Gastric Resection for Malignancy (Gastrectomy)
Gastric Resection for Malignancy (Gastrectomy)
Introduction
Gastric cancer represents a significant global health challenge, ranking as the fifth most common cancer worldwide and the third leading cause of cancer-related mortality.[1] Annually, there are over 1 million new cases globally, with approximately 27,500 new diagnoses in the United States alone.[2][3] The reported incidence stands at 5.6%, with a mortality rate of 7.7%, underscoring the need for effective management strategies.[4] Advanced gastric cancer accounts for 50% to 80% of all gastric cancer cases, with many patients (35%–51%) failing to achieve desired responses to neoadjuvant chemotherapy and 15% experiencing tumor progression.[5][6]
In Western populations, a multimodal approach has become the standard response to these challenges, combining innovative combinations of chemotherapeutic agents, radiotherapies, and immunomodulatory drugs tailored to individual patient and tumor characteristics.[7][8] This personalized approach aims to minimize treatment-related toxicities while maximizing the effectiveness of conventional therapeutic strategies.[9] However, despite these advancements, radical en bloc surgical resection of the tumor with concomitant lymph node dissection remains the cornerstone of management.[10][11]
Surgical options for gastric cancer resection include total, proximal, distal, and pylorus-preserving distal gastrectomies. The choice of surgical approach for gastric adenocarcinoma depends on factors such as where the epicenter of the tumor resides, the extent of stomach involvement, histological subtype, and genomic etiology. Given that gastric cancer is primarily a locoregional disease, the primary objective of surgery is to remove the primary tumor with a clear longitudinal and circumferential resection margin, preferably with a minimum distance of 5 cm from the palpable edge of the tumor. This involves achieving R0 resection, which may require combined organ resection, if necessary, along with lymph node dissection. Subsequently, the surgery aims to restore intestinal and biliary continuity safely to ensure sufficient nutritional intake. In cases of more extensive disease, selected patients may benefit from multivisceral resection (MVR) or cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC).
Traditionally, open gastrectomy has been the predominant method for gastric cancer resection. However, in recent years, minimally invasive surgical (MIS) techniques, such as laparoscopic gastrectomy and robotic-assisted gastrectomy, have gained popularity.[12] Proponents of MIS tout its benefits, including decreased morbidity, accelerated recovery, and improved cosmetic outcomes.[13] However, the selection of surgical approach remains multifactorial and is influenced by patient characteristics, disease pathology, and institutional expertise. Although MIS techniques offer compelling advantages, open gastrectomy retains its role in specific scenarios, underscoring the importance of individualized care in gastric cancer management.[14]
Since its inception in 1994, laparoscopic gastrectomy has evolved into a well-established surgical modality for early gastric carcinoma. Numerous multicenter, prospective, randomized clinical trials have demonstrated long-term oncological and survival outcomes comparable to open gastrectomy. Therefore, laparoscopic gastrectomy is considered a well-established surgical approach to managing early gastric carcinoma.[15][16] Moreover, laparoscopic gastrectomy is increasingly recognized as a feasible, safe, and effective approach for radical resection of locally advanced distal gastric cancer.[17][18] Despite these advancements, debates persist regarding disparities in postoperative and oncological outcomes between laparoscopic and open gastrectomy, alongside the technical intricacies and learning curve associated with laparoscopic techniques.[19][20]
Robotic-assisted surgery presents a promising solution to address the limitations of conventional laparoscopy in managing gastric cancers. Advantages include 3-dimensional vision, enhanced skill acquisition, increased dexterity, improved mobility, and better ergonomics for surgeons.[14] However, despite these benefits, the adoption of robotic-assisted gastrectomy in upper gastrointestinal surgery, particularly for gastric cancer resections, has been slower compared to other specialties. Limited high-quality data, primarily from retrospective studies, hinders a comprehensive evaluation of robotic-assisted gastrectomy's role in gastric tumor resection, highlighting the need for further research to elucidate its long-term oncological outcomes and efficacy.[21]
Despite numerous randomized controlled trials and standard pairwise meta-analyses, consensus on the oncological and surgical safety of laparoscopic and robotic-assisted gastrectomy compared to open gastrectomy for gastric carcinoma resection remains elusive.[22][23][24] Recent trials have reported short-term postoperative and survival outcomes following robotic-assisted gastrectomy, sparking optimism among gastroesophageal surgeons that these minimally invasive approaches may enhance patient outcomes.[22][23] However, further research is necessary to establish a definitive consensus on the efficacy and safety of laparoscopic gastrectomy and robotic-assisted gastrectomy relative to open gastrectomy in managing gastric cancer.
Another emerging modality for treating early gastric cancer is endoscopic submucosal dissection (ESD), particularly when lymph node metastasis risk is low. In contrast to surgical gastrectomy, ESD offers a minimally invasive approach with significant benefits, such as preserving the entire stomach and maintaining the patient's quality of life. Despite some drawbacks, this technique signifies a notable advancement in the management of early gastric cancer, offering patients effective treatment while minimizing the impact on their overall well-being.[25]
The evolution from traditional open procedures to minimally invasive techniques reflects a significant advancement in the surgical management of gastric malignancies, offering patients improved outcomes and a better quality of life. This activity explores the various surgical approaches for treating gastric cancer, discussing their advantages, limitations, and emerging trends in the field.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
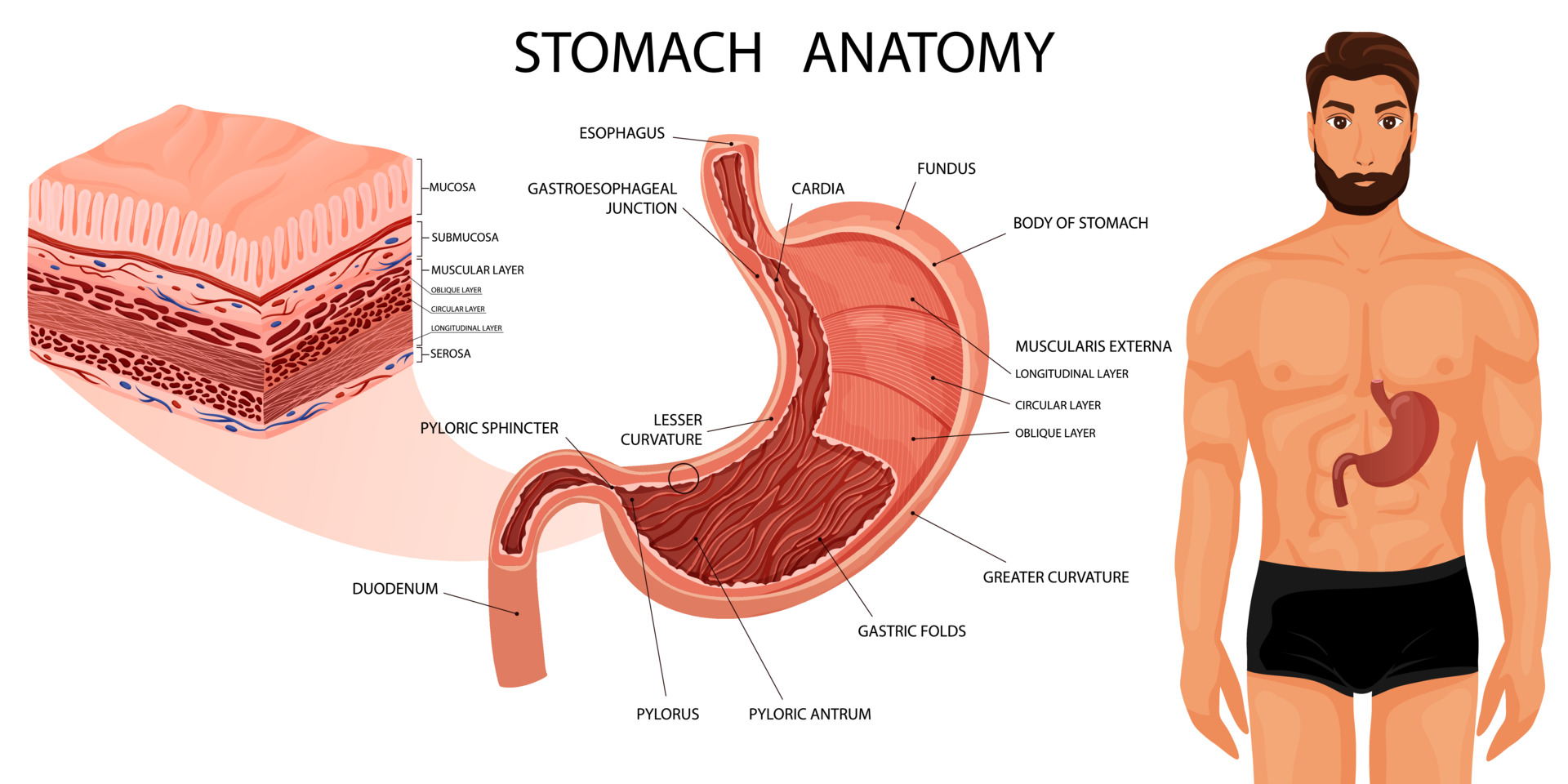
A comprehensive understanding of the stomach's anatomy, including its structure, vascular supply, and lymphatic drainage, is essential for successful gastric resection (see Image. Stomach Anatomy).
Stomach Structure
The stomach, a vital organ for digestion, comprises 4 primary regions:
- Cardia: This is the entry point for food from the esophagus into the stomach.
- Fundus: This dome-shaped region lies above and to the left of the cardia.
- Body: This is the largest portion of the stomach and lies below the fundus.
- Pylorus: This funnel-shaped region connects the stomach to the duodenum.
- The pyloric antrum connects to the stomach's body, leading to the narrower pyloric canal, which further connects to the duodenum. The smooth muscle pyloric sphincter regulates stomach emptying.
The stomach wall has 5 layers:
- Mucosa: This is the innermost layer where nearly all stomach cancers originate. The mucosa has 3 parts—epithelial cells, a layer of connective tissue (the lamina propria), and a thin layer of muscle known as the muscularis mucosa.
- Submucosa: This is a supporting layer beneath the mucosa.
- Muscularis propria: This is a thick muscle layer responsible for moving and mixing the stomach contents.
- Subserosa
- Serosa: This is the outermost wrapping layer of the stomach.
In its relaxed state, the stomach's mucosa and submucosa form folds known as rugae.
Ligamentous Attachments
The following ligaments are attached to the stomach to anchor it in place:
- Gastrocolic ligament: This portion of the greater omentum connects the stomach's greater curvature to the transverse colon and forms part of the anterior wall of the lesser sac.
- Gastrosplenic ligament: This portion of the greater omentum connects the stomach's greater curvature to the splenic hilum and contains the left gastroepiploic and short gastric arteries.
- Gastrohepatic ligament: This peritoneal attachment connects the liver to the stomach's lesser curvature, forms part of the anterior wall of the lesser sac, and contains the right and left gastric arteries.
- Gastrophrenic ligament: This peritoneal attachment connects the diaphragm to the superior portion of the stomach.
Vascular Supply
The arterial blood supply to the stomach includes:
- Celiac trunk: This originates from the abdominal aorta and has 3 major branches—the left gastric, common hepatic, and splenic arteries.
- Left gastric artery: This arises from the celiac trunk, runs along the superior portion of the stomach's lesser curvature, and anastomoses with the right gastric artery.
- Right gastric artery: This originates from the proper hepatic artery, which runs along the inferior portion of the stomach's lesser curvature and anastomoses with the left gastric artery.
- Left gastroepiploic artery: This arises from the splenic artery, runs along the superior portion of the stomach's greater curvature, and anastomoses with the right gastroepiploic artery.
- Right gastroepiploic artery: This arises from the gastroduodenal artery, travels along the inferior portion of the stomach's greater curvature, and anastomoses with the left gastroepiploic artery.
- Short gastric arteries: These short arteries originate from the splenic and left gastroepiploic arteries to supply the stomach's greater curvature.
Lymphatic Drainage
The lymphatic drainage of the stomach is anatomically elaborate and includes 16 regional lymphatic stations. Lymph node stations are categorized into N1, N2, N3, or N4 groups concerning their location to the primary tumor.[26][27]
The groups are as follows:
- N1 nodes: These include perigastric lymph nodes (stations 1-6).
- N2 nodes: These include lymph nodes along the major vessels originating from the celiac trunk, including the left gastric, common hepatic, and splenic arteries (stations 7-11).
- N3 nodes: These lymph nodes are located in the hepatoduodenal ligament (station 12), retropancreatic region (station 13), and the root of the mesentery (station 14).
- N4 nodes: These lymph nodes are along the middle colic vein (station 15) and the paraaortic region (station 16).
Lymph node dissection is characterized by the removal of specific lymph node stations. A D1 lymphadenectomy entails removing the nodes in stations 1 to 6, while a D2 lymphadenectomy involves removing the nodes in stations 1 to 11. A D2+ lymphadenectomy further includes the removal of nodes in stations 1 to 16.[28][29]
Indications
The indications for each type of gastric resection and associated procedures are mentioned below.
Endoscopic Submucosal Dissection
Endoscopic resection is widely used as a standard modality for the curative treatment of early gastric cancer when the risk of lymph node metastasis is negligible.[30][31] Endoscopic mucosal resection (EMR) and ESD are the primary techniques used for this purpose. ESD, in particular, addresses the limitations of EMR by enabling the removal of larger lesions and providing more precise assessments of tumor invasion depth and vascular involvement. Consequently, ESD has increasingly supplanted EMR as the preferred treatment modality for early gastric cancer.[32] Compared with surgical gastrectomy, ESD is a minimally invasive procedure with additional benefits, including preserving the entire stomach and maintaining the patient's quality of life.[25]
Thin, early-stage gastric cancers are infrequently detected in Western populations to allow for endoscopic resection. As these lesions are rare within the US population, it is often difficult for endoscopic practitioners to obtain and maintain proficiency in advanced techniques such as endoscopic mucosal or submucosal resection.[11] Conversely, Asia, particularly China, recorded the highest number of gastric cancer cases in 2020, with an incidence rate of 22.4 per 100,000 people.[33] The heightened incidence in Asia has led to the majority of guidelines for endoscopic resection originating from this region.
The Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition) specify the following as absolute indications for ESD:
- Differentiated intramucosal carcinoma without ulcer lesions and tumor diameter ≤3 cm.
- Differentiated intramucosal carcinoma with ulcer lesions ≤3 cm.
In addition, according to the same guidelines, the following is considered an expanded indication for ESD:
- Undifferentiated intramucosal carcinoma without ulcer and with a diameter ≤2 cm.[34]
Tumor lesions with a risk of lymph node metastasis lower than 1%, wherein endoscopic resection is deemed to be equally effective as radical surgery, are classified as absolute indications for ESD therapy.[35]
Although ESD achieves en bloc/R0 resection in more than 90% of cases, it does not guarantee cure in up to 20%. This may result from previously undetected submucosal invasion or horizontal extension, alterations in histopathological type (particularly toward the undifferentiated-predominant mixed type), or identification of lymphovascular invasion upon histopathological examination. Therefore, accurate evaluation of the size, depth of invasion, horizontal extent, and histopathological type of early gastric cancer is imperative to enhance patient selection for successful ESD.[25]
Distal Gastrectomy
Distal gastrectomy is the preferred treatment for middle and distal-third gastric cancers in which a 4- to 6-cm proximal margin can be obtained while maintaining an adequately sized remnant pouch.[36] However, the indication for conventional distal gastrectomy varies based on several factors, including the need for a more extensive procedure in advanced stages and the preference for conservative treatments in early gastric cancer.[26]
Pylorus-Preserving Distal Gastrectomy
Some surgeons perform the pylorus-preserving distal gastrectomy with gastro-gastrostomy, citing reduced risks of bile reflux, dumping syndrome, gallstone formation, and weight loss.[37] However, some concern prevails regarding potential compromises in lymphadenectomy thoroughness due to the necessity of preserving the infrapyloric vessels. This preservation could restrict access to lymph nodes in the infrapyloric region, which is essential for precise staging and optimal oncological outcomes.
Consequently, Japanese guidelines advocate for pylorus-preserving distal gastrectomy in cases of gastric cancer where the tumor has started to grow into the stomach wall. However, this recommendation applies only when there are no affected lymph nodes or metastasis present (cT1N0M0) located in the middle of the stomach and when achieving a macroscopically negative 4-cm distal margin is feasible.[38] Further studies are being conducted to clarify the risks and benefits of pylorus-preserving distal gastrectomy concerning lymphadenectomy completeness and oncological outcomes.
Proximal Gastrectomy
For gastric cancer located in the upper third of the stomach, proximal gastrectomy is a viable option to preserve the physiological function of the distal stomach and pylorus. Both American and Japanese guidelines recommend proximal gastrectomy for patients with early gastric cancer (cT1N0M0).[39]
Total Gastrectomy
Total gastrectomy is recommended for tumors involving a significant portion of the proximal stomach, especially those extending along most of the lesser or greater curvature. This approach ensures a negative proximal margin, which is crucial for complete resection. Additionally, expansive tumors hindering the attainment of adequate macroscopic margins (4-6 cm) necessitate total gastrectomy. Signet ring cell histology, which indicates diffuse submucosal seeding, presents challenges for achieving an R0 resection without total gastrectomy. Furthermore, individuals with inactivating germline mutations of the CDH1 gene, associated with multifocal gastric cancer, may require prophylactic total gastrectomy.[36]
Lymphadenectomy
A comprehensive lymphadenectomy is crucial for accurate pathologic staging during gastrectomy. Historically, 3 types of lymphadenectomy performed with gastrectomy have been described. D1 lymphadenectomy involves circumferential dissection along the stomach to retrieve nodes from stations 1 to 6. D2 lymphadenectomy includes lymph nodes retrieved with a D1 dissection and those along the celiac trunk and its branches (common hepatic artery, left gastric artery, and splenic artery), comprising stations 7 to 11. Finally, a D3 lymphadenectomy includes additional nodes along the portal tract, hepatic artery, and adjacent to the aorta (stations 12–16). Multiple retrospective reports have highlighted an association between improved survival and increased lymph nodes retrieved in the gastrectomy specimen.[40]
Trials investigating D3 lymphadenectomy have shown a survival advantage compared to D1; however, no survival benefit has been observed compared to D2 dissection.[41] For adequate pathological staging of gastric cancer patients, it is widely recommended to perform a pancreas-sparing D2 lymphadenectomy, adhering to the guidelines set forth by the American Joint Committee on Cancer. This approach should aim to retrieve at least 15 lymph nodes during the gastrectomy procedure.[42]
Multivisceral Resection
MVR for locally advanced gastric cancer presents a challenging yet potentially beneficial surgical approach to achieving negative margins and improving oncologic outcomes. Early studies reported wide ranges of perioperative morbidity and mortality; however, more recent evidence suggests a decline in these rates over time, possibly reflecting the learning curve effect and improvements in surgical techniques. For example, a Taiwanese study demonstrated reduced postoperative morbidity and mortality over 12 years, indicating improved surgical outcomes with increasing experience.[43] Similarly, a multicenter Italian cohort study reported relatively lower perioperative morbidity and mortality rates, further supporting the feasibility and safety of MVR for gastric cancer.[44]
Although MVR is associated with increased perioperative morbidity, particularly with extensive resections such as pancreatectomy, studies have shown comparable perioperative mortality rates across different extents of resection.[45] However, 5-year overall survival rates significantly decreased with the extent of resection, with MVR with pancreatectomy emerging as an independent predictor of poor survival on multivariate analysis. Despite the associated risks, MVR presents a potentially curative option for specific patients with locally advanced gastric cancer who show responsiveness to neoadjuvant therapy. Extensive preoperative counseling is crucial in educating patients about the potential benefits and risks of MVR, facilitating shared decision-making, and optimizing treatment outcomes.
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
Nearly 40% of newly diagnosed gastric cancer patients are found to have metastatic disease, with around one-third of these cases involving peritoneal metastases.[46] Additionally, postgastrectomy patients may develop peritoneal lesions in up to 46% of cases. The peritoneal-blood barrier restricts the efficacy of conventional systemic agents in the adjuvant setting, prompting the need for more direct therapeutic approaches. CRS, combined with HIPEC, has emerged as a strategy to target peritoneal metastases in various oncologic diseases, including gastric cancer.[47]
Multiple randomized trials shed light on the roles of CRS and HIPEC in the management of gastric cancer in patients with peritoneal metastases. The results suggest that combined CRS and HIPEC may offer survival benefits and reduce peritoneal recurrences compared to CRS alone or systemic chemotherapy alone. The ongoing PERISCOPE II trial seeks to further investigate the advantages of combined treatment modalities, which include systemic chemotherapy, gastrectomy, CRS, and HIPEC, in gastric cancer patients exhibiting limited peritoneal dissemination and positive peritoneal cytology. This trial is anticipated to provide valuable insights into the optimal management approach for this specific patient population.
As ongoing trials, such as PERISCOPE II, continue to contribute to the body of research in this area, we can anticipate the development of improved patient selection criteria and treatment strategies for advanced therapies such as CRS and hyperthermic intraperitoneal chemotherapy (HIPEC). These advancements hold the potential to significantly enhance outcomes for gastric cancer patients with peritoneal metastases.
Contraindications
Absolute contraindications to gastric resection involve patients deemed unfit for general anesthesia. Relative contraindications include advanced age, severe cardiopulmonary dysfunction, or diminished life expectancy due to comorbidities. In addition, conducting a comprehensive risk-benefit assessment is imperative before proceeding with gastric resection in such cases.
Total gastrectomy is contraindicated when achieving wide negative margins (4-6 cm), which is feasible with partial gastrectomy. Partial gastrectomy presents significantly improved safety and long-term functional outcomes, especially in patients with advanced age, malnutrition, and extensive comorbidities.[36]
Equipment
Performing a gastrectomy, whether through an open or laparoscopic approach, requires specialized equipment tailored to the complexity of the procedure and the surgeon's preferences. A list of equipment required for both methods is mentioned below.
Open Gastrectomy
The equipment required to perform an open gastrectomy typically includes:
- Self-retaining table-mounted retractor
- Surgical instruments, including a variety of scalpels, forceps, retractors, scissors, and clamps
- Electrocautery device
- Sutures
- Staplers
- Sterile drapes, gowns, and gloves.
Laparoscopic Gastrectomy
The equipment required to perform a laparoscopic gastrectomy typically includes:
- Laparoscopic instruments, including trocars, graspers, dissectors, scissors, and retractors
- Laparoscopes (0° and 30°) with a light source
- Monitors
- Insufflation system
- CO2 absorption system
- Laparoscopic staplers
- Laparoscopic suturing devices and sutures
- Vessel sealing device
- Electrocautery
- Sterile drapes, gowns, and gloves.
Personnel
The surgeon undertaking a gastric resection should possess adequate experience in foregut and oncological surgery. Essential personnel, in addition to the operating surgeon, should include a surgical assistant, an anesthesiologist, a surgical technician, and a circulating nurse.
Preparation
Unfortunately, the majority of gastric carcinoma cases are diagnosed in advanced stages, often presenting with symptoms such as weight loss, cachexia, anorexia, early satiety, dyspepsia, gastric outlet obstruction, or malnutrition. The diagnostic workup for gastric cancer entails a range of tests aimed at identifying candidates for gastric resection versus other treatment options. This includes routine laboratory assessments such as baseline hemoglobin, platelet count, and a complete metabolic profile. Serum markers such as albumin and prealbumin can be used to assess a patient's nutritional status, aiding in patient evaluation and treatment planning.
Diagnostic evaluation usually begins with an esophagogastroduodenoscopy (EGD) if a gastric neoplasm is suspected. Esophagogastroduodenoscopy provides a histopathologic diagnosis and an insight into the location and extent of the tumor.[48] Endoscopic ultrasound is used to assess for tumor depth (T stage) and possible nodal involvement.[48] Computed tomography (CT) of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained to evaluate for the presence of distant metastatic disease or bulky lymphadenopathy. In addition, positron emission tomography (PET) is an additional imaging modality that can be used as an adjunct for staging.[48]
An interprofessional approach is essential to determine surgical resectability, loan preoperatively, and consider the role of neoadjuvant chemotherapy or radiation. A thorough evaluation of the patient's comorbidities is required for medical optimization, including assessing the patient's performance status and ability to tolerate surgery. Moreover, addressing any modifiable risk factors before surgical intervention is crucial. For instance, preoperative smoking cessation has been shown to enhance outcomes following gastrectomy for malignancy.[49]
Technique or Treatment
For all techniques, the patient is positioned supine on the operating room table and prepared and draped using standard sterile procedures. Preoperative antibiotics are administered, and a nasogastric tube is inserted for gastric decompression. The outline below delineates the essential steps for gastric resection procedures.[36][48][50]
Minimally Invasive Surgical Technique Versus Open Procedures
Gastric resection may be performed using an open, laparoscopic, or robotic-assisted approach. Randomized controlled trials comparing laparoscopic and open gastrectomy have shown oncologic equivalency and favorable outcomes in postoperative recovery with minimally invasive approaches.[48] Minimally invasive gastrectomy has been shown to have decreased hospital length of stay, fewer perioperative complications, and less intraoperative bleeding than open procedures.[48][50] Considerations for open gastrectomy include surgeon preference, increased operative difficulty, port site recurrence, and decreased adequacy of lymph node dissection.[51]
The KLASS-02 randomized control trial demonstrated that laparoscopic surgery was noninferior to open surgery based on 3-year recurrence-free survival rates and associated with a lower rate of complications in patients with locally advanced gastric cancers.[18] Follow-up at 5 years revealed no significant differences in recurrence-free survival and overall survival rates between the laparoscopic and open surgery groups. Moreover, the laparoscopic approach showed a lower incidence of late complications, indicating its superiority in surgical outcomes over open surgery. These findings underscore the clinical relevance of laparoscopic surgery for patients with locally advanced gastric cancers, supported by favorable long-term oncological and surgical outcomes.[52]
Staging Laparoscopy
Initially, a diagnostic laparoscopy is essential to exclude occult metastatic disease in the liver or peritoneum. If no evidence of grossly metastatic disease is detected, gastric resection can proceed. To facilitate access, a liver retractor may be utilized to mobilize and retract the left lobe of the liver.
Endoscopic Submucosal Dissection
Patients undergoing ESD typically receive conscious sedation or general anesthesia, depending on the complexity of the procedure and patient preference. Before dissection, the lesion's margins are marked using argon plasma coagulation or electrocautery to delineate resection borders, guiding dissection for complete lesion removal with negative margins. A solution containing a lifting agent (such as saline with epinephrine or glycerol) is injected into the submucosal layer beneath the lesion, creating a cushion that lifts the mucosa from the muscular layer. This facilitates dissection, enhancing safety by reducing perforation risk.
An initial mucosal incision is made using specialized electrosurgical knives, such as a needle knife or insulated-tip knife, outside the marking, establishing a starting point for dissection. Subsequently, after the initial incision, the submucosal layer is dissected away from the muscularis propria using precise cutting and coagulation techniques. The dissection progresses layer by layer, gradually exposing and resecting the underlying lesion. Meticulous hemostasis is maintained throughout the procedure using electrocautery or hemostatic forceps to control bleeding from small vessels encountered during dissection. Once the lesion is fully dissected, it undergoes careful inspection to ensure complete removal en bloc with negative margins. Specialized retrieval devices or snares are utilized to retrieve the resected specimen for histopathological evaluation. Any visible defects or perforations in the mucosa are closed post-procedure using endoscopic clips or natural wound healing techniques. Patients are closely monitored for potential complications such as bleeding or perforation, and hospitalization for observation and supportive care may be necessary.
Total Gastrectomy
The surgical approaches for total gastrectomy typically involve an upper midline or left thoracoabdominal incision, depending on the location and extent of the tumor.
- Upper midline incision: This method involves making a midline cut from the xiphoid to the umbilicus, providing effective access to the upper abdomen and generally suitable for most gastric tumors. Nevertheless, if the tumor extends into the cardia or fundus and approaches the esophagus, a left thoracoabdominal incision may be preferable.
- Left thoracoabdominal incision: This incision starts from the seventh intercostal space on the left side of the chest and extends to the upper midline. This incision is used for tumors involving the cardia or fundus that extend toward the esophagus. This method facilitates exposure of the supradiaphragmatic distal esophagus up to the inferior pulmonary ligament level. Deflation of the left lung using a double-lumen endotracheal tube may assist in proximal dissection.
Upon abdominal access, a comprehensive exploration ensues to detect metastatic disease, primarily in the liver, peritoneum, hepatoduodenal ligament, and root of the mesentery. The presence of metastases may preclude gastrectomy. For cases involving high-risk or locally advanced tumors, diagnostic laparoscopy may be utilized to assess metastatic dissemination, as previously discussed.
With the patient positioned in the right semi-lateral decubitus position by tilting the operating room bed, the left triangular ligament of the liver is divided. This maneuver mobilizes the left lateral segment of the liver, allowing exposure of the gastroesophageal junction for further dissection and tumor removal. The greater omentum is separated from the transverse colon and epiploic appendages. The right gastroepiploic vessels are ligated at their origin from the gastroduodenal artery and the gastrocolic trunk of the superior mesenteric vein. The short gastric arteries are divided close to the spleen, followed by the division of the left gastroepiploic artery close to where it originates off the splenic artery. The right gastric artery is ligated before the duodenum division, usually accomplished with a linear stapler. The gastrohepatic ligament is divided carefully to prevent damage to any replaced or accessory left hepatic artery. The stomach is retracted superiorly to facilitate the dissection of lymph nodes near the porta hepatis, hepatic artery, and celiac trunk. Structures lateral to the left hepatic artery are thinned before dividing the left gastric artery near its origin from the celiac trunk. Division of the phrenoesophageal ligament permits circumferential dissection of the distal esophagus. Paracardial lymph nodes are excised, followed by division of the distal esophagus.
After a total gastrectomy, reconstruction with a Roux-en-Y esophagojejunostomy or a Hunt-Lawrence jejunal pouch may be performed.[36] Usually, the preferred method of reconstruction is typically a Roux-en-Y esophagojejunostomy. Randomized controlled trials have shown Roux-en-Y reconstruction has a decreased rate of long-term postoperative outcomes following gastric resection.[53] This procedure involves creating a 40- to 60-cm Roux limb from the jejunojejunostomy to minimize alkaline reflux proximal to the anastomosis. The procedural steps include the jejunum, located 30 to 50 cm from the ligament of Treitz, which is divided using a linear GIA stapler to form the Roux limb proximally and the biliopancreatic limb distally. A jejunojejunostomy is created 60 to 70 cm along the Roux limb. The antimesenteric borders of the biliopancreatic limb and the Roux limb are aligned, and enterotomies are made for the linear GIA stapler. The anastomosis is finalized using a TA stapler. Closure of the mesenteric defect with an absorbable suture is performed to prevent internal hernias. The Roux limb is brought up to the proximal stomach or esophagus in either an antecolic or retrocolic manner. Subsequently, a stapled or hand-sewn end-to-end esophagojejunostomy or gastrojejunostomy anastomosis is created.
The esophagojejunostomy can be performed using various techniques:
- Hand-sewn: This method involves suturing the esophagus to the jejunum using interrupted or running absorbable sutures.
- EEA circular stapler: An alternative method involves using an EEA circular stapler. In this approach, an anvil stapler is placed into the distal end of the esophagus, and the esophagus is closed with a purse-string suture over the anvil. The anvil can be inserted using an orogastric tube delivered by the anesthesiologist.
After the anastomosis is completed, a water bubble test can be conducted to detect any leaks. In addition, it is crucial to keep the anastomosis defunctionalized during the early postoperative period, permitting only saliva to pass through. This strategy fosters optimal healing and diminishes the likelihood of complications.
The routine use of drains following total gastrectomy has undergone extensive scrutiny, with numerous trials and studies consistently demonstrating no discernible benefit.[54][55] Some institutions reserve drain placement for cases where extravisceral extension necessitates pancreatectomy for oncologic clearance.[39] However, feeding jejunostomy placement is a common practice to facilitate extended enteral feeding, particularly for patients with risk factors for esophagogastric leak or preoperative weight loss. Nonetheless, recent research has raised doubts about the universal benefits of routine feeding jejunostomy tube placement, citing concerns about heightened infectious complications without a corresponding increase in the receipt of adjuvant therapy.[48][56] Several institutions conduct a fluoroscopic upper gastrointestinal study by the fifth day after surgery to assess for anastomotic leaks before initiating oral intake.[36] This approach allows for early detection and appropriate management of potential complications, thereby optimizing patient outcomes.
Distal Gastrectomy
When performing a distal gastrectomy, preserving certain short gastric vessels is paramount, unlike in total gastrectomy. The blood supply to the residual stomach can entirely depend on these short gastric vessels. For staging purposes, it is crucial to dissect the cephalad branch of the left gastric artery, which bifurcates high along the lesser curvature. This dissection is essential for obtaining adjacent lymph nodes, including those near the distal 2 to 3 cm of the esophagus.
Following gastric resection, 2 common modalities for reconstruction are Billroth II or Roux-en-Y gastrojejunostomy. While a Billroth I reconstruction is often not feasible due to the duodenum's fixed position, which typically cannot reach the gastric stump, a Billroth II reconstruction is a viable alternative. In a Billroth II reconstruction, the jejunal limb is brought up either antecolic to the transverse colon to meet the gastric stump or retrocolic through a defect in the transverse mesocolon. Typically, a proximal loop of the jejunum located just beyond the ligament of Treitz is brought up to form an anastomosis without tension or angulation. Creating a long afferent limb should be avoided, as it could lead to complications such as kinking or occlusion, resulting in afferent loop syndrome. Thus, careful attention to the length and positioning of the jejunal limb is essential to ensure optimal outcomes following reconstruction.
Despite best efforts, Billroth II anastomoses are commonly fraught with alkaline reflux gastritis. Additionally, malabsorption of fat-soluble vitamins can be associated with loss of duodenal continuity. Due to these issues, multiple institutions opt for a Roux-en-Y gastrojejunostomy utilizing a Roux limb of 40 to 50 cm to mitigate the risk of alkaline reflux. When comparing reconstruction methods, superior long-term outcomes have been observed with Roux-en-Y gastrojejunostomy, including reduced bile reflux or esophagitis, enhanced quality of life, and fewer abnormal findings on upper endoscopy.
Pylorus-Preserving Distal Gastrectomy
The procedure mirrors that of a distal gastrectomy, with the distinction that the stomach is transected proximal to the pylorus, preserving the infrapyloric vessels. The preservation of these vessels in pylorus-preserving distal gastrectomy has raised questions about the adequacy of oncological lymph node dissection. Reports have indicated relatively fewer lymph nodes retrieved from infrapyloric and suprapyloric nodal stations with this procedure, prompting concerns.
Proximal Gastrectomy
Several methods for reconstruction have been described following proximal gastrectomy, including esophagogastrostomy, jejunal interposition, and double tract reconstruction. Esophagogastrostomy is the most common reconstruction after proximal gastrectomy. Among the different techniques such as esophagogastric end-to-end anastomosis and posterior gastric wall end-to-side esophagogastrostomy, anterior gastric wall end-to-side esophagogastrostomy is preferred due to its association with reduced cases of reflux, enhanced meal intake, and increased postoperative weight. However, a notable drawback of esophagogastrostomy is the heightened risk of bile reflux compared to other reconstruction methods.[57]
A jejunal interposition is generally performed using a 10- to 20-cm jejunal limb delivered either antecolic or retrocolic to create an end-to-side esophagojejunal anastomosis and an end-to-side or side-to-side gastrojejunostomy with the anterior gastric wall. Alternatively, a 25- to 35-cm jejunal limb can be reversed and brought retrocolic to fashion a U-shaped 10- to 15-cm jejunal pouch, which is connected in a similar manner.
Finally, a double tract reconstruction entails forming a Roux-en-Y esophagojejunostomy, a side-to-side gastrojejunostomy 10 cm below, and an end-to-side jejunojejunostomy 20 cm distal to the gastrojejunostomy.
Extent of Lymphadenectomy
The extent of lymphadenectomy in gastric carcinoma typically follows the D2 nodal dissection, considered the gold standard. Stations 1 to 7 encompass perigastric nodes, which are taken en bloc with the gastric specimen. Lymphadenectomy proceeds along the proper hepatic artery and extends along the common hepatic artery (station 8) toward the celiac axis (station 9) and splenic artery (stations 11p and 11d). Dissection then progresses down to the hepatoduodenal ligament to harvest associated lymph nodes (station 12a).
Multivisceral Resection
In the context of gastric cancer surgery, MVR entails the removal not only of the stomach but also of adjacent organs or tissues affected or at risk of involvement by the tumor. This extensive surgical approach aims to achieve complete oncological clearance and is typically reserved for cases where gastric cancer has invaded neighboring structures or metastasized to adjacent organs. The specific organs or tissues that may be included in an MVR for gastric cancer can vary depending on the extent of tumor invasion and the individual patient's anatomy.
Commonly resected organs or structures may include:
- Pancreas: Portions of the pancreas may require resection if the tumor has invaded the pancreatic capsule or if there is involvement of the pancreatic head.
- Spleen: Partial or total splenectomy may be necessary if the tumor has invaded the greater curvature of the stomach or the splenic hilum.
- Liver: Segments of the liver may be resected in cases of hepatic metastases or direct liver invasion to achieve complete tumor clearance.
- Colon: Partial colectomy may be performed if the tumor extends into the transverse colon or involves the gastrocolic ligament to ensure complete resection.
- Duodenum: Partial or total duodenectomy may be necessary if tumors located in the proximal stomach involve the duodenum.
- Pancreaticoduodenal ligament: Resection of the ligament containing the common bile duct and the main pancreatic duct may be required if involved in the tumor.
- Peritoneum: Portions of the peritoneum may need to be removed if there is evidence of peritoneal metastasis or carcinomatosis.
MVR for gastric cancer is a complex procedure that requires careful preoperative planning, extensive surgical expertise, and multidisciplinary collaboration. While it allows for aggressive tumor removal, it also carries a higher risk of complications compared to standard gastric resections. Therefore, patient selection and thorough preoperative evaluation are crucial to ensure optimal outcomes.
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
CRS and HIPEC are employed to target peritoneal metastases. CRS involves the meticulous removal of visible tumor nodules from the peritoneal surfaces and adjacent organs. The goal of CRS is to debulk the tumor burden as extensively as possible, aiming for complete macroscopic removal of all visible tumor deposits. This procedure requires extensive surgical expertise and may involve the resection of multiple organs or tissues affected by tumor invasion, such as the peritoneum, omentum, spleen, and parts of the gastrointestinal tract. The extent of surgery depends on the location and extent of the peritoneal spread of the gastric cancer.
HIPEC is a specialized form of chemotherapy administered directly into the peritoneal cavity following CRS. Common agents used in HIPEC for gastric cancer include mitomycin C, 5-fluorouracil, oxaliplatin, cisplatin, or doxorubicin. The chemotherapy solution is heated to a hyperthermic temperature (typically between 41 °C and 43 °C) to enhance its cytotoxic effects on remaining cancer cells while minimizing systemic toxicity. Elevated temperature also improves drug penetration into tumor nodules and enhances chemotherapy efficacy. HIPEC is administered for a predetermined duration, usually 60 to 90 minutes, during which the abdomen is continuously perfused with the heated chemotherapy solution. Upon completion of HIPEC, the chemotherapy solution is drained from the peritoneal cavity, and the abdominal incision is closed.
The combination of CRS and HIPEC aims to achieve complete cytoreduction of visible tumor deposits, followed by eradicating any remaining microscopic disease with intraperitoneal chemotherapy. This approach offers several potential benefits, including improved local tumor control, reduced risk of peritoneal recurrence, and potentially prolonged survival in select patients with gastric cancer and peritoneal metastases. However, CRS and HIPEC are complex procedures that require careful patient selection, extensive surgical expertise, and a multidisciplinary team approach involving surgical oncologists, medical oncologists, and other specialists. Additionally, patients undergoing CRS and HIPEC may experience perioperative complications and require careful postoperative monitoring and supportive care.
Complications
High-risk patients are more susceptible to complications, which can lead to considerable morbidity or even mortality. Risk factors such as tobacco use, preoperative malnutrition, total gastrectomy, nonmalignant indications for resection, and blood transfusions have been associated with a higher risk of morbidity.[49] As with any surgical procedure, gastric resection carries inherent intraoperative risks, including bleeding and potential injury to surrounding structures, such as iatrogenic spleen injury.[58]
Despite significant advancements in surgical techniques, anesthesiology, postoperative care, and interventional radiology for gastric cancer, gastrectomy still carries risks of severe postoperative complications such as anastomotic leakage and intraabdominal abscess. These complications can impede recovery, delay the initiation of adjuvant chemotherapy, and compromise quality of life. Moreover, postoperative complications have been shown to adversely affect the overall and recurrence-free survival of patients after curative gastrectomy for gastric cancer. Hence, certain complications can have catastrophic effects on both short- and long-term outcomes. Recently reported overall morbidity rates after resection for gastric cancer range from 17.4% to 24.5% in East Asia, with slightly higher rates of 13.6% to 46% in Western countries.[59]
Complications Associated with Gastric Resection
The most common postgastrectomy complications following gastric resection include nutritional deficiencies, dumping syndrome, small gastric remnant, postvagotomy diarrhea, delayed gastric emptying, afferent or efferent loop syndrome, Roux stasis, and bile reflux gastritis.[60]
Postoperative complications can be categorized as either early (occurring within days to weeks) or late (after 6 weeks), as described below.
Early complications: These complications include anastomotic leak, bowel obstruction, postoperative ileus, duodenal stump blowout, delayed gastric emptying, surgical site infection, and intraabdominal infection.
Late complications: Late complications arising after gastric resection encompass various challenges that can significantly impact a patient's well-being and postoperative management.
- Bile reflux gastritis: This condition occurs due to the chronic exposure of the gastric remnant to biliopancreatic secretions caused by the loss of the pylorus. Symptoms include epigastric pain, nausea with vomiting, and pain that is only partially associated with meals. Diagnosis is often made via endoscopy, revealing bile and inflammation in the distal stomach, or through a hepatobiliary iminodiacetic acid (HIDA) scan showing bile pooling in severe cases. Surgical correction is the mainstay of treatment and consists of conversion to Roux-en-Y gastrojejunostomy with at least a 60-cm Roux limb to divert biliopancreatic contents away from the gastric remnant.[60]
- Dumping syndrome: This condition encompasses a constellation of gastrointestinal and vasomotor symptoms triggered by the rapid emptying of hyperosmolar gastric contents into the proximal intestine. Early dumping begins within 30 minutes of food consumption and manifests with both gastrointestinal and vasomotor symptoms, including abdominal pain, diarrhea, bloating, nausea, flushing, palpitations, diaphoresis, tachycardia, syncope, and hypertension. Late dumping occurs 2 to 4 hours after a meal and consists primarily of vasomotor symptoms associated with hypoglycemia. Treatment modalities include dietary adjustments, medical treatment with somatostatin analogs, or surgical interventions for refractory cases.[60]
- Afferent and efferent limb syndrome: These are well-established complications of gastric resection. Afferent loop syndrome, albeit rare, arises from various causes such as internal hernia, marginal ulceration, adhesions, recurrent cancer, or intussusception, particularly in patients with Billroth II gastrectomy. Symptoms include immediate postprandial pain and cramping, followed by vomiting that completely relieves symptoms. Detecting acute afferent loop syndrome within 1 to 2 weeks postoperatively is crucial, given its potential to lead to a duodenal stump leak. Conversely, efferent loop syndrome, characterized by mechanical obstruction at the gastrojejunostomy, can stem from various factors such as anastomotic stricture, marginal ulceration, recurrent cancer, or adhesions. The symptoms of this condition typically include bilious emesis or delayed gastric emptying.[60]
- Internal hernia or Peterson hernia: These are a known cause of acute abdominal pain in patients with gastric resection and Roux-en-Y reconstruction. In these cases, 3 common types of transmesenteric hernias are observed. Transmesocolic hernias involve herniation through the surgical defect in the transverse mesocolon, where the alimentary limb descends. Peterson hernias occur through the potential space between the Roux limb mesentery and the mesocolon, situated behind the alimentary limb. Lastly, bowel herniation can occur through the small bowel mesentery, particularly at the jejunostomy site.[61]
- Additional complications include anastomotic stricture, malnutrition and nutritional deficiencies, marginal ulcers, and cancer recurrence.[61]
Clinical Significance
Gastric resection for malignancy is of paramount clinical significance as it serves as a cornerstone in the management of gastric cancer—a leading cause of cancer-related mortality globally. Surgical resection aims to achieve complete removal of the tumor and associated lymph nodes, offering a potential cure for early-stage disease and improving survival outcomes in locally advanced cases. Furthermore, gastric resection is critical in palliating symptoms such as obstruction, bleeding, and pain, thereby improving the patient's quality of life.
Advancements in endoscopic and surgical techniques, including minimally invasive approaches such as laparoscopic and robotic-assisted gastrectomy, have led to a decrease in the morbidity associated with gastric resection. This underscores its clinical significance as both a curative and palliative treatment option for gastric malignancy. Early detection, careful patient selection, and multidisciplinary collaboration are essential for optimizing the clinical outcomes of gastric resection in managing gastric cancer.
Enhancing Healthcare Team Outcomes
Enhancing patient-centered care, outcomes, safety, and team performance related to gastric resection for malignancy requires a collaborative effort among physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals. Physicians and advanced practitioners must possess strong surgical skills, including proficiency in various gastrectomy techniques and assessing tumor extent and patient suitability for surgery. They should implement evidence-based perioperative management strategies, such as optimizing nutrition and pain control, to enhance patient outcomes. Effective interprofessional communication among healthcare team members is essential for ensuring seamless care coordination, sharing pertinent patient information, and promptly addressing any concerns or changes in the patient's condition.
Nurses are critical in providing holistic care to patients undergoing gastric resection. They provide essential patient education, prepare patients for surgery, oversee postoperative care, and monitor for complications. Pharmacists contribute to ensuring appropriate medication management, including pain control and infection prevention postoperatively. Collaboration among all healthcare team members is essential for effective care coordination, ensuring that each patient receives comprehensive, individualized care tailored to their needs and preferences. Through cohesive teamwork, healthcare professionals can optimize patient-centered care, improve outcomes, enhance patient safety, and elevate team performance in managing gastric malignancies through gastric resection
Nursing, Allied Health, and Interprofessional Team Interventions
Effective communication and coordination among the interprofessional healthcare team are essential to optimize gastric resection outcomes and reduce morbidity and mortality associated with gastric resection. Nursing staff must communicate effectively during handoff to report a history of gastric resection and associated comorbidities. The availability of specialized nurses for support and education is also crucial. Nurses should possess a thorough understanding of potential complications such as bowel obstruction, anastomotic leak, postoperative bleeding, duodenal stump blowout, delayed gastric emptying, and malnutrition. Additionally, promptly identifying patients presenting with acute abdominal pain, nausea, or vomiting can prompt nurses to contact surgical services promptly for further evaluation and intervention.
Media
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May:71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4 [PubMed PMID: 33538338]
Hu Y, Zaydfudim VM. Quality of Life After Curative Resection for Gastric Cancer: Survey Metrics and Implications of Surgical Technique. The Journal of surgical research. 2020 Jul:251():168-179. doi: 10.1016/j.jss.2020.02.005. Epub 2020 Mar 7 [PubMed PMID: 32151826]
Level 2 (mid-level) evidenceSiegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019 Jan:69(1):7-34. doi: 10.3322/caac.21551. Epub 2019 Jan 8 [PubMed PMID: 30620402]
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London, England). 2020 Aug 29:396(10251):635-648. doi: 10.1016/S0140-6736(20)31288-5. Epub [PubMed PMID: 32861308]
Han X, Kong D, He X, Xie S, Li C. A meta-analysis of the effect of laparoscopic gastric resection on the surgical site wound infection in patients with advanced gastric cancer. International wound journal. 2023 Dec:20(10):4300-4307. doi: 10.1111/iwj.14332. Epub 2023 Jul 26 [PubMed PMID: 37493021]
Level 1 (high-level) evidenceXu Q, Sun Z, Li X, Ye C, Zhou C, Zhang L, Lu G. Advanced gastric cancer: CT radiomics prediction and early detection of downstaging with neoadjuvant chemotherapy. European radiology. 2021 Nov:31(11):8765-8774. doi: 10.1007/s00330-021-07962-2. Epub 2021 Apr 28 [PubMed PMID: 33909133]
Ng SP, Leong T. Role of Radiation Therapy in Gastric Cancer. Annals of surgical oncology. 2021 Aug:28(8):4151-4157. doi: 10.1245/s10434-021-09639-y. Epub 2021 Mar 10 [PubMed PMID: 33689079]
Donlon NE, Davern M, Hayes C, Power R, Sheppard AD, Donohoe CL, Lysaght J, Reynolds JV. The immune response to major gastrointestinal cancer surgery and potential implications for adjuvant immunotherapy. Critical reviews in oncology/hematology. 2022 Jul:175():103729. doi: 10.1016/j.critrevonc.2022.103729. Epub 2022 May 31 [PubMed PMID: 35662586]
Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019 Sep:68(9):1545-1575. doi: 10.1136/gutjnl-2018-318126. Epub 2019 Jul 5 [PubMed PMID: 31278206]
Jin T, Liu HD, Yang K, Chen ZH, Zhang YX, Hu JK. Effectiveness and safety of robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of 12,401 gastric cancer patients. Updates in surgery. 2022 Feb:74(1):267-281. doi: 10.1007/s13304-021-01176-3. Epub 2021 Oct 16 [PubMed PMID: 34655427]
Level 1 (high-level) evidenceJoshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA: a cancer journal for clinicians. 2021 May:71(3):264-279. doi: 10.3322/caac.21657. Epub 2021 Feb 16 [PubMed PMID: 33592120]
Caruso S, Giudicissi R, Mariatti M, Cantafio S, Paroli GM, Scatizzi M. Laparoscopic vs. Open Gastrectomy for Locally Advanced Gastric Cancer: A Propensity Score-Matched Retrospective Case-Control Study. Current oncology (Toronto, Ont.). 2022 Mar 9:29(3):1840-1865. doi: 10.3390/curroncol29030151. Epub 2022 Mar 9 [PubMed PMID: 35323351]
Level 2 (mid-level) evidenceBobo Z, Xin W, Jiang L, Quan W, Liang B, Xiangbing D, Ziqiang W. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: meta-analysis and trial sequential analysis of prospective observational studies. Surgical endoscopy. 2019 Apr:33(4):1033-1048. doi: 10.1007/s00464-018-06648-z. Epub 2019 Feb 4 [PubMed PMID: 30719561]
Level 1 (high-level) evidenceDavey MG, Temperley HC, O'Sullivan NJ, Marcelino V, Ryan OK, Ryan ÉJ, Donlon NE, Johnston SM, Robb WB. Minimally Invasive and Open Gastrectomy for Gastric Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Annals of surgical oncology. 2023 Sep:30(9):5544-5557. doi: 10.1245/s10434-023-13654-6. Epub 2023 Jun 1 [PubMed PMID: 37261563]
Level 1 (high-level) evidenceClaassen YHM, van Amelsfoort RM, Hartgrink HH, Dikken JL, de Steur WO, van Sandick JW, van Grieken NCT, Cats A, Boot H, Trip AK, Jansen EPM, Kranenbarg EM, Braak JPBM, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH. Effect of Hospital Volume With Respect to Performing Gastric Cancer Resection on Recurrence and Survival: Results From the CRITICS Trial. Annals of surgery. 2019 Dec:270(6):1096-1102. doi: 10.1097/SLA.0000000000002940. Epub [PubMed PMID: 29995679]
Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2019 Jan:22(1):214-222. doi: 10.1007/s10120-018-0864-4. Epub 2018 Aug 20 [PubMed PMID: 30128720]
Level 1 (high-level) evidenceYu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019 May 28:321(20):1983-1992. doi: 10.1001/jama.2019.5359. Epub [PubMed PMID: 31135850]
Level 1 (high-level) evidenceHyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU, Korean Laparoendoscopic Gastrointestinal Surgery Study Group. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Oct 1:38(28):3304-3313. doi: 10.1200/JCO.20.01210. Epub 2020 Aug 20 [PubMed PMID: 32816629]
Level 1 (high-level) evidenceWang Z, Xing J, Cai J, Zhang Z, Li F, Zhang N, Wu J, Cui M, Liu Y, Chen L, Yang H, Zheng Z, Wang X, Gao C, Wang Z, Fan Q, Zhu Y, Ren S, Zhang C, Liu M, Ji J, Su X. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surgical endoscopy. 2019 Jan:33(1):33-45. doi: 10.1007/s00464-018-6391-x. Epub 2018 Nov 1 [PubMed PMID: 30386984]
Level 1 (high-level) evidenceLi Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA surgery. 2019 Dec 1:154(12):1093-1101. doi: 10.1001/jamasurg.2019.3473. Epub [PubMed PMID: 31553463]
Level 1 (high-level) evidenceMa J, Li X, Zhao S, Zhang R, Yang D. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis. World journal of surgical oncology. 2020 Nov 24:18(1):306. doi: 10.1186/s12957-020-02080-7. Epub 2020 Nov 24 [PubMed PMID: 33234134]
Level 1 (high-level) evidenceMuaddi H, Hafid ME, Choi WJ, Lillie E, de Mestral C, Nathens A, Stukel TA, Karanicolas PJ. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Annals of surgery. 2021 Mar 1:273(3):467-473. doi: 10.1097/SLA.0000000000003915. Epub [PubMed PMID: 32398482]
Level 1 (high-level) evidenceChen X, Feng X, Wang M, Yao X. Laparoscopic versus open distal gastrectomy for advanced gastric cancer: A meta-analysis of randomized controlled trials and high-quality nonrandomized comparative studies. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2020 Nov:46(11):1998-2010. doi: 10.1016/j.ejso.2020.06.046. Epub 2020 Jul 11 [PubMed PMID: 32758382]
Level 1 (high-level) evidenceAiolfi A, Lombardo F, Matsushima K, Sozzi A, Cavalli M, Panizzo V, Bonitta G, Bona D. Systematic review and updated network meta-analysis of randomized controlled trials comparing open, laparoscopic-assisted, and robotic distal gastrectomy for early and locally advanced gastric cancer. Surgery. 2021 Sep:170(3):942-951. doi: 10.1016/j.surg.2021.04.014. Epub 2021 May 20 [PubMed PMID: 34023140]
Level 1 (high-level) evidenceKim GH. Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes. World journal of gastroenterology. 2023 Nov 21:29(43):5800-5803. doi: 10.3748/wjg.v29.i43.5800. Epub [PubMed PMID: 38074917]
Level 2 (mid-level) evidenceSantoro R, Ettorre GM, Santoro E. Subtotal gastrectomy for gastric cancer. World journal of gastroenterology. 2014 Oct 14:20(38):13667-80. doi: 10.3748/wjg.v20.i38.13667. Epub [PubMed PMID: 25320505]
Rosa F, Costamagna G, Doglietto GB, Alfieri S. Classification of nodal stations in gastric cancer. Translational gastroenterology and hepatology. 2017:2():2. doi: 10.21037/tgh.2016.12.03. Epub 2017 Jan 17 [PubMed PMID: 28217752]
Dinescu VC, Gheorman V, Georgescu EF, Paitici Ș, Bică M, Pătrașcu Ș, Bunescu MG, Popa R, Berceanu MC, Pătrașcu AM, Gheorman LM, Dinescu SN, Udriștoiu I, Gheorman V, Forțofoiu MC, Cojan TȚ. Uncovering the Impact of Lymphadenectomy in Advanced Gastric Cancer: A Comprehensive Review. Life (Basel, Switzerland). 2023 Aug 18:13(8):. doi: 10.3390/life13081769. Epub 2023 Aug 18 [PubMed PMID: 37629625]
Marano L, Carbone L, Poto GE, Restaino V, Piccioni SA, Verre L, Roviello F, Marrelli D. Extended Lymphadenectomy for Gastric Cancer in the Neoadjuvant Era: Current Status, Clinical Implications and Contentious Issues. Current oncology (Toronto, Ont.). 2023 Jan 8:30(1):875-896. doi: 10.3390/curroncol30010067. Epub 2023 Jan 8 [PubMed PMID: 36661716]
Palacios-Salas F, Benites-Goñi H, Marin-Calderón L, Bardalez-Cruz P, Vásquez-Quiroga J, Alva-Alva E, Medina-Morales B, Asencios-Cusihuallpa J. Efficacy and Safety of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasms: A Latin American Cohort Study. Clinical endoscopy. 2022 Mar:55(2):248-255. doi: 10.5946/ce.2021.192. Epub 2021 Nov 12 [PubMed PMID: 34763382]
Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intestinal research. 2021 Apr:19(2):127-157. doi: 10.5217/ir.2020.00020. Epub 2020 Oct 13 [PubMed PMID: 33045799]
Level 1 (high-level) evidenceZheng Z, Yin J, Liu XY, Yan XS, Xu R, Li MY, Cai J, Chen GY, Zhang J, Zhang ZT. Current indications for endoscopic submucosal dissection of early gastric cancer. World journal of gastrointestinal oncology. 2021 Jun 15:13(6):560-573. doi: 10.4251/wjgo.v13.i6.560. Epub [PubMed PMID: 34163573]
Ilic M, Ilic I. Epidemiology of stomach cancer. World journal of gastroenterology. 2022 Mar 28:28(12):1187-1203. doi: 10.3748/wjg.v28.i12.1187. Epub [PubMed PMID: 35431510]
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2021 Jan:24(1):1-21. doi: 10.1007/s10120-020-01042-y. Epub 2020 Feb 14 [PubMed PMID: 32060757]
Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2020 Jan:32(2):180-190. doi: 10.1111/den.13531. Epub 2019 Sep 17 [PubMed PMID: 31529716]
Level 3 (low-level) evidenceMakris EA, Poultsides GA. Surgical Considerations in the Management of Gastric Adenocarcinoma. The Surgical clinics of North America. 2017 Apr:97(2):295-316. doi: 10.1016/j.suc.2016.11.006. Epub [PubMed PMID: 28325188]
Imada T, Rino Y, Takahashi M, Suzuki M, Tanaka J, Shiozawa M, Kabara K, Hatori S, Ito H, Yamamoto Y, Amano T. Postoperative functional evaluation of pylorus-preserving gastrectomy for early gastric cancer compared with conventional distal gastrectomy. Surgery. 1998 Feb:123(2):165-70 [PubMed PMID: 9481402]
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017 Jan:20(1):1-19. doi: 10.1007/s10120-016-0622-4. Epub 2016 Jun 24 [PubMed PMID: 27342689]
Narayan RR, Poultsides GA. Advances in the surgical management of gastric and gastroesophageal junction cancer. Translational gastroenterology and hepatology. 2021:6():16. doi: 10.21037/tgh.2020.02.06. Epub 2021 Jan 5 [PubMed PMID: 33409410]
Level 3 (low-level) evidenceGholami S, Janson L, Worhunsky DJ, Tran TB, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber SM, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Efron B, Norton JA, Poultsides GA. Number of Lymph Nodes Removed and Survival after Gastric Cancer Resection: An Analysis from the US Gastric Cancer Collaborative. Journal of the American College of Surgeons. 2015 Aug:221(2):291-9. doi: 10.1016/j.jamcollsurg.2015.04.024. Epub 2015 May 5 [PubMed PMID: 26206635]
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. The British journal of surgery. 2004 Mar:91(3):283-7 [PubMed PMID: 14991627]
Level 1 (high-level) evidenceSasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K, Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. The New England journal of medicine. 2008 Jul 31:359(5):453-62. doi: 10.1056/NEJMoa0707035. Epub [PubMed PMID: 18669424]
Level 1 (high-level) evidenceLo SS, Wu CW, Shen KH, Hsieh MC, Lui WY. Higher morbidity and mortality after combined total gastrectomy and pancreaticosplenectomy for gastric cancer. World journal of surgery. 2002 Jun:26(6):678-82 [PubMed PMID: 12053218]
Pacelli F, Cusumano G, Rosa F, Marrelli D, Dicosmo M, Cipollari C, Marchet A, Scaringi S, Rausei S, di Leo A, Roviello F, de Manzoni G, Nitti D, Tonelli F, Doglietto GB, Italian Research Group for Gastric Cancer. Multivisceral resection for locally advanced gastric cancer: an Italian multicenter observational study. JAMA surgery. 2013 Apr:148(4):353-60. doi: 10.1001/2013.jamasurg.309. Epub [PubMed PMID: 23715879]
Level 2 (mid-level) evidenceTran TB, Worhunsky DJ, Norton JA, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber S, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Poultsides GA. Multivisceral Resection for Gastric Cancer: Results from the US Gastric Cancer Collaborative. Annals of surgical oncology. 2015 Dec:22 Suppl 3():S840-7. doi: 10.1245/s10434-015-4694-x. Epub 2015 Jul 7 [PubMed PMID: 26148757]
Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. International journal of cancer. 2014 Feb 1:134(3):622-8. doi: 10.1002/ijc.28373. Epub 2013 Aug 5 [PubMed PMID: 23832847]
Guner A, Yildirim R. Surgical management of metastatic gastric cancer: moving beyond the guidelines. Translational gastroenterology and hepatology. 2019:4():58. doi: 10.21037/tgh.2019.08.03. Epub 2019 Aug 19 [PubMed PMID: 31559339]
Gholami S, Cassidy MR, Strong VE. Minimally Invasive Surgical Approaches to Gastric Resection. The Surgical clinics of North America. 2017 Apr:97(2):249-264. doi: 10.1016/j.suc.2016.11.003. Epub 2017 Feb 14 [PubMed PMID: 28325185]
Martin AN, Das D, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and Mortality After Gastrectomy: Identification of Modifiable Risk Factors. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2016 Sep:20(9):1554-64. doi: 10.1007/s11605-016-3195-y. Epub 2016 Jun 30 [PubMed PMID: 27364726]
Brenkman HJ, Correa-Cote J, Ruurda JP, van Hillegersberg R. A Step-Wise Approach to Total Laparoscopic Gastrectomy with Jejunal Pouch Reconstruction: How and Why We Do It. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2016 Nov:20(11):1908-1915 [PubMed PMID: 27561635]
Higgins RM, Kubasiak JC, Jacobson RA, Janssen I, Myers JA, Millikan KW, Deziel DJ, Luu MB. Outcomes and Use of Laparoscopic Versus Open Gastric Resection. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2015 Oct-Dec:19(4):. doi: 10.4293/JSLS.2015.00095. Epub [PubMed PMID: 26941544]
Son SY, Hur H, Hyung WJ, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Yang HK, Han SU, Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: 5-Year Outcomes of the KLASS-02 Randomized Clinical Trial. JAMA surgery. 2022 Oct 1:157(10):879-886. doi: 10.1001/jamasurg.2022.2749. Epub [PubMed PMID: 35857305]
Level 1 (high-level) evidenceHe L, Zhao Y. Is Roux-en-Y or Billroth-II reconstruction the preferred choice for gastric cancer patients undergoing distal gastrectomy when Billroth I reconstruction is not applicable? A meta-analysis. Medicine. 2019 Nov:98(48):e17093. doi: 10.1097/MD.0000000000017093. Epub [PubMed PMID: 31770192]
Level 1 (high-level) evidenceLiu HP, Zhang YC, Zhang YL, Yin LN, Wang J. Drain versus no-drain after gastrectomy for patients with advanced gastric cancer: systematic review and meta-analysis. Digestive surgery. 2011:28(3):178-89. doi: 10.1159/000323954. Epub 2011 May 4 [PubMed PMID: 21540606]
Level 1 (high-level) evidenceAlvarez Uslar R, Molina H, Torres O, Cancino A. Total gastrectomy with or without abdominal drains. A prospective randomized trial. Revista espanola de enfermedades digestivas. 2005 Aug:97(8):562-9 [PubMed PMID: 16266223]
Level 1 (high-level) evidenceSun Z, Shenoi MM, Nussbaum DP, Keenan JE, Gulack BC, Tyler DS, Speicher PJ, Blazer DG 3rd. Feeding jejunostomy tube placement during resection of gastric cancers. The Journal of surgical research. 2016 Jan:200(1):189-94. doi: 10.1016/j.jss.2015.07.014. Epub 2015 Jul 16 [PubMed PMID: 26248478]
Wang S, Lin S, Wang H, Yang J, Yu P, Zhao Q, Li M. Reconstruction methods after radical proximal gastrectomy: A systematic review. Medicine. 2018 Mar:97(11):e0121. doi: 10.1097/MD.0000000000010121. Epub [PubMed PMID: 29538208]
Level 1 (high-level) evidenceZhang X, Wei Z, Fu H, Hu Z, Wang W, Yan R. Predictors of iatrogenic splenic injury in radical gastrectomy for gastric cancer. Frontiers in oncology. 2024:14():1361185. doi: 10.3389/fonc.2024.1361185. Epub 2024 Mar 27 [PubMed PMID: 38601758]
Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection. Surgery today. 2020 Jan:50(1):3-11. doi: 10.1007/s00595-019-01877-8. Epub 2019 Sep 18 [PubMed PMID: 31535226]
Davis JL, Ripley RT. Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. The Surgical clinics of North America. 2017 Apr:97(2):277-293. doi: 10.1016/j.suc.2016.11.005. Epub [PubMed PMID: 28325187]
Patel RY, Baer JW, Texeira J, Frager D, Cooke K. Internal hernia complications of gastric bypass surgery in the acute setting: spectrum of imaging findings. Emergency radiology. 2009 Jul:16(4):283-9. doi: 10.1007/s10140-008-0781-7. Epub 2008 Dec 17 [PubMed PMID: 19089479]