Introduction
The fallopian tubes are bilateral conduits between the ovaries and the uterus in the female pelvis. They function as channels for oocyte transport and fertilization. Given this role, the fallopian tubes are a common etiology of infertility as well as the target of purposeful surgical sterilization. They can also be sites of ascending infection or neoplasms.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Fallopian tubes, otherwise called oviducts or uterine tubes, are hollow seromuscular organs that originate at the uterine horns, extend laterally within the superior edge of the mesosalpinx of the broad ligament, and terminate near the ipsilateral ovary. They are 11 to 12 cm in length and have a lumen diameter of less than 1 mm.[1] The fallopian tube comprises four anatomical regions: uterine, isthmus, ampulla, and infundibulum. Most medially, the uterine part includes the uterine ostium and a short segment nearest to the uterine horn. The isthmus is adjacent to the uterine part. Lateral to the isthmus is the ampulla, the most common site of fertilization. Most distal from the uterus, the infundibulum ends at an abdominal ostium opening up into the peritoneal cavity and fimbriae, which catch the released oocyte during each menstrual cycle. One fimbria, named the fimbria ovarica, serves to connect the infundibulum to the ovary nearby. In addition to providing a space for fertilization to occur, the fallopian tubes function as a passageway for the ovum or gamete from the ovary to the uterus.[2]
Embryology
The paramesonephric ducts, otherwise known as the Mullerian ducts, form in female embryos at around week five or six due to the absence of anti-mullerian hormone (AMH). With estrogen stimulation, the cranial ends of these ducts eventually form the fallopian tubes. Caudally, the paramesonephric ducts fuse in the midline and ultimately form the uterus and superior vagina. Most cranially, a remnant of the paramesonephric duct may persist as a vestigial structure called the vesicular appendage or the hydatid cyst of Morgagni.[3]
Blood Supply and Lymphatics
The arterial supply to the fallopian tube arises from anastomoses between the ovarian and tubal branches of the ovarian artery and ascending branches of the uterine artery. On each side, the ovarian arteries branch off the abdominal aorta inferior to the origin of the renal arteries. The uterine arteries stem from the internal iliac arteries, and the ascending branches travel superiorly towards the uterine horns while its descending branches travel inferiorly towards the superior vagina. The ovarian arteries supply the lateral fallopian tube, while the ascending branches of the uterine artery supply the medial fallopian tube; however, anastomoses between the two generally ensures that compromise of either will not cause ischemia of any portion of the tube.
The venous drainage goes to the tubal branches of the uterine and ovarian veins, in a similar configuration as the arterial supply. The inferior vena cava (IVC) receives the drainage from the right ovarian vein, while the left renal vein receives the drainage from the left ovarian vein. The uterine veins drain into the internal iliac veins, which drain into the IVC.
Lymph drainage of the fallopian tubes is similar to that of the ovaries. Lymph flows from the fallopian tubes to both the para-aortic (lumbar) lymph nodes and the pelvic lymph nodes.[2][4]
Nerves
Afferent pain fibers for the fallopian tubes travel along the same pathway as sympathetic efferent innervation, which originates from T11, T12, and L1. By contrast, minor parasympathetic innervation is shared between vagal fibers of the ovarian plexus for the lateral part of the fallopian tube and medially from the pelvic splanchnic nerve from S1, S2, and S3. The most medial isthmus receives the densest innervation, and both innervation and musculature become sparser from proximal to the distal fallopian tube.[5]
Muscles
The muscular layer of the fallopian tubes gets sandwiched between the inner mucosa, which contains longitudinal cilia extending into the lumen, and the outermost serosa. This layer is also known as the muscularis mucosa and comprises an inner circular layer and outer longitudinal layer.
The contractions of this muscular layer work in conjunction with cilia movements and tubal secretory fluids to achieve ovum or gamete transport along the length of the fallopian tube. Short, frequent contractions help mix tubal fluid, while continuous tonic contractions not only contribute to anterograde transport of ovum or gamete but also allow the admission of an embryo across the utero-tubal junction at the most hormonally optimal point in time during the menstrual cycle.[5]
Physiologic Variants
Benign para tubal cysts may be found incidentally on ultrasound or during surgeries. These cysts appear most often in middle age and are usually asymptomatic. The previously mentioned hydatid of Morgagni, an embryologic remnant, may also be present at the distal-most part of the fallopian tube.
There have been rare reported cases of fallopian tube diverticula, agenesis, and duplication. Diverticula or duplications increase the risk of tubal pathologies such as hydrosalpinx or torsion, as well as ectopic pregnancies. Tubal abnormalities leading to occlusion or tubal agenesis may contribute to infertility. Diethylstilbestrol (DES) exposure in utero, in particular, has been linked to abnormal development of the fallopian tube.[6][7]
Surgical Considerations
Commonly performed surgical procedures involving the fallopian tubes include[8][9]:
- Salpingo-oophorectomy: removal of the ovary and fallopian tube
- Salpingectomy: removal of the fallopian tube
- Salpingostomy: surgical incision into the fallopian tube to create an opening
- Tubal ligation: an umbrella term for female surgical sterilization by occlusion, removal, or ligation of fallopian tubes
Clinical Significance
Fallopian tubes are involved in several gynecologic diseases that necessitate the procedures mentioned earlier. The ampulla is the most common site of ectopic pregnancies, which are either treated medically or removed surgically with a salpingostomy (creating an opening in the tube) or salpingectomy (tube excision and removal). Removal of the fallopian tubes may be necessary for malignancies involving the ovary, fallopian tube, or uterus, as well as benign conditions, including hydrosalpinx and tubo-ovarian abscess.
Fallopian tubes have a unique significance in the context of ovarian cancer. High grade serous ovarian cancer (HGSOC), the most common type of ovarian cancer, is often diagnosed at an advanced stage, leading to poor prognosis. Recent evidence has shown that most cases of HGSOC originate in the fallopian tubes years before the presentation of ovarian cancer.[10]
A tubal ligation is an option for patients desiring permanent sterilization. This procedure is regarded as very safe and highly effective. Patients should, however, understand that it is permanent, and clinicians should be aware of the risk factors for post-procedure regret, including age less than 30, lower parity, sterilization performed in the immediate postpartum period, and low socioeconomic status.[11][8][9]
Other Issues
Abnormalities of the fallopian tubes have been implicated in up to one-third of infertility cases. Causes that may negatively impact fallopian tube structure or function include congenital absence or malformation, infections such as Chlamydia trachomatis, Neisseria gonorrhea, genital tuberculosis, and endometriosis adhesions, causing tubal distortion. As such, a history of recurrent pelvic inflammatory disease related to these infections or a history of intra-abdominal surgery leading to adhesions correlates with a higher risk of tubal factor infertility. An increased number of past infections also correlates with increased severity of damage to the fallopian tubes.[12]
Tubal occlusion investigation can be by hysterosalpingography or hysteroscopy. Interventions for occlusion specific to the fallopian tube include selective salpingography, recanalization, re-anastomosis, and neosalpingostomy.[1]
Media
(Click Image to Enlarge)
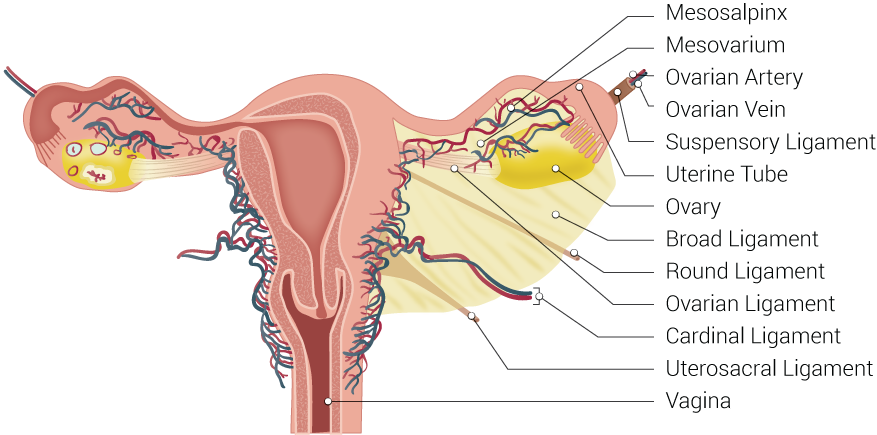
Uterine Tubal Anatomy and Ligaments. Shown in this illustration are anatomical structures surrounding the uterus and fallopian or uterine tubes, including the mesosalpinx, mesovarium, ovarian artery, ovarian vein, suspensory ligament, uterine tube, ovary, broad ligament, round ligament, ovarian ligament, cardinal ligament, uterosacral ligament, and vagina.
Contributed by B Palmer
References
Thurmond AS, Machan LS, Maubon AJ, Rouanet JP, Hovsepian DM, Moore A, Zagoria RJ, Dickey KW, Bass JC. A review of selective salpingography and fallopian tube catheterization. Radiographics : a review publication of the Radiological Society of North America, Inc. 2000 Nov-Dec:20(6):1759-68 [PubMed PMID: 11112827]
Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clinical obstetrics and gynecology. 1980 Dec:23(4):1177-93 [PubMed PMID: 7004702]
Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. The journal of obstetrics and gynaecology research. 2010 Oct:36(5):929-37. doi: 10.1111/j.1447-0756.2010.01272.x. Epub 2010 Sep 16 [PubMed PMID: 20846260]
Ajithkumar TV, Minimole AL, John MM, Ashokkumar OS. Primary fallopian tube carcinoma. Obstetrical & gynecological survey. 2005 Apr:60(4):247-52 [PubMed PMID: 15795632]
Level 2 (mid-level) evidenceEzzati M, Djahanbakhch O, Arian S, Carr BR. Tubal transport of gametes and embryos: a review of physiology and pathophysiology. Journal of assisted reproduction and genetics. 2014 Oct:31(10):1337-47. doi: 10.1007/s10815-014-0309-x. Epub 2014 Aug 13 [PubMed PMID: 25117646]
Level 3 (low-level) evidenceBriceag I, Costache A, Purcarea VL, Cergan R, Dumitru M, Briceag I, Sajin M, Ispas AT. Fallopian tubes--literature review of anatomy and etiology in female infertility. Journal of medicine and life. 2015 Apr-Jun:8(2):129-31 [PubMed PMID: 25866566]
Gandhi KR, Siddiqui AU, Wabale RN, Daimi SR. The accessory fallopian tube: A rare anomaly. Journal of human reproductive sciences. 2012 Sep:5(3):293-4. doi: 10.4103/0974-1208.106344. Epub [PubMed PMID: 23532389]
Level 3 (low-level) evidenceMarino S, Canela CD, Nama N. Tubal Sterilization. StatPearls. 2023 Jan:(): [PubMed PMID: 29262077]
Venturella R, Morelli M, Zullo F. The Fallopian Tube in the 21st Century: When, Why, and How to Consider Removal. The oncologist. 2015 Nov:20(11):1227-9. doi: 10.1634/theoncologist.2015-0172. Epub 2015 Sep 17 [PubMed PMID: 26382741]
Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, Bhattacharya R, Novak M, Jones S, Phallen J, Hruban CA, Hirsch MS, Lin DI, Schwartz L, Maire CL, Tille JC, Bowden M, Ayhan A, Wood LD, Scharpf RB, Kurman R, Wang TL, Shih IM, Karchin R, Drapkin R, Velculescu VE. High grade serous ovarian carcinomas originate in the fallopian tube. Nature communications. 2017 Oct 23:8(1):1093. doi: 10.1038/s41467-017-00962-1. Epub 2017 Oct 23 [PubMed PMID: 29061967]
Dawood MY. Laparoscopic surgery of the fallopian tubes and ovaries. Seminars in laparoscopic surgery. 1999 Jun:6(2):58-67 [PubMed PMID: 10459057]
Khalaf Y. ABC of subfertility. Tubal subfertility. BMJ (Clinical research ed.). 2003 Sep 13:327(7415):610-3 [PubMed PMID: 12969933]