Introduction
Gastric atrophy (GA) and intestinal metaplasia of the gastric mucosa (GIM) are collectively known as chronic atrophic gastritis (CAG). These early conditions can lead to the development of gastric adenocarcinoma (GC). This review focuses on the current evidence and guidelines in diagnosis, management, and surveillance of chronic atrophic gastritis to identify those at risk of progression to gastric adenocarcinoma.[1]
Chronic atrophic gastritis is considered a precursor lesion for gastric cancer, which is the fifth most common cancer globally and carries third-highest cancer-related mortality in the world. This aggressive cancer presents late in most countries with no screening program and leads to numerous deaths due to late diagnosis.
The common etiologies of this premalignant lesion are Helicobacter pylori (H. pylori) and autoimmune gastritis. Chronic inflammation leads to the loss of gastric mucosa leading to an acid depleted environment hypothesized as an early precursor to distal gastric cancer.[2]
H. pylori is a microaerophilic gram-negative bacterial pathogen. Its role has been implicated in not only atrophic gastritis but also peptic ulcers, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (MALT). Identification and eradication of the pathogen have a significant role in reducing the risk of CAG.[3][4]
It is of utmost importance to identify the precancerous lesions by identifying those at risk. It is also crucial to follow-up with surveillance endoscopy and, if needed, endoscopically intervening to avoid major resection surgery in advanced stage gastric cancer.
The popular Correa Cascade suggested the linear progression from chronic atrophic gastritis (CAG) with metaplastic intestinal epithelium to low-grade dysplasia (LGD), high-grade dysplasia (HGD), and eventually gastric adenocarcinoma.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Although the etiology of atrophic gastritis is still being debated, we know H. pylori bacteria are the main culprit for chronic atrophic gastritis. Over time, this can lead to progressive loss and destruction of gastric glands described as multifocal atrophic gastritis. The prevalence of the infection is almost 50% of the world population. H. pylori were initially found to be the cause of gastric and duodenal ulcers in the early 1980s. The difficulty arises in the management of individuals infected with the pathogen that can then adversely affect the management of esophageal disease.[3][5]
Autoimmune gastritis is due to the destruction of oxyntic mucosa cells from autoantibodies against the H+/K+ ATPase antigen protein resulting in mucosal atrophy. This autoimmune reaction predominantly attacks the body and fundus of the stomach where the oxyntic cells are located (parietal and chief cells), sparing the antrum.[6][7]
Epidemiology
Chronic atrophic gastritis is more prevalent in the older population, although it is variable in different regions of the world. The difficulty arises due to the asymptomatic nature of the condition in most individuals.[8]
Age
In a population-based cohort study in Germany diagnosed on the serological assessment of pepsinogen 1 and H. pylori testing, the prevalence increased from 4.8% in the 50 to 54 age group to 8.7% in the 70 to 74 age group. This difference was observed to be higher in Japan, rising from 2.7 to 9.1%.[9][10]
Autoimmune atrophic gastritis is prevalent in the older women of Northern European and Scandinavian origin but has now been found to exist in every ethnicity, region, and age.[11]
Age and Helicobacter Pylori
There is a higher prevalence of CAG in older individuals with H. pylori infection and slightly more common in men. Prevalence was observed in serological pepsinogen and endoscopic biopsy testing. In higher incidence areas like Japan and China, this varies to as high as 33% to 84%.[12][9]
Overall there is a wide variation in the risk of gastric adenocarcinoma developing in individuals with premalignant conditions like CAG (GA/IM) with a <1% annual incidence (person-year) irrespective of whether the individual is from a low risk or high-risk group.[13]
There are two different types of malignancies associated with CAG, gastric adenocarcinoma linked to H pylori infection and a neuroendocrine gastrin dependent tumor (type 1 gastric carcinoid) secondary to hyperplasia of the enterochromaffin-like cells.[2]
Atrophic Gastritis and Cancer Risk
Endoscopic surveillance was performed in another cohort study for individuals with H pylori AG to identify the risks of gastric cancer. The mean duration of follow-up endoscopy was 6.2 +/- 4.8 years. The cumulative 5-year incidence of gastric adenocarcinoma ranged between 0.7% in no or mild AG to 10% in severe AG.[14][15]
Pathophysiology
The risk of gastric cancer in individuals is related to a combination of three different sets of etiologies: the primary H. pylori infection and the host’s ability of an immune response, the host’s susceptibility to atrophic gastritis from chronic inflammation, and environmental factors like cigarette smoking and high salt intake.
H. pylori may cause non-atrophic gastritis (NAG) initially that may be susceptible to cure by clearing the infection; however, severe NAG might lead to atrophy of the mucosa and pseudopyloric metaplasia. At this stage, progression to atrophy depends on the virulence of the bacteria like the presence of cag-positive vacA s1m1 strains vs. cag-negative vacA s2m2 strains in non-atrophic gastritis (NAG).[16]
Multifocal Atrophic Gastritis (MAG) or Type B Gastritis
A prolonged inflammatory process, mostly due to H. pylori, leads to a gradual loss of glandular oxyntic mucosa. This starts in the body and antrum and then spreads throughout the stomach, and it is called multifocal atrophic gastritis (MAG). The reducing levels of pepsinogen 1 are an indicator of the degree of atrophy of the oxyntic cells. Pepsinogen 2 levels rise due to their contrasting stimulation by the inflammation by H. pylori and hyperplasia of the foveolar cells. Pepsinogen levels and pepsinogen 1/pepsinogen 2 ratio can predict extensive atrophic gastritis.[17]
Autoimmune Metaplastic Atrophic Gastritis (AMAG) or Type A Gastritis
This elusive syndrome is uncommon and may also have concomitant H pylori infection. It is largely asymptomatic in the early stages. It involves severe diffuse atrophy of the oxyntic cells (parietal cells) in the body and fundus but not in the antrum due to an autoimmune reaction from anti-parietal cell antibodies. This causes hypochlorhydria raising the pH in the stomach. The intrinsic factor, which is essential for vitamin B12 absorption, is secreted from the parietal cells. Lack of intrinsic factor leads to pernicious anemia, the hematological manifestation of this autoimmune process.[18][6][19]
Intestinal Metaplasia
The lost gastric epithelial mucosa is eventually replaced by intestinal-type mucosa, which may be small intestinal or colonic type depending on the morphology and immunohistochemistry. There may be small intestinal goblet cells amongst enterocytes and may have microvilli giving a ‘brush border’ appearance.[20]
The colonic type of intestinal metaplasia shows a large bowel phenotype and mucin expression. The digestive enzymes are not detectable all the time and hence are termed ‘incomplete.’ This mucosa does not have microvilli and has a variety of mucin cells. Incomplete mucosa and a large area of involvement of this type of intestinal metaplasia carry a higher risk of being pre-malignant.[10]
Histopathology
H. pylori are the most frequent cause of gastritis with the infiltration of polymorphonuclear neutrophils and mononuclear leukocytes in the mucosa and lamina propria. The severity of the inflammation depends on the strain of H. pylori, and marked acute inflammation can predispose to microabscesses. In chronic gastritis, there is an accumulation of mononuclear leukocytes in the lamina propria and the presence of lymphoid aggregates with germinal centers.[21]
The histological features include pallor, loss of gastric folds, prominent vessels, and an atrophic border (demarcation of pale atrophy where normal pink border ends).[22]
It is suggested that the extent of the atrophic border with the normal pink mucosa is directly related to the prediction of CAG progression to GC risk. In certain circumstances, corpus biopsies can provide valuable information as prolonged proton pump inhibitor use can have almost no infiltration of H. pylori, and the oxyntic mucosa affected is more proximal to the antrum.[23]
On standard white light endoscopy, IM appears as small grey-white slightly elevated plaques with surrounded uneven patchy pale and pink mucosa.[24] When IM spreads, straight glands become elongated and in a groove pattern like those in the antrum.[25]
Metaplasia is defined as the replacement of the differentiated mucosa type into another differentiated cell type, not normal for the organ. Intestinal metaplasia involves the replacement of gastric mucosa with intestinal mucosa characteristically, goblets cells, enterocytes, and colonocytes.[26]
History and Physical
The classical presentation would suggest individuals presenting with epigastric pain, nausea, and occasionally vomiting; however, this is rare. The most common presentation is anemia. This can manifest as generalized weakness, tiredness, headaches, and palpitations.[27]
We know that most of the individuals with H. pylori-induced multifocal atrophic gastritis are asymptomatic. The symptoms, if present, like nausea and vomiting, are short-lived and recurrent.[28]
Similarly, autoimmune metaplastic atrophic gastritis usually has nonspecific signs and symptoms in mostly females over 60 years of age.[29]
Pernicious anemia can develop in AMAG with an overall prevalence of 0.1%. It can present with fatigue, dizziness, irritability, depression, insomnia, and mood swings. Occasionally, it can present with normal vitamin B12 levels.[30]
Most individuals are already treated for their iron, folate, or vitamin B12 deficiency without a confirmed underlying diagnosis.[11]
The presentation can be variable and can lead to diagnostic difficulty when individuals have associated autoimmune conditions such as hypothyroidism, myelodysplastic syndromes, sideroblastic anemia, or coexisting iron deficiency anemia and thalassemia.[30]
The physical examination is of limited use and is usually nonspecific with rare evidence of pallor, anemia, and emaciation due to poor appetite, but these are uncommon.
In AMAG, there may be stigmata of associated autoimmune disease with jaundice, tachycardia, and a systolic murmur.[30]
Evaluation
It is evident from all guidelines worldwide that chronic atrophic gastritis or intestinal metaplasia patients are at risk of gastric adenocarcinoma. The aim is to identify advanced stages of gastritis with atrophy and intestinal metaplasia in the antrum and the corpus mucosa to prevent progression to high-grade dysplasia and invasive carcinoma. High-quality endoscopy is the gold standard.
Pepsinogen Levels
Low pepsinogen 1 levels and a low pepsinogen 1/pepsinogen 2 ratio can predict advanced atrophic gastritis and should be followed by endoscopy. It was concluded that a combination of pepsinogen, H. pylori status, and gastrin-17 levels is a good diagnostic test for the diagnosis of atrophic gastritis in populations at high risk of GC.[31]
In a meta-analysis of a study population of more than 32000 individuals to identify individuals at risk of GC and CAG, the risk of development of GC from a positive pepsinogen test
A value of .3+ had a sensitivity of 57% and specificity of 76%.[32]
Endoscopy
The recent update in guidance on the investigation and management of gastric precancerous lesions (GPL) relate to publications from the European Society of Gastroenterological Endoscopy in association with European Societies (management of epithelial precancerous conditions and lesions in the stomach (MAPS II) and the American Gastroenterological Association (AGA).[17]
The European and British guidelines focus on the diagnosis, treatment, and surveillance of atrophic gastritis, IM, and dysplasia, whereas the American guidelines focus on the management of individuals with IM.
First-time biopsies should be followed by mapping biopsies according to the Sydney protocol for gastritis from at least two topographic sites, antrum x2 (A1 lesser curve of the antrum & A2 greater curve of the antrum), incisura angularis (A3), anterior and posterior corpus x2 and for H. pylori. This is because of the irregular spread of gastritis. Also, any morphologically abnormal lesion like an ulcer, raised surface, or polyp adjacent to the atrophic areas should be biopsied separately and sent for histological assessment in a separate vial.[33]
Endoscopy Techniques
Conventional chromoendoscopy (CE) with the application of dyes like indigo carmine, methylene blue, acetic acid, and hematoxylin is highly accurate in the detection of preneoplastic gastric lesions.[34]
High definition chromo-endoscopy is better than high definition white light endoscopy for the diagnosis and the mapping of the extent of gastric precancerous lesions (GPLs).
White light endoscopy (WLE) is not good enough, but HD WLE has shown promising results but not reliable enough to offer as protocol.[35]
Narrowband imaging is shown to be more sensitive for detecting AG and IM compared to HD white light endoscopy and also the better visual diagnostic ability for detecting early cancer. Some centers are using a combined HD WLE with narrowband imaging to achieve a better yield of targeted biopsies.[36][37]
The Updated Sydney protocol showed the incisura angularis region has the highest incidence and severity of IM. Incisura increased the high-risk stages of OLGA/OLGIM stages. Incisura and more extensive biopsies could be useful where CE is not available.[38][39]
Low-Grade and High-Grade Dysplasia
High-quality chromo-endoscopy is recommended if biopsies show low-grade or high-grade dysplasia. The individual may need intensive surveillance endoscopy or referral for endoscopic resection.[17]
It is important to note that the OLGA system evaluates the whole spectrum from gastric atrophy, fibrosis, pseudo-pyloric metaplasia, and intestinal metaplasia in contrast to the OLGIM system that only evaluates IM.
OLGA and OLGIM both advise testing for and treating H. pylori in patients with GPL. This also applies after the endoscopic excision of GPLs. Both assessment systems also agree IM, whether it is extensive/ colonic (incomplete) type or IM with a family history of GC, are considered high risk for GC. In both assessment techniques, IM subtyping is also not essential but would be useful for surveillance.
European guidelines advise short interval repeat endoscopy with Sydney Protocol of biopsies in those accidentally found to have CAG. In contrast, the American Gastroenterological Association (AGA) does not recommend a short interval endoscopy. This should be high-quality guide-targeted biopsies and virtual chromo-endoscopy.[40]
The guidance by the AGA is based on shared decision making between clinician and patient for routine surveillance endoscopy of 'high risk for GC' patients and takes into consideration race/ethnicity as a risk factor. The rationale by AGA is that none of the randomized controlled trials suggest endoscopic surveillance reduces the risk of GC in individuals with GPL. The rationale of European Societies is that there is sufficient data from observational cohort studies to justify more stringent surveillance.[41][42]
Treatment / Management
Helicobacter Pylori Eradication
There have been numerous studies assessing the role of eradication therapy on chronic atrophic and non-atrophic gastritis and gastric cancer. In one meta-analysis, eradication therapy significantly improved the histological (OLGA/OLGIM) grading of gastric atrophy in the corpus and antrum and intestinal metaplasia in the antrum (but not corpus).[43](A1)
There may be a significant improvement in the risk of developing intestinal GC by H. pylori eradication. In two other meta-analyses, H. pylori eradication significantly reduced the risk of GC but not chronic atrophic gastritis and IM.[44][45] H. pylori eradication therapy is recommended in individuals endoscopically treated for superficial gastric neoplasia. This reduces the metachronous recurrence of GC by almost half.[4](A1)
In summary, H. pylori eradication has an essential role in histological downstaging and may have a role in the reduction of progression to GC.
COX Inhibitors
Even though cyclo-oxygenase inhibitors (COX-1 or COX-2) have been shown to slow down the progression of precancerous gastric conditions, and in theory, reduce the risk of GC, there is not enough statistical evidence from trials to recommend them for prevention.[46](A1)
Non-steroidal Anti-inflammatory Drugs
There is some evidence of the beneficial effect of NSAIDs and aspirin in slowing down the progression of gastric precancerous lesions after H. pylori eradiation from previous trials. Still, the effect is small to recommend it as a guideline. Further research is required to study the effects of NSAIDs on GPLs. Low dose aspirin may be safer to use and provide other systemic benefits, for example, in cardiovascular disease, but any benefit should be reviewed against the known gastrointestinal effects.[47](A1)
Other Drugs
Moluodan, a Chinese herbal medicine, reported in an RCT in individuals with dysplasia downgraded to non-dysplastic tissue by 24.6% on histological testing after six months of treatment.[48] Rebamipide reduced inflammation and the incidence of atrophic gastritis and intestinal metaplasia in an RCT from China.[49](A1)
Vitamin A, C, and E have been shown to reduce the risk of gastric cancer by a third in the general population when ingested in low supplemental doses.[50](A1)
Endoscopic Treatment
The treatment of chronic atrophic gastritis and intestinal metaplasia is surveillance to reduce progression to more advanced GPLs like dysplasia. The frequency of surveillance depends on histological findings.
Endoscopic resection is recommended for dysplastic lesions and early gastric adenocarcinoma. Endoscopic mucosal resection (EMR) technique is required for en-bloc excision of less than 10 mm lesions. Endoscopic submucosal dissection (ESD) may be required for lesions larger than 10 mm.[51][52](B2)
The protocols for more advanced intestinal gastric adenocarcinoma lesions are beyond the scope of this article and should follow gastric cancer protocols to ensure R0 resections.
Differential Diagnosis
Based on the clinical presentation, the following conditions can present with upper gastrointestinal symptoms and signs, making diagnosis reliant on invasive investigations like endoscopy:
Esophageal
Inflammatory
- Reflux esophagitis
- Eosinophilic esophagitis
- Esophageal stricture
Infective
- Bacterial/fungal/viral esophagitis
- Chagas disease
- Candidiasis
Motility Disorders
- Achalasia of the esophagus
- Diffuse esophageal spasm
- Nutcracker esophagus
Neoplastic
- Adenocarcinoma
- Squamous cell carcinoma
- Lymphoma
- Gastrointestinal stromal tumor
- Neuroendocrine tumor
Stomach
Inflammatory
- Gastric ulcer (H. pylori, corticosteroids, stress, anastomotic ulcers)
- Acute erosive gastritis (NSAIDs, aspirin)
- Chemo toxic
- Crohn disease
- Biliary reflux gastritis
- Menetrier gastritis
- Radiation-induced
- Foreign body (trichobezoar, swallowed objects)
Infective
- Bacterial gastroenteritis
- Viral gastroenteritis
- Fungal gastroenteritis
Neoplastic
- Gastric adenocarcinoma
- Gastric lymphoma, Non-Hodgkin
- Gastrointestinal stromal tumor
- Neuroendocrine tumor
Duodenum
Inflammatory
- Duodenitis
- Duodenal ulcers
- Infective
- Bacterial gastroenteritis
- Viral gastroenteritis
- Fungal gastroenteritis
Neoplastic
- Malignant neoplasm of the small intestine
- Gastrointestinal stromal tumor
- Neuroendocrine tumor
Pancreas
- Pancreatitis
Gallbladder
- Cholelithiasis
Staging
The progressive changes in the gastric mucosa can lead to the development of the intestinal type of gastric adenocarcinomas. The histological changes from superficial gastritis to malignant mucosal transformation are described below:
- Helicobacter pylori gastritis
- Non-atrophic gastritis (NAG)
- Duodenal ulcer or multi-focal atrophic gastritis (MAG) without metaplasia
- MAG with metaplasia (small intestinal type) or intestinal metaplasia of the incomplete colonic type
- Low-grade dysplasia
- High-grade dysplasia
- Intraepithelial mucosal carcinoma
- Invasive intestinal adenocarcinoma
Staging of Chronic Atrophic Gastritis/Intestinal Metaplasia
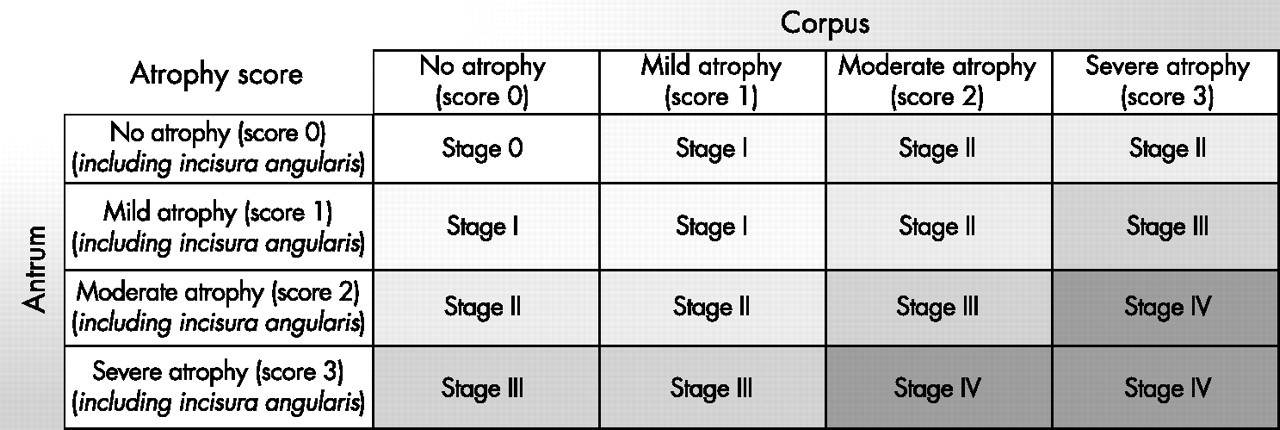
It was the success of hepatitis severity staging that encouraged a group of pathologists and gastroenterologists 'The Operative Link on Gastritis Assessment (OLGA)' to organize a similar reporting system. This is based on cancer risk from lowest (Stage 0) to highest (Stage IV) based on the severity of atrophy and the extent of the spread of the atrophy in the stomach.[53]
The abnormal mucosal areas identified using a visual analog scale are compared with standardized biopsy samples of at least five different topographic areas. The staging is based on the combined scoring of the severity of atrophy and the location and extent of CAG in the stomach. The extent of mucosal atrophy is calculated on histological specimens as the percentage proportion of atrophic/metaplastic mucosa compared to normal mucosa in the antrum and corpus. Separate 'mean' atrophy severity scores are calculated of the antrum and corpus and then compared with the topographic areas involved resulting in stages 1, 2, 3, and 4 of the OLGA classification.
Atrophy Severity (antrum and corpus)
- No atrophy 0% - score 0
- Mild 0-30% - score 1
- Moderate 30-60% - score 2
- Severe >60% - score 3
Location
Antral Atrophy Score
- A1 (lesser curve of antrum)
- A2 (greater curve of the antrum)
- A3 (incisura angularis)
Corpus Atrophy Score
- C1 (lesser curve of the corpus)
- C2 (greater curve of the corpus)
The mean antral score will be calculated as A1+A2+A3 and the mean corpus score C1+ C2. The overall OLGA score is classified between stages 1 to 4 based on a combination of the severity of atrophy and the location of spread in the antrum and/or corpus. Stages 0, 1, and 2 are considered low risk and stage 3 and 4, high risk. In summary, advanced stage 3 and 4 have more extensive mucosal atrophy and more widespread topographic involvement of the stomach mucosa of the antrum and corpus and warrant definitive surveillance. It is of note that an individual can have stage 3 gastritis with severe atrophy scoring 3 that only involves the antrum but will be stage 2 if it only involves the corpus.
GA and GIM have higher risks for progression to gastric cancer when involving corpus, antrum, and incisura (pan gastritis stage 3 or 4).
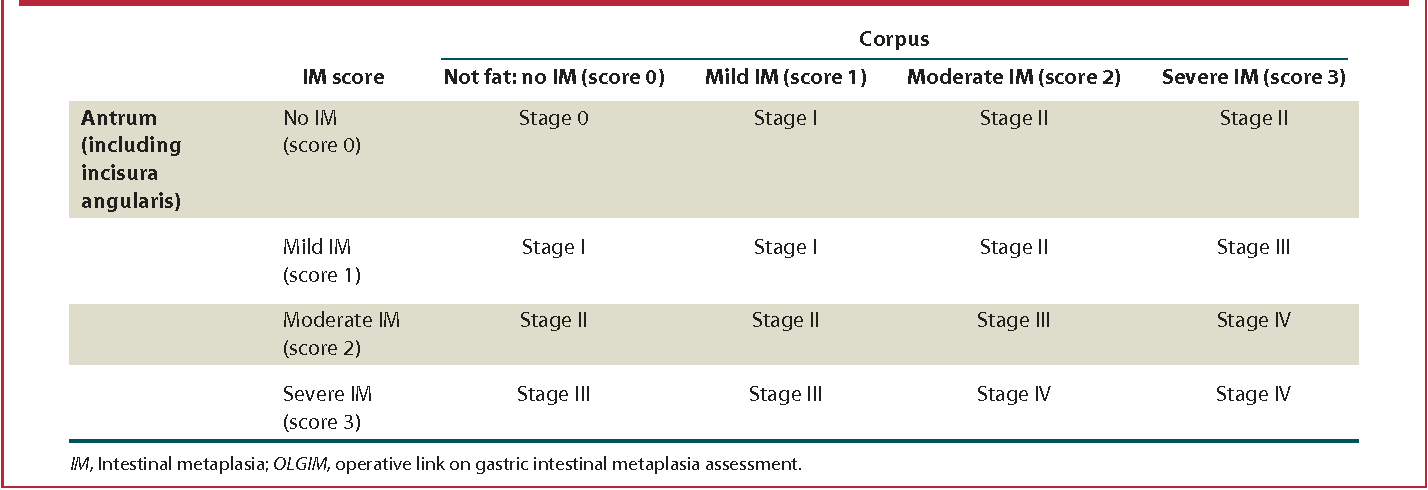
The Operative Link on Gastric Intestinal Metaplasia uses the same configuration of biopsies at five regions in individuals with intestinal metaplasia and classifies low-risk OLGIM stage 0, 1, 2, and high-risk OLGIM stage 3, 4 based on the severity and extent of spread of IM in the stomach. This system is recommended by the American Gastroenterological Association (AGA), whereas European and British centers follow the OLGA classification for mapping and surveillance. The severity of metaplasia is graded as mild, moderate, and severe intestinal metaplasia in the antrum and the corpus. More advanced stages (3/4) have a progressive spread of moderate to severe intestinal metaplasia in the corpus and the antrum.[54]
The OLGA system of histological classification evaluates all chronic atrophic changes, including intestinal metaplasia, whereas, in OLGIM, only intestinal metaplasia is considered. The proponents of OLGIM suggest a higher likelihood of progression to gastric cancer in high stage OLGIM vs. high stage OLGA cases.[55]
A recent meta-analysis of studies comprising more than 2700 subjects showed a strong association between OLGA/OLGIM stages 3/4 and gastric adenocarcinoma vs. stage 1/2. It is essential that individuals with OLGA/OLGIM stages 3/4 have more intensive surveillance to reduce the risk of GC.[56]
Gastric dysplasia is the turning point in the progression of gastric carcinogenesis. Dysplasia should, without a doubt, be treated as a malignant lesion with endoscopic or surgical resection.[57]
The staging of gastric adenocarcinoma is based on the Lauren classification into diffuse and intestinal-type gastric adenocarcinoma.[58]
Prognosis
Gastric adenocarcinoma is the fifth most common cancer in the world.[1]
Chronic atrophic gastritis is often diagnosed late, as many individuals can be asymptomatic for years and diagnosed incidentally. It is essential to investigate those with risk factors, such as the new onset of upper gastrointestinal symptoms in those over 50 years of age, pernicious anemia, or with a family history.[27]
According to one of the largest meta-analysis of numerous studies carried out worldwide, the incidence of gastric cancer in individuals with gastric atrophy varies widely from 0.53 percent to 15.24 per 1000 person-years depending on region and ethnicity, with the highest numbers in Asian countries. The incidence of GC in individuals with intestinal metaplasia was even more varied from 0.38 to 17.08 per 1000 person-years. The highest incidence rate, which was above 15 per 1000 person-years, was in Asia and the United States of America.[13]
In terms of prognosis, the cumulative five-year incidence of gastric adenocarcinoma increases from 0.7% in mild to 10% in severe atrophic gastritis in H. pylori-associated gastritis in a more recent Japanese cohort study.[14]
It is essential to identify individuals from certain ethnic minorities at risk and investigate them. In another study, Americans of Hispanic and Asian heritage had a higher prevalence of H. pylori infection and gastric precancerous lesions (GPLs). Patient ethnicity should be taken into consideration as a risk factor in the detection of GPLs.[3][59]
Complications
Complications associated with gastric mucosal atrophy can typically be:
- Achlorhydria (loss of acid production results in hypergastrinemia)
- Macrocytic anemia (intrinsic factor in autoimmune gastritis)
- Chronic iron deficiency anemia (due to loss of iron absorption)
- Duodenal/gastric ulcer
- Benign strictures of the pylorus
- Hemorrhagic gastritis
- Gastric adenocarcinoma
- Mucosa-associated lymphoid tissue (MALT)
- Gastric carcinoid tumor (enterochromaffin cell hyperplasia)
Deterrence and Patient Education
- Identification of those at risk and regular surveillance is essential to avoid the progression of gastric adenocarcinoma.
- Although there is controversy in the long-term management of atrophic gastritis and intestinal metaplasia, an accurate endoscopic diagnosis can help in coming to a mutually acceptable agreement on surveillance between the clinician and patient.
- The incidental finding of anemia (iron deficiency or B12) should be investigated alongside medical treatment to avoid delay in diagnosis.
- Helicobacter pylori eradication in earlier gastritis and resection of gastric precancerous lesions can reduce the risk of primary and metachronous malignancy.
Enhancing Healthcare Team Outcomes
Most individuals are asymptomatic or may present with the clinical picture of anemia (tiredness, headaches, palpitations, etc.) or vague upper gastrointestinal signs and symptoms like heartburn, epigastric pain, nausea, and rarely vomiting. Individuals with pernicious anemia might have sequelae of autoimmune conditions like associated thyroid disease, Diabetes Mellitus, dermatological, or joint disease.
Intestinal metaplasia is the most reliable marker for the progression of chronic atrophic gastritis. Specialized endoscopy with chromoendoscopy or high definition white light endoscopy with or without narrowband imaging is useful to map and take targetted biopsies. Though intestinal metaplasia at a focal location carries a higher risk of gastric cancer compared to no IM or CAG, the risk appears to be too small to justify surveillance for all individuals. It should be decided on individual risk factors already discussed.[55]
- Single location IM with incomplete colonic type IM with persistent H. pylori or with a family history will require high-quality endoscopy in 3 years.[33][60][61] [Level 2, Level 1]
- Advanced stages of atrophic gastritis or IM in both antrum and corpus should have an endoscopy in 3 years.[17]
- Individuals with pernicious anemia have a seven-fold relative risk of GC with a 0.27% per person-years incidence rate. Individuals with autoimmune atrophic gastritis should have an endoscopy in 3 to 5 years.[19][62]
- Dysplasia, whether high grade or low grade, will need six-monthly and 12 monthly endoscopies, respectively, with the aim for resection of the dysplastic lesions.[10]
In summary, chronic atrophic gastritis (gastric atrophy and gastric intestinal metaplasia) is a gastric precancerous lesion. There is evidence that advanced stages of CAG need surveillance in all the above higher risk groups of individuals to prevent progression to malignancy. High-quality endoscopy for the initial diagnosis and a multidisciplinary approach involving the primary care provider, nurse, and gastroenterologist is required for the appropriate investigation, staging, and long-term follow-up of these individuals. Primary care physicians could help to identify individuals with unclear etiology of anemia or other gastrointestinal symptoms. Nursing colleagues could help with obtaining a detailed history, including any family history or history of H. pylori infection. A gastroenterologist can help with endoscopy and refer patients back to the primary care provider with a plan for future surveillance and identification of signs and symptoms of complications, including gastric cancer.
Media
References
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020 Jul:159(1):335-349.e15. doi: 10.1053/j.gastro.2020.02.068. Epub 2020 Apr 2 [PubMed PMID: 32247694]
Correa P. The biological model of gastric carcinogenesis. IARC scientific publications. 2004:(157):301-10 [PubMed PMID: 15055303]
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. 2006 Jul:19(3):449-90 [PubMed PMID: 16847081]
Level 3 (low-level) evidenceChoi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. The New England journal of medicine. 2018 Mar 22:378(12):1085-1095. doi: 10.1056/NEJMoa1708423. Epub [PubMed PMID: 29562147]
Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World journal of gastroenterology. 2014 Sep 28:20(36):12781-808. doi: 10.3748/wjg.v20.i36.12781. Epub [PubMed PMID: 25278678]
Level 3 (low-level) evidencePittman ME, Voltaggio L, Bhaijee F, Robertson SA, Montgomery EA. Autoimmune Metaplastic Atrophic Gastritis: Recognizing Precursor Lesions for Appropriate Patient Evaluation. The American journal of surgical pathology. 2015 Dec:39(12):1611-20. doi: 10.1097/PAS.0000000000000481. Epub [PubMed PMID: 26291507]
Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmunity reviews. 2014 Apr-May:13(4-5):459-62. doi: 10.1016/j.autrev.2014.01.048. Epub 2014 Jan 11 [PubMed PMID: 24424193]
Annibale B, Esposito G, Lahner E. A current clinical overview of atrophic gastritis. Expert review of gastroenterology & hepatology. 2020 Feb:14(2):93-102. doi: 10.1080/17474124.2020.1718491. Epub 2020 Jan 24 [PubMed PMID: 31951768]
Level 3 (low-level) evidenceWeck MN, Stegmaier C, Rothenbacher D, Brenner H. Epidemiology of chronic atrophic gastritis: population-based study among 9444 older adults from Germany. Alimentary pharmacology & therapeutics. 2007 Sep 15:26(6):879-87 [PubMed PMID: 17767472]
Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019 Sep:68(9):1545-1575. doi: 10.1136/gutjnl-2018-318126. Epub 2019 Jul 5 [PubMed PMID: 31278206]
Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nature reviews. Gastroenterology & hepatology. 2013 Sep:10(9):529-41. doi: 10.1038/nrgastro.2013.101. Epub 2013 Jun 18 [PubMed PMID: 23774773]
Namekata T, Miki K, Kimmey M, Fritsche T, Hughes D, Moore D, Suzuki K. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. American journal of epidemiology. 2000 Apr 15:151(8):820-30 [PubMed PMID: 10965979]
Level 2 (mid-level) evidenceSpence AD, Cardwell CR, McMenamin ÚC, Hicks BM, Johnston BT, Murray LJ, Coleman HG. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: a systematic review. BMC gastroenterology. 2017 Dec 11:17(1):157. doi: 10.1186/s12876-017-0708-4. Epub 2017 Dec 11 [PubMed PMID: 29228909]
Level 1 (high-level) evidenceShichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointestinal endoscopy. 2016 Oct:84(4):618-24. doi: 10.1016/j.gie.2016.03.791. Epub 2016 Mar 16 [PubMed PMID: 26995689]
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. The New England journal of medicine. 2001 Sep 13:345(11):784-9 [PubMed PMID: 11556297]
Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical microbiology reviews. 2010 Oct:23(4):713-39. doi: 10.1128/CMR.00011-10. Epub [PubMed PMID: 20930071]
Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019 Apr:51(4):365-388. doi: 10.1055/a-0859-1883. Epub 2019 Mar 6 [PubMed PMID: 30841008]
Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World journal of gastroenterology. 2017 Jan 28:23(4):563-572. doi: 10.3748/wjg.v23.i4.563. Epub [PubMed PMID: 28216963]
Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Alimentary pharmacology & therapeutics. 2013 Feb:37(4):375-82. doi: 10.1111/apt.12177. Epub 2012 Dec 10 [PubMed PMID: 23216458]
Level 1 (high-level) evidenceKato Y, Kitagawa T, Yanagisawa A, Kubo K, Utsude T, Hiratsuka H, Tamaki M, Sugano H. Site-dependent development of complete and incomplete intestinal metaplasia types in the human stomach. Japanese journal of cancer research : Gann. 1992 Feb:83(2):178-83 [PubMed PMID: 1372886]
Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. Journal of clinical pathology. 1980 Aug:33(8):711-21 [PubMed PMID: 7430384]
Uedo N, Yao K. Endoluminal Diagnosis of Early Gastric Cancer and Its Precursors: Bridging the Gap Between Endoscopy and Pathology. Advances in experimental medicine and biology. 2016:908():293-316. doi: 10.1007/978-3-319-41388-4_14. Epub [PubMed PMID: 27573777]
Level 3 (low-level) evidenceSugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Internal medicine (Tokyo, Japan). 2017:56(6):579-586. doi: 10.2169/internalmedicine.56.7775. Epub 2017 Mar 17 [PubMed PMID: 28321054]
Nagata N, Shimbo T, Akiyama J, Nakashima R, Kim HH, Yoshida T, Hoshimoto K, Uemura N. Predictability of Gastric Intestinal Metaplasia by Mottled Patchy Erythema Seen on Endoscopy. Gastroenterology research. 2011 Oct:4(5):203-209 [PubMed PMID: 27957016]
Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S, Tatsuta M. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006 Aug:38(8):819-24 [PubMed PMID: 17001572]
Crafa P, Russo M, Miraglia C, Barchi A, Moccia F, Nouvenne A, Leandro G, Meschi T, De' Angelis GL, Di Mario F. From Sidney to OLGA: an overview of atrophic gastritis. Acta bio-medica : Atenei Parmensis. 2018 Dec 17:89(8-S):93-99. doi: 10.23750/abm.v89i8-S.7946. Epub 2018 Dec 17 [PubMed PMID: 30561425]
Li Y, Xia R, Zhang B, Li C. Chronic Atrophic Gastritis: A Review. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2018:37(3):241-259. doi: 10.1615/JEnvironPatholToxicolOncol.2018026839. Epub [PubMed PMID: 30317974]
White JR, Winter JA, Robinson K. Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. Journal of inflammation research. 2015:8():137-47. doi: 10.2147/JIR.S64888. Epub 2015 Aug 13 [PubMed PMID: 26316793]
Level 2 (mid-level) evidencePark JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. The American journal of surgical pathology. 2010 Nov:34(11):1591-8. doi: 10.1097/PAS.0b013e3181f623af. Epub [PubMed PMID: 20975338]
Oo TH, Rojas-Hernandez CM. Challenging clinical presentations of pernicious anemia. Discovery medicine. 2017 Sep:24(131):107-115 [PubMed PMID: 28972879]
Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Alimentary pharmacology & therapeutics. 2017 Oct:46(7):657-667. doi: 10.1111/apt.14248. Epub 2017 Aug 7 [PubMed PMID: 28782119]
Level 1 (high-level) evidenceTerasawa T, Nishida H, Kato K, Miyashiro I, Yoshikawa T, Takaku R, Hamashima C. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PloS one. 2014:9(10):e109783. doi: 10.1371/journal.pone.0109783. Epub 2014 Oct 14 [PubMed PMID: 25314140]
Level 1 (high-level) evidenceHwang YJ, Kim N, Lee HS, Lee JB, Choi YJ, Yoon H, Shin CM, Park YS, Lee DH. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Alimentary pharmacology & therapeutics. 2018 Feb:47(3):380-390. doi: 10.1111/apt.14424. Epub 2017 Nov 29 [PubMed PMID: 29193217]
Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. Journal of gastroenterology and hepatology. 2016 Sep:31(9):1539-45. doi: 10.1111/jgh.13313. Epub [PubMed PMID: 26860924]
Level 2 (mid-level) evidenceAnagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C, Ragunath K. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007 Mar:39(3):202-7 [PubMed PMID: 17273960]
Level 2 (mid-level) evidenceAng TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. European journal of gastroenterology & hepatology. 2015 Dec:27(12):1473-8. doi: 10.1097/MEG.0000000000000478. Epub [PubMed PMID: 26426836]
Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011 Dec:141(6):2017-2025.e3. doi: 10.1053/j.gastro.2011.08.007. Epub 2011 Aug 19 [PubMed PMID: 21856268]
Level 1 (high-level) evidenceIsajevs S, Liepniece-Karele I, Janciauskas D, Moisejevs G, Funka K, Kikuste I, Vanags A, Tolmanis I, Leja M. The effect of incisura angularis biopsy sampling on the assessment of gastritis stage. European journal of gastroenterology & hepatology. 2014 May:26(5):510-3. doi: 10.1097/MEG.0000000000000082. Epub [PubMed PMID: 24625520]
Varbanova M, Wex T, Jechorek D, Röhl FW, Langner C, Selgrad M, Malfertheiner P. Impact of the angulus biopsy for the detection of gastric preneoplastic conditions and gastric cancer risk assessment. Journal of clinical pathology. 2016 Jan:69(1):19-25. doi: 10.1136/jclinpath-2015-202858. Epub 2015 Jul 10 [PubMed PMID: 26163538]
ASGE Standards of Practice Committee, Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointestinal endoscopy. 2015 Jul:82(1):1-8. doi: 10.1016/j.gie.2015.03.1967. Epub 2015 Apr 29 [PubMed PMID: 25935705]
Matysiak-Budnik T, Camargo MC, Piazuelo MB, Leja M. Recent Guidelines on the Management of Patients with Gastric Atrophy: Common Points and Controversies. Digestive diseases and sciences. 2020 Jul:65(7):1899-1903. doi: 10.1007/s10620-020-06272-9. Epub [PubMed PMID: 32356261]
Isajevs S, Liepniece-Karele I, Janciauskas D, Moisejevs G, Putnins V, Funka K, Kikuste I, Vanags A, Tolmanis I, Leja M. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Archiv : an international journal of pathology. 2014 Apr:464(4):403-7. doi: 10.1007/s00428-014-1544-3. Epub 2014 Jan 30 [PubMed PMID: 24477629]
Kong YJ, Yi HG, Dai JC, Wei MX. Histological changes of gastric mucosa after Helicobacter pylori eradication: a systematic review and meta-analysis. World journal of gastroenterology. 2014 May 21:20(19):5903-11. doi: 10.3748/wjg.v20.i19.5903. Epub [PubMed PMID: 24914352]
Level 1 (high-level) evidenceChen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016 Jan:19(1):166-75. doi: 10.1007/s10120-015-0462-7. Epub 2015 Jan 22 [PubMed PMID: 25609452]
Level 1 (high-level) evidenceRokkas T, Rokka A, Portincasa P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Annals of gastroenterology. 2017:30(4):414-423. doi: 10.20524/aog.2017.0144. Epub 2017 Apr 7 [PubMed PMID: 28655977]
Level 1 (high-level) evidenceWong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, Liu WD, Feng GS, Zhang XD, Li J, Lu AP, Xia HH, Lam S, You WC. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012 Jun:61(6):812-8. doi: 10.1136/gutjnl-2011-300154. Epub 2011 Sep 13 [PubMed PMID: 21917649]
Level 1 (high-level) evidenceHuang XZ, Chen Y, Wu J, Zhang X, Wu CC, Zhang CY, Sun SS, Chen WJ. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget. 2017 Jan 17:8(3):4781-4795. doi: 10.18632/oncotarget.13591. Epub [PubMed PMID: 27902474]
Level 1 (high-level) evidenceTang XD, Zhou LY, Zhang ST, Xu YQ, Cui QC, Li L, Lu JJ, Li P, Lu F, Wang FY, Wang P, Bian LQ, Bian ZX. Randomized double-blind clinical trial of Moluodan () for the treatment of chronic atrophic gastritis with dysplasia. Chinese journal of integrative medicine. 2016 Jan:22(1):9-18. doi: 10.1007/s11655-015-2114-5. Epub 2015 Oct 1 [PubMed PMID: 26424292]
Level 1 (high-level) evidenceHan X, Jiang K, Wang B, Zhou L, Chen X, Li S. Effect of Rebamipide on the Premalignant Progression of Chronic Gastritis: A Randomized Controlled Study. Clinical drug investigation. 2015 Oct:35(10):665-73. doi: 10.1007/s40261-015-0329-z. Epub [PubMed PMID: 26369655]
Level 1 (high-level) evidenceKong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, Xu D. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PloS one. 2014:9(12):e116060. doi: 10.1371/journal.pone.0116060. Epub 2014 Dec 30 [PubMed PMID: 25549091]
Level 1 (high-level) evidenceJapanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017 Jan:20(1):1-19. doi: 10.1007/s10120-016-0622-4. Epub 2016 Jun 24 [PubMed PMID: 27342689]
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2009:12(3):148-52. doi: 10.1007/s10120-009-0515-x. Epub 2009 Nov 5 [PubMed PMID: 19890694]
Level 2 (mid-level) evidenceRugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007 May:56(5):631-6 [PubMed PMID: 17142647]
Level 2 (mid-level) evidenceCapelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointestinal endoscopy. 2010 Jun:71(7):1150-8. doi: 10.1016/j.gie.2009.12.029. Epub 2010 Apr 9 [PubMed PMID: 20381801]
Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM Jr, Correa P, Wilson KT, Piazuelo MB. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018 Jul:67(7):1239-1246. doi: 10.1136/gutjnl-2016-311685. Epub 2017 Jun 24 [PubMed PMID: 28647684]
Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2018 Jul:21(4):579-587. doi: 10.1007/s10120-018-0812-3. Epub 2018 Feb 19 [PubMed PMID: 29460004]
Level 1 (high-level) evidenceCorrea P. Clinical implications of recent developments in gastric cancer pathology and epidemiology. Seminars in oncology. 1985 Mar:12(1):2-10 [PubMed PMID: 3975643]
Level 3 (low-level) evidenceLAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta pathologica et microbiologica Scandinavica. 1965:64():31-49 [PubMed PMID: 14320675]
Choi CE, Sonnenberg A, Turner K, Genta RM. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Digestive diseases and sciences. 2015 Jul:60(7):2070-6. doi: 10.1007/s10620-015-3591-2. Epub 2015 Feb 28 [PubMed PMID: 25724165]
González CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. International journal of cancer. 2013 Sep 1:133(5):1023-32. doi: 10.1002/ijc.28003. Epub 2013 Feb 5 [PubMed PMID: 23280711]
Level 2 (mid-level) evidenceConchillo JM, Houben G, de Bruïne A, Stockbrügger R. Is type III intestinal metaplasia an obligatory precancerous lesion in intestinal-type gastric carcinoma? European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2001 Aug:10(4):307-12 [PubMed PMID: 11535872]
Level 2 (mid-level) evidenceLahner E, Zagari RM, Zullo A, Di Sabatino A, Meggio A, Cesaro P, Lenti MV, Annibale B, Corazza GR. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2019 Dec:51(12):1621-1632. doi: 10.1016/j.dld.2019.09.016. Epub 2019 Oct 19 [PubMed PMID: 31635944]