Definition/Introduction
The Ghon complex is named after Anton Ghon (1866-1936), an Austrian pathologist who described primary tuberculosis caused by Mycobacterium tuberculosis as having a pulmonary lesion with regional lymph involvement.[1] Chest radiography in patients with primary pulmonary tuberculosis (TB) commonly reveals one of the following findings: parenchymal disease, lymphadenopathy, pleural effusion, or miliary tuberculosis; these findings may also present in combination.[2] The hallmark of primary parenchymal disease is a pulmonary lesion, also known as a Ghon lesion or Ghon focus, and affected draining lymphadenopathy forming the Ghon complex.[3]
The Ghon complex is a non-pathognomonic finding on chest radiography that is significant for pulmonary tuberculosis.[1] The location of the Ghon complex is usually subpleural and predominantly in the upper part of the lower lobe or lower part of the middle or upper lobe.[2] However, the Ghon complex can be found in any part of the lung.[1] It should also be noted that a single patient may have multiple Ghon complexes. The original work described the autopsy findings of patients with primary pulmonary tuberculosis; a single complex was seen in 72.4%, and two complexes were noted in 14.7%; at the other end of the spectrum, 3.5% of patients had five or more Ghon complexes.[1]
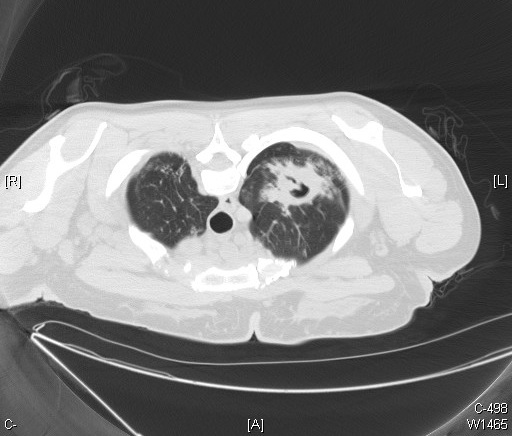
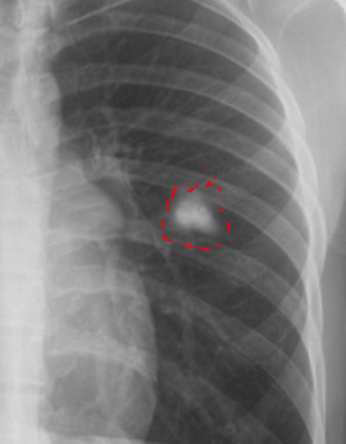
The most frequently encountered route of infection with M. tuberculosis is through the respiratory tract. The predilection of M. tuberculosis for the upper lobes is possibly due to the ventilatory physiology of this region. [4] Radiographic findings of the Ghon complex may include parenchymal scarring of the lung tissue, lesion cavitation, or lobar consolidation, as seen in Figure 1, Figure 2, and Figure 3, respectively.
The Ghon complex should not be confused with the Ranke complex. The Ghon complex precedes the development of the Ranke complex. The Ghon complex is seen in untreated primary pulmonary tuberculosis infection with Ghon lesion fibrosis. The Ranke complex results from a primary tuberculosis Ghon complex undergoing calcification during the inflammatory process and is characterized by a calcified Ghon lesion and calcified mediastinal lymph nodes.[5]
After primary infection, M. tuberculosis can progress in one of three ways. The primary infection can be eradicated from the body by the immune system. The infection may persist in a dormant, controlled state, sometimes called latent tuberculosis; latency may last indefinitely. The primary infection may bypass latency and progress to cause symptomatic disease, sometimes referred to as active tuberculosis.[4]
Patients with latent tuberculosis are at risk of reactivation to active tuberculosis; this risk is highly dependent on the immune status of the host.[2][4] In the developing world, human immunodeficiency virus (HIV) infection plays a large role in the progression of tuberculosis via increasing immunosuppression as the infection continues without effective antiretroviral therapy.[6] Additionally, tuberculosis infection increases the levels of HIV replication.[6] Therefore, co-infection worsens both infections.
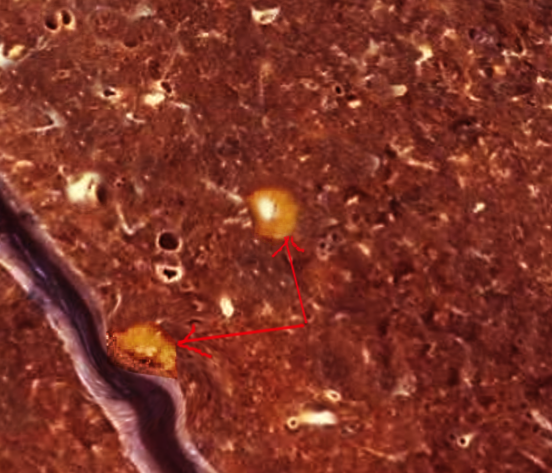
On pathological examination, the Ghon complex usually measures approximately 1.0 to 1.5 centimeters in area.[4] A fibroblastic rim surrounds caseous necrosis.[4] Histological examination reveals granulomas with surrounding macrophages and Langerhans giant cells.[4]
Cavitation of Ghon complexes occurs with greater frequency in children when compared with adults, and the presence of these lesions correlates with positive gastric aspirates and sputum cultures in children.[1] It is thought that the propensity of the Ghon complex to cavitate may also be responsible for the increased incidence of extrapulmonary tuberculosis in children.[1]
The Ghon complex is not unique to pulmonary tuberculosis and can also be seen in other infections, such as pulmonary histoplasmosis and paracoccidioidomycosis.[2][7] Therefore, it is important to consider the patient's travel, occupational, and exposure history when encountering the Ghon complex.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
In 2016, TB was estimated to be responsible for 1.3 million deaths among HIV-negative individuals and remained one of the leading infectious causes of death globally.[8] Approximately one-quarter of the global population has latent tuberculosis.[9] There is a higher prevalence of pulmonary TB among individuals in developing nations of the eastern hemisphere due to disparities in access to healthcare for diagnostic evaluation and access to medications.[10] Although less common in the western hemisphere, pulmonary TB continues to affect individuals in developed countries, specifically patients living with HIV and acquired immune deficiency syndrome (AIDS) as an opportunistic infection, those with chronic kidney disease, exposure to silica dust without silicosis, treatment with immunosuppressive medications (such as tumor necrosis factor-alpha inhibitors), and patients with tobacco, alcohol, or drug use disorders.[8]
Approximately 1.7 billion people are estimated to have latent pulmonary TB, but only 5% to 15% of these individuals may develop active TB.[8][11] In 2015, the World Health Organization (WHO) set out to end the global burden of TB-related deaths.[12] The WHO’s “End TB Strategy” aims to reduce the incidence and death rate of TB by 90% and 95%, respectively.[12] This plan aligns with reducing the global burden of TB to the burden of incidence in developed countries today by 2035.[12] Unfortunately, in 2020 the number of estimated deaths secondary to TB was noted to increase for the first time in nine years.[13] The COVID-19 pandemic is thought to have resulted in this setback.[13] Half of the people estimated to be infected with TB were not tested or treated.[13]
Imaging interpretation can be challenging, as tuberculosis can mimic many conditions, including malignancy and sarcoidosis.[5] Computers have been used to aid in detecting tuberculosis on chest radiography in specific settings, and more research is needed in this area.[14]
Clinical Significance
The diagnosis of primary pulmonary tuberculosis heavily relies on the culture of M. tuberculosis from the sputum,[15] nasopharyngeal aspirate,[16] gastric aspirate,[17] pleural fluid,[18] or pleural biopsy.[18] Nucleic acid amplification tests (such as the Xpert MTB/RIF assay), serological tests, and chest radiography support the diagnosis of pulmonary TB.[19] The Ghon complex may be absent in patients with primary pulmonary TB.[20] Researchers estimate that in about 15% of cases of primary TB, a Ghon focus may develop, while 9% of cases of primary TB may develop tuberculomas (Figure 4), and lymphadenopathy presents in children and adults in only 96% and 43% of cases, respectively.[20]
In an estimated two-thirds of cases of primary TB, a parenchymal focus resolves independently. Overall, about 15% of patients with primary TB may present with normal chest radiography.[20][21] Due to the ability of M. tuberculosis to spread from the respiratory tract to distant parts of the body by lymphatic and hematogenous routes, untreated primary pulmonary TB can result in extrapulmonary infection of the pericardium, myocardium, central nervous system, head and neck, intra-abdominal organs, peritoneum, and the genitourinary tract or present as spondylitis, osteomyelitis, tuberculous arthritis.[22] Pulmonary tuberculosis is a curable disease that requires extended use of antibiotics. Multiple drug-resistant (MDR) or extensively drug-resistant (XDR) tuberculosis is a growing concern and requires careful management.[23][24]
The Ghon complex highlights the role of lymphatics in disseminating M. tuberculosis. Lymph nodes are the site of antigen presentation but also play a role in the persistence of M. tuberculosis infections.[1] Additionally, macrophages may disseminate the organism to the bloodstream and enable the development of extrapulmonary tuberculosis.[1] Recent research continually highlights the role of lymphatics in the pathogenesis of this disease.[1]
Nursing, Allied Health, and Interprofessional Team Interventions
The recognition of radiologic signs, such as the Ghon complex, can be the first alert that pulmonary tuberculosis is present; radiologists play a critical role in the diagnosis of pulmonary tuberculosis. In addition, alerting other healthcare team members to the presence of a Ghon focus allows for initiating treatment and enacting isolation precautions to protect hospital workers and other patients from potential exposure. However, radiologists are often not the first clinicians that evaluate the radiograph. Therefore, the ability of clinicians to recognize the radiographic signs of possible tubercular infection helps to ensure patient safety. Additionally, members of the infection prevention teams coordinate with treating providers to ensure that patients with potentially active tuberculosis are placed under appropriate isolation precautions to halt transmission.
Healthcare workers are at a ten times higher risk of becoming infected by M. tuberculosis when compared to the general population.[25][26] Respiratory droplets transmit pulmonary TB from an infected host with active TB. M. tuberculosis aerosolizes into the air around an infected patient with regular respiratory activity, but coughing can connote greater infectivity.[27] These aerosolized particles remain suspended in the air for an extended period. Clinicians, nurses, and auxiliary staff must practice the necessary precautions when caring for and treating patients with pulmonary TB. In the acute care setting, nurses spend more time interacting with patients infected with primary TB when compared to other clinicians.
Nurses should know the signs and symptoms of active TB to minimize their risk of exposure to infected patients. The signs and symptoms of active TB include but are not limited to cough, hemoptysis, unintentional weight loss, fever and chills, night sweats, loss of appetite, and fatigue.[28] Clinical histories, such as travel to an endemic area, history of incarceration, intravenous drug use, housing instability, living in crowded conditions such as shelters, exposure to someone with diagnosed tuberculosis, or history of untreated latent tuberculosis, can help assess risk.[29]
After identifying a patient with potentially active TB, nurses and healthcare workers should follow their healthcare facilities' protocols for minimizing their occupational exposure to TB and place patients in airborne precautions to protect other hospitalized patients.[26] Where available, such as in the middle to high-income areas of the world, healthcare facilities should provide personal protective equipment such as N-95 masks or CAPRs (controlled air-purifying respirators).
In addition to minimizing the healthcare workers' risk of exposure to TB, the risk of TB exposure to the inpatient population should be minimized by using airborne isolation rooms equipped with negative pressure air circulation; this limits exposure to contaminated room air to those outside of the patient isolation area.[26] Nurses, pathologists, pulmonologists, and laboratory workers are at the highest risk of nosocomial TB infection.[26] Qualitative studies have described that nursing interventions enhance patient adherence to treatment and community-based treatment of MDR TB.[30][31]
Nursing, Allied Health, and Interprofessional Team Monitoring
A diagnosis of tuberculosis requires long-term follow-up.[14] An infectious disease physician should monitor these patients for medication compliance and clinical improvement. Due to the highly infectious nature of active pulmonary tuberculosis, patients with this condition are also often followed by government agencies to protect the health of the public by ensuring adherence to isolation protocols and treatment. Directly observed therapy (DOT) is often utilized to ensure that patients with active tuberculosis can access and adhere to treatment.[32] Steady adherence to treatment also decreases the risk of developing antimicrobial resistance.[32] However, this disease affects many people, often in low-resource countries, so this modality is not always feasible or possible. An entire course of treatment is at least six to nine months, and the radiological improvements often lag behind the clinical symptoms.[33]
Media
(Click Image to Enlarge)
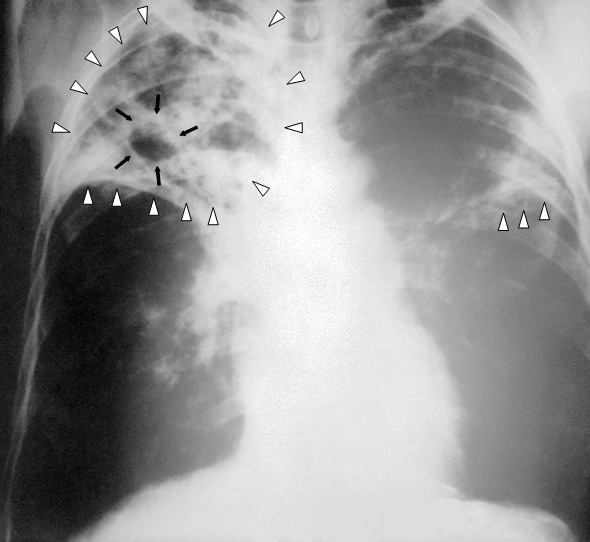
Pulmonary Tuberculosis. This image shows an anteroposterior chest x-ray of a patient diagnosed with advanced bilateral pulmonary tuberculosis. The x-ray reveals the presence of bilateral pulmonary infiltrate (white triangles), and "caving formation" (black arrows) present in the right apical region. The findings suggest far-advanced tuberculosis.
Centers for Disease Control and Prevention
(Click Image to Enlarge)
References
Donald PR, Diacon AH, Thee S. Anton Ghon and His Colleagues and Their Studies of the Primary Focus and Complex of Tuberculosis Infection and Their Relevance for the Twenty-First Century. Respiration; international review of thoracic diseases. 2021:100(7):557-567. doi: 10.1159/000509522. Epub 2020 Dec 15 [PubMed PMID: 33321506]
Lyon SM, Rossman MD. Pulmonary Tuberculosis. Microbiology spectrum. 2017 Jan:5(1):. doi: 10.1128/microbiolspec.TNMI7-0032-2016. Epub [PubMed PMID: 28185620]
Concepcion NDP, Laya BF, Andronikou S, Daltro PAN, Sanchez MO, Uy JAU, Lim TRU. Standardized radiographic interpretation of thoracic tuberculosis in children. Pediatric radiology. 2017 Sep:47(10):1237-1248. doi: 10.1007/s00247-017-3868-z. Epub 2017 Aug 29 [PubMed PMID: 29052771]
Stephenson L, Byard RW. An atlas overview of characteristic features of tuberculosis that may be encountered at autopsy. Forensic science, medicine, and pathology. 2020 Mar:16(1):143-151. doi: 10.1007/s12024-019-00161-y. Epub 2019 Aug 30 [PubMed PMID: 31471869]
Level 3 (low-level) evidenceSkoura E, Zumla A, Bomanji J. Imaging in tuberculosis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2015 Mar:32():87-93. doi: 10.1016/j.ijid.2014.12.007. Epub [PubMed PMID: 25809762]
Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nature reviews. Microbiology. 2018 Feb:16(2):80-90. doi: 10.1038/nrmicro.2017.128. Epub 2017 Nov 7 [PubMed PMID: 29109555]
de Campos EP, Bertoli CJ, Barbosa KS. [Pulmonary lymph node in acute juvenile paracoccidioidomycosis (a case report)]. Revista da Sociedade Brasileira de Medicina Tropical. 1992 Jul-Sep:25(3):195-200 [PubMed PMID: 1308953]
Level 3 (low-level) evidenceGlaziou P, Floyd K, Raviglione MC. Global Epidemiology of Tuberculosis. Seminars in respiratory and critical care medicine. 2018 Jun:39(3):271-285. doi: 10.1055/s-0038-1651492. Epub 2018 Aug 2 [PubMed PMID: 30071543]
Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The Diagnosis and Treatment of Tuberculosis. Deutsches Arzteblatt international. 2019 Oct 25:116(43):729-735. doi: 10.3238/arztebl.2019.0729. Epub [PubMed PMID: 31755407]
Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. The Lancet. Respiratory medicine. 2018 Apr:6(4):299-314. doi: 10.1016/S2213-2600(18)30057-2. Epub [PubMed PMID: 29595511]
Level 3 (low-level) evidenceVynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. American journal of epidemiology. 2000 Aug 1:152(3):247-63 [PubMed PMID: 10933272]
Lönnroth K, Raviglione M. The WHO's new End TB Strategy in the post-2015 era of the Sustainable Development Goals. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2016 Mar:110(3):148-50. doi: 10.1093/trstmh/trv108. Epub [PubMed PMID: 26884490]
Chakaya J, Petersen E, Nantanda R, Mungai BN, Migliori GB, Amanullah F, Lungu P, Ntoumi F, Kumarasamy N, Maeurer M, Zumla A. The WHO Global Tuberculosis 2021 Report - not so good news and turning the tide back to End TB. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2022 Nov:124 Suppl 1():S26-S29. doi: 10.1016/j.ijid.2022.03.011. Epub 2022 Mar 20 [PubMed PMID: 35321845]
Furin J, Cox H, Pai M. Tuberculosis. Lancet (London, England). 2019 Apr 20:393(10181):1642-1656. doi: 10.1016/S0140-6736(19)30308-3. Epub 2019 Mar 20 [PubMed PMID: 30904262]
Datta S, Shah L, Gilman RH, Evans CA. Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. The Lancet. Global health. 2017 Aug:5(8):e760-e771. doi: 10.1016/S2214-109X(17)30201-2. Epub 2017 Jun 15 [PubMed PMID: 28625793]
Level 1 (high-level) evidenceNicol MP, Zar HJ. Advances in the diagnosis of pulmonary tuberculosis in children. Paediatric respiratory reviews. 2020 Nov:36():52-56. doi: 10.1016/j.prrv.2020.05.003. Epub 2020 May 25 [PubMed PMID: 32624357]
Level 3 (low-level) evidenceStockdale AJ, Duke T, Graham S, Kelly J. Evidence behind the WHO guidelines: hospital care for children: what is the diagnostic accuracy of gastric aspiration for the diagnosis of tuberculosis in children? Journal of tropical pediatrics. 2010 Oct:56(5):291-8. doi: 10.1093/tropej/fmq081. Epub 2010 Sep 3 [PubMed PMID: 20817689]
Shaw JA, Irusen EM, Diacon AH, Koegelenberg CF. Pleural tuberculosis: A concise clinical review. The clinical respiratory journal. 2018 May:12(5):1779-1786. doi: 10.1111/crj.12900. Epub [PubMed PMID: 29660258]
Schito M, Migliori GB, Fletcher HA, McNerney R, Centis R, D'Ambrosio L, Bates M, Kibiki G, Kapata N, Corrah T, Bomanji J, Vilaplana C, Johnson D, Mwaba P, Maeurer M, Zumla A. Perspectives on Advances in Tuberculosis Diagnostics, Drugs, and Vaccines. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Oct 15:61Suppl 3(Suppl 3):S102-18. doi: 10.1093/cid/civ609. Epub [PubMed PMID: 26409271]
Level 3 (low-level) evidenceBurrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007 Sep-Oct:27(5):1255-73 [PubMed PMID: 17848689]
Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR. American journal of roentgenology. 1986 Mar:146(3):497-506 [PubMed PMID: 3484866]
Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Nov-Dec:39(7):2023-2037. doi: 10.1148/rg.2019190109. Epub [PubMed PMID: 31697616]
Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, Boulabhal F, Paramasivan CN, Kam KM, Mitarai S, Nunn P, Raviglione M, Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet (London, England). 2009 May 30:373(9678):1861-73. doi: 10.1016/S0140-6736(09)60331-7. Epub 2009 Apr 15 [PubMed PMID: 19375159]
Caminero JA, Cayla JA, García-García JM, García-Pérez FJ, Palacios JJ, Ruiz-Manzano J. Diagnosis and Treatment of Drug-Resistant Tuberculosis. Archivos de bronconeumologia. 2017 Sep:53(9):501-509. doi: 10.1016/j.arbres.2017.02.006. Epub 2017 Mar 27 [PubMed PMID: 28359606]
Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS medicine. 2006 Dec:3(12):e494 [PubMed PMID: 17194191]
Level 1 (high-level) evidenceMcGowan JE Jr. Nosocomial tuberculosis: new progress in control and prevention. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1995 Sep:21(3):489-505 [PubMed PMID: 8527533]
Patterson B, Wood R. Is cough really necessary for TB transmission? Tuberculosis (Edinburgh, Scotland). 2019 Jul:117():31-35. doi: 10.1016/j.tube.2019.05.003. Epub 2019 May 28 [PubMed PMID: 31378265]
Jilani TN, Avula A, Zafar Gondal A, Siddiqui AH. Active Tuberculosis. StatPearls. 2024 Jan:(): [PubMed PMID: 30020618]
Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Social science & medicine (1982). 2009 Jun:68(12):2240-6. doi: 10.1016/j.socscimed.2009.03.041. Epub 2009 Apr 23 [PubMed PMID: 19394122]
Carlsson M, Johansson S, Eale RP, Kaboru BB. Nurses' Roles and Experiences with Enhancing Adherence to Tuberculosis Treatment among Patients in Burundi: A Qualitative Study. Tuberculosis research and treatment. 2014:2014():984218. doi: 10.1155/2014/984218. Epub 2014 Aug 19 [PubMed PMID: 25215232]
Level 2 (mid-level) evidencePalacios E, Guerra D, Llaro K, Chalco K, Sapag R, Furin J. The role of the nurse in the community-based treatment of multidrug-resistant tuberculosis (MDR-TB). The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2003 Apr:7(4):343-6 [PubMed PMID: 12729339]
Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. The Cochrane database of systematic reviews. 2015 May 29:2015(5):CD003343. doi: 10.1002/14651858.CD003343.pub4. Epub 2015 May 29 [PubMed PMID: 26022367]
Level 1 (high-level) evidenceNahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Oct 1:63(7):853-67. doi: 10.1093/cid/ciw566. Epub [PubMed PMID: 27621353]
Level 1 (high-level) evidence