Introduction
Gastric cancer is the fourth leading cause of cancer deaths worldwide and the fifth most frequently diagnosed cancer.[1] The incidence of gastric cancer shows significant geographic variation and has declined over the past 3 decades.[2] Risk factors include dietary habits, genetic syndromes, Helicobacter pylori infection, and age.[2] High-risk populations may benefit from screening programs, although such programs are uncommon in Western countries. Gastric cancer primarily arises in 2 histologic forms: intestinal and diffuse-type cancers.[3] Gastric cancers are more common in the distal stomach (antrum and body), but proximal gastric cancers are increasing in frequency.[4]
Often, gastric cancer is advanced at diagnosis due to the late onset of symptoms. Endoscopy is the diagnostic test of choice, allowing for visual inspection, tissue sampling, and resection of early tumors.[5] Gastrectomy remains the mainstay of curative treatment, with the extent of gastric resection tailored to tumor location and size. A comprehensive lymphadenectomy is also recommended. Patient outcomes improve with perioperative chemotherapy and potentially with radiotherapy.
Recently, targeted agents and immunotherapy have significantly improved outcomes in patients with actionable mutations. Despite these advancements, the overall prognosis for gastric cancer remains poor, especially in less developed regions.[5] Gastroesophageal junction (GEJ) tumors have separate staging, treatment, and prognosis. Please see StatPearls' companion resource, "Esophageal Cancer," for more information on these malignancies.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Gastric Cancer Risk Factors
Gastric cancers are typically sporadic, but 5% to 10% of cases have a familial or genetic association.[4] The key risk factors for gastric cancer include:
- Helicobacter pylori: Chronic and recurrent H pylori infections are well-established risk factors for gastric adenocarcinoma. The duration of infection, other risk factors, and host factors likely play significant roles in the pathogenesis.[4] Please see StatPearls' companion resource, "Helicobacter Pylori," for more information.
- Epstein-Barr virus: This viral infection has been increasingly noted in association with gastric cancer, usually affecting the proximal stomach.[6] Please see StatPearls' companion resource, "Epstein-Barr Virus," for more information.
- Dietary factors: Risk factors include high salt intake (salt-preserved foods), consumption of N-nitroso compounds, smoking, a diet low in vitamins A and C, high consumption of smoked or cured foods, lack of refrigerated foods, and contaminated drinking water.[7]
- Obesity: A high body mass index increases the risk of proximal gastric and GEJ cancers.[7]
- Smoking: Smoking increases the risk of gastric cancer, particularly in men.[7]
- Gastroesophageal reflux: Chronic reflux is associated with a higher rate of GEJ adenocarcinomas. Additionally, bile reflux into the stomach, as seen in patients after certain gastric surgeries, increases the risk of gastric cancer.[7]
- Environmental factors: Exposure to rubber manufacturing, tin mining, metal processing, and coal increases the risk of gastric cancer.[7]
- Host factors: Individuals with type A blood have approximately a 20% higher incidence of gastric cancer compared to those with blood groups O, B, or AB, particularly with the diffuse type. Pernicious anemia and chronic atrophic gastritis increase the risk of intestinal-type gastric cancer up to 6-fold. Please see StatPearls' companion resources, "Pernicious Anemia" and "Atrophic Gastritis," for more information. Other conditions associated with an increased risk include benign gastric ulcers, hypertrophic gastropathy, and gastric polyps.[7]
- Protective factors: A high intake of fiber, fruits, and vegetables likely provides a protective benefit against gastric cancer.[7]
- Genetic associations: A subset of gastric cancers are associated with genetic syndromes, including:
- Hereditary nonpolyposis colon cancer has a 13% lifetime risk, predominantly intestinal type. Please see StatPearls' companion resource, "Hereditary Nonpolyposis Colorectal Cancer," for more information.
- Familial adenomatous polyposis has a 10% risk. Please see StatPearls' companion resource, "Familial Adenomatous Polyposis," for more information.
- Peutz-Jeghers syndrome has a 29% risk. Please see StatPearls' companion resource, "Peutz-Jeghers Syndrome," for more information.
- Juvenile polyposis syndrome has a 21% risk.
- Li-Fraumeni syndrome is associated with gastric cancer. Please see StatPearls' companion resource, "Li-Fraumeni Syndrome," for more information.
- Hereditary breast and ovarian cancer syndrome are associated with gastric cancer.
- Phosphatase and tensin homolog or hamartoma tumor (Cowden) syndrome are also associated with gastric cancer. Please see StatPearls' companion resource, "Cowden Disease," for more information.
Epidemiology
Gastric cancer is the fifth most common and the fourth most lethal malignancy worldwide.[7] Although incidence and mortality rates have declined, they remain significantly higher in low-resource countries and East Asia. In the United States, approximately 26,890 newly detected cases of gastric cancer are expected to be diagnosed in 2024. Gastric cancer is more prevalent among men than women.
In developed countries, the rate of distal gastric cancers has declined dramatically due to better nutrition, access to clean water, and the early identification and treatment of H pylori infection. However, the rates of proximal gastric and GEJ cancers have been increasing. The overall rates of intestinal and diffuse-type gastric cancers have declined, with the decline being more marked for intestinal-type cancers. As a result, diffuse-type cancers now constitute a significant proportion of all gastric cancers. Intestinal-type cancers are more common in men, whereas diffuse-type cancers occur equally in both sexes.[7]
Pathophysiology
According to the Lauren classification, gastric adenocarcinoma has 2 main histologic variants.[8] The most common variant is the intestinal type, characterized by gland formation and similar in appearance to other intestinal adenocarcinomas. The less common diffuse-type gastric cancer lacks intercellular adhesion molecules and does not typically have gland formation. Intestinal-type cancers may be associated with signet-ring cells.
The molecular drivers for intestinal-type cancers are likely multifactorial. H pylori is the most common inciting factor for carcinogenesis, and evidence suggests that these lesions progress from dysplasia to carcinoma over time. Precancerous lesions and conditions predisposing to cancer, such as gastric ulcers, atrophic gastritis, and pernicious anemia, often precede the development of cancer.[8] In diffuse-type cancers, no defined precancerous lesions have been identified.
Histopathology
Gross Pathology Features
On gross examination, gastric cancer typically presents as a mass or a nonhealing ulcer. Diffuse gastric cancers, however, may lack a noticeable mass due to extensive submucosal infiltration. This extensive infiltration leads to a nondistended stomach characterized by the absence of rugal folds. This distinctive appearance is called "leather bottle stomach" or linitis plastica.[9]
Histopathology Findings
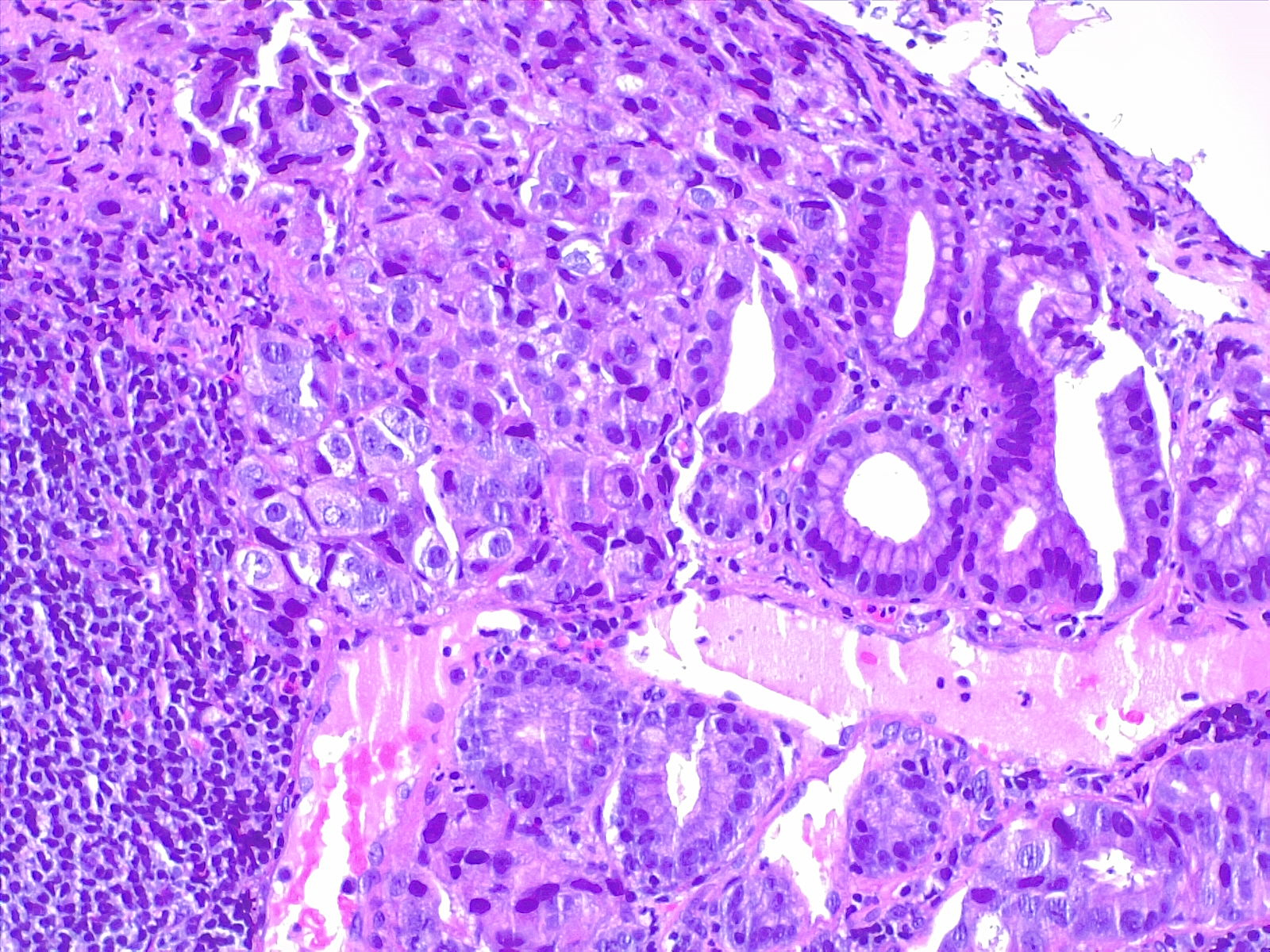
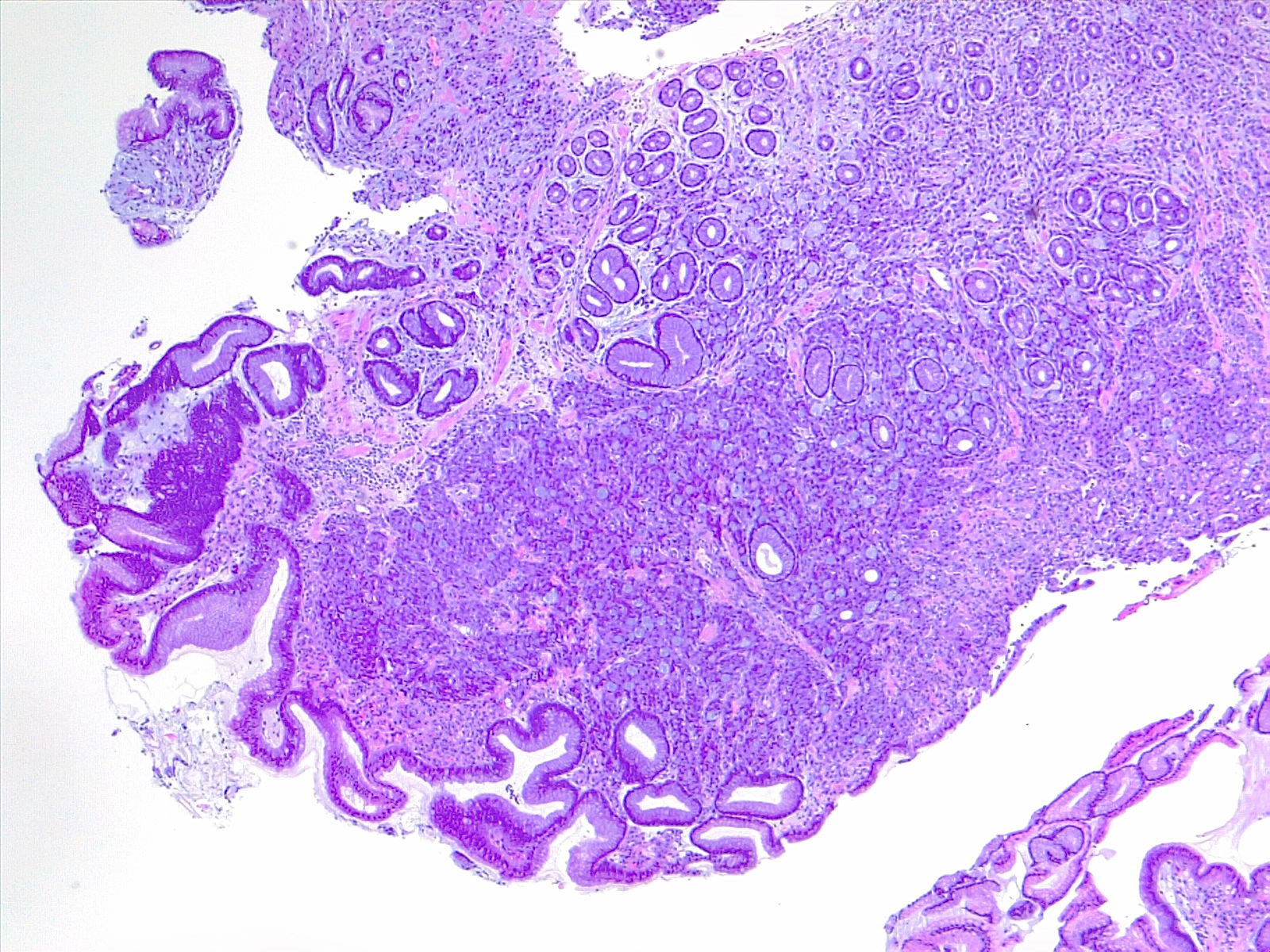
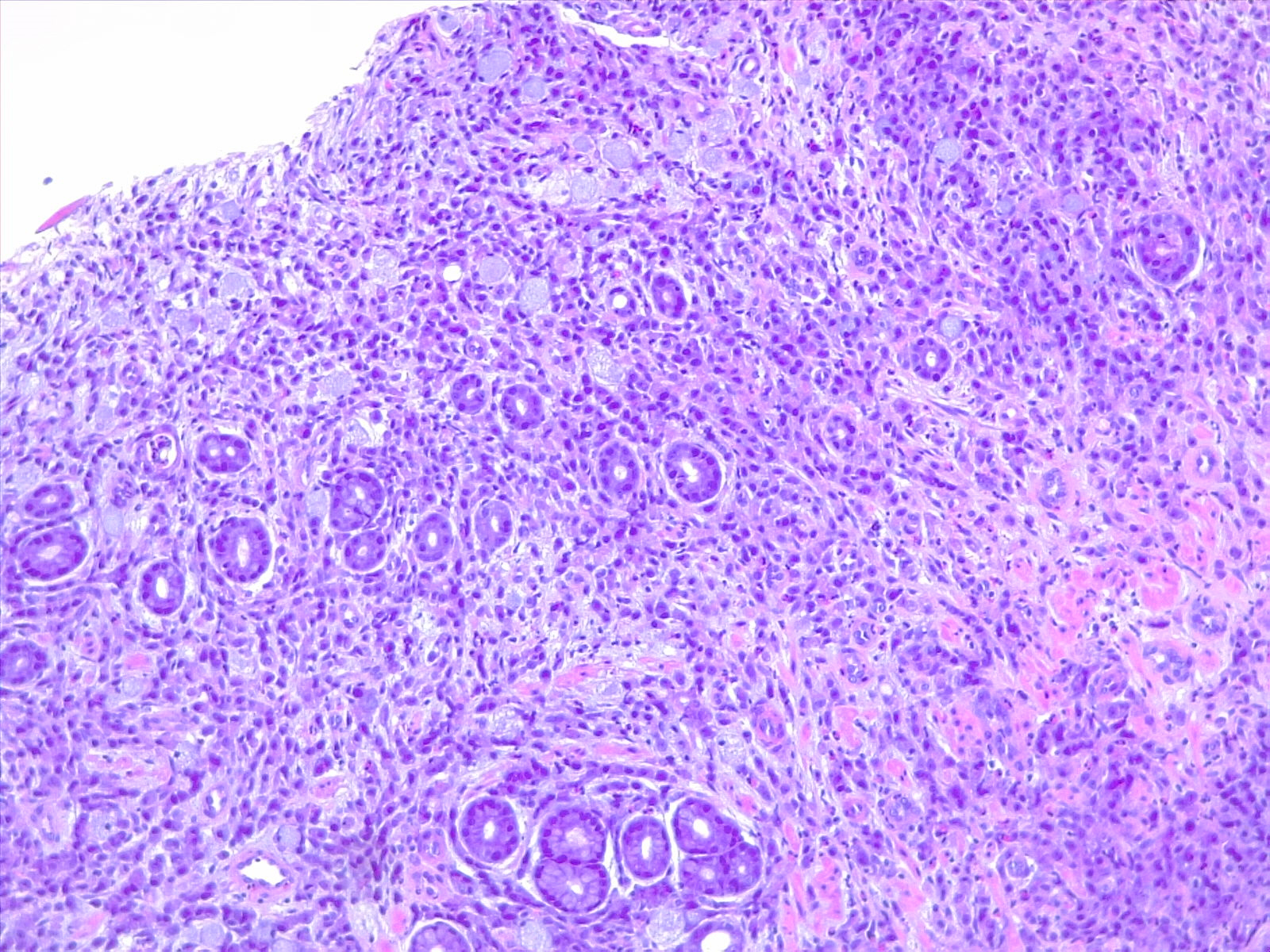
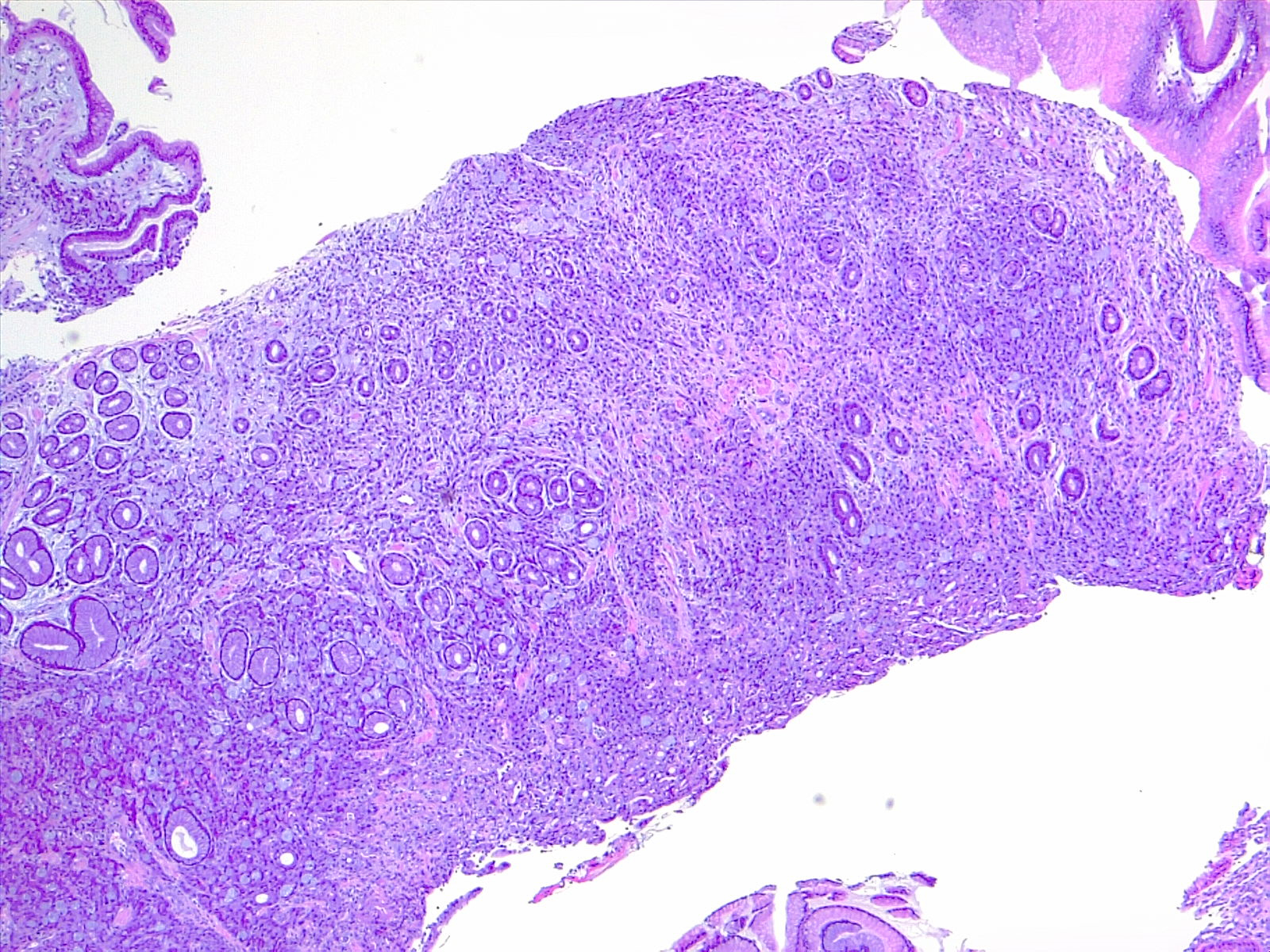
Microscopically, the histologic appearance of gastric cancer has traditionally been classified under the Lauren classification, which divides gastric cancers into diffuse-type and intestinal-type tumors (see Image. Gastric Carcinoma).[10] Diffuse-type tumors exhibit markedly aberrant cells with little or no gland formation and occasionally contain signet ring cells (see Image. Diffuse Carcinoma of the Stomach) characterized by cytoplasmic mucin with an eccentrically placed nucleus (see Images. Diffuse Gastric Cancer and Signet Ring Carcinoma). Depending on tumor differentiation, intestinal-type tumors demonstrate gland formation, ranging from well-formed to markedly aberrant glands. Due to the cohesive nature of these cells, they often present as mass lesions. More recent classifications, eg, the World Health Organization classification, are more detailed and subdivide adenocarcinomas into several distinct subtypes. The impact of these subtypes on overall prognosis remains unclear.
Molecular Pathology
Identifying molecular drivers of carcinoma is increasingly important in determining prognosis and treatment. The Cancer Genome Atlas-based molecular classification [11] of gastric cancer incorporates molecular diagnostics and clinical and histologic features, including:
- Epstein-Bar Virus-associated: These tumors, located in the proximal stomach, are characterized by CIMP (CpG island methylator phenotype), mutations in PIK3CA, ARID1A, and BCOR, and amplifications of programmed death protein 1 and L2.
- Microsatellite instability: Resulting from defects in mismatch repair genes, these tumors are hypermutated and typically found in more distal cancers.
- Genomically stable: Genomic stability is associated with diffuse-type cancers, which have the lowest overall mutational burden, with mutations in CDH1 often present.
- Chromosomal instability: Carcinomas with chromosomal instability are the most common group of gastric cancers. They occur in the gastroesophageal junction and cardia and have multiple associated mutations, the most common being TP53.
- HER2 gene amplification: HER2 gene amplification is reported in 12% to 27% of gastric cancer cases, with protein overexpression in 9% to 23% of cases. HER2 positivity, more frequent in the intestinal subtype (33%) compared to the diffuse subtype (8%), has been associated with tumor invasion, lymph node metastasis, and poorer survival. HER2 testing is recommended for all metastatic gastric cancer, using immunohistochemistry scoring: negative for 0 or 1+, positive for 3+, with reflex fluorescent in situ hybridization for an equivocal 2+ score to confirm (see Image. HER2 Analysis)
The United States Food and Drug Administration has approved immunotherapy for patients with microsatellite instability in solid tumors, including gastric cancer, allowing the evaluation of immunotherapy potential in patients with metastatic disease who have progressed on standard therapy. Epstein-Bar (EBV)positive gastric tumors generally have a better prognosis, although routine EBV staining is not yet recommended in clinical care.[12][13][14][15][16]
History and Physical
Clinical History
Gastric cancer often presents with a variety of symptoms that can be subtle and easily mistaken for less severe conditions in its early stages. Common symptoms include persistent indigestion, stomach discomfort, and a feeling of fullness or bloating after small meals. As the disease progresses, patients may experience more severe symptoms such as unexplained weight loss, persistent nausea or vomiting, difficulty swallowing, and noticeable fatigue. There may be visible signs of gastrointestinal bleeding, such as black or bloody stools, and in advanced stages, a palpable mass in the abdomen. Due to the nonspecific nature of these symptoms, gastric cancer is frequently diagnosed at a later stage, emphasizing the importance of early medical evaluation for persistent gastrointestinal issues. In high-risk populations that undergo screening endoscopy (most commonly seen in Japan and South Korea), gastric cancer is often diagnosed at an early stage.[10]
Physical Examination Findings
Physical examination findings in gastric cancer are typically evident only in the advanced stages of the disease. These may include an abdominal mass or a dilated stomach suggestive of gastric outlet obstruction. Metastatic gastric cancer can present with several distinctive signs, including the Virchow node (ie, left supraclavicular adenopathy), Sister Mary Joseph node (ie, periumbilical nodule), and Irish node (ie, left axillary node). Direct metastasis to the peritoneum may manifest as a Krukenberg tumor (ovarian mass), Blumer shelf (cul-de-sac mass), ascites (peritoneal carcinomatosis), and hepatomegaly, often indicating a diffuse disease burden.[10] Paraneoplastic manifestations, although rare and nonspecific to gastric cancer, can include various dermatological conditions (eg, diffuse seborrheic keratosis or acanthosis nigricans), hematological disorders (eg, microangiopathic hemolytic anemia and a hypercoagulable state known as Trousseau syndrome), renal disorders (eg, membranous nephropathy), and autoimmune diseases (eg, polyarteritis nodosa).[17]
Evaluation
Diagnostic Evaluation
Patients exhibiting symptoms indicative of gastric cancer should undergo an upper endoscopy as a primary diagnostic tool.[4] Although upper endoscopy is more invasive and costly than traditional barium or gastrografin swallow studies, it offers the critical advantage of direct tissue diagnosis through biopsy of esophageal, gastric, or duodenal lesions. Any suspicious gastric ulcer should be biopsied multiple times to improve diagnostic accuracy significantly; sensitivity increases from approximately 70% with a single biopsy to 98% with 7 biopsies. Meticulous documentation of the lesion's location, size, and characteristics is essential.[18] Advanced techniques such as chromoendoscopy and narrow-band imaging should be utilized if there is a suspicion of more subtle mucosal lesions. Also, multiple mucosal biopsies should be obtained if H pylori infection, atrophic gastritis, or other pathologies are suspected. Upper endoscopy has been highly effective in detecting early-stage gastric cancer in high-incidence areas like Japan and South Korea, where it has led to higher cure rates following resection. However, its practice is less common in other regions.
Following upper endoscopy, an endoscopic ultrasound (EUS) is often warranted for a more detailed assessment of gastric cancer lesions.[4] EUS is instrumental in determining the T stage of the tumor and evaluating the perigastric lymph nodes, which can be biopsied via EUS-guided fine-needle aspiration. The pooled sensitivity and specificity of EUS for distinguishing between T1 and T2 tumors are 0.85 and 0.90, respectively, with confidence intervals of 0.78 to 0.91 and 0.85 to 0.93, respectively. This distinction is crucial, as T1 gastric cancers might be eligible for endoscopic resection. However, the accuracy of EUS is highly dependent on the operator's skill, and less experienced clinicians may have compromised precision in assessing gastric wall invasion.[19]
Staging Evaluation
For comprehensive preoperative staging, chest and abdominal imaging are necessary to exclude metastasis and determine surgical resectability.[4] Early abdominopelvic computed tomography (CT) is performed to rule out gross metastatic disease. However, it has limitations in assessing T and N stages and detecting small peritoneal metastases, with an overall accuracy ranging from 42% to 82%. Biopsy of any suspicious solitary or oligometastatic sites should be performed, and paracentesis is recommended if malignant ascites is suspected. Chest CT is preferred over plain radiographs for better evaluation. In cases where initial staging is negative for metastatic disease, positron emission tomography combined with CT may be used to assess resectability. Serum markers, including carcinoembryonic antigen, cancer antigen 125, carbohydrate antigen 19-9, and cancer antigen 72-4, have limited diagnostic value and may be elevated due to other conditions.
Staging laparoscopy with peritoneal washings for cytology is advised for patients with potentially resectable disease before starting chemotherapy.[4] During diagnostic laparoscopy, up to 40% of patients may be found to have peritoneal disease, detected through direct biopsy or cytology from peritoneal washings, which might not be identified by other preoperative imaging.[4] Patients with detected peritoneal disease are generally considered to have metastatic disease and typically do not undergo surgery. However, a small number may be candidates for aggressive cytoreduction and intraperitoneal chemotherapy.
Treatment / Management
The treatment modalities used for treating gastric cancer include endoscopic resection, surgery, chemotherapy, radiation, and targeted therapies. Often, these techniques are combined based on tumor location, stage, and patient-related factors. Endoscopic techniques are reserved for tumors limited to the mucosa or the very superficial submucosa without high-risk factors. Most patients with tumors greater than T2 or who have any evidence of nodal involvement should undergo perioperative chemotherapy followed by surgery. Patients with advanced or nonresectable tumors should undergo chemotherapy with palliative intent. Radiation is typically reserved for palliation or when surgical resection is inadequate. Each modality is discussed under the relevant section.
Differential Diagnosis
Differential diagnoses that should be considered when evaluating gastric cancer include:
- Acute gastritis
- Atrophic gastritis
- Bacterial gastroenteritis
- Chronic gastritis
- Esophageal cancer
- Esophageal stricture
- Esophagitis
- Non-Hodgkin lymphoma
- Peptic ulcer disease
- Viral gastroenteritis
Surgical Oncology
Endoscopic Resection for Early Local Disease
Endoscopic techniques for treating gastric cancer include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). These minimally invasive procedures are utilized for early-stage gastric cancer, mainly when the tumors are confined to the mucosa or superficial submucosa.[20] EMR involves the removal of the target lesion using a snare device.[21] The snare, often equipped with electrocautery, excises the lesion from the gastric wall. This technique is generally suited for small, well-defined lesions and can be performed either with or without electrocautery, which helps achieve hemostasis and reduce bleeding during the procedure. EMR is typically used for tumors less than 2 cm in diameter confined to the mucosal layer. The procedure offers diagnostic and therapeutic benefits, allowing for the removal and immediate evaluation of the lesion.
Endoscopic submucosal dissection (ESD) is more sophisticated and complex than EMR. ESD involves dissecting the submucosa around the target lesion using either sharp dissection or cautery.[21] This technique removes larger lesions and those with a more invasive pathology. The procedure starts with creating a submucosal lift, followed by careful dissection of the lesion from the underlying muscle layer. ESD is preferred for lesions that are not amenable to EMR due to their size or depth of invasion. This procedure allows for en-bloc resection, where the entire lesion is removed in 1 piece, which is critical for accurate staging and reducing the risk of local recurrence. Endoscopic resection is considered a viable therapeutic option when specific criteria are met.[21] These criteria include:
- Lesion size: The lesion should be <2 cm in diameter.
- Histological characteristics: The lesion should be moderate to well-differentiated, indicating a lower likelihood of aggressive behavior.
- Clear margins: The margins of the resected specimen must be free of cancer cells, ensuring that the tumor has been completely removed.
- Absence of high-risk features: The lesion should not exhibit high-risk features, such as lymphovascular or perineural invasion. These features indicate a higher risk of metastasis and a poorer prognosis.
Clinicians should note if the tumors extend beyond the superficial submucosa, as tumors that invade the deeper submucosa or beyond are associated with significantly higher rates of lymph node metastasis and are generally not suitable for endoscopic resection alone. When performed by skilled clinicians, endoscopic dissection is typically done as an en-bloc resection, which improves the accuracy of staging and reduces the risk of residual cancer. The success rates of endoscopic resection are high, with 5-year overall survival rates ranging from 84% to 96%, depending on the tumor's depth of invasion and other pathological factors. These rates compare favorably with those for gastrectomy, which can offer up to 98% survival for early-stage disease. However, no randomized controlled trials directly compare endoscopic resection with gastrectomy; therefore, treatment decisions should be based on individual patient factors, tumor characteristics, and available expertise. In summary, endoscopic techniques, including EMR and ESD, provide effective, minimally invasive options for managing early gastric cancer. The choice between EMR and ESD depends on the size, depth, and characteristics of the lesion, as well as the expertise of the performing team.[22][23][24]
Gastric Resection
Surgery for gastric cancer has made remarkable advancements over recent decades and remains the only definitive cure for patients without metastatic disease. The integration of perioperative or neoadjuvant chemotherapy before surgical resection has significantly improved survival rates for those with locally advanced gastric cancer. Experts now recommend it for most patients with this stage of disease. For patients with T1a and T1b gastric cancers, where endoscopic resection is not feasible, upfront surgical resection remains the standard approach. For tumors at T2 or higher, diagnostic laparoscopy is often employed to assess peritoneal involvement, including peritoneal biopsies and cytology. This is followed by perioperative chemotherapy, with surgical resection performed upon completion of the chemotherapy regimen. The chemotherapy regimen's choice and duration are individualized based on patient-specific factors detailed in the medical oncology section.[10]
The surgical approach varies depending on the tumor's location within the stomach. The fundamental principles of surgery involve achieving an adequate proximal and distal margin, typically 3 to 5 cm, and performing a complete lymphadenectomy of the perigastric lymph nodes (D2 lymphadenectomy) along with appropriate reconstruction. Distal gastric cancers involving the stomach's antrum or distal body are generally treated with distal subtotal gastrectomy. For tumors located in the proximal body or fundus of the stomach, total gastrectomy is often required. For smaller and less advanced tumors, a proximal gastrectomy may be considered. Gastric reconstruction is typically performed using Roux-en-Y gastrojejunostomy for distal subtotal gastrectomy and Roux-en-Y esophagojejunostomy for total gastrectomy. In addition, feeding jejunostomy tubes may be placed to provide nutritional support post-surgery.[10][25]
The extent of lymphadenectomy has been a subject of extensive debate. D1 lymphadenectomy involves the removal of only the perigastric lymph nodes, whereas D2 lymphadenectomy includes these nodes and those along the major vascular axis surrounding the stomach. Two large trials by the Dutch Cancer Group and the Medical Research Council, which compared D1 with D2 lymphadenectomy, were initially flawed and underpowered to conclusively demonstrate the benefits of D2 lymphadenectomy. However, a long-term follow-up study from the Dutch trial, with a median of 15 years and involving 1078 patients, showed results indicating that D2 lymphadenectomy was associated with reduced locoregional recurrence (12% versus 22%), regional recurrence (13% versus 19%), and gastric cancer-related mortality (37% versus 48%) compared to D1 lymphadenectomy.[26] Despite these benefits, D2 lymphadenectomy was associated with significantly higher operative morbidity (10% versus 4%), complication rates (43% versus 25%), and reoperation rates (18% versus 8%). Nevertheless, advances in surgical techniques, such as spleen-preserving D2 resection, have mitigated some of these risks, making D2 lymphadenectomy the recommended approach for resectable gastric cancer.[10][25]
D3 super-extended lymphadenectomy, which includes periaortic dissection, was studied in the Japan Clinical Oncology Group trial 9501. This approach showed no added survival benefit and was associated with significantly higher perioperative complication rates.[27] Current guidelines recommend sampling at least 15 lymph nodes, which has been shown to provide a survival benefit. Minimally invasive techniques, such as laparoscopic surgery, may be employed by experienced surgeons with outcomes comparable to those of open surgery.[4]
In highly selected patients with gastric cancer and peritoneal metastases and no evidence of other distant metastasis, cytoreduction with or without hyperthermic intraperitoneal chemotherapy may be indicated. Patients should undergo preoperative chemotherapy to ensure that there is no disease progression before undergoing cytoreduction. Phase 3 randomized control trials on the role of cytoreduction and hyperthermic intraperitoneal chemotherapy are ongoing.[28]
For patients with gastric cancer who experience obstruction or uncontrolled bleeding, palliative resection may be necessary. However, chemotherapy and radiation therapy are often effective in managing symptoms and should be considered as first-line palliative treatments. Surgery should be reserved for cases where other modalities fail to provide adequate palliation.
Radiation Oncology
The role of radiation therapy (RT) in gastric cancer has evolved. Adjuvant chemoradiation was recommended as the treatment of choice in patients who had not undergone preoperative chemotherapy. This was based primarily on the SWOG 9008/INT0116, a randomized phase 3 trial that assigned 556 patients with stages I to III gastroesophageal (20%) or gastric cancers (80%) to receive either surgery alone or surgery followed by adjuvant combined chemoradiotherapy. There was an approximate 20% improvement in survival for the group receiving the combined-modality therapy. The median overall survival in the surgery-only group was 27 months compared with 36 months in the chemoradiation group; the HR for death in the surgery-only arm was 1.35 (95% CI, 1.09 to 1.66; P = .005), and relapse HR was 1.52 (95% CI, 1.23 to 1.86; P < .001).[29] The study has been criticized for the meager rate of D1 (<54%) or D2 (<10%) lymph node dissection, which was mandated per protocol. The rate of distant metastasis was not reduced in the adjuvant chemoradiation group (16% versus 18%), suggesting that adjuvant therapy could have mainly compensated for inferior surgery.
CALGB Intergroup C80101 tried to improve the results obtained with INT0116 protocol regimen therapy by randomly selecting patients with resected gastric or GEJ cancer to receive chemoradiotherapy with epirubicin, cisplatin, 5-fluorouracil. This study was not adequately powered to assess noninferiority and did not demonstrate any outcome difference between both arms. The Korean ARTIST trial provided further combined therapy evidence. This trial evaluated the addition of radiation therapy to adjuvant chemotherapy in patients who underwent gastrectomy with D2 lymph node dissection. Patients were randomly selected to receive 6 cycles of chemotherapy with cisplatin and capecitabine (CX) or 2 cycles of CX followed by chemoradiation, followed by 2 more cycles of CX. At a median follow-up of 84 months, neither disease-free survival (DFS) nor overall survival differed between the 2 arms. However, unplanned subsets of patients with node-positive disease and intestinal-type gastric cancer did have a significantly improved DFS with the addition of radiation therapy.[30]
The ARTIST 2 trial sought to clarify this benefit, randomizing patients who underwent a D2 lymphadenectomy to adjuvant chemotherapy alone (S1 alone or S1 with oxaliplatin) or adjuvant chemoradiation. There was no benefit to adding radiation, with 3-year overall survival actually being higher in the chemotherapy arms.[31] The current role of radiation therapy is mostly in patients who have had inadequate surgery (ie, less than D2 dissection), are unable to tolerate chemotherapy, or in bulky tumors that are symptomatic or those that have local invasion.
Medical Oncology
Neoadjuvant and Adjuvant Therapy for Locally Advanced Resectable Disease
Surgical resection alone can potentially cure early-stage gastric cancer, with a 5-year overall survival rate of 75% for stage I, declining significantly to 35% for stage II and 25% or less for stage III. Most patients who recur after surgery do so distally, which has focused research on neoadjuvant (preoperative) and adjuvant (postoperative) therapies. Perioperative chemotherapy combines the 2 approaches, with chemotherapy administered before and after surgery.
Perioperative chemotherapy
Perioperative chemotherapy aims to downstage primary tumors and regional lymph nodes, increasing the likelihood of long-term curative resections. There is a recommendation for high-risk patients (eg, those with bulky T3/T4 tumors, perigastric nodes, linitis plastica, or positive peritoneal cytology) to avoid unnecessary surgery if metastases appear. The MAGIC trial was a significant study in this area, involving 503 patients with resectable stomach cancer, GEJ cancer, and distal esophagus cancer. The trial compared perioperative chemotherapy (3 preoperative and 3 postoperative cycles of epirubicin, cisplatin, and fluorouracil) to surgery alone, finding significant improvements in median overall survival (36% versus 23% 5-year survival) and progression-free survival for the chemotherapy group. The trial suggested that preoperative treatment was the main contributor to improved outcomes since only 55% of patients completed postoperative therapy.[32] The French FNLCC/FFCD ACCORD trial also reported benefits for perioperative chemotherapy, showing better R0 resection rates, reduced disease-free survival, and lower mortality risk. Conversely, the EORTC trial 40954 failed to show survival benefits due to limited accrual.[33]
The FLOT4-AIO trial compared the FLOT regimen (docetaxel, oxaliplatin, leucovorin, and fluorouracil) to an epirubicin-containing regimen, showing higher pathological complete response rates and less toxicity except for neutropenia in the FLOT regimen. Preliminary results favored the FLOT regimen with a median overall survival of 50 months compared to 35 months for the epirubicin-containing regimen.[34][35] Future research aims to determine the benefits of neoadjuvant chemoradiation over chemotherapy alone. Patients undergoing perioperative chemotherapy are restaged after completing 3 to 4 cycles of chemotherapy. If no evidence of disease progression is noted, then surgery is performed. Most patients then receive postoperative chemotherapy for another 3 to 4 cycles, although this depends on patient tolerance and their recovery from surgery.
Adjuvant chemotherapy
Adjuvant chemotherapy is recommended after surgery in patients who have not received preoperative therapy. Adjuvant chemotherapy alone was evaluated in the Japanese ACTS-GC phase 3 trial with S1 (an oral fluoropyrimidine 80 to 120 mg daily for 4 weeks, repeated every 6 weeks for 1 year) as postoperative D2-resection adjuvant therapy compared to surgery alone in 1059 patients with stage II or III gastric cancer and demonstrated a survival benefit with a 5-year overall survival rate of 72% in the S-1 group and 61% in the surgery only group.[36] Similar results were reported by the Korean phase 3 multicenter CLASSIC trial and in a meta-analysis of several trials, where chemotherapy was associated with a significant overall survival benefit with an HR of 0.85 (95% CI, 0.80 to 0.90) and an improvement in DFS (hazard ratio 0.79; 95% CI, 0.72 to 0.87) versus surgery alone.[37] In general, the adjuvant therapy approach is favored in East Asia, while perioperative chemotherapy, usually with the FLOT regimen, is favored in the West.
Palliative Therapy for Locally Advanced Unresectable and Advanced Metastatic Disease
The treatment of unresectable locally advanced gastric cancer typically involves regimens used for advanced metastatic disease. The primary goals of medical therapy for advanced gastric cancer are palliative: to alleviate symptoms, enhance quality of life, and modestly extend survival. Several chemotherapy agents are effective, including fluoropyrimidines (eg, fluorouracil and capecitabine), anthracyclines (eg, epirubicin), platinum compounds (eg, cisplatin, oxaliplatin), taxanes (eg, paclitaxel and docetaxel), irinotecan, and targeted therapies like trastuzumab for HER2-positive cancers and ramucirumab, a vascular endothelial growth factor 2 antibody. Combination regimens have a higher response rate (up to 65%) than single-agent therapies (up to 40%).
Patients eligible for chemotherapy should be tested for HER2 overexpression. Patients that are HER2-positive can receive trastuzumab combined with chemotherapy (commonly fluoropyrimidine plus platinum). For HER2-negative patients in good health, doublet therapy is recommended over triplet regimens due to the higher toxicity of the latter, which is reserved for select patients with excellent performance status. Older or poor-performance individuals may be treated with single agents like fluorouracil, capecitabine, irinotecan, or low-dose taxanes. Docetaxel and irinotecan have shown benefits in second-line chemotherapy, but no optimal standard of care exists.
Fluorouracil-based combination trials have established the benefits of adding anthracyclines, platinum agents, or taxanes over older treatments. For example, the German trial compared FLP (fluorouracil, leucovorin, cisplatin) with FLO (fluorouracil, leucovorin, oxaliplatin) and found superior survival with FLO in patients older than 65. The TAX-325 trial demonstrated the superiority of adding docetaxel to cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone (CF). However, DCF had higher toxicity, including febrile neutropenia, limiting its use to patients with good performance status.[38] The REAL-2 trial substituted capecitabine for fluorouracil and oxaliplatin for cisplatin, showing that EOX (epirubicin, oxaliplatin, capecitabine) was less toxic and provided modestly more prolonged survival than ECF (epirubicin, cisplatin, fluorouracil). FOLFIRI (fluorouracil, leucovorin, irinotecan) has shown similar efficacy to other regimens with less toxicity, making it an alternative for patients unable to tolerate platinum-based treatments.
Targeted agents like trastuzumab have shown efficacy in advanced gastric cancer. The ToGA trial added trastuzumab to chemotherapy in HER2-positive individuals, improving median overall survival, progression-free survival, and overall response rate without significant toxicity differences.[39] Consequently, trastuzumab with chemotherapy is the standard for HER2-positive cancers. However, in clinical trials, lapatinib, another HER2/EGFR inhibitor, did not show survival benefits. Similarly, cetuximab and panitumumab, targeting EGFR, worsened toxicity and overall survival in trials.[10]
Angiogenesis inhibitors have yielded mixed results. In the AVAGAST trial, bevacizumab improved progression-free survival and overall response rate but not overall survival. At the same time, ramucirumab showed modest survival benefits in second-line settings, leading to its approval. However, adding ramucirumab to first-line therapy did not show benefits.[40] After first-line chemotherapy failure, second-line options include irinotecan or paclitaxel, which improve overall survival compared to the best supportive care. Radiation therapy can provide symptomatic relief but is rarely used alone. Novel agents under investigation include olaparib, which showed significant benefits in combination with paclitaxel, and pembrolizumab, an anti-programmed death protein-1 antibody showing promising results in programmed death-ligand 1–positive tumors. Other promising agents include apatinib and regorafenib, which have demonstrated survival benefits in clinical trials.[41]
Staging
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) Eighth Edition 2017 staging system for gastric cancer (see Table. The American Joint Committee on Cancer Gastric Cancer Staging) stratifies 5-year overall survival (5-y OS) according to pathological stage and intervention (surgery only IA-93.6%, IIA-81.8%, and IIIA-54.2% or with neoadjuvant I-76.5%, II-46.3%, III-18.3%, and IV-5.7%). The following TMN classification is utilized for grading:
- T, Tumor
- TX: Primary tumor cannot be assessed
- T0: No evidence of primary tumor
- Tis: Carcinoma in situ; intraepithelial tumor without invasion of the lamina propria
- T1: Tumor invades the lamina propria, muscularis mucosae, or submucosa
- T1a: Tumor invades the lamina propria or muscularis mucosae
- T1b: Tumor invades the submucosa
- T2: Tumor invades the muscularis propria
- T3: Tumor penetrates the subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures.
- T4: Tumor invades the serosa (visceral peritoneum) or adjacent structures
- T4a: Tumor invades the serosa
- T4b: Tumor invades adjacent structures
- N, Nodes
- NX: Regional lymph nodes cannot be assessed
- N0: No regional lymph node metastasis
- N1: Metastasis in 1 to 2 regional lymph nodes
- N2: Metastasis in 3 to 6 regional lymph nodes
- N3: Metastasis in 7 or more regional lymph nodes
- N3a: Metastasis in 7 to 15 regional lymph nodes
- N3b: Metastasis in ≥16 regional lymph nodes
- M, Metastasis
- M0: No distant metastasis.
- M1: Distant metastasis present.
Table. The American Joint Committee on Cancer Gastric Cancer Staging
| 0 | Tis: Carcinoma in situ; intraepithelial tumor without invasion of the lamina propria | N0: No regional lymph node metastasis | M0: No distant metastasis |
| IA | T1: Tumor invades lamina propria, muscularis mucosae, or submucosa | N0: No regional lymph node metastasis | M0: No distant metastasis |
| IB | T2: Tumor invades muscularis propria | N0: No regional lymph node metastasis | M0: No distant metastasis |
| T1: Tumor invades lamina propria, muscularis mucosae, or submucosa | N1: Metastasis in 1-2 regional lymph nodes | M0: No distant metastasis | |
| IIA | T3: Tumor penetrates subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures | N0: No regional lymph node metastasis | M0: No distant metastasis |
| T2: Tumor invades muscularis propria | N1: Metastasis in 1-2 regional lymph nodes | M0: No distant metastasis | |
| T1: Tumor invades lamina propria, muscularis mucosae, or submucosa | N2: Metastasis in 3-6 regional lymph nodes | M0: No distant metastasis | |
| IIB | T4a: Tumor invades serosa (visceral peritoneum) | N0: No regional lymph node metastasis | M0: No distant metastasis |
| T3: Tumor penetrates subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures | N1: Metastasis in 1-2 regional lymph nodes | M0: No distant metastasis | |
| T2: Tumor invades muscularis propria | N2: Metastasis in 3-6 regional lymph nodes | M0: No distant metastasis | |
| T1: Tumor invades lamina propria, muscularis mucosae, or submucosa | N3a: Metastasis in 7-15 regional lymph nodes | M0: No distant metastasis | |
| IIIA | T4a: Tumor invades serosa (visceral peritoneum) | N1: Metastasis in 1-2 regional lymph nodes | M0: No distant metastasis |
| T3: Tumor penetrates subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures | N2: Metastasis in 3-6 regional lymph nodes | M0: No distant metastasis | |
| T2: Tumor invades muscularis propria | N3a: Metastasis in 7-15 regional lymph nodes | M0: No distant metastasis | |
| T1: Tumor invades lamina propria, muscularis mucosae, or submucosa | N3b: Metastasis in 16 or more regional lymph nodes | M0: No distant metastasis | |
| IIIB | T4b: Tumor invades adjacent structures | N0: No regional lymph node metastasis | M0: No distant metastasis |
| T4a: Tumor invades serosa (visceral peritoneum) | N2: Metastasis in 3-6 regional lymph nodes | M0: No distant metastasis | |
| T3: Tumor penetrates subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures | N3a: Metastasis in 7-15 regional lymph nodes | M0: No distant metastasis | |
| T2: Tumor invades muscularis propria | N3b: Metastasis in 16 or more regional lymph nodes | M0: No distant metastasis | |
| IIIC | T4b: Tumor invades adjacent structures | N1-N2: Metastasis in 1-6 regional lymph nodes | M0: No distant metastasis |
| T4a: Tumor invades serosa (visceral peritoneum) | N3a: Metastasis in 7-15 regional lymph nodes | M0: No distant metastasis | |
| T4b: Tumor invades adjacent structures | N3a: Metastasis in 7-15 regional lymph nodes | M0: No distant metastasis | |
| T4a: Tumor invades serosa (visceral peritoneum) | N3b: Metastasis in 16 or more regional lymph nodes | M0: No distant metastasis | |
| T4b: Tumor invades adjacent structures | N3b: Metastasis in 16 or more regional lymph nodes | M0: No distant metastasis | |
| IV | Any T | Any N | M1: Distant metastasis present |
Prognosis
The prognosis of gastric cancer is closely linked to the extent of the tumor, including nodal involvement and direct tumor invasion beyond the gastric wall. Tumor grade may also offer additional prognostic insight. More than 50% of patients with localized distal gastric cancer can achieve a cure. However, early-stage disease constitutes only 10% to 20% of all gastric cancer cases diagnosed in the United States.
Most patients present with metastatic disease, either regional or distant. The overall 5-year survival rate varies significantly, ranging from nearly 0 for patients with disseminated disease to approximately 50% for those with distal, resectable regional disease. For patients with localized proximal gastric cancer, the 5-year survival rate is only 10% to 15%. Although treatment for disseminated gastric cancer can provide symptomatic relief and some extension of life, long-term remissions are rare.
Complications
Gastric cancer's complications are diverse. Complications related to the primary tumor include bleeding, obstruction, perforation, and fistulization to surrounding organs. Metastatic disease can present with different symptoms based on the site of metastasis. Complications of surgery include bleeding, surgical site and organ space infections, anastomotic leaks, injury to surrounding structures like the spleen and duodenum, and longer-term complications like incisional hernias. (Please refer to the Medical Oncology section for more information on the adverse effects of chemotherapy).
Deterrence and Patient Education
Gastric cancer is the fifth most common malignancy in both men and women and the fourth most common cause of death. Chronic infection with H. Pylori, smoking, and obesity are correctable risk factors that individuals should be aware of. Alarm symptoms that require urgent evaluation with an upper endoscopy include upper or lower gastrointestinal bleeding, weight loss, or dysphagia. Those with a strong family history of gastric cancer should begin screening with upper endoscopy at an early age. Most local and regional diseases are treated with chemotherapy and surgery. Metastatic disease is best managed with chemotherapy, surgery, and radiation reserved for symptoms or palliation.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals with gastric cancer requires a coordinated, collaborative approach among healthcare professionals, including oncologists, surgeons, nurses, pharmacists, and other specialists. Clinicians must have strong skills in diagnosing, evaluating, and treating gastric cancer, interpreting complex imaging, and choosing appropriate treatments. Depending on the cancer's stage, collaboration with gastroenterologists and surgical and radiation oncologists is essential.
An evidence-based, individualized treatment plan tailored to each patient's needs is crucial. Ethical considerations and respecting patient autonomy must guide treatment decisions. Clearly defined roles and responsibilities within the interprofessional team are critical to ensure that each specialist contributes effectively. Effective communication is necessary to share information, address concerns, and resolve challenges. Coordinated care is vital for managing the patient's journey, from diagnosis to follow-up, to prevent errors, avoid delays, and prioritize patient safety. This comprehensive approach enhances outcomes and ensures patient satisfaction, leading to more effective management of gastric cancer.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
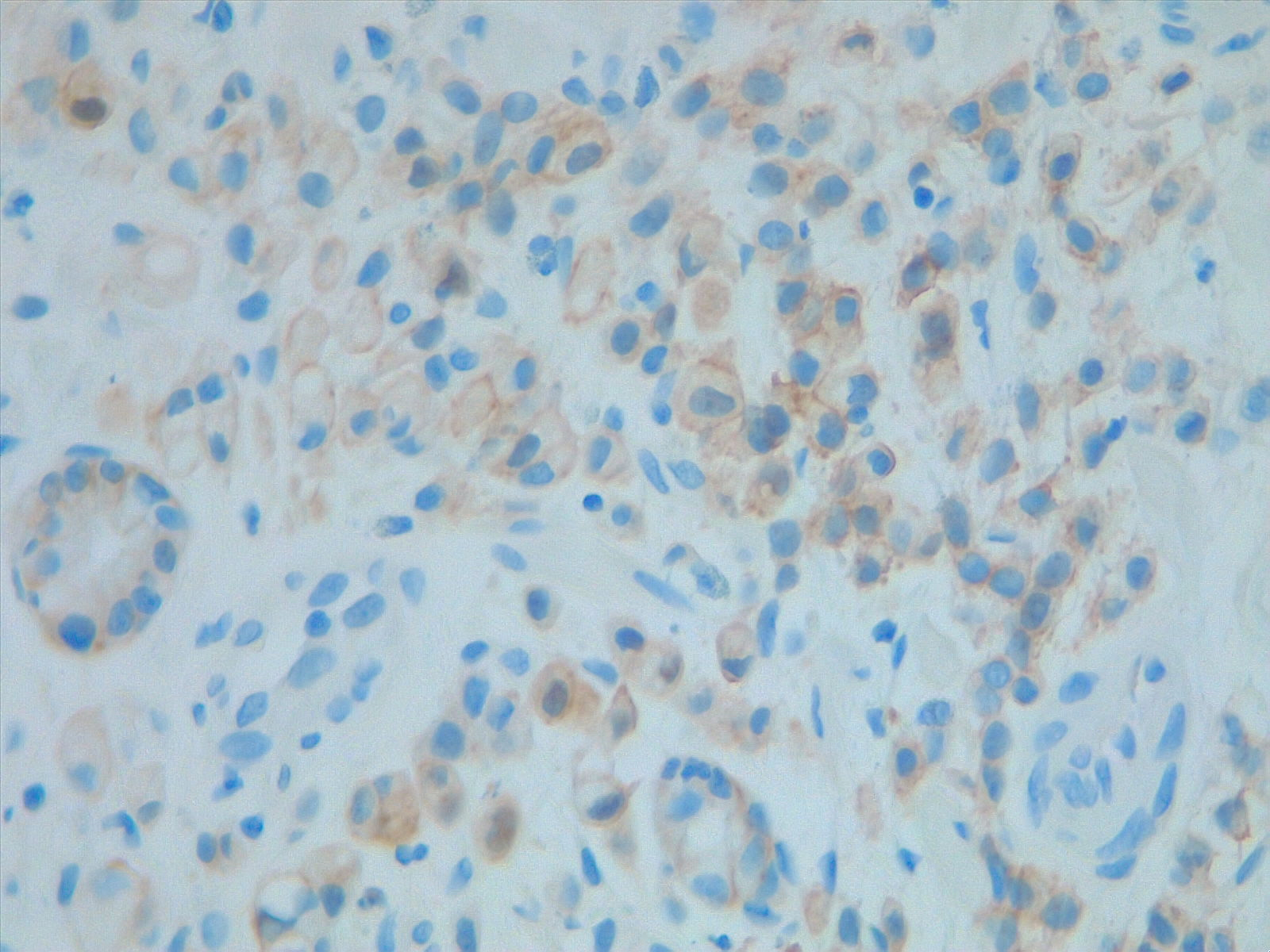
HER2 Analysis. Prognostic and predictive HER2 analysis is crucial for gastric cancer treatment. Correct HER2 evaluation can select patients for target treatment. Here, immunohistochemistry is performed and graded as 1+ ("staining is weak or detected in only one part of the membrane of at least 5 cohesive cells").
Contributed by F Farci, MD
References
Ilic M, Ilic I. Epidemiology of stomach cancer. World journal of gastroenterology. 2022 Mar 28:28(12):1187-1203. doi: 10.3748/wjg.v28.i12.1187. Epub [PubMed PMID: 35431510]
Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020 Mar:18(3):534-542. doi: 10.1016/j.cgh.2019.07.045. Epub 2019 Jul 27 [PubMed PMID: 31362118]
Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, Pu J, Lv J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World journal of gastroenterology. 2023 Apr 28:29(16):2452-2468. doi: 10.3748/wjg.v29.i16.2452. Epub [PubMed PMID: 37179585]
Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2022 Feb:20(2):167-192. doi: 10.6004/jnccn.2022.0008. Epub [PubMed PMID: 35130500]
Level 1 (high-level) evidenceLordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2022 Oct:33(10):1005-1020. doi: 10.1016/j.annonc.2022.07.004. Epub 2022 Jul 29 [PubMed PMID: 35914639]
Level 1 (high-level) evidenceSun K, Jia K, Lv H, Wang SQ, Wu Y, Lei H, Chen X. EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives. Frontiers in oncology. 2020:10():583463. doi: 10.3389/fonc.2020.583463. Epub 2020 Dec 14 [PubMed PMID: 33381453]
Level 3 (low-level) evidenceMachlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. International journal of molecular sciences. 2020 Jun 4:21(11):. doi: 10.3390/ijms21114012. Epub 2020 Jun 4 [PubMed PMID: 32512697]
Ning FL, Zhang NN, Wang J, Jin YF, Quan HG, Pei JP, Zhao Y, Zeng XT, Abe M, Zhang CD. Prognostic value of modified Lauren classification in gastric cancer. World journal of gastrointestinal oncology. 2021 Sep 15:13(9):1184-1195. doi: 10.4251/wjgo.v13.i9.1184. Epub [PubMed PMID: 34616522]
Hamid K, Hamza M, Rezac L, Shrestha A. Linitis Plastica. South Dakota medicine : the journal of the South Dakota State Medical Association. 2022 Aug:75(8):375 [PubMed PMID: 36745987]
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London, England). 2020 Aug 29:396(10251):635-648. doi: 10.1016/S0140-6736(20)31288-5. Epub [PubMed PMID: 32861308]
Wang Q, Liu G, Hu C. Molecular Classification of Gastric Adenocarcinoma. Gastroenterology research. 2019 Dec:12(6):275-282. doi: 10.14740/gr1187. Epub 2019 Nov 21 [PubMed PMID: 31803306]
Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology. 1982 Feb:82(2):228-31 [PubMed PMID: 7054024]
Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017 Mar:20(2):217-225. doi: 10.1007/s10120-016-0601-9. Epub 2016 Feb 20 [PubMed PMID: 26897166]
Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. European journal of radiology. 2002 Feb:41(2):161-7 [PubMed PMID: 11809546]
Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepato-gastroenterology. 2004 Sep-Oct:51(59):1544-7 [PubMed PMID: 15362797]
Simon M, Mal F, Perniceni T, Ferraz JM, Strauss C, Levard H, Louvet C, Fuks D, Gayet B. Accuracy of staging laparoscopy in detecting peritoneal dissemination in patients with gastroesophageal adenocarcinoma. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2016 Apr:29(3):236-40. doi: 10.1111/dote.12332. Epub 2015 Mar 10 [PubMed PMID: 25758761]
Level 2 (mid-level) evidenceWanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Annals of surgery. 1993 Nov:218(5):583-92 [PubMed PMID: 8239772]
Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2021 Jan:33(1):4-20. doi: 10.1111/den.13883. Epub 2020 Dec 9 [PubMed PMID: 33107115]
Ungureanu BS, Sacerdotianu VM, Turcu-Stiolica A, Cazacu IM, Saftoiu A. Endoscopic Ultrasound vs. Computed Tomography for Gastric Cancer Staging: A Network Meta-Analysis. Diagnostics (Basel, Switzerland). 2021 Jan 16:11(1):. doi: 10.3390/diagnostics11010134. Epub 2021 Jan 16 [PubMed PMID: 33467164]
Level 1 (high-level) evidenceDeprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Apr:54(4):412-429. doi: 10.1055/a-1751-5742. Epub 2022 Feb 18 [PubMed PMID: 35180797]
Landin MD, Guerrón AD. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection. The Surgical clinics of North America. 2020 Dec:100(6):1069-1078. doi: 10.1016/j.suc.2020.07.004. Epub 2020 Sep 8 [PubMed PMID: 33128880]
Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World journal of gastroenterology. 2014 Apr 28:20(16):4566-73. doi: 10.3748/wjg.v20.i16.4566. Epub [PubMed PMID: 24782609]
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2000 Dec:3(4):219-225 [PubMed PMID: 11984739]
Level 3 (low-level) evidenceMin BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, Kim JJ. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015 Sep:47(9):784-93. doi: 10.1055/s-0034-1392249. Epub 2015 Jun 25 [PubMed PMID: 26111362]
Barreto SG, Sirohi B. Why should we perform a D2 lymphadenectomy in gastric cancer? Future oncology (London, England). 2017 Oct:13(23):2009-2012. doi: 10.2217/fon-2017-0282. Epub 2017 Oct 6 [PubMed PMID: 28984466]
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. The Lancet. Oncology. 2010 May:11(5):439-49. doi: 10.1016/S1470-2045(10)70070-X. Epub 2010 Apr 19 [PubMed PMID: 20409751]
Level 1 (high-level) evidenceSasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K, Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. The New England journal of medicine. 2008 Jul 31:359(5):453-62. doi: 10.1056/NEJMoa0707035. Epub [PubMed PMID: 18669424]
Level 1 (high-level) evidenceSugarbaker PH, Van der Speeten K. Adjuvant HIPEC for gastric cancer. Journal of gastrointestinal oncology. 2021 Apr:12(Suppl 1):S18-S19. doi: 10.21037/jgo-2020-08. Epub [PubMed PMID: 33970159]
Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM, Stemmermann GN, Blanke CD, Macdonald JS. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jul 1:30(19):2327-33. doi: 10.1200/JCO.2011.36.7136. Epub 2012 May 14 [PubMed PMID: 22585691]
Level 1 (high-level) evidenceLee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jan 20:30(3):268-73. doi: 10.1200/JCO.2011.39.1953. Epub 2011 Dec 19 [PubMed PMID: 22184384]
Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK, ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Annals of oncology : official journal of the European Society for Medical Oncology. 2021 Mar:32(3):368-374. doi: 10.1016/j.annonc.2020.11.017. Epub 2020 Dec 3 [PubMed PMID: 33278599]
Level 1 (high-level) evidenceCunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006 Jul 6:355(1):11-20 [PubMed PMID: 16822992]
Level 1 (high-level) evidenceYchou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1715-21. doi: 10.1200/JCO.2010.33.0597. Epub 2011 Mar 28 [PubMed PMID: 21444866]
Level 1 (high-level) evidenceAl-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD, FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (London, England). 2019 May 11:393(10184):1948-1957. doi: 10.1016/S0140-6736(18)32557-1. Epub 2019 Apr 11 [PubMed PMID: 30982686]
Level 1 (high-level) evidenceAl-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. The Lancet. Oncology. 2016 Dec:17(12):1697-1708. doi: 10.1016/S1470-2045(16)30531-9. Epub 2016 Oct 22 [PubMed PMID: 27776843]
Level 1 (high-level) evidenceSakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. The New England journal of medicine. 2007 Nov 1:357(18):1810-20 [PubMed PMID: 17978289]
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. The Lancet. Oncology. 2014 Nov:15(12):1389-96. doi: 10.1016/S1470-2045(14)70473-5. Epub 2014 Oct 15 [PubMed PMID: 25439693]
Level 1 (high-level) evidenceVan Cutsem E,Moiseyenko VM,Tjulandin S,Majlis A,Constenla M,Boni C,Rodrigues A,Fodor M,Chao Y,Voznyi E,Risse ML,Ajani JA, Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Nov 1; [PubMed PMID: 17075117]
Level 1 (high-level) evidenceBang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010 Aug 28:376(9742):687-97. doi: 10.1016/S0140-6736(10)61121-X. Epub 2010 Aug 19 [PubMed PMID: 20728210]
Level 1 (high-level) evidenceOhtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Oct 20:29(30):3968-76. doi: 10.1200/JCO.2011.36.2236. Epub 2011 Aug 15 [PubMed PMID: 21844504]
Level 1 (high-level) evidenceIlson DH. Advances in the treatment of gastric cancer. Current opinion in gastroenterology. 2018 Nov:34(6):465-468. doi: 10.1097/MOG.0000000000000475. Epub [PubMed PMID: 30303856]
Level 3 (low-level) evidence