Introduction
As the oldest cerebellar nucleus, the fastigial nucleus (FN) plays essential roles in motor control by sending signals to the brainstem and eye movement nuclei and the maintenance of many activities within the human body, such as feeding, immune, and cardiovascular and respiratory functions.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
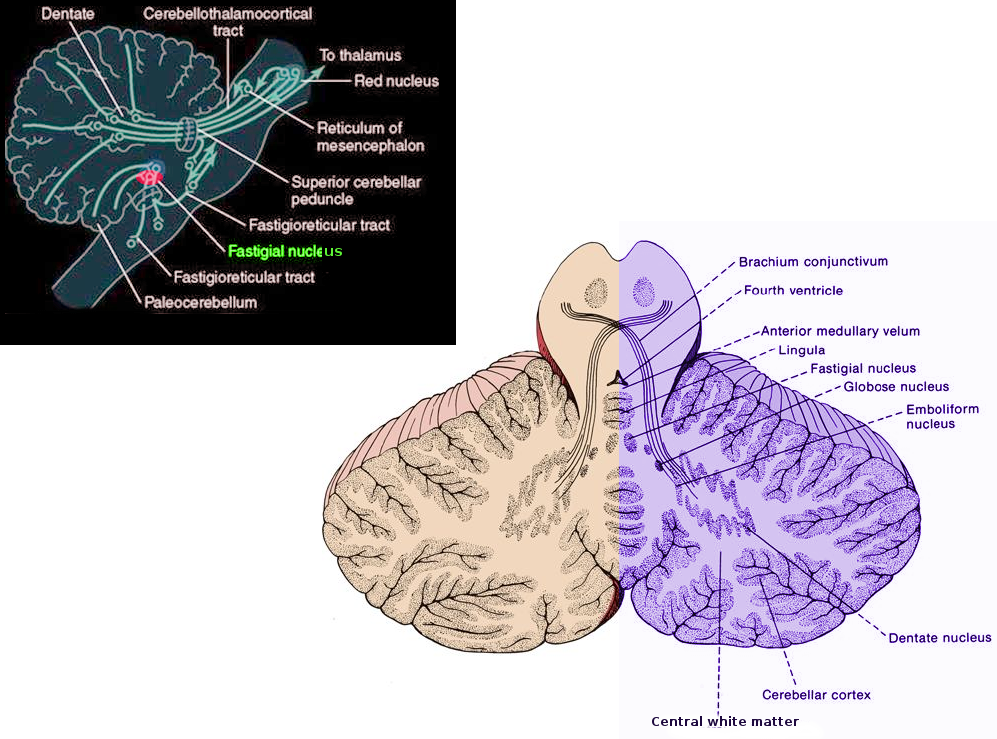
The fastigial nucleus constitutes one of the primary cerebellar nuclei and is situated closest to the midline toward the anterior segment of the superior vermis and over the roof of the fourth ventricle.[2][3] Compared to the dentate and interposed nuclei, it is the smallest and the oldest.[1] Different types of neurons that differ according to their morphology, projection patterns, and firing properties get distributed within the fastigial nucleus. Based on their projection patterns, fastigial nucleus neurons can categorize into projection neurons and interneurons.[2][4] Projection neurons have cerebellar nuclei that transmit long axons while interneurons have short axons that join with neurons only inside the fastigial nucleus. Glutamatergic, GABAergic and glycinergic neurons are also present in the fastigial nucleus, and neurons with different electrophysiological features have also been found within the fastigial nucleus as well.[4][2] Generally, fastigial nucleus neurons subdivide into five categories[1]:
- Large glutamatergic projection neurons that transmit their axons to different parts of the brain
- Large glycinergic projection neurons that are situated in the rostral portion of the fastigial nucleus and transmit signals to ipsilateral vestibular nuclei and the caudal brainstem
- Medium-sized GABAergic projection neurons that offer feedback to the inferior olive
- Small GABA/glycine interneurons that restrict their axon terminals in the nucleus and are responsible for signaling and integration in the fastigial nucleus
- Small non-GABAergic interneurons that have faster repolarization and spontaneous firing than GABAergic neurons
As previously mentioned, the fastigial nucleus is essential in motor control by sending signals to the medial descending systems and eye movement nuclei and the maintenance of many activities within the human body, such as feeding, immune, defecation and micturition, and cardiovascular and respiratory functions.[1]
Embryology
Among the evolution of animals, the three cerebellar nuclei were first discovered in the elasmobranch[5] and remained preserved in amphibians, reptiles, and birds.[6] In mammals, the fastigial nucleus and interposed nucleus are homologous with the medial and lateral nuclei of lower tetrapods.[6] Unlike the interposed nucleus, which further separates into the emboliform and globose nuclei in humans, the structure of the fastigial nucleus is retained in mammals, which suggests its essential role in the cerebellum.[2]
Blood Supply and Lymphatics
One of the arteries that contribute blood to the cerebellum is the superior cerebellar artery (SCA). Another artery that imparts blood to the cerebellum is the anterior inferior cerebellar artery. Finally, the posterior inferior cerebellar artery (PICA) also contributes blood to the cerebellum.[7] While the blood supply of the fastigial nucleus is not entirely clear, for the dentate nucleus, anatomical research has found that its blood supply mostly derives from the SCA and the PICA. The SCA is found to be more prominent on the dorsal, rostral, and lateral sides while the PICA is found to be more prominent on the ventral and medial sides of the dentate nucleus.[8]
Nerves
Within the cerebellar vermis, Purkinje cells, which constitute the main afferent pathway for the fastigial nucleus, transmit inhibitory GABAergic axons that contain integrated information within the cerebellar vermis to the fastigial nucleus.[2][9] The fastigial nucleus is found to play an important role in the spinocerebellum. Previous studies have also discovered that in monkeys, the fastigial nucleus receives afferent pathways from the flocculus, which plays an important role in mediating visual-vestibular interactions.[10] The caudal portion within the medial and dorsal segments of the inferior olive along with the mossy fibers within structures such as the nucleus reticularis tegmenti pointis, and medullary reticular formation transmits excitatory glutamatergic signals to the fastigial nucleus.[1] The fastigial nucleus also accepts serotonergic signals from the medullary/pontine reticular formation along with the raphe nuclei and noradrenergic signals originating from the locus coeruleus.[11] Structures such as the lateral, dorsal, and posterior hypothalamic areas, dorsomedial hypothalamus nucleus, and periventricular hypothalamic nucleus send signals to the fastigial nucleus as well.[12][13][14] While the neurotransmitters emitted by these projections are unknown, histamine along with orexin are possible candidates.[1]
The fastigial nucleus also has many efferent pathways. First, it sends descending pathways to the vestibular nuclei along with medullary/pontine reticular formations.[2][9] It also transmits signals to different brainstem structures that regulate movements of the head, face, and eyes, including nuclei of the trochlear, abducens, and facial nerves, perihypoglossal nucleus, rostral interstitial nucleus, oculomotor nuclei, and paramedian pontine reticular formation.[1] In studies involving primates apart from humans, research demonstrates that fastigial nucleus neurons can target the opposite side of the body and reach the primary motor cortex through the ventral lateral nucleus in the thalamus.[15][16]
The fastigial nucleus also sends signals to many nonmotor regions in the brain. Many animal studies revealed that the fastigial nucleus could reach structures within the medullary/pontine reticular formations, including the gigantocellular nucleus and nucleus ambiguus.[17][18] The fastigial nucleus also targets the hypothalamus via the superior cerebellar peduncle,[1] and transmits signals to the limbic system to regulate emotional activities.[19][20] Finally, the fastigial nucleus also targets the thalamus, nigra, and ventral tegmentum within the midbrain.[1]
Muscles
As mentioned previously, the fastigial nucleus is involved in axial along with proximal motor control. Studies involving patients with lesions in the fastigial nucleus have found that these patients lose control in the muscles of the axial and trunk regions in antigravity posture.[21] Animal studies have also found that animals with inactivation of the fastigial nucleus have serious ataxia and equilibrium disturbances that do not affect them when they reach and grasp objects.[22][23] Rats with lesions in the fastigial nucleus also demonstrated deficiencies when walking on narrow beams, grid runways, and rota-rods.[1] Further studies also showed that only the rostral fastigial nucleus is involved in motor functions of the axial along with proximal motor regions.[1] The rostral fastigial nucleus integrates spatial information with head and body motion to regulate one’s gait and posture.[24][25][26] Also, the fact that the fastigial nucleus targets the primary motor cortex suggests the fastigial nucleus is also involved with movement initiation.[15][16]
Afferents involving serotonin and histamine have also been shown to be involved in fastigial nucleus-related motor behavior. For instance, serotonin and histamine have excitatory outcomes on the fastigial nucleus.[1] Histamine also has been shown to only depolarize projection neurons in the fastigial nucleus, which explains how selective histaminergic afferents can be in the fastigial nucleus.[27] Microinjections of histamine within bilateral fastigial nuclei have also been shown to enhance motor performances of rats on structures such as balance beams.[28][29]
Surgical Considerations
Previous studies demonstrated that patients with cerebellar lesions in the fastigial nucleus often have saccadic hypermetria.[30] Neurons in the caudal fastigial nucleus often encode the start and termination of saccades and provide a presaccadic burst to implement contraversive saccades.[1] Lesions in the unilateral portion of the fastigial nucleus have also been shown to lead to deficiencies in the contralateral pursuit movement.[1] Bilateral lesions of the paramedian reticular nucleus or the rostral ventrolateral reticular nucleus in the medulla can destroy the integrity of the fastigial nucleus. Electrical stimulation near the region of the fastigial nucleus has also been shown to result in respiratory tachypnea during surgery. Fastigial nucleus ablation has also been shown not to change eupneic breathing to a significant degree and significantly attenuate the response to hypercapnia and hypoxia.[1]
Clinical Significance
As stated previously, the fastigial nucleus plays important roles in motor control by sending signals to the medial descending systems and eye movement nuclei and the maintenance of many activities within the human body, such as feeding, immune, and cardiovascular and respiratory functions. The caudal fastigial nucleus accepts inputs from the oculomotor vermis to predict the real-time motion of the eyes and transmits information to structures in the lower brainstem that are involved in saccades and smooth pursuit movements.[1] Patients with cerebellar lesions in the fastigial nucleus often have saccadic hypermetria as neurons in the caudal fastigial nucleus encode the start and termination of saccades and provide a presaccadic burst to implement contraversive saccades.[31]
The fastigial nucleus is also involved in feeding control with the hypothalamus, as its stimulation can alter movements within the jejunum, ileum, and colon. Gastric control is also under the regulation of the fastigial nucleus through adrenergic, adrenal catecholamine, and vagal cholinergic release.[1] Stimulating the fastigial nucleus has also resulted in elicit postsynaptic responses and regulate LHA glucose-sensitive neurons.[32][33] The fastigial nucleus also integrates information with other feeding signals, such as gastric vagal and leptin inputs.[34] The fastigial nucleus also may be involved in cardiovascular functions, as the fastigial pressor response has been shown to lead to a rapid increase in arterial pressure for cats. While animals with lesions of the fastigial nucleus may not have disorders involving resting blood pressure or heart rate, they may have significant deficits in compensatory responses to hemorrhage or endotoxic shock. Electrical stimulation near the region of the fastigial nucleus has also been shown to result in respiratory tachypnea during surgery. Fastigial nucleus ablation has also been shown not significantly to change eupneic breathing and significantly attenuate the response to hypercapnia and hypoxia. Therefore, the fastigial nucleus may play an important role in respiratory control.[1]
The fastigial nucleus has also been shown to regulate defecation and micturition functions. Stimulating the fastigial nucleus suppresses the defecation reflex and alters bladder motility in a bidirectional manner.[1] In rats, research has also shown that fastigial nucleus lesions can lead to an increase in T lymphocytes and natural killer cells, suggesting the fastigial nucleus is important in immune regulation.[35] Stimulating the fastigial nucleus in cats has also been shown to lead to fits of anger, which suggests the fastigial nucleus is important in emotional control.[1]
Fastigial nucleus lesions are related to many syndromes and disorders. For instance, previous studies have shown that patients with lesions after cerebellar tumor resections demonstrate gait and posture ataxia that often involve the fastigial nucleus. The fastigial nucleus is also related to the pathology of congenital central hypoventilation syndrome (CCHS). CCHS is a disorder that causes deficits in the ventilatory response to hypercarbia and hypoxemia. Patients with CCHS often have a defective fastigial nucleus and do not respond well to ventilatory or blood pressure changes. Moreover, stimulating the fastigial nucleus may result in a protective result toward gastric mucosal injury and acute myocardial infarction. Patients with autism and cerebellar cognitive affective syndrome have also been shown to have a dysfunctional fastigial nucleus.[1]
Media
References
Zhang XY, Wang JJ, Zhu JN. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum & ataxias. 2016:3():9. doi: 10.1186/s40673-016-0047-1. Epub 2016 May 3 [PubMed PMID: 27144010]
Ito M. Cerebellar circuitry as a neuronal machine. Progress in neurobiology. 2006 Feb-Apr:78(3-5):272-303 [PubMed PMID: 16759785]
Level 3 (low-level) evidenceDiedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. NeuroImage. 2011 Feb 1:54(3):1786-94. doi: 10.1016/j.neuroimage.2010.10.035. Epub 2010 Oct 18 [PubMed PMID: 20965257]
Uusisaari MY, Knöpfel T. Diversity of neuronal elements and circuitry in the cerebellar nuclei. Cerebellum (London, England). 2012 Jun:11(2):420-1. doi: 10.1007/s12311-011-0350-6. Epub [PubMed PMID: 22278661]
Level 3 (low-level) evidenceIkenaga T, Yoshida M, Uematsu K. Cerebellar efferent neurons in teleost fish. Cerebellum (London, England). 2006:5(4):268-74 [PubMed PMID: 17134989]
Level 3 (low-level) evidenceArends JJ, Zeigler HP. Organization of the cerebellum in the pigeon (Columba livia): II. Projections of the cerebellar nuclei. The Journal of comparative neurology. 1991 Apr 8:306(2):245-72 [PubMed PMID: 1711054]
Level 3 (low-level) evidenceAdigun OO, Reddy V, Sevensma KE. Anatomy, Head and Neck: Basilar Artery. StatPearls. 2023 Jan:(): [PubMed PMID: 29083786]
Kim S, Lee H, Lee Y, Lee J, Yang J, Lee M, Yang H. Blood Supply by the Superior Cerebellar Artery and Posterior Inferior Cerebellar Artery to the Motor and Nonmotor Domains of the Human Dentate Nucleus. World neurosurgery. 2019 Feb:122():e606-e611. doi: 10.1016/j.wneu.2018.10.111. Epub 2018 Oct 26 [PubMed PMID: 31108077]
Ito M. The modifiable neuronal network of the cerebellum. The Japanese journal of physiology. 1984:34(5):781-92 [PubMed PMID: 6099855]
Level 3 (low-level) evidenceFuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. Journal of neurophysiology. 1994 Dec:72(6):2714-28 [PubMed PMID: 7897484]
Level 3 (low-level) evidenceBishop GA, Ho RH, King JS. A temporal analysis of the origin and distribution of serotoninergic afferents in the cerebellum of pouch young opossums. Anatomy and embryology. 1988:179(1):33-48 [PubMed PMID: 3213954]
Level 3 (low-level) evidenceHaines DE, Dietrichs E, Mihailoff GA, McDonald EF. The cerebellar-hypothalamic axis: basic circuits and clinical observations. International review of neurobiology. 1997:41():83-107 [PubMed PMID: 9378614]
Level 3 (low-level) evidenceZhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain research reviews. 2006 Aug 30:52(1):93-106 [PubMed PMID: 16497381]
Level 3 (low-level) evidenceDietrichs E. Cerebellar autonomic function: direct hypothalamocerebellar pathway. Science (New York, N.Y.). 1984 Feb 10:223(4636):591-3 [PubMed PMID: 6198719]
Level 3 (low-level) evidenceAllen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiological reviews. 1974 Oct:54(4):957-1006 [PubMed PMID: 4370744]
Level 3 (low-level) evidenceKelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003 Sep 10:23(23):8432-44 [PubMed PMID: 12968006]
Level 3 (low-level) evidenceAndrezik JA, Dormer KJ, Foreman RD, Person RJ. Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neuroscience. 1984 Feb:11(2):497-507 [PubMed PMID: 6201783]
Level 3 (low-level) evidenceHomma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neuroscience research. 1995 Aug:23(1):89-102 [PubMed PMID: 7501304]
Level 3 (low-level) evidenceHarper JW, Heath RG. Anatomic connections of the fastigial nucleus to the rostral forebrain in the cat. Experimental neurology. 1973 Mar-Apr:39(2):285-92 [PubMed PMID: 4573973]
Level 3 (low-level) evidenceHeath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Experimental neurology. 1974 Nov:45(2):268-87 [PubMed PMID: 4422320]
Level 3 (low-level) evidenceIlg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain : a journal of neurology. 2008 Nov:131(Pt 11):2913-27. doi: 10.1093/brain/awn246. Epub 2008 Oct 3 [PubMed PMID: 18835866]
Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annual review of neuroscience. 1992:15():403-42 [PubMed PMID: 1575449]
Level 3 (low-level) evidenceMartin JH, Cooper SE, Hacking A, Ghez C. Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. Journal of neurophysiology. 2000 Apr:83(4):1886-99 [PubMed PMID: 10758100]
Level 3 (low-level) evidenceKleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Büttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. Journal of neurophysiology. 2004 May:91(5):2090-100 [PubMed PMID: 15069099]
Level 3 (low-level) evidenceShaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 May 12:24(19):4491-7 [PubMed PMID: 15140919]
Level 3 (low-level) evidenceBrooks JX, Cullen KE. Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Aug 26:29(34):10499-511. doi: 10.1523/JNEUROSCI.1937-09.2009. Epub [PubMed PMID: 19710303]
Level 3 (low-level) evidenceZhang J, Zhuang QX, Li B, Wu GY, Yung WH, Zhu JN, Wang JJ. Selective Modulation of Histaminergic Inputs on Projection Neurons of Cerebellum Rapidly Promotes Motor Coordination via HCN Channels. Molecular neurobiology. 2016 Mar:53(2):1386-1401. doi: 10.1007/s12035-015-9096-3. Epub 2015 Jan 30 [PubMed PMID: 25633097]
Level 3 (low-level) evidenceTang B, Zhang J, Yu L, Li HZ, Zhu JN, Wang JJ. Excitation of histamine on neuronal activity of cerebellar fastigial nucleus in rat. Inflammation research : official journal of the European Histamine Research Society ... [et al.]. 2008:57 Suppl 1():S41-2. doi: 10.1007/s00011-007-0637-8. Epub [PubMed PMID: 18345481]
Level 3 (low-level) evidenceHe YC, Wu GY, Li D, Tang B, Li B, Ding Y, Zhu JN, Wang JJ. Histamine promotes rat motor performances by activation of H(2) receptors in the cerebellar fastigial nucleus. Behavioural brain research. 2012 Mar 1:228(1):44-52. doi: 10.1016/j.bbr.2011.11.029. Epub 2011 Nov 29 [PubMed PMID: 22146592]
Level 3 (low-level) evidenceRamat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Experimental brain research. 2005 Jan:160(1):89-106 [PubMed PMID: 15289966]
Kleine JF, Guan Y, Buttner U. Saccade-related neurons in the primate fastigial nucleus: what do they encode? Journal of neurophysiology. 2003 Nov:90(5):3137-54 [PubMed PMID: 12853435]
Level 3 (low-level) evidenceMin BI, Oomura Y, Katafuchi T. Responses of rat lateral hypothalamic neuronal activity to fastigial nucleus stimulation. Journal of neurophysiology. 1989 Jun:61(6):1178-84 [PubMed PMID: 2746318]
Level 3 (low-level) evidenceZhang YP, Ma C, Wen YQ, Wang JJ. Convergence of gastric vagal and cerebellar fastigial nuclear inputs on glycemia-sensitive neurons of lateral hypothalamic area in the rat. Neuroscience research. 2003 Jan:45(1):9-16 [PubMed PMID: 12507719]
Level 3 (low-level) evidenceLi B, Guo CL, Tang J, Zhu JN, Wang JJ. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neuro-Signals. 2009:17(2):132-43. doi: 10.1159/000197913. Epub 2009 Feb 2 [PubMed PMID: 19182493]
Level 3 (low-level) evidencePeng YP, Qiu YH, Chao BB, Wang JJ. Effect of lesions of cerebellar fastigial nuclei on lymphocyte functions of rats. Neuroscience research. 2005 Mar:51(3):275-84 [PubMed PMID: 15710491]
Level 3 (low-level) evidence