Introduction
The spleen is the largest lymphoid organ in the body, playing a crucial role in the immune system and fostering the production and maturation of essential components such as immunoglobulin M, B lymphocytes, and opsonins within its confines. One critical function of the spleen is to protect the body against infections from polysaccharide-encapsulated bacteria, such as Streptococcus pneumoniae and other Streptococcal species (eg, Haemophilus influenzae type b, Neisseria meningitidis, Escherichia coli, Salmonella, Klebsiella).
Additionally, the spleen acts as the primary reservoir for platelets and serves as a filter for red blood cells, eliminating damaged or malformed cells from circulation. Further, the spleen facilitates extramedullary hematopoiesis, a process crucial for blood cell production.[1][2][3] Functional asplenism, also known as "functional hyposplenism" or "splenic hypofunction," is a medical condition where the spleen is structurally intact but does not function properly. The term "functional asplenia" was coined by Pearson et al in 1969 while studying patients with sickle cell anemia.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Functional asplenism is characterized by the absence of phagocytic activity while maintaining the presence of splenic tissue, and occurs as a result of:
- Autoimmune disorders such as systemic lupus erythematosus, Hashimoto thyroiditis, antiphospholipid syndrome, rheumatoid arthritis, or autoimmune polyglandular syndrome type 1, also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy [5][6]
- Inflammatory conditions such as multiple sclerosis, celiac disease, inflammatory bowel disease, hepatic cirrhosis, sickle cell disease, beta thalassemia, chronic graft-versus-host disease, and nephrotic syndrome [7][8]
- Advanced human immunodeficiency virus infection [9]
- Hematologic-oncogenic disorders like acute leukemias, non-Hodgkin lymphoma, and advanced breast cancer
- Therapies including bone marrow transplantation, high-dose corticosteroids, splenic irradiation, and total parenteral nutrition
- Other disorders like amyloidosis, primary biliary cirrhosis, and primary pulmonary hypertension [10]
Epidemiology
The incidence of functional asplenism is contingent upon the prevalence and incidence rates of the precipitating disease. Below are several epidemiological studies on functional asplenia:
- In contrast to hypersplenism typically observed in alcoholic liver disease, hyposplenism may also occur in some instances. In a study of 82 patients with alcoholic liver disease, the effects of hyposplenism were monitored over 2 years. Among the patients, 16% died, and 6% had pitted erythrocyte counts similar to splenectomised patients. Abstinence from alcohol for 2 months led to a decrease in pitted erythrocyte count in most patients, while in 2 patients, 1 abstaining and 1 continuing heavy drinking, the count increased.[11]
- The prevalence of functional asplenia among individuals with sickle cell disease ranges from 20% to 30% in children and up to 50% in adults. An estimated 1 million individuals in the United States are hyposplenic or asplenic, with approximately 100,000 being attributed to sickle cell disease.[12]
- The prevalence of functional asplenia in thalassemia can vary depending on the type and severity of the disease, as well as the patient's age. Results from a 2013 study published in the Journal of Pediatric Hematology/Oncology revealed that functional asplenia was present in approximately 50% to 75% of patients diagnosed with beta-thalassemia major, the most severe form of the disease.[13]
Pathophysiology
Functional asplenism is a gradual process that begins with hyposplenism. Researchers theorize that in diseases like sickle cell, the entrapment of red blood cells in the spleen, followed by splenic infarction, leads to hyposplenism. Hyposplenism eventually progresses to functional asplenism through auto-splenectomy. In other hematologic disorders, functional asplenism occurs due to splenic tissue infiltration caused by several mechanisms, including tumor cell infiltration, sarcoidosis, amyloid deposition, or vascular occlusion.[8]
Further research exploring and understanding the underlying mechanisms and intricate processes leading to functional asplenism is essential to develop more effective treatments and targeted therapies to improve the overall management of patients affected by these complex hematologic conditions and enhance the quality of life for those experiencing functional asplenism.
Histopathology
Histopathology proves invaluable in diagnosing and managing functional asplenism. This detailed analysis provides crucial insights, aiding healthcare professionals in accurate diagnosis and effective treatment strategies tailored to individual patients. Early recognition and appropriate management of functional asplenism are essential to preventing life-threatening infections.[14]
One of the key features of functional asplenism is the absence or reduction of white pulp in the spleen due to a decrease in the size and number of lymphoid follicles—the basic structural units of the white pulp. The lymphoid follicles contain B- and T-lymphocytes, critical immune system components. The white pulp filters the blood and removes foreign particles, such as bacteria and viruses. Without a functional white pulp, individuals with functional asplenism are at increased risk of developing infections, particularly from encapsulated bacteria such as S pneumoniae, H influenzae type b, and N meningitidis. These infections can be serious and life-threatening and can lead to the overwhelming post-splenectomy infection syndrome.[15]
In addition to a decrease in lymphoid follicles, there may be an increase in the red pulp, which is responsible for filtering and storing red blood cells. Histopathology can provide essential insights into the underlying pathology of functional asplenism. In addition, the characteristic decrease in white pulp and increase in red pulp can help diagnose and manage various conditions associated with functional asplenism, including sickle cell disease, thalassemia, and autoimmune disorders.
History and Physical
When conducting a history and physical examination on a patient with functional asplenism, the clinician will typically ask about the patient's medical history, including any underlying conditions, such as sickle cell disease or other disorders that can affect spleen function. They may also ask about any previous infections or episodes of fever, which can be more common in patients with functional asplenism.
During the physical examination, the clinician checks for signs of an enlarged spleen, such as tenderness or a palpable mass in the left upper abdomen. They may also look for signs of infection, such as fever or an elevated white blood cell count. Other physical findings include jaundice (yellowing of the skin and eyes), anemia (low red blood cell count), and a low platelet count.
Patients with asplenia or hyposplenism who are bitten by an animal, particularly a dog, should seek immediate medical care. These patients are at risk of developing fulminant sepsis from Capnocytophaga canimorsus infection, which can be fatal.[16] There is also an increased risk of infection by intra-erythrocyte parasites such as Malaria and Babesia.[17] A rare case of lethal Waterhouse-Friderichsen syndrome due to Capnocytophaga canimorsus has also been reported in a patient who is asplenic.[18]
Evaluation
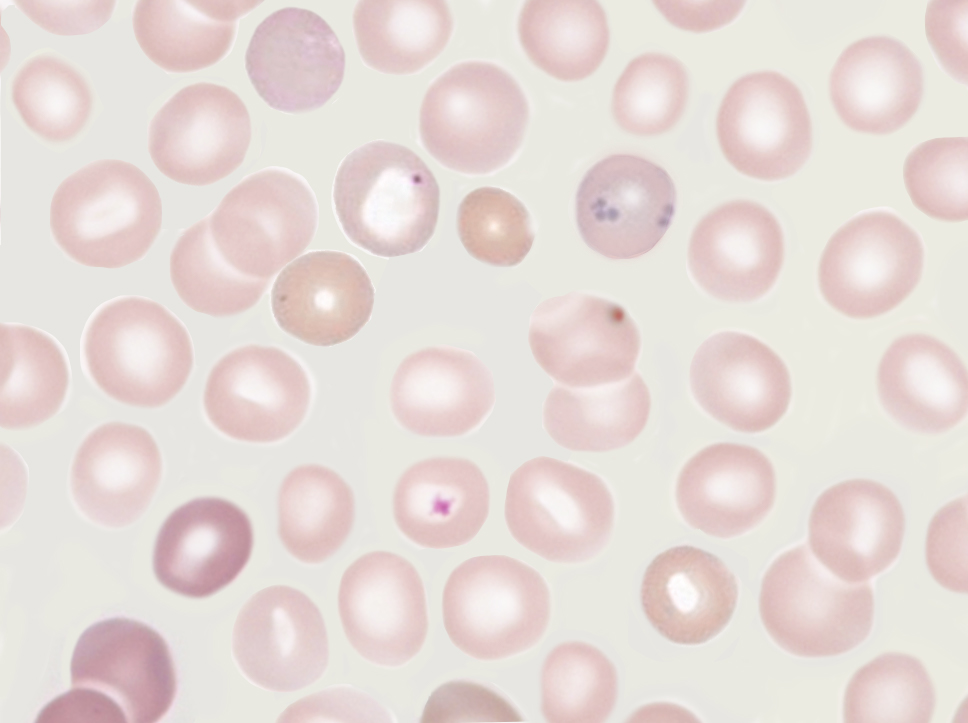
A functional hyposplenism diagnosis may be based on hematology, nuclear medicine, ultrasound imaging, or immunological parameters. The peripheral blood smear is vital for evaluating functional asplenism, as various cells may help identify functional asplenism. Howell-Jolly bodies on a peripheral blood smear indicate abnormal spleen function.[19] Howell-Jolly bodies are nuclear remnants of old red blood cells (RBCs) typically removed by the spleen (see Image. Howell-Jolly Body). Although they are typically found on a peripheral blood smear, a flow cytometry-based assay for Howell-Jolly bodies is currently under investigation. Thrombocytosis, lymphocytosis, and monocytosis are additional hematological signs of functional asplenism. Acanthocytes, giant cells, Heinz bodies, and iron granules may also be observed on a peripheral blood smear. Demonstration of many of these abnormalities requires special dyes and microscopy. In addition, the presence and quantitation of "pitted" RBCs have been used to determine the function of remaining splenic tissue.[11]
A technetium-99m-labeled radiocolloid scan helps evaluate splenic function by measuring the uptake of injected radio-labeled particles by the splenic endothelium relative to the liver.[2] This scan provides information regarding the phagocytic function of the spleen. Liver-spleen scintigraphy has the disadvantage of being invasive and time-consuming for patients. Newer methods currently in use include immune response upon vaccination or evaluating the functionality of specific B-cell subsets. Functional asplenia is also associated with small, avascular spleens on Doppler sonography.[20] Immunological methods such as measuring tuftsin levels are of little clinical significance for evaluating spleen phagocytic function.[21]
Treatment / Management
Treating and managing functional asplenism reduces the risk of infections and complications associated with this condition. Some of the recommended strategies include:
- Vaccinations: Vaccination is an essential aspect of managing functional asplenism. Patients should receive vaccinations against the bacteria that commonly cause infections in people with impaired spleen function, including vaccines against S pneumoniae, H influenzae type b, and N meningitidis. Patients should also receive annual influenza vaccination to reduce the risk of influenza-related complications. Study results have shown a significant reduction in the percentage of severe infections in patients after immunization with pneumococcal vaccines. The commonly used pneumococcal polysaccharide vaccine is ineffective in those who are asplenic because it requires the presence of immunoglobulin M memory B cells. The pneumococcal conjugate vaccine is more effective in functionally asplenic patients because it employs a T-cell-dependent mechanism and is the preferred vaccine. In addition, vaccines are thought to act better when the spleen is intact. Hence, the vaccine should be given 2 weeks before surgery in patients undergoing splenectomy. Moreover, patients with impaired splenic function should receive 3 doses of messenger ribonucleic acid COVID-19 vaccines, just like other patients who are immunocompromised. This is supported by the notion that a subset of memory B cells in the spleen could exert a protective role against this virus.[22]
- Antibiotics: Patients with functional asplenia may be prescribed long-term antibiotic prophylaxis to prevent infections. Antibiotics such as penicillin or amoxicillin are typically used for this purpose, but local microorganism resistance should be considered. In addition, antimalarial prophylaxis should be given to patients traveling to malaria-endemic areas.[17]
- Antibiotic prophylaxis: This is generally reserved for patients who are asplenic or hyposplenic who undergo procedures like endoscopic sinus surgery or bronchoscopy, as encapsulated organisms (such as S pneumoniae and H influenzae type b) can colonize these areas, leading to a fatal infection. For adults, 2 grams of amoxicillin is given orally 30 to 60 minutes before the procedure. For children, 3 months of age and older, and weighing less than 40 kg, amoxicillin 50 mg/kg is given an hour before the procedure (maximum dose 2 g). Alternatives include cephalosporins (eg, cephalexin), clindamycin, and macrolides such as azithromycin or erythromycin. Levofloxacin and moxifloxacin can also be used for adults.
- Treatment for animal bites in patients who are asplenic should involve thorough wound irrigation and antibiotic therapy that targets Capnocytophaga species. In the event of an animal bite, the first line of treatment consists of a beta-lactam-beta-lactamase inhibitor combination antibiotic, such as piperacillin-tazobactam (4.5 g every 6 hours) or meropenem (2 g every 8 hours) in combination with vancomycin to target Capnocytophaga species. However, meropenem is usually preferred over piperacillin-tazobactam when meningitis is suspected due to its higher penetration into the cerebrospinal fluid.
- Education and awareness: Patients with functional asplenism should be educated on the signs and symptoms of infection and when to seek medical attention. Patients should also be encouraged to carry a medical alert card or bracelet that identifies their condition and advises on appropriate management in an emergency.
- Regular medical follow-up: Patients with functional asplenism should receive regular medical follow-up to monitor for complications and adjust management as necessary.
Managing functional asplenia is individualized and may vary depending on the underlying condition causing it. Therefore, consulting a healthcare professional with experience managing functional asplenia is essential.
Differential Diagnosis
The differential diagnosis for functional asplenism includes:
- Chronic lymphocytic leukemia [23]
- Lymphomas
- Amyloidosis
- Bone marrow transplant
- Celiac disease
- Glomerulonephritis
- Graves disease [24]
- Hereditary hemoglobinopathies
- Inflammatory bowel disease
- Isolated congenital asplenia
- Post splenectomy
- Rheumatoid arthritis
- Sarcoidosis [25]
- Splenic irradiation
- Splenic vein thrombosis
- Tumors, such as lymphoma
- B-Thalassemia
Prognosis
When recognized and managed adequately with vaccines and proper prophylaxis, people with functional asplenism can lead a long, relatively healthy life despite being immunocompromised. Mortality varies widely in patients who are functionally asplenic depending on the infecting pathogen, underlying comorbidities, and access to vaccines and prophylactic antibiotics, in addition to other factors. Recognition of early sepsis with appropriate treatment can substantially reduce overall mortality.
Complications
Overwhelming Post-Splenectomy Infection
Overwhelming post-splenectomy infection (OPSI) is a syndrome encountered in patients who have functional asplenia. Bacteremia caused by encapsulated microorganisms characterizes the syndrome, with the most commonly reported pathogen in OPSI being Streptococcus pneumoniae. The syndrome might present without a visible primary source of infection and with a short prodromal phase; clinical presentations include septic shock, disseminated intravascular coagulopathy, and bilateral adrenal hemorrhage. OPSI is a medical emergency, and antibiotics should be administered immediately, along with supportive measures.[26]
Patients who are hyposplenic might already be taking prophylactic antibiotics, and hence, cultures might be negative; however, antibiotic therapy should continue. Empiric parental antibiotic therapy should include vancomycin and either cefotaxime or ceftriaxone. Although the efficacy of intravenous immunoglobulin treatment for asplenic sepsis has not been proven, some experts recommend its administration in this situation. The mortality of OPSI is high, with overall mortality rates of 50% to 70%, and death may result in 24 to 48 hours.
This syndrome has been observed from 10 years and up to 30 years after diagnosis with hyposplenism. Some studies indicate that the risk of OPSI decreases with the time elapsed since splenectomy, while other studies do not show a significant reduction.
Thrombocytosis
Functional asplenism is almost always associated with secondary elevation of platelet count because the spleen is the leading site of platelets’ destruction and provokes reflexive thrombocytosis. However, this is not seen in patients with liver cirrhosis and portal hypertension; these patients present with thrombocytopenia rather than thrombocytosis due to impaired liver function. Thrombocytosis increases the risk of thrombotic events.
Venous thrombosis in patients with asplenism is associated with platelet counts greater than 600 to 800 k/µL and occurs in approximately 5% of patients. Thrombocytosis can also cause arterial thrombosis, leading to myocardial infarction and cerebrovascular accidents. Some patients take aspirin or even a cytoreductive agent such as hydroxyurea or anagrelide to reduce the risk of thrombotic events.
Acute infectious purpura fulminans
Acute infectious purpura fulminans is a rapidly progressive syndrome of hemorrhagic skin necrosis associated with acute infection due to encapsulated organisms (usually Streptococcus pneumoniae or group A streptococci) resulting in disseminated intravascular coagulation with a strong predilection for patients with asplenism or functional hyposplenism.[27]
Deterrence and Patient Education
Patients should be instructed to have an asplenic alert card to inform the healthcare professional that they do not have a functional spleen. They should be current with their vaccinations and have access to prophylactic antibiotics. They must be educated to avoid dog or tick bites and take caution when traveling to malaria-endemic areas.
Pearls and Other Issues
Knowledge of the spleen's crucial and protective functions shows the importance of recognizing functional asplenism, a condition most commonly seen in patients with autoimmune diseases and various hematological disorders. OPSI, as well as thrombotic events, are serious medical emergencies that require prompt intervention. Prompt recognition of the illness's high-risk nature may limit morbidity.The simple interventions of immunizations and prophylactic antibiotics are expected to reduce the frequency of OPSI and decrease its mortality despite the controversy over antibiotic use in patients.
Enhancing Healthcare Team Outcomes
Patients with functional asplenia require a coordinated care approach involving multiple healthcare professionals, especially hematology and infectious disease teams. Healthcare professionals who care for patients with functional asplenia should have a comprehensive understanding of the immune system and the complications associated with this condition; it is essential to know how to diagnose and manage infections and prevent them through vaccination and other preventive measures. A comprehensive care plan is essential for patients with functional asplenia. The care plan should include follow-up appointments at regular intervals to monitor the patient's condition and a plan for managing potential complications. Healthcare professionals should collaborate with patients and their families to develop a care plan tailored to their needs and preferences. The care plan should also consider the patient's cultural and religious beliefs.Healthcare professionals should take responsibility for their professional development and stay current with the latest research and best practices. In addition, effective communication is essential for ensuring a coordinated approach to care. Collaboration among healthcare professionals ensures that the patient receives comprehensive, coordinated care through sharing information about the patient's condition, treatments, and medications and working together to manage potential complications.
Media
(Click Image to Enlarge)
References
Balaphas A, Meyer J, Harbarth S, Amzalag G, Buhler LH, Morel P. [Patient management after splenectomy in 2015: state of the art and recommendations]. Revue medicale suisse. 2015 Jun 17:11(479):1345-50 [PubMed PMID: 26255496]
Scheuerman O, Bar-Sever Z, Hoffer V, Gilad O, Marcus N, Garty BZ. Functional hyposplenism is an important and underdiagnosed immunodeficiency condition in children. Acta paediatrica (Oslo, Norway : 1992). 2014 Sep:103(9):e399-403. doi: 10.1111/apa.12697. Epub 2014 Jul 31 [PubMed PMID: 24850471]
Halbertsma FJ, Neeleman C, Weemaes CM, van Deuren M. The absent and vanishing spleen: congenital asplenia and hyposplenism--two case reports. Acta paediatrica (Oslo, Norway : 1992). 2005 Mar:94(3):369-71 [PubMed PMID: 16028659]
Level 3 (low-level) evidencePearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. The New England journal of medicine. 1969 Oct 23:281(17):923-6 [PubMed PMID: 5811425]
Bello MO, Garla VV. Polyglandular Autoimmune Syndrome Type I. StatPearls. 2024 Jan:(): [PubMed PMID: 30725896]
Starzyk J, Kumorowicz-Kopiec M, Kowalczyk M, Starzyk B, Rybakowa M, Dziatkowiak H. Natural history of asplenism in APECED--patient report. Journal of pediatric endocrinology & metabolism : JPEM. 2001 Apr:14(4):443-9 [PubMed PMID: 11327379]
Level 3 (low-level) evidenceLioté F, Angle J, Gilmore N, Osterland CK. Asplenism and systemic lupus erythematosus. Clinical rheumatology. 1995 Mar:14(2):220-3 [PubMed PMID: 7789066]
Level 3 (low-level) evidenceComenzo R, Malachowski M, Berkman E. Clinical correlation of positive direct antiglobulin tests in patients with sickle cell disease. Immunohematology. 1992:8(1):13-6 [PubMed PMID: 15946047]
Carrillo J, Negredo E, Puig J, Molinos-Albert LM, Rodríguez de la Concepción ML, Curriu M, Massanella M, Navarro J, Crespo M, Viñets E, Millá F, Clotet B, Blanco J. Memory B cell dysregulation in HIV-1-infected individuals. AIDS (London, England). 2018 Jan 14:32(2):149-160. doi: 10.1097/QAD.0000000000001686. Epub [PubMed PMID: 29112067]
Suzuki K, Ino K, Mizutani M, Ohishi K, Sekine T, Katayama N. [Multiple myeloma accompanying splenic amyloidosis and overwhelming pneumococcemia]. [Rinsho ketsueki] The Japanese journal of clinical hematology. 2007 Nov:48(11):1503-7 [PubMed PMID: 18080510]
Level 3 (low-level) evidenceMuller AF, Toghill PJ. Functional hyposplenism in alcoholic liver disease: a toxic effect of alcohol? Gut. 1994 May:35(5):679-82 [PubMed PMID: 8200565]
Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. The New England journal of medicine. 2014 Jul 24:371(4):349-56. doi: 10.1056/NEJMcp1314291. Epub [PubMed PMID: 25054718]
Musallam KM, Taher AT, Cappellini MD, Sankaran VG. Clinical experience with fetal hemoglobin induction therapy in patients with β-thalassemia. Blood. 2013 Mar 21:121(12):2199-212; quiz 2372. doi: 10.1182/blood-2012-10-408021. Epub 2013 Jan 11 [PubMed PMID: 23315167]
Peretz S, Livshits L, Pretorius E, Makhro A, Bogdanova A, Gassmann M, Koren A, Levin C. The protective effect of the spleen in sickle cell patients. A comparative study between patients with asplenia/hyposplenism and hypersplenism. Frontiers in physiology. 2022:13():796837. doi: 10.3389/fphys.2022.796837. Epub 2022 Aug 29 [PubMed PMID: 36105295]
Level 2 (mid-level) evidenceKirkineska L, Perifanis V, Vasiliadis T. Functional hyposplenism. Hippokratia. 2014 Jan:18(1):7-11 [PubMed PMID: 25125944]
Vignon G, Combeau P, Violette J, Cognée AS, Méglio S, Carrère F, Aucher P, Lellouche F. [A fatal septic shock due to Capnocytophaga canimorsus and review of literature]. La Revue de medecine interne. 2018 Oct:39(10):820-823. doi: 10.1016/j.revmed.2018.03.384. Epub 2018 Apr 26 [PubMed PMID: 29706238]
Luu S, Spelman D, Woolley IJ. Post-splenectomy sepsis: preventative strategies, challenges, and solutions. Infection and drug resistance. 2019:12():2839-2851. doi: 10.2147/IDR.S179902. Epub 2019 Sep 12 [PubMed PMID: 31571940]
Schuler F, Padberg JS, Hullermann C, Kümpers P, Lepper J, Schulte M, Uekötter A, Schaumburg F, Kahl BC. Lethal Waterhouse-Friderichsen syndrome caused by Capnocytophaga canimorsus in an asplenic patient. BMC infectious diseases. 2022 Aug 17:22(1):696. doi: 10.1186/s12879-022-07590-1. Epub 2022 Aug 17 [PubMed PMID: 35978295]
Nakagami Y, Uchino K, Okada H, Suzuki K, Enomoto M, Mizuno S, Yamamoto H, Hanamura I, Nakayama T, Tani H, Takami A. Potential role of Howell-Jolly bodies in identifying functional hyposplenism: a prospective single-institute study. International journal of hematology. 2020 Oct:112(4):544-552. doi: 10.1007/s12185-020-02925-7. Epub 2020 Jun 23 [PubMed PMID: 32572828]
Joshpe G, Rothenberg SP, Baum S. Transient functional asplenism in sickle cell-C disease. The American journal of medicine. 1973 Nov:55(5):720-2 [PubMed PMID: 4749210]
Level 3 (low-level) evidenceWilliam BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology (Amsterdam, Netherlands). 2007 Feb:12(1):1-13 [PubMed PMID: 17364987]
Rossi CM, Lenti MV, Merli S, Di Sabatino A. Role of IgM Memory B Cells and Spleen Function in COVID-19. Frontiers in immunology. 2022:13():889876. doi: 10.3389/fimmu.2022.889876. Epub 2022 Jun 30 [PubMed PMID: 35844543]
Montague AM, Pathak S. Chronic Lymphocytic Leukemia With Variant Genetics. StatPearls. 2024 Jan:(): [PubMed PMID: 36251840]
Pokhrel B, Bhusal K. Graves Disease. StatPearls. 2024 Jan:(): [PubMed PMID: 28846288]
Bokhari SRA, Zulfiqar H, Mansur A. Sarcoidosis. StatPearls. 2024 Jan:(): [PubMed PMID: 28613460]
Abe Y, Itagaki H, Endo T. Overwhelming Post-splenectomy Infection Caused by Escherichia coli 20 Years After Splenectomy: A Case Report. Cureus. 2023 Jul:15(7):e42184. doi: 10.7759/cureus.42184. Epub 2023 Jul 20 [PubMed PMID: 37602031]
Level 3 (low-level) evidenceWard KM, Celebi JT, Gmyrek R, Grossman ME. Acute infectious purpura fulminans associated with asplenism or hyposplenism. Journal of the American Academy of Dermatology. 2002 Oct:47(4):493-6 [PubMed PMID: 12271289]
Level 3 (low-level) evidence