise from various causes such as surgery or neovascularization, leading to blockage of the outflow tracts and subsequent elevation of IOP, potentially resulting in glaucomatous optic nerve damage if left untreated.
History and Physical
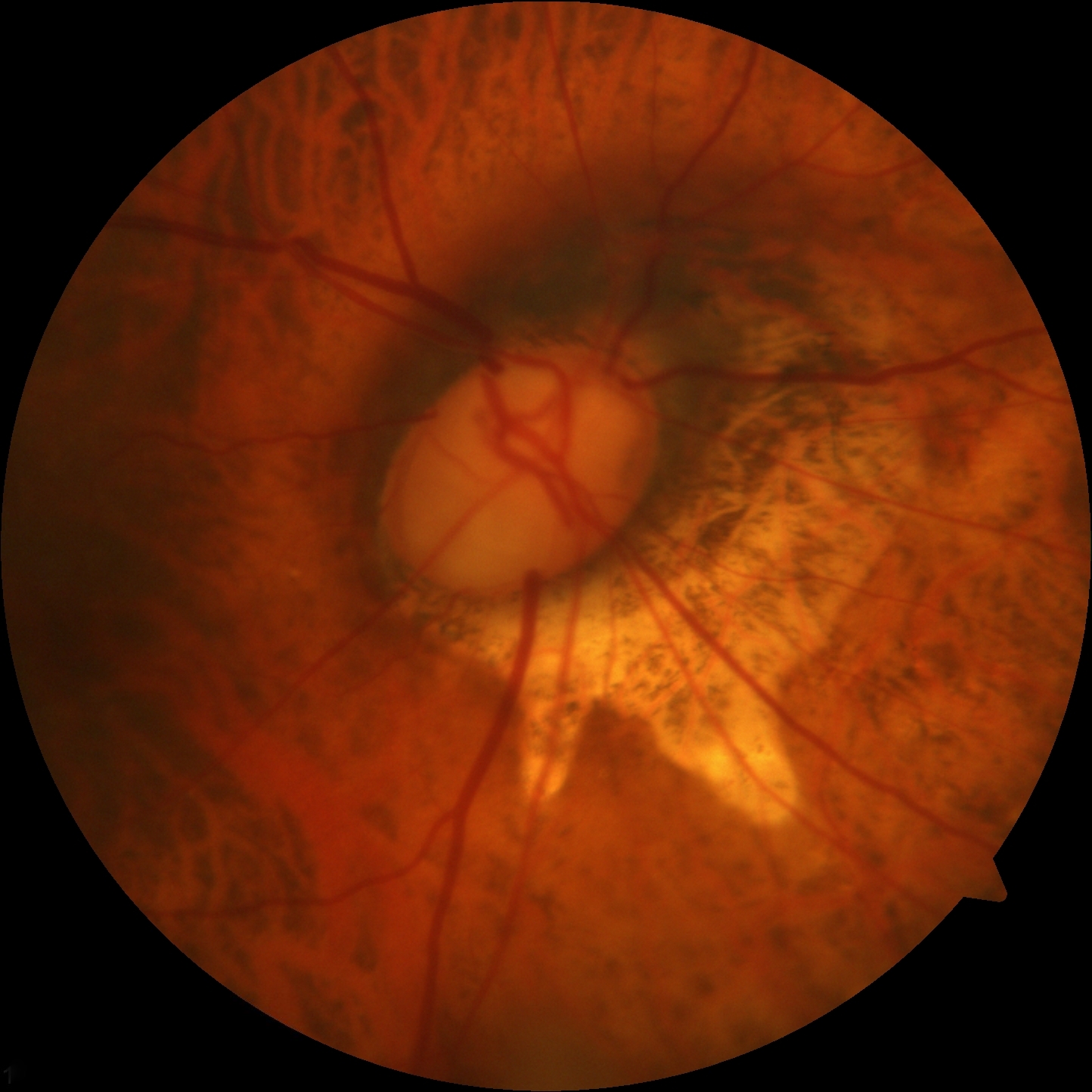
Many patients with glaucoma, especially early in the disease, are unaware they have this condition until it is detected during a routine eye examination. Numerous meta-analyses and systematic reviews have indicated that the prevalence of undetected glaucoma in adults exceeds 50% globally, with higher rates observed in Asia and Africa.[72] Individuals typically experience a gradual loss of peripheral vision while retaining central vision until the disease progresses significantly. This can manifest as a classic arcuate pattern on Humphrey visual field testing. During a comprehensive eye examination, optic nerves may exhibit a focally notched neural retinal rim or diffuse cup enlargement, a reduction in peripheral vision detected through visual field testing, and, although not necessary for diagnosis, an elevated IOP reading on tonometry. Usually, changes are observed bilaterally but may progress asymmetrically, leading to an asymmetric optic nerve cup. A cup-to-disc ratio greater than 0.5 is often associated with glaucoma, with initial loss typically observed in the inferotemporal and superotemporal poles of the optic disc (see Image. Optic Nerve Cup-to-Disc Ratio of 0.75).[73]
Patients with normal-tension glaucoma will typically be asymptomatic and have an IOP of less than 21 mmHg, making it challenging to detect and often leading to underdiagnosis due to the normal IOP values. Slit-lamp examination reveals changes in the optic disc, such as an increased cup-to-disc ratio, along with possible disc hemorrhage in the nerve fiber layer.[74] Patients may also have a medical history that includes vasospasm, coagulopathies, nocturnal hypotension, autoimmune diseases, vascular diseases, thyroid dysfunction, or sleep apnea.
In acute angle-closure glaucoma, patients usually experience sudden severe ocular pain, redness, blurry vision or decreased visual acuity, headache, nausea, and vomiting, as well as may report seeing halos of light. On examination, patients have an unresponsive mid-dilated pupil and a firm feeling of eyeball upon palpation. These attacks are often triggered by pupillary dilation from weak mydriatic or dilating drops. IOP is typically significantly elevated and ranges from 30 to 50 mmHg. Predisposing risk factors for acute angle-closure glaucoma include elevated hypermetropia, an angle between the iris and the cornea around 20° or less observed during gonioscopy, and/or an anterior chamber depth of less than 2.5 mm.[75] Patients with these reports should be advised to avoid dilating medications to reduce their risk of experiencing an acute attack. On slit-lamp examination, signs such as a large optic cup with narrowing of the neuroretinal rim and splinter hemorrhages may also be present.[76]
Patients with secondary glaucoma typically have a history of recent ophthalmic procedures, trauma, or underlying health conditions causing neovascularization, such as diabetic retinopathy or previous retinal vascular occlusions. Patients may sometimes not recall a specific precipitating factor, but subtle clinical examination findings can point to the cause of elevated IOP. Examination findings may include exfoliative material on the anterior lens capsule, pigment deposition on the corneal endothelial cells, flare in the anterior chamber indicative of uveitis, abnormal blood vessels on the iris, or signs of trauma, depending on the underlying etiology.
Evaluation
Assessment includes a fundoscopic examination, visual field testing, tonometry, optical coherence tomography (OCT), and gonioscopy. Of these, IOP is the greatest risk factor, thereby making tonometry of utmost importance.[77] Goldmann applanation tonometry is the gold standard for patients with risk factors, elevated IOP, and glaucoma, although several other types of tonometers are available.[78][79][80] Alternative tonometers can be considered when Goldmann applanation tonometry is impractical, including bedridden patients, noncollaborative individuals, children, or those allergic to anesthetic drops.[81][82]
Additional helpful tests in glaucoma evaluation include assessing visual acuity to determine any impact on vision, pachymetry to measure corneal thickness, and retinal scans to monitor progressive changes in the retinal nerve fiber layer. Regular visual field testing using full-threshold strategies is crucial for individuals with risk factors, ocular hypertension, and those undergoing glaucoma treatment.[83][84][85] OCT is valuable for monitoring morphological changes in the optic nerve and retinal nerve fiber layer, especially in patients with ocular hypertension and early-to-moderate glaucoma.[86]
Glaucoma diagnosis relies on identifying progressive optic neuropathy and/or visual field defects, often in conjunction with elevated IOP. Ocular hypertension is diagnosed in individuals with IOP levels exceeding 21 mmHg without signs of glaucomatous optic neuropathy or functional visual field defects. Research indicates that around 20% of people with ocular hypertension may progress to glaucoma, highlighting the importance of regular testing, tonometry, and comprehensive eye examinations to initiate appropriate treatment aimed at reducing IOP in the presence of initial glaucomatous damage.[87]
A single gold standard test does not exist for diagnosing glaucoma. Typically, glaucoma is diagnosed during routine eye examinations, as the disease is often asymptomatic without noticeable vision loss. Clinicians rely on recognizing the characteristic appearance of the optic nerve, assessing risk factors, and interpreting ancillary test results to establish an accurate diagnosis and stage of glaucoma.[88] Currently, the American Academy of Ophthalmology recommends routine comprehensive eye examinations for individuals with glaucoma risk factors, with the examination frequency tailored to factors such as age, race, family history, and specific risk factors.[89]
Treatment / Management
Managing glaucoma involves personalized strategies based on the type and severity of the condition. Available treatments cannot reverse vision loss; they aim to lower IOP, a key risk factor, to prevent further damage and vision loss. Therapeutic options such as eye drops, laser procedures, and surgeries are focused on reducing IOP. Monitoring disease progression involves using tools like tonometry, visual field tests, OCT, and vision loss mapping.
Open-angle glaucoma is typically managed initially with medications aimed at reducing eye pressure. Common medication classes include prostaglandin analogs, β-blockers, carbonic anhydrase inhibitors, α-2 agonists, miotic agents, and more recently, Rho-kinase inhibitors and nitric oxide-donating medications.[90][91] Laser trabeculoplasty, such as argon laser trabeculoplasty, selective laser trabeculoplasty, and multipulse laser trabeculoplasty, may also be considered in certain cases. However, the benefits of lowering IOP with laser trabeculoplasty often last several months, and retreatments are commonly necessary.[92] (A1)
If medical and/or laser management is unsuccessful, procedures such as trabeculectomy, deep sclerectomy, canaloplasty, insertion of a drainage valve/tube shunt, and laser treatment to the ciliary body to reduce aqueous production can help achieve better control of IOP.[93][94][95] Minimally invasive glaucoma surgery (MIGS) is an emerging option for individuals with mild-to-moderate glaucoma.[96] Compared to traditional trabeculectomy and tube shunts, MIGS offers a more favorable safety profile, quicker recovery time, and effective reduction of IOP to the mid-high teens. Studies also indicate that MIGS placement can decrease the number of pressure-lowering medications needed to maintain target IOP levels.[97](A1)
Normal-tension glaucoma can be managed with medications to reduce IOP and address any underlying medical conditions. Treatment options include prostaglandin analogs, α-2 agonists, carbonic anhydrase inhibitors, and miotics. The use of β-blockers is debated due to concerns about reduced optic nerve head perfusion, particularly regarding the potential exacerbation of the early morning nadir in blood pressure. If medical therapy proves ineffective, laser trabeculoplasty or filtration surgery may be necessary, especially in cases of progressive vision loss. The collaborative normal-tension glaucoma study demonstrated that patients with this condition can slow or stabilize their field loss after achieving a 30% reduction in IOP.[98](B3)
Angle-closure glaucoma is considered a medical emergency due to the potential for elevated pressures leading to glaucomatous optic nerve damage, ischemic nerve damage, or retinal vascular occlusion. Patients can take medications to reduce eye pressure as quickly as possible but usually require a laser procedure called laser peripheral iridotomy. This procedure involves creating a small hole in the iris to alleviate pupillary blockage. By equalizing the pressure gradient between the posterior and anterior chambers, laser iridotomy resolves the iris bombe and opens up the drainage angle in the anterior chamber, relieving the condition. The peripheral iris can be flattened with laser iridoplasty and, less commonly, with laser pupilloplasty.
A decrease in IOP does not always indicate that the angle has reopened. Ischemic damage to the ciliary body during an attack can reduce aqueous humor production for several weeks. Therefore, it is crucial to perform a follow-up gonioscopy to confirm angle patency. This evaluation also helps assess the percentage of the angle with peripheral anterior synechia from acute or prior subacute attacks. After resolving the acute crisis, patients are at a high risk of experiencing an attack in the contralateral eye. Therefore, they should undergo gonioscopy to assess the angle and consider prophylactic iridotomy in the other eye if the angle is narrow.[99] Treatment for secondary glaucoma should focus on addressing the underlying cause along with the possible inclusion of medications to reduce IOP.[100]
Differential Diagnosis
When diagnosing POAG, it is crucial to rule out other conditions that can cause optic neuropathy. Differential diagnoses to consider include previous ischemic optic neuropathy, optic atrophy, and compressive non-glaucomatous optic neuropathy, as they can exhibit similar visual field loss patterns and, in some cases, lead to "pseudo-cupping" of the optic nerve.[101] If elevated IOP or characteristic glaucomatous optic nerve changes are observed, performing a gonioscopy is essential to assess the anterior chamber's status (open, narrow, or closed). Moreover, clinicians should be vigilant for subtle signs of various secondary glaucomas, review the patient's medication history for potential idiosyncratic drug reactions or steroid responses, and gather a comprehensive history of prior ocular trauma and surgeries.
With an acute presentation resembling acute angle-closure glaucoma, clinicians should also consider other potential diagnoses such as iritis, traumatic hyphema, conjunctivitis, episcleritis, migraine, cluster headache, subconjunctival hemorrhage, corneal abrasion, endophthalmitis, orbital compartment syndrome, corneal ulcer, periorbital infections, and infectious keratitis. A thorough history-taking and detailed slit-lamp examination are crucial for narrowing down the differentials and arranging appropriate examinations or referrals as needed.[102][103][104]
Prognosis
Glaucoma is not a benign disorder, and if left untreated, it can lead to permanent vision loss. The higher the pressure and the duration of elevated IOP, the greater the risk of damaging the optic nerve. Timely diagnosis plays a critical role in mitigating glaucomatous damage, and early intervention is crucial in preventing and slowing down vision loss progression. Effective treatment, especially in maintaining low IOP levels, can often lead to positive outcomes, preserving visual field integrity and halting disease advancement.
Complications
Complications of glaucoma include visual field loss and can lead to eventual complete blindness, with a progression to no light perception vision in the affected eye.[105]
Deterrence and Patient Education
Standard screening during a complete eye examination includes checking the IOP of both eyes. Alongside tonometry, periodic eye examinations should encompass a thorough anterior segment evaluation and a fundoscopic exam, with special attention to the optic nerve. These initial screening tests are crucial for identifying glaucoma suspects early in the disease course. High-risk glaucoma suspects require further diagnostic testing such as visual field examination, OCT, pachymetry, and gonioscopy to confirm the diagnosis.
Patients should be educated about the causes, risk factors, and treatment for glaucoma. They should understand that most cases of glaucoma result in slow, progressive vision loss that often goes unnoticed until a significant portion of the visual field is affected. Therefore, regular eye exams are highly recommended to identify high-risk patients promptly and prevent irreversible vision loss.
Enhancing Healthcare Team Outcomes
Glaucoma represents a chronic and severe condition that may lead to irreversible vision loss if detected late in its progression. Effective management of the condition involves an interprofessional team focused on addressing patients' visual health concerns. A crucial aspect of treatment lies in patient education. Pharmacists should emphasize the importance of medication compliance and the necessity of regular follow-ups with the eye specialist. Ophthalmic technicians must collaborate closely with clinicians to oversee scheduled visual field and OCT assessments. Ophthalmic nurses are responsible for monitoring IOP and communicating these findings promptly to the ophthalmologist for further evaluation and management. This collaborative approach ensures comprehensive care and better outcomes for patients with glaucoma.
Regular eye appointments and compliance with medication are vital to lessening disease progression in glaucoma patients. Given its hereditary nature, educating family members about the increased risk of developing glaucoma is important, prompting them to undergo regular screenings for early detection. Notably, healthcare team members must consult the ophthalmologist before making any changes to medication dosage or frequency. Effective communication among healthcare providers is essential to reduce the morbidity associated with glaucoma.[106]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
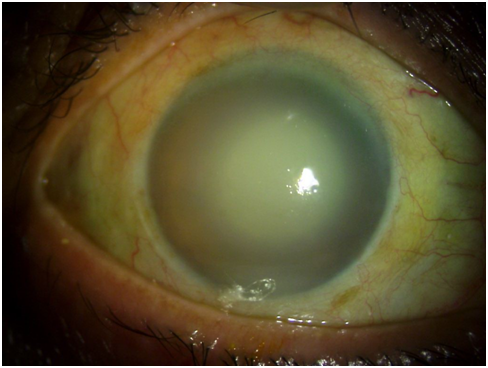
Hypermature Morgagnian Cataract. This image captures the outcome of treatment for an older male patient who initially presented with severe corneal edema, congestion, and elevated intraocular pressure. Following the administration of antiglaucoma medications and topical steroids, a remarkable reduction in corneal edema was observed, exposing a hypermature Morgagnian cataract.
Sridhar U. Cornea Illustrated: A Guide to Clinical Diagnosis. Kolkata, West Bengal, India: New Central Book Agency; 2017.
(Click Image to Enlarge)
References
Ezinne NE, Shittu O, Ekemiri KK, Kwarteng MA, Tagoh S, Ogbonna G, Mashige KP. Visual Impairment and Blindness among Patients at Nigeria Army Eye Centre, Bonny Cantonment Lagos, Nigeria. Healthcare (Basel, Switzerland). 2022 Nov 18:10(11):. doi: 10.3390/healthcare10112312. Epub 2022 Nov 18 [PubMed PMID: 36421637]
Mahabadi N, Foris LA, Tripathy K. Open Angle Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28722917]
Wagner IV, Stewart MW, Dorairaj SK. Updates on the Diagnosis and Management of Glaucoma. Mayo Clinic proceedings. Innovations, quality & outcomes. 2022 Dec:6(6):618-635. doi: 10.1016/j.mayocpiqo.2022.09.007. Epub 2022 Nov 16 [PubMed PMID: 36405987]
Level 2 (mid-level) evidenceCook C, Foster P. Epidemiology of glaucoma: what's new? Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2012 Jun:47(3):223-6. doi: 10.1016/j.jcjo.2012.02.003. Epub [PubMed PMID: 22687296]
Gallo Afflitto G, Aiello F, Cesareo M, Nucci C. Primary Open Angle Glaucoma Prevalence in Europe: A Systematic Review and Meta-Analysis. Journal of glaucoma. 2022 Oct 1:31(10):783-788. doi: 10.1097/IJG.0000000000002083. Epub 2022 Aug 9 [PubMed PMID: 35980843]
Level 1 (high-level) evidenceSingh K, Bhushan P, Mishra D, Kaur K, Gurnani B, Singh A, Pandey S. Assessment of optic disk by disk damage likelihood scale staging using slit-lamp biomicroscopy and optical coherence tomography in diagnosing primary open-angle glaucoma. Indian journal of ophthalmology. 2022 Dec:70(12):4152-4157. doi: 10.4103/ijo.IJO_1113_22. Epub [PubMed PMID: 36453304]
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet (London, England). 2017 Nov 11:390(10108):2183-2193. doi: 10.1016/S0140-6736(17)31469-1. Epub 2017 May 31 [PubMed PMID: 28577860]
Parab A, Kavitha S, Odayappan A, Venkatesh R. Clinical and demographic profile of patients less than 40 years of age presenting to glaucoma services at a tertiary care eye hospital in South India. Indian journal of ophthalmology. 2022 Dec:70(12):4186-4192. doi: 10.4103/ijo.IJO_963_22. Epub [PubMed PMID: 36453311]
Khazaeni B, Zeppieri M, Khazaeni L. Acute Angle-Closure Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28613607]
Foster PJ, Luben R, Khawaja AP. Association, Risk, and Causation-Examining the Role of Systemic Medications in the Onset of Acute Angle-Closure Episodes. JAMA ophthalmology. 2022 Nov 1:140(11):1064-1065. doi: 10.1001/jamaophthalmol.2022.3724. Epub [PubMed PMID: 36136324]
Xu KM, Cho R, Chan TYB. Retrospective Analysis of Switching Bimatoprost 0.01% to Bimatoprost 0.03% in Patients with Various Types of Glaucoma and Ocular Hypertension. Clinical ophthalmology (Auckland, N.Z.). 2022:16():2385-2390. doi: 10.2147/OPTH.S368214. Epub 2022 Jul 29 [PubMed PMID: 35936971]
Level 2 (mid-level) evidenceGosling D, Meyer JJ. Normal Tension Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 35015402]
Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Igo RP Jr, Hysi PG, Glastonbury CA, Ashley-Koch A, Brilliant M, Brown AA, Budenz DL, Buil A, Cheng CY, Choi H, Christen WG, Curhan G, De Vivo I, Fingert JH, Foster PJ, Fuchs C, Gaasterland D, Gaasterland T, Hewitt AW, Hu F, Hunter DJ, Khawaja AP, Lee RK, Li Z, Lichter PR, Mackey DA, McGuffin P, Mitchell P, Moroi SE, Perera SA, Pepper KW, Qi Q, Realini T, Richards JE, Ridker PM, Rimm E, Ritch R, Ritchie M, Schuman JS, Scott WK, Singh K, Sit AJ, Song YE, Tamimi RM, Topouzis F, Viswanathan AC, Verma SS, Vollrath D, Wang JJ, Weisschuh N, Wissinger B, Wollstein G, Wong TY, Yaspan BL, Zack DJ, Zhang K, Study EN, ANZRAG Consortium, Weinreb RN, Pericak-Vance MA, Small K, Hammond CJ, Aung T, Liu Y, Vithana EN, MacGregor S, Craig JE, Kraft P, Howell G, Hauser MA, Pasquale LR, Haines JL, Wiggs JL. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nature genetics. 2016 Feb:48(2):189-94. doi: 10.1038/ng.3482. Epub 2016 Jan 11 [PubMed PMID: 26752265]
Level 2 (mid-level) evidenceWu X, Yang X, Liang Q, Xue X, Huang J, Wang J, Xu Y, Tong R, Liu M, Zhou Q, Shi J. Drugs for the treatment of glaucoma: Targets, structure-activity relationships and clinical research. European journal of medicinal chemistry. 2021 Dec 15:226():113842. doi: 10.1016/j.ejmech.2021.113842. Epub 2021 Sep 11 [PubMed PMID: 34536672]
Buffault J, Labbé A, Hamard P, Brignole-Baudouin F, Baudouin C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal francais d'ophtalmologie. 2020 Sep:43(7):e217-e230. doi: 10.1016/j.jfo.2020.05.002. Epub 2020 Jun 16 [PubMed PMID: 32561029]
Shen WC, Huang BQ, Yang J. Regulatory mechanisms of retinal ganglion cell death in normal tension glaucoma and potential therapies. Neural regeneration research. 2023 Jan:18(1):87-93. doi: 10.4103/1673-5374.344831. Epub [PubMed PMID: 35799514]
Kaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. StatPearls. 2025 Jan:(): [PubMed PMID: 34662067]
Yeung HH, Kumar-Singh R, Walton DS. Infantile Aphakic Glaucoma: A Proposed Mechanism. Journal of pediatric ophthalmology and strabismus. 2022 Jul-Aug:59(4):236-242. doi: 10.3928/01913913-20210929-02. Epub 2021 Dec 20 [PubMed PMID: 34928772]
Jafer Chardoub AA, Blair K. Juvenile Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 32965934]
Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG). Seminars in ophthalmology. 2008 Jan-Feb:23(1):19-25. doi: 10.1080/08820530701745199. Epub [PubMed PMID: 18214788]
Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. American journal of ophthalmology. 2001 Jun:131(6):699-708 [PubMed PMID: 11384564]
Terelak-Borys B, Czechowicz-Janicka K. Investigation into the vasospastic mechanisms in the pathogenesis of glaucomatous neuropathy. Klinika oczna. 2011:113(7-9):201-8 [PubMed PMID: 22256559]
Reinehr S, Guntermann A, Theile J, Benning L, Grotegut P, Kuehn S, Serschnitzki B, Dick HB, Marcus K, Joachim SC, May C. Proteomic Analysis of Retinal Tissue in an S100B Autoimmune Glaucoma Model. Biology. 2021 Dec 23:11(1):. doi: 10.3390/biology11010016. Epub 2021 Dec 23 [PubMed PMID: 35053014]
Nowrouzi A, Benitez-Del-Castillo J, Kafi-Abasabadi S, Rodriguez-Calzadilla M, Diaz-Ramos A, Rodriguez-Suarez A, Mota-Chozas I. Peripheral vascular disease - a new vascular disease associated with normal tension glaucoma: a case report. Journal of medical case reports. 2020 Nov 19:14(1):224. doi: 10.1186/s13256-020-02533-3. Epub 2020 Nov 19 [PubMed PMID: 33208187]
Level 3 (low-level) evidenceSung MS, Ji YS, Heo H, Park SW. Comparison of the Structure-Function Relationship Between Advanced Primary Open Angle Glaucoma and Normal Tension Glaucoma. Journal of glaucoma. 2022 Jul 1:31(7):574-583. doi: 10.1097/IJG.0000000000002053. Epub 2022 May 16 [PubMed PMID: 35583511]
Dave SD, Meyer JJ. Chronic Closed Angle Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 32644524]
Sener H, Evereklioglu C, Horozoglu F, Sener ABG. Optic nerve head vessel density using OCTA in patients with primary angle closure disease: A systematic review and network meta-analysis. Photodiagnosis and photodynamic therapy. 2023 Mar:41():103209. doi: 10.1016/j.pdpdt.2022.103209. Epub 2022 Dec 6 [PubMed PMID: 36493693]
Level 1 (high-level) evidenceSamokhvalov NV, Sorokin EL, Marchenko AN, Pashentsev IE. [Anatomical and morphometric features of anterior eye segment structures in hyperopia and the risk of developing primary angle-closure glaucoma]. Vestnik oftalmologii. 2022:138(5):22-28. doi: 10.17116/oftalma202213805122. Epub [PubMed PMID: 36288414]
Cai JC, Chen YL, Cao YH, Babenko A, Chen X. Numerical study of aqueous humor flow and iris deformation with pupillary block and the efficacy of laser peripheral iridotomy. Clinical biomechanics (Bristol, Avon). 2022 Feb:92():105579. doi: 10.1016/j.clinbiomech.2022.105579. Epub 2022 Jan 19 [PubMed PMID: 35085976]
Douglas GR, Drance SM, Schulzer M. The visual field and nerve head in angle-closure glaucoma. A comparison of the effects of acute and chronic angle closure. Archives of ophthalmology (Chicago, Ill. : 1960). 1975 Jun:93(6):409-11 [PubMed PMID: 1131080]
Izquierdo NJ, Traboulsi EI, Enger C, Maumenee IH. Glaucoma in the Marfan syndrome. Transactions of the American Ophthalmological Society. 1992:90():111-7; discussion 118-22 [PubMed PMID: 1494814]
Kondo K, Isono H. A case of angle-closure glaucoma caused by spontaneous lens dislocation. Clinical case reports. 2022 Dec:10(12):e6670. doi: 10.1002/ccr3.6670. Epub 2022 Dec 5 [PubMed PMID: 36483875]
Level 3 (low-level) evidenceShah SS, Meyer JJ. Lens-Induced Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 34662038]
Kale Y, Isik DU, Celik U, Hekimoglu E, Celik IH, Bas AY, Demirel N. Neonatal Marfan syndrome with angle-closure glaucoma, tricuspid and mitral insufficiency. Genetic counseling (Geneva, Switzerland). 2015:26(1):95-8 [PubMed PMID: 26043516]
Alshomar K, Alsirhy E, Mirza A, Osman M, Alobaidan A, Osman EA. Prevalence of Plateau Iris Syndrome among Patients Presenting with Primary Angle Closure and Primary Angle-Closure Glaucoma in a Tertiary Eye Care Hospital. Middle East African journal of ophthalmology. 2021 Oct-Dec:28(4):221-225. doi: 10.4103/meajo.meajo_232_21. Epub 2022 Apr 30 [PubMed PMID: 35719280]
Kiuchi Y, Kanamoto T, Nakamura T. Double hump sign in indentation gonioscopy is correlated with presence of plateau iris configuration regardless of patent iridotomy. Journal of glaucoma. 2009 Feb:18(2):161-4. doi: 10.1097/IJG.0b013e31817d23b5. Epub [PubMed PMID: 19225356]
Level 2 (mid-level) evidenceLaganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1992 Mar:110(3):346-50 [PubMed PMID: 1543451]
Level 2 (mid-level) evidenceD'cruz RP, Rao A. 'Progressive peripheral anterior synechiae in iridocorneoendothelial syndrome- a crawling disaster'. European journal of ophthalmology. 2023 May:33(3):NP40-NP44. doi: 10.1177/11206721211070095. Epub 2021 Dec 29 [PubMed PMID: 34964381]
Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001 Oct:108(10):1767-76; quiz1777, 1800 [PubMed PMID: 11581047]
Level 1 (high-level) evidenceMishra C, Meyer JJ. Neovascular Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 35015418]
Perez RN, Phelps CD, Burton TC. Angel-closure glaucoma following scleral buckling operations. Transactions. Section on Ophthalmology. American Academy of Ophthalmology and Otolaryngology. 1976 Mar-Apr:81(2):247-52 [PubMed PMID: 936397]
Aminlari A, East M, Wei W, Quillen D. Topiramate induced acute angle closure glaucoma. The open ophthalmology journal. 2008 Mar 28:2():46-7. doi: 10.2174/1874364100802010046. Epub 2008 Mar 28 [PubMed PMID: 19478906]
Shivkumar C, Gadiwan M, Rout M, Ghosh A, Haroon S, Ramakrishnan R. Visual outcomes and complications of manual small-incision cataract surgery in patients with pseudoexfoliation. Indian journal of ophthalmology. 2022 Nov:70(11):3912-3917. doi: 10.4103/ijo.IJO_1581_22. Epub [PubMed PMID: 36308126]
Level 2 (mid-level) evidenceCollao V, Morris J, Chauhan MZ, Abdelrahman L, Martínez-de-la-Casa JM, Vidal-Villegas B, Burgos-Blasco B, Bhattacharya SK. Analyses of pseudoexfoliation aqueous humor lipidome. Molecular omics. 2022 Jun 13:18(5):387-396. doi: 10.1039/d1mo00495f. Epub 2022 Jun 13 [PubMed PMID: 35485348]
Tuteja S, Zeppieri M, Chawla H. Pseudoexfoliation Syndrome and Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 34662036]
Gurnani B, Kaur K. Pigment Dispersion Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 35593834]
Plateroti P, Plateroti AM, Abdolrahimzadeh S, Scuderi G. Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma: A Review of the Literature with Updates on Surgical Management. Journal of ophthalmology. 2015:2015():370371. doi: 10.1155/2015/370371. Epub 2015 Oct 29 [PubMed PMID: 26605078]
Zeppieri M. Pigment dispersion syndrome: A brief overview. Journal of clinical and translational research. 2022 Oct 31:8(5):344-350 [PubMed PMID: 36518550]
Level 3 (low-level) evidenceZeppieri M, Tripathy K. Pigment Dispersion Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 35593820]
Feroze KB, Zeppieri M, Khazaeni L. Steroid-Induced Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28613653]
Roll P, Benedikt O. [Electronmicroscopic studies of the trabecular meshwork in corticosteroid glaucoma]. Klinische Monatsblatter fur Augenheilkunde. 1979 Mar:174(3):421-8 [PubMed PMID: 480814]
Level 3 (low-level) evidenceKhurana M, Alam MS, Balekudaru S, Vijaya L, Madhuri MB, Halbe SV, Noronha VO, George RJ, Mukherjee B. Intraocular Pressure in the Eyes of Patients With Carotid-Cavernous Fistulas: Profile, Intereye Asymmetry, and Treatment Outcomes. Journal of glaucoma. 2019 Dec:28(12):1074-1078. doi: 10.1097/IJG.0000000000001392. Epub [PubMed PMID: 31658226]
Calafiore S, Perdicchi A, Scuderi G, Contestabile MT, Abdolrahimzadeh S, Recupero SM. Glaucoma Management in Carotid Cavernous Fistula. Case reports in ophthalmology. 2016 May-Aug:7(2):296-302. doi: 10.1159/000446151. Epub 2016 Jun 2 [PubMed PMID: 27462258]
Level 3 (low-level) evidenceGreslechner R, Helbig H, Spiegel D. [Secondary open-angle glaucoma: uveitic secondary glaucoma, steroid-induced glaucoma, posttraumatic and postoperative glaucoma, tumor-related glaucoma and glaucoma due to elevated episcleral venous pressure]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2022 May:119(5):533-546. doi: 10.1007/s00347-022-01630-6. Epub 2022 Apr 26 [PubMed PMID: 35471612]
Sen S, Tripathy K. Uveitis Glaucoma Hyphema Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 35593837]
Okonkwo ON, Tripathy K. Posner-Schlossman Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 35015437]
Choong YF, Irfan S, Menage MJ. Acute angle closure glaucoma: an evaluation of a protocol for acute treatment. Eye (London, England). 1999 Oct:13 ( Pt 5)():613-6 [PubMed PMID: 10696311]
POSNER A, SCHLOSSMAN A. Further observations on the syndrome of glaucomatocyclitic crises. Transactions - American Academy of Ophthalmology and Otolaryngology. American Academy of Ophthalmology and Otolaryngology. 1953 Jul-Aug:57(4):531-6 [PubMed PMID: 13090248]
Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus. 2020 Nov 24:12(11):e11686. doi: 10.7759/cureus.11686. Epub 2020 Nov 24 [PubMed PMID: 33391921]
Imrie C, Tatham AJ. Glaucoma: the patient's perspective. The British journal of general practice : the journal of the Royal College of General Practitioners. 2016 May:66(646):e371-3. doi: 10.3399/bjgp16X685165. Epub [PubMed PMID: 27127293]
Level 3 (low-level) evidenceBourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S, Taylor HR, Vision Loss Expert Group. Causes of vision loss worldwide, 1990-2010: a systematic analysis. The Lancet. Global health. 2013 Dec:1(6):e339-49. doi: 10.1016/S2214-109X(13)70113-X. Epub 2013 Nov 11 [PubMed PMID: 25104599]
Level 1 (high-level) evidenceLeung DYL, Tham CC. Normal-tension glaucoma: Current concepts and approaches-A review. Clinical & experimental ophthalmology. 2022 Mar:50(2):247-259. doi: 10.1111/ceo.14043. Epub 2022 Feb 7 [PubMed PMID: 35040248]
Heijl A, Bengtsson B, Hyman L, Leske MC, Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009 Dec:116(12):2271-6. doi: 10.1016/j.ophtha.2009.06.042. Epub 2009 Oct 24 [PubMed PMID: 19854514]
Level 1 (high-level) evidenceReina-Torres E, Baptiste TMG, Overby DR. Segmental outflow dynamics in the trabecular meshwork of living mice. Experimental eye research. 2022 Dec:225():109285. doi: 10.1016/j.exer.2022.109285. Epub 2022 Oct 21 [PubMed PMID: 36273576]
Gupta V, Singh A, Pandya I, Sofi R, Sen S, Somarajan BI, Gupta S, Nag TC. Differences in outflow channels between two eyes of unilateral primary congenital glaucoma. Acta ophthalmologica. 2021 Mar:99(2):187-194. doi: 10.1111/aos.14540. Epub 2020 Jul 23 [PubMed PMID: 32701215]
Tan S, Yu M, Baig N, Chan PP, Tang FY, Cheung CY, Tham CCY. Association of Ultra-Short-Term Intraocular Pressure Fluctuation With Disease Progression in Primary Angle Closure Glaucoma: The CUPAL Study. Journal of glaucoma. 2022 Nov 1:31(11):874-880. doi: 10.1097/IJG.0000000000002103. Epub 2022 Aug 16 [PubMed PMID: 35980863]
Omodaka K, Kikawa T, Kabakura S, Himori N, Tsuda S, Ninomiya T, Takahashi N, Pak K, Takeda N, Akiba M, Nakazawa T. Clinical characteristics of glaucoma patients with various risk factors. BMC ophthalmology. 2022 Sep 19:22(1):373. doi: 10.1186/s12886-022-02587-5. Epub 2022 Sep 19 [PubMed PMID: 36123604]
Wolvaardt E, Stevens S. Measuring intraocular pressure. Community eye health. 2019:32(107):56-57 [PubMed PMID: 32123477]
Arora T, Bali SJ, Arora V, Wadhwani M, Panda A, Dada T. Diurnal versus office-hour intraocular pressure fluctuation in primary adult onset glaucoma. Journal of optometry. 2015 Oct-Dec:8(4):239-43. doi: 10.1016/j.optom.2014.05.005. Epub 2014 Jun 16 [PubMed PMID: 26386536]
Levene RZ. Low tension glaucoma: a critical review and new material. Survey of ophthalmology. 1980 May-Jun:24(6):621-64 [PubMed PMID: 7414505]
Level 3 (low-level) evidenceDrance SM, Sweeney VP, Morgan RW, Feldman F. Factors involved in the production of low tension glaucoma. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 1974 Oct:9(4):399-403 [PubMed PMID: 4423185]
Soh Z, Yu M, Betzler BK, Majithia S, Thakur S, Tham YC, Wong TY, Aung T, Friedman DS, Cheng CY. The Global Extent of Undetected Glaucoma in Adults: A Systematic Review and Meta-analysis. Ophthalmology. 2021 Oct:128(10):1393-1404. doi: 10.1016/j.ophtha.2021.04.009. Epub 2021 Apr 16 [PubMed PMID: 33865875]
Level 1 (high-level) evidenceJonas JB, Fernández MC, Stürmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993 Jan:100(1):63-8 [PubMed PMID: 8433829]
Chuangsuwanich T, Tun TA, Braeu FA, Wang X, Chin ZY, Panda SK, Buist M, Strouthidis N, Perera S, Nongpiur M, Aung T, Girard MJA. Differing Associations between Optic Nerve Head Strains and Visual Field Loss in Patients with Normal- and High-Tension Glaucoma. Ophthalmology. 2023 Jan:130(1):99-110. doi: 10.1016/j.ophtha.2022.08.007. Epub 2022 Aug 11 [PubMed PMID: 35964710]
Moghimi S, Ramezani F, He M, Coleman AL, Lin SC. Comparison of Anterior Segment-Optical Coherence Tomography Parameters in Phacomorphic Angle Closure and Acute Angle Closure Eyes. Investigative ophthalmology & visual science. 2015 Dec:56(13):7611-7. doi: 10.1167/iovs.15-17336. Epub [PubMed PMID: 26624492]
Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. The British journal of ophthalmology. 2003 Apr:87(4):450-4 [PubMed PMID: 12642309]
Bader J, Zeppieri M, Havens SJ. Tonometry. StatPearls. 2024 Jan:(): [PubMed PMID: 29630277]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27 [PubMed PMID: 34501306]
Brusini P, Salvetat ML, Zeppieri M. It Is All about Pressure. Journal of clinical medicine. 2022 Jun 23:11(13):. doi: 10.3390/jcm11133640. Epub 2022 Jun 23 [PubMed PMID: 35806926]
Zeppieri M, Gurnani B. Applanation Tonometry. StatPearls. 2024 Jan:(): [PubMed PMID: 35881737]
Salvetat ML, Zeppieri M, Tosoni C, Brusini P. Repeatability and accuracy of applanation resonance tonometry in healthy subjects and patients with glaucoma. Acta ophthalmologica. 2014 Feb:92(1):e66-73. doi: 10.1111/aos.12209. Epub 2013 Jul 10 [PubMed PMID: 23837834]
Level 2 (mid-level) evidenceSalvetat ML, Zeppieri M, Tosoni C, Brusini P. Comparisons between Pascal dynamic contour tonometry, the TonoPen, and Goldmann applanation tonometry in patients with glaucoma. Acta ophthalmologica Scandinavica. 2007 May:85(3):272-9 [PubMed PMID: 17488456]
Salvetat ML, Zeppieri M, Tosoni C, Felletti M, Grasso L, Brusini P. Corneal Deformation Parameters Provided by the Corvis-ST Pachy-Tonometer in Healthy Subjects and Glaucoma Patients. Journal of glaucoma. 2015 Oct-Nov:24(8):568-74. doi: 10.1097/IJG.0000000000000133. Epub [PubMed PMID: 25318572]
Level 2 (mid-level) evidenceZeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. European journal of ophthalmology. 2005 Mar-Apr:15(2):196-201 [PubMed PMID: 15812759]
Salvetat ML, Zeppieri M, Tosoni C, Parisi L, Brusini P. Non-conventional perimetric methods in the detection of early glaucomatous functional damage. Eye (London, England). 2010 May:24(5):835-42. doi: 10.1038/eye.2009.216. Epub 2009 Aug 21 [PubMed PMID: 19696803]
Level 2 (mid-level) evidenceMahmoudinezhad G, Moghimi S, Proudfoot JA, Brye N, Nishida T, Yarmohammadi A, Kamalipour A, Zangwill LM, Weinreb RN. Effect of Testing Frequency on the Time to Detect Glaucoma Progression With Optical Coherence Tomography (OCT) and OCT Angiography. American journal of ophthalmology. 2023 Jan:245():184-192. doi: 10.1016/j.ajo.2022.08.030. Epub 2022 Sep 10 [PubMed PMID: 36096181]
Kelly SR, Khawaja AP, Bryan SR, Azuara-Blanco A, Sparrow JM, Crabb DP. Progression from ocular hypertension to visual field loss in the English hospital eye service. The British journal of ophthalmology. 2020 Oct:104(10):1406-1411. doi: 10.1136/bjophthalmol-2019-315052. Epub 2020 Mar 25 [PubMed PMID: 32217541]
Tan C, Hou Y, Qiao YS, Chen JY, Sun XH. [The study of the correlation between age and the pathogenic factors of primary glaucoma: a review]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2022 Dec 11:58(12):1106-1110. doi: 10.3760/cma.j.cn112142-20220616-00297. Epub [PubMed PMID: 36480899]
Level 2 (mid-level) evidenceSalvetat ML, Zeppieri M, Tosoni C, Brusini P, Medscape. Baseline factors predicting the risk of conversion from ocular hypertension to primary open-angle glaucoma during a 10-year follow-up. Eye (London, England). 2016 Jun:30(6):784-95. doi: 10.1038/eye.2016.86. Epub 2016 May 13 [PubMed PMID: 27174381]
Buffault J, Brignole-Baudouin F, Reboussin É, Kessal K, Labbé A, Mélik Parsadaniantz S, Baudouin C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. Journal of clinical medicine. 2022 Feb 15:11(4):. doi: 10.3390/jcm11041001. Epub 2022 Feb 15 [PubMed PMID: 35207274]
Walters TR, Kothe AC, Boyer JL, Usner DW, Lopez K, Duquesroix B, Fechtner RD, Navratil T. A Randomized, Controlled Comparison of NCX 470 (0.021%, 0.042%, and 0.065%) and Latanoprost 0.005% in Patients With Open-angle Glaucoma or Ocular Hypertension: The Dolomites Study. Journal of glaucoma. 2022 Jun 1:31(6):382-391. doi: 10.1097/IJG.0000000000002030. Epub 2022 Apr 6 [PubMed PMID: 35394456]
Level 1 (high-level) evidenceZhou R, Sun Y, Chen H, Sha S, He M, Wang W. Laser Trabeculoplasty for Open-Angle Glaucoma: A Systematic Review and Network Meta-Analysis. American journal of ophthalmology. 2021 Sep:229():301-313. doi: 10.1016/j.ajo.2020.07.046. Epub 2020 Sep 2 [PubMed PMID: 32888900]
Level 1 (high-level) evidenceMuralidharan S, Kumar S, Ichhpujani P, Dhillon HK. Quality of life in glaucoma patients: Comparison of medical therapy, trabeculectomy, and glaucoma drainage device surgery. Indian journal of ophthalmology. 2022 Dec:70(12):4206-4211. doi: 10.4103/ijo.IJO_667_22. Epub [PubMed PMID: 36453315]
Level 2 (mid-level) evidenceBrusini P, Papa V, Zeppieri M. Canaloplasty in Pseudoexfoliation Glaucoma. Can It Still Be Considered a Good Choice? Journal of clinical medicine. 2022 Apr 30:11(9):. doi: 10.3390/jcm11092532. Epub 2022 Apr 30 [PubMed PMID: 35566656]
Rojananuangnit K, Jiaranaisilawong P, Rattanaphaithun O, Sathim W. Surgical Outcomes of Glaucoma Drainage Device Implantation in Refractory Glaucoma Patients in Thailand. Clinical ophthalmology (Auckland, N.Z.). 2022:16():4163-4178. doi: 10.2147/OPTH.S393730. Epub 2022 Dec 14 [PubMed PMID: 36540897]
Gurnani B, Tripathy K. Minimally Invasive Glaucoma Surgery. StatPearls. 2025 Jan:(): [PubMed PMID: 35881761]
Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. Journal of cataract and refractive surgery. 2012 Aug:38(8):1339-45. doi: 10.1016/j.jcrs.2012.03.025. Epub [PubMed PMID: 22814041]
Level 1 (high-level) evidenceAnderson DR, Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Current opinion in ophthalmology. 2003 Apr:14(2):86-90 [PubMed PMID: 12698048]
Level 3 (low-level) evidenceWright C, Tawfik MA, Waisbourd M, Katz LJ. Primary angle-closure glaucoma: an update. Acta ophthalmologica. 2016 May:94(3):217-25. doi: 10.1111/aos.12784. Epub 2015 Jun 27 [PubMed PMID: 26119516]
Bai HQ, Yao L, Wang DB, Jin R, Wang YX. Causes and treatments of traumatic secondary glaucoma. European journal of ophthalmology. 2009 Mar-Apr:19(2):201-6 [PubMed PMID: 19253235]
Musa MJ, Zeppieri M. Foster Kennedy Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 35881754]
Leibowitz HM. The red eye. The New England journal of medicine. 2000 Aug 3:343(5):345-51 [PubMed PMID: 10922425]
Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Seminars in ophthalmology. 1999 Jun:14(2):95-108 [PubMed PMID: 10758217]
Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Current opinion in ophthalmology. 2011 Mar:22(2):124-32. doi: 10.1097/ICU.0b013e328343c1a3. Epub [PubMed PMID: 21307679]
Level 3 (low-level) evidenceHarwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Jun:124(6):853-9 [PubMed PMID: 16769839]
Level 3 (low-level) evidencePelčić G, Ljubičić R, Barać J, Biuk D, Rogoić V. Glaucoma, depression and quality of life: multiple comorbidities, multiple assessments and multidisciplinary plan treatment. Psychiatria Danubina. 2017 Sep:29(3):351-359. doi: 10.24869/psyd.2017.351. Epub [PubMed PMID: 28949316]
Level 2 (mid-level) evidence