Introduction
Facial nerve palsy is a common presenting complaint in primary care offices, emergency departments, otolaryngology, and neurology clinics. Trauma accounts for 6-27% of all facial nerve palsies, depending upon whether anticipated iatrogenic injuries (such as radical parotidectomy for oncological resection) are considered "trauma" or not.[1] The most common traumatic causes of facial paralysis are resection of tumors, temporal bone fractures, and penetrating trauma to the facial nerve, including iatrogenic injury.[1][2] Facial paralysis has implications for patients' quality of life due to the facial nerve's role in myriad activities of daily living.[3]
Alongside the emotional impact of impaired facial expression, facial nerve palsy can produce ophthalmological, otological, rhinological, gustatory, and articulation sequelae. Ophthalmological consequences from impaired lacrimation, ectropion, and epiphora include exposure to keratopathy, which can ultimately lead to blindness if not recognized and addressed promptly. Otological consequences include hyperacusis and numbness of a portion of the external auditory canal. Impaired muscular support to the nasal valve can lead to nasal obstruction. Impact on the perioral musculature can result in altered speech and oral incompetence, while damage to the chorda tympani branch of the facial nerve can lead to dysgeusia.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Trauma to the facial nerve with resulting facial paralysis has been described in various scenarios. These include:
- Basilar skull fracture (temporal bone, predominantly)
- Penetrating trauma to the extratemporal aspect of the facial nerve (such as a knife and gunshot wounds)
- Birth trauma (most often with forceps delivery)
- Iatrogenic (typically during tumor excision but also during facial or mastoid surgery)
- Barotrauma (generally from scuba diving or airplane travel)
- Lightning strike [2][4]
Epidemiology
It is challenging to report with any precision the frequency of facial nerve trauma in the general population, but when viewed as a subset of facial paralysis as a whole, the epidemiology of traumatic facial palsy becomes easier to describe. In a 2014 study of 1,989 patients with facial nerve palsy, Hohman and Hadlock reported that 5.6% of cases resulted from accidental trauma, but this figure excluded iatrogenic injury; including iatrogenic cases, the figure approached 27%.[1]
A 2009 study published by Junior and colleagues reviewed the charts of 54 patients with facial paralysis and determined that 24% of patients had traumatic etiologies, while a 1996 paper written by Bleicher and colleagues, however, indicated that only 8% of facial paralysis were traumatic in origin.[5] Most recently, Chávez-Serna and co-authors reported that 35% of facial paralysis cases resulted from trauma in their 2021 series of 108 patients.[6] The most common traumatic etiologies are acoustic neuroma resection, parotid tumor resection, temporomandibular joint surgery, middle ear surgery, temporal bone fractures, and birth trauma.[1][5][6] The frequency with which each of these is seen varies greatly depending on the patient population and the healthcare setting.
For higher-volume trauma centers, temporal bone fractures may be the most common cause of traumatic facial palsy; facial paralysis occurs in 7 to 10% of all temporal bone fractures. The pyramidal shape and high density of the temporal bone mean that a significant force is necessary to cause a fracture.[7] Therefore, 31% of temporal bone fractures occur secondary to motor vehicle collisions. The next most common causes of temporal bone fractures are assaults and falls.[4][8] Consequently, approximately 90% of temporal bone fractures are associated with intracranial injuries, and 10% are associated with cervical spine injuries. Therefore, evaluating patients with temporal bone fractures for concomitant injuries is crucial.[9]
Pathophysiology
A broad range of injury severity exists in facial nerve trauma due to the various mechanisms by which injuries may be produced. Temporal bone fractures tend to cause crush injuries that evolve over hours to days, and sometimes even weeks, although they certainly can, in rare cases, result in transection of the nerve and immediate hemifacial paralysis.[7] Similarly, iatrogenic injuries tend to result from traction or thermal damage from nearby electrocautery but may also involve partial or complete nerve transection, as may be the case when drilling in the mastoid cavity. Likewise, penetrating facial injuries may stretch or transect the affected nerve branch, either partially or completely. Frustratingly, the extent of the trauma is often unclear at the time of injury. When conceptualizing nerve injury, 2 classification schemes are commonly used to describe severity: the Seddon and Sunderland scales (see Table. Management of Traumatic Facial Paralysis Algorithm).[10][11]
The Seddon scale is simpler and focused primarily on implications for management, while the Sunderland scale characterizes the extent of injury with respect to the microanatomy of the nerve involved. At its most basic, the functional unit of a motor nerve is the axon, which is the long filament that connects the cell body, located in the pons, to the motor end plate at the neuromuscular junction. The motor axons of the facial nerve, of which there are approximately 6,000, are insulated in myelin produced by Schwann cells.[12] Surrounding the myelin sheath is the endoneurium, a thin connective tissue membrane. These individually wrapped axons are bundled into fascicles, which are, in turn, surrounded by perineurial membranes. Lastly, the nerve is made up of fascicles surrounded by epineurium.
Seddon Classification
- Neurapraxia: a localized conduction block with focal demyelination and no Wallerian degeneration - complete recovery is expected.
- Axonotmesis: a variable amount of axonal injury with Wallerian degeneration and an intact epineurium - synkinesis may occur in more severe cases.
- Neurotmesis: complete nerve transection with Wallerian degeneration - flaccidity persists unless the nerve is repaired.
Sunderland Classification
- Class I: equivalent to Seddon's neurapraxia - complete recovery is expected,
- Class II: mild axonotmesis in which the endoneurium remains intact, but Wallerian degeneration occurs - complete recovery is expected.
- Class III: moderate axonotmesis in which the endoneurium is disrupted and Wallerian degeneration occurs, but the perineurium remains intact - mild to moderate synkinesis is expected.
- Class IV: severe axonotmesis in which the endoneurium and perineurium are disrupted, and Wallerian degeneration occurs, but the epineurium remains intact - severe synkinesis is expected.
- Class V: equivalent to Seddon neurotmesis - flaccidity persists unless the nerve is repaired.
To understand either classification system, it is also critical to be familiar with the concept of Wallerian degeneration. Wallerian degeneration is the process by which axons and Schwann cells disintegrate and are removed to make room for the regeneration of a new axon.[13] This process begins at the node of Ranvier just proximal to the site of injury and proceeds distally out to the neuromuscular junction, thus clearing the way for the injured axon to grow back to the neuromuscular junction at a rate of approximately 1 mm per day.[14] In cases of total nerve transection, Wallerian degeneration takes roughly 72 hours, but the process may take days or weeks to finish in cases of crush injuries.[15] Once Wallerian degeneration is complete, at least in the case of nerve transection injuries, the distal stump of the nerve is no longer stimulable, which may make exploration and repair more challenging; for this reason, most surgeons prefer to explore the facial nerve early if there is a high index of suspicion for transection.
In cases of nerve transection, the gold standard treatment is primary repair of the epineurium, which effectively converts a Sunderland class V injury into a class IV injury. Regardless, the injury remains severe, and any facial nerve injury of Sunderland class III or higher likely results in synkinesis due to the disruption of internal neural architecture. In Sunderland class I and II injuries, the epineurium - the membrane that surrounds the myelin covering the axon - is preserved; with this membrane intact, the affected axons regrow back to their original motor endplates, and complete recovery should occur. Sunderland class III-V injuries involve progressively greater degrees of disruption of the internal neural architecture, with correspondingly greater potential for misdirection of regenerating axons and resulting aberrant reinnervation. This aberrant reinnervation may take the form of involuntary movements accompanying voluntary movements or greater resting tension in the affected muscles; these symptoms are termed "synkinesis." Unfortunately, there is no way to correlate a clinical or laboratory examination with the Sunderland classification, making precise prognostication of functional recovery difficult. Electroneuronography, a type of evoked electromyography, can tell the difference between neurapraxia and anything more severe but cannot provide more anatomical detail than that.
Beyond the issue of the severity of the nerve injury itself, there remains the question of what risk factors and mechanisms of injury are likely to produce facial nerve trauma. For temporal bone fractures, the amount of force and the vector with which it is applied are the primary determinants of the type of injury sustained and whether or not facial paralysis results. The Ulrich classification of temporal bone fractures was suggested in 1926 and describes the orientation of the fracture relative to the petrous ridge of the temporal bone.[8] Longitudinal fractures (approximately 80%) run parallel to the long axis of the petrous ridge. They are typically produced by blows directed laterally towards the skull, as may be obtained during a physical altercation. These fractures rarely involve the otic capsule, as the fracture line follows the path of least resistance towards the petrous apex of the temporal bone; however, ossicular chain disruption, hemotympanum, tympanic membrane perforation, and otorrhea, as well as conductive hearing loss, are often associated with these injuries. Fortunately, facial nerve palsy occurs in only about 20% of longitudinal temporal bone fractures.[8]
Transverse fractures (approximately 20%) run perpendicular to the long axis of the petrous ridge and often straight through the labyrinthine capsule. They are often caused by force-directed anteroposteriorly, such as a motor vehicle accident. These fractures originate near the jugular foramen or foramen magnum and extend into the middle cranial fossa. Consequently, these fractures commonly result in sensorineural hearing loss, which may result from cochlear nerve transection, labyrinthine structures damage, or stapes footplate injury. They are associated with perilymphatic fistula (with nystagmus away from the fractured side) and facial palsy in 50% of cases.[8] Many fractures, however, do not fit well into this classification scheme, which led to the development of the otic capsule-sparing vs disrupting system described by Brodie and Thompson in 1997.[16]
In this scheme, temporal bone fractures are categorized based on whether or not they spare or disrupt the bone of the otic capsule. Otic capsule-sparing fractures (approximately 95%) tend to result from laterally-directed blows to the skull and are only associated with facial paralysis in about 6% of cases. Otic capsule-disrupting fractures only account for 5% of temporal bone fractures; however, up to half of these injuries result in facial paralysis.[16] The facial paralysis that accompanies temporal bone fractures is somewhat unique when compared to other types of facial nerve trauma because the bony Fallopian canal, which encases the facial nerve during its intratemporal course, makes it difficult for the injured nerve to swell without compromising its perfusion.[17]
In the case of extratemporal facial nerve trauma, the edematous nerve is unlikely to become compressed and can, therefore, maintain its perfusion. With extratemporal injuries, even delayed paralysis is likely present within a matter of several hours to a few days. Within the temporal bone, however, even comparatively minor facial nerve insults can result in edema that progresses very slowly for days to weeks before reaching a point at which perfusion is compromised and nerve function is impaired.
From the cerebellopontine angle, where the facial nerve exits the pons, to the stylomastoid foramen, where it exits the skull, the facial nerve takes a wandering course through the temporal bone. It enters the bone through the internal auditory meatus, along with the cochlear and vestibular nerves, occupying the anterosuperior position within the internal auditory canal, where it travels for 8 to 10 mm before separating from the other nerves and arriving at the labyrinthine segment. Here, it enters the Fallopian canal and makes its first turn, or "genu," at the geniculate ganglion, where the greater superficial petrosal nerve is given off to provide parasympathetic innervation to the lacrimal gland and the mucous glands of the nose and palate. In the ~4 mm long labyrinthine segment, the overall diameter of the bony canal narrows to approximately 0.7 mm, its narrowest portion, and therefore, the area most likely to be affected in temporal bone fractures.[18][19]
The tympanic or "horizontal" segment follows, roughly 9 to 10 mm in length, where the facial nerve courses superior to the stapes footplate within the middle ear and may occasionally be incompletely covered by bone, rendering it vulnerable to otological pathologies, such as infection or barotrauma. Finally, the nerve turns again at its second genu to travel inferiorly through the mastoid cavity, where it gives off the chorda tympani nerve to provide taste sensation to the anterior two-thirds of the tongue; this portion is known as the mastoid or "vertical" segment, and it is 11 to 12 mm long, ending where the nerve passes through the stylomastoid foramen.[20]
After exiting the skull, the main trunk of the facial nerve turns anteriorly to enter the parotid gland, where it divides into the main extratemporal branches at the "pes anserinus," so named because the separation of branches resembles the foot of a goose. These branches serve as the division between the superficial and deep lobes of the parotid gland. From superior to inferior, the branches are the frontal (which controls the muscles of the forehead and upper eyelid), the zygomatic (which controls the muscles of the eyelids and midface), the buccal (which controls the muscles of the midface and mouth), the marginal mandibular (which controls the depressors of the lower lip), and the cervical (which controls the platysma and some depressors of the lower lip as well). Branching patterns and control of specific muscles vary somewhat between individuals, but the facial nerve branches run deep to the superficial musculoaponeurotic system of the face and the temporoparietal fascia of the scalp as well as deep to all the mimetic muscles other than the mentalis, buccinator, and levator anguli oris. Additionally, the facial nerve innervates the auricular muscles, the stapedius muscle, the stylohyoid muscle, and the posterior belly of the digastric muscle.
As mentioned above, the facial nerve is anatomically susceptible to barotrauma in some patients, which is a comparatively uncommon occurrence. Case reports have been published detailing the stories of a handful of patients who have suffered acute facial paralysis due to SCUBA diving or traveling at high altitudes in an airplane or a car driving through a mountain pass.[21][22][23][24] The pathogenesis of the palsy has not been elucidated, but it may result from dehiscence of the facial nerve within the middle ear, making it susceptible to pressure changes, particularly in the setting of Eustachian tube dysfunction. In some cases, hearing loss and subdural air on neuroimaging have also been reported with facial paralysis.[22]
History and Physical
When evaluating a patient with acute, traumatic facial paralysis, the 2 most important pieces of information to elucidate are the timeline of the paralysis and its severity. When the paralysis is immediate and complete, there should be a high index of suspicion for a facial nerve transection; however, if the paralysis is delayed or incomplete, the nerve must be at least partially intact. If nerve transection is suspected, exploration should be undertaken to repair the injury. If the nerve is partially intact, the patient can typically be observed closely with the general expectation of a good recovery. The caveat to this last point is that facial paralysis from temporal bone fractures often takes days to weeks to reach its peak because the edema produced within the Fallopian canal of the facial nerve may develop very slowly. If the paralysis becomes complete, however, patients with temporal bone fractures may be candidates for facial nerve decompression, which is why it is critical to follow them closely until they begin to recover function, meet decompression criteria or 2 months have passed since the injury.
While it may seem very straightforward to determine the timeline and the severity of the paralysis in most cases, there are occasions when it may not be. Examples include long surgeries in which an iatrogenic injury caused early on may be immediately apparent on emergence from anesthesia but could still represent a delayed onset and polytrauma patients (particularly involving the temporal bone) that present intubated and sedated without the ability to follow commands or to provide a history of paralysis onset. Nevertheless, it is incumbent upon the clinician to obtain the best history possible, sometimes from relatives, friends, paramedics, or bystanders, and to assess facial nerve function as systematically as possible. Many classification systems are used to describe impaired facial nerve function, but the most common is that popularized by House and Brackmann in 1984.[25] This classification is:
- Grade I: normal function
- Grade II: mild dysfunction, symmetric at rest, slight asymmetry with movement but complete eye closure with gentle effort, possible mild synkinesis
- Grade III: moderate dysfunction, symmetric at rest, moderate asymmetry with movement but complete eye closure with full effort, possible moderate synkinesis
- Grade IV: moderate dysfunction, symmetric at rest, moderate asymmetry with movement, and incomplete eye closure with full effort
- Grade V: severe dysfunction, grossly asymmetric at rest, marked asymmetry with movement, and incomplete eye closure with full effort
- Grade VI: severe dysfunction, grossly asymmetric at rest, no movement at all (see Video. House-Brackmann Grade VI Facial Paralysis).
The House-Brackmann scale is simple and rapid to employ and is, therefore, quite popular. However, it was created to characterize facial paralysis of otologic or otological iatrogenic origin, which is really only useful for describing hemifacial flaccid paralysis. Its description of synkinesis is limited, and it cannot describe segmental abnormalities. Other classification systems, such as the Yanigahara, Sidney, Sunnybrook, House-Brackmann 2.0, and eFACE scales, provide more granularity when describing facial paralysis, but for the sake of documentation, it may be both simpler and more precise to convey the findings in a detailed and systematic way that describes the functional status of each major extratemporal facial nerve branch.[26][27][28][29] These findings should also be documented photographically and videographically, which are the most effective means of assessing recovery progress.
- Frontal: elevate brows, furrow brows (scowl).
- Zygomatic: close eyes gently (or watch for natural blinks), close eyes tightly - the distinction between the ability to close the eye with normal effort vs. with full effort is the difference between House-Brackmann grades II and III.
- Buccal: smile, pucker lips.
- Marginal mandibular: depress lower lip to show bottom teeth.
- Cervical: contract platysma.
- Overall: look for asymmetry at rest, particularly in brow height, lower eyelid position, nasolabial fold depth and orientation, the position of the upper lip philtrum, and the position of the oral commissures.
It is important to consider the non-emotive functions of the facial nerve as well, which are generally evaluated during the history by asking questions about hearing, taste, nasal breathing, eye dryness or pain, and eye tearing. When patients present after skull base surgery or injury, the corneal sensation should be assessed with a wisp of cotton to rule out corneal hypesthesia. Patients who undergo acoustic neuroma resections are at particular risk for corneal hypesthesia, which can mask the progress of exposure keratopathy and increase the risk of long-term ocular surface problems. These patients are candidates for early eyelid weight placement, as are patients who are less able to protect their corneas due to a poor Bell's phenomenon (upward rotation of the globe with eye closure). Lastly, the emotional status of any patient with facial paralysis should be assessed, as the psychosocial impact on patients, particularly young females, can be profound and may require behavioral health intervention.[3] In the case of polytrauma patients, particularly temporal bone fracture patients, it is critical to look for other injuries, particularly cervical spine fractures, cerebrospinal fluid leaks, and other intracranial pathology, which accompany temporal bone fractures in up to 90% of cases.[30]
Evaluation
Once a thorough history has been taken and a physical examination performed, followed by photo- and video documentation, additional studies may be performed, if necessary. In cases of traumatic facial paralysis, the most common additional tests include non-contrast computed tomography scanning for temporal bone fractures and electrodiagnostic studies to determine the severity of nerve injury. When other injuries accompany the facial paralysis, further workup may be prudent, such as β-2 transferrin testing of clear rhinorrhea or otorrhea to rule out a cerebrospinal fluid leak, a neck film to determine whether or not there is cervical spine fracture, and an audiogram to evaluate for hearing loss.
Electrodiagnostic Studies
Electroneuronography (ENoG) is an evoked form of electromyography in which the facial nerve is stimulated where it exits the temporal bone at the stylomastoid foramen using a transcutaneous electrical signal. The resulting compound action potentials of the facial muscles, usually the orbicularis oris and orbicularis oculi, are then measured via surface electrodes.[31] The amplitude of the action potential is compared between the injured and uninjured sides to determine the severity of the trauma; this requires that the injury be unilateral. If the injury was mild enough that no Wallerian degeneration occurs, or if the ENoG is performed before 72 hours have passed since the injury and Wallerian degeneration has not had time yet to occur, the action potentials may not differ significantly between the left and right sides, even though there may not be any appreciable facial movement. Typically, however, ENoG is only performed for patients with complete (House-Brackmann grade VI) facial paralysis to determine if the injury is as severe as it appears on the physical examination. If there is a loss of >90% of the action potential amplitude on the injured side relative to the normal side, the injury is considered severe, and the patient may be a candidate for facial nerve decompression in the case of temporal bone fracture. Because of the slowly progressive nature of some paralyses after a temporal bone fracture, ENoG testing is often repeated weekly for 1 to 2 months in these patients.[18]
Patients who meet decompression criteria essentially have the equivalent of a Sunderland class V injury; if they do not undergo decompression, they are likely to experience worse outcomes than those who do not meet decompression criteria.[8] The ENoG may also be used to assess the severity of the injury for patients in whom the extent of the injury is unclear, for example, a patient who undergoes acoustic neuroma resection and emerges from anesthesia with a complete hemifacial paralysis despite the surgeon being under the impression that the facial nerve was intact throughout the operation.
Voluntary electromyography (EMG) is another form of electrodiagnostic testing that is often employed later in the course of facial paralysis. It is used to assess the status of the muscles and determine whether they are denervated but viable, in the process of recovering their innervation, or are no longer capable of receiving a neural input. Multiple waveforms may be visible on EMG, including fibrillation, positive sharp waves, polyphasic potentials, flat lines, and normal voluntary motor unit potentials. Muscles that are denervated but viable, ie, targets for reinnervation via either facial nerve repair or nerve transfer, generally demonstrate fibrillation and positive sharp waves with insertional activity during recording electrode needle placement. Muscles whose innervation is returning but may not yet have contraction visible on physical examination or detectable on ENoG demonstrate polyphasic potentials. Muscles that are no longer viable due to prolonged denervation (12 to 24 months), atrophy, and fibrosis show a flat line with no electrical activity on EMG, while muscles with normal stimulability have visible voluntary motor unit potentials. These potentials may still be visible even if the ENoG demonstrates >90% action potential amplitude loss; in that case, facial nerve decompression or repair may not be necessary.
Treatment / Management
In essence, the goal of treating traumatic facial paralysis must be to preserve or restore as much facial mimetic function as possible. This can be accomplished in myriad ways, depending upon the severity of the injury and the interval between the injury and the patient's seeking care.
Acute injury: <12 Months Since Injury
Medical Management
For the majority of patients with acute traumatic facial paralysis, similar to patients suffering from viral reactivation facial paralyses, a course of high-dose oral steroids (prednisone 60 mg PO daily x 5 days, with an additional 5-day taper) can improve outcomes by reducing inflammation and edema of the facial nerve.[2][32][33] Care should be taken when prescribing corticosteroids to patients with diabetes, patients with gastric/peptic ulcers, patients already taking steroids, patients taking fluoroquinolones, and others due to the potential adverse effects of steroids. In addition to systemic corticosteroids, there is some evidence that dihydropyridine calcium channel blockers, specifically nimodipine, can improve outcomes after facial nerve trauma by reducing apoptosis and improving axonal sprouting.[34] Nifedipine has been investigated as well, but the evidence is less clear that this medication improves facial nerve outcomes clinically, although animal studies in which nifedipine was applied topically to the injured nerve do demonstrate better histological recovery with nifedipine than control subjects had.(A1)
Eye Care
Patients with acute facial paralysis of any etiology require close follow-up to ensure the health of the corneal surface, particularly patients with intracranial injuries to the facial nerve that may have also affected the trigeminal nerve and caused corneal hypesthesia. Most patients with paralytic lagophthalmos are acutely aware of any ocular issues that occur, such as corneal abrasions or ulcerations, because of the pain that accompanies them, but patients lacking the ability to feel corneal injuries are at a high risk of developing ocular surface complications.[1](B2)
Likewise, patients with poor Bell's phenomena should be considered high-risk, followed more carefully, or treated more aggressively. Standard treatment for paralytic lagophthalmos should include artificial tear drops as needed during the day, eyelid stretching exercises and ocular lubricant at night, with eyelid taping if necessary.[35] For patients at high risk for ocular surface injury who continue to have ocular discomfort despite the conservative management previously mentioned, fitting for a scleral contact lens or placement of an upper eyelid weight should be considered.[36][37] Additional periocular procedures may also be required, such as lateral tarsal strip canthopexy, tarsorrhaphy, or tarsoconjunctival flap transfer.(B2)
Nerve Transection
When the facial nerve is known to have been transected, or there is a high suspicion that this has occurred, an effort should be made to locate the injury site and repair the nerve. Ideally, the exploration occurs within the first 72 hours after injury so that the process of Wallerian degeneration not have been completed yet; this keeps the distal stump stimulable and, therefore, easier to find in the wound bed. The proximal stump can always be located via antegrade dissection from the main trunk of the facial nerve or the pes anserinus. For cases of very proximal injury, the nerve may be located within the mastoid and followed distally through the stylomastoid foramen, where it exits the skull base. Other methods of identifying the facial nerve's main trunk include following the tragal cartilage's anterior surface until reaching the tip (tragal pointer) and then continuing the dissection deeper for 1 cm and 1 cm more inferiorly. The tympanomastoid suture line is also palpable, and this can be followed to within about 3 mm of the stylomastoid foramen.[38]
The approach to the facial nerve exploration varies depending on the suspected location of the injury, but in the face itself, the nerve is typically visualized via the preauricular portion of a Blair incision, which provides access to the extratemporal main truck of the facial nerve as well as the pes anserinus and the main branches. If the injury is medial to the lateral canthus, as may occur with a facial laceration, the nerve branch does not usually need to be repaired because of the degree of redundancy among distal midfacial branches and the high likelihood of spontaneous functional recovery. When preparing for surgery, it is important to avoid injecting local anesthetics into the area (in some patients, lidocaine may diffuse farther than expected and last longer than 2 hours; injection of plain 1:100,000 epinephrine may be preferable) and to request that the anesthesia provider not administer a long-acting paralytic medication, which interferes with the use of a nerve stimulator to find the distal stump.
In some cases, rather than encountering a transection injury, the surgeon may find the nerve intact but non-stimulable and surrounded by scar tissue. External neurolysis - removing the scar tissue from around the epineurium - may reduce inflammation and improve function. If the scar tissue appears to penetrate the epineurium and involve the interior of the nerve, the nerve should be divided and trimmed back to normal neural tissue, which should demonstrate distinct fascicles and pinpoint bleeding. Significant scarring within the nerve itself may indicate a severe crush or shearing injury or even a transection injury that failed to distract the nerve stumps far enough away from each other to prevent coaptation during healing. Segmental resection of the nerve is also recommended when a portion of the nerve has had >50% of its diameter disrupted, which would likely result in extensive scarring; a common clinical scenario resulting in this type of injury is mastoidectomy, in which a drill is inadvertently raked along the facial nerve. Identifying scarring within the nerve is critical because scarring prevents proper axonal regeneration, resulting in suboptimal recovery. Internal neurolysis and removal of scar tissue from within the nerve must be performed to optimize outcomes in these cases. Occasionally, after debriding the nerve stumps back to normal tissue, the ends may not be sewn back together easily without tension. Tension-free coaptation of the nerve ends is critical because stretching the nerves compromises perfusion, which compromises healing and subsequent functional recovery. A gap of up to 6 mm between nerve ends is acceptable to relieve tension, provided some form of the sheath is applied to contain the regenerating axons.[39][40](A1)
If the gap between nerve ends is going to be >6 mm, placement of an interposition graft ("cable graft") is preferable to applying excessive tension to the nerve ends or excessively dissecting around the nerve stumps to mobilize them, both of which decrease perfusion. The most common source of cable graft material is the greater auricular nerve, which is a good size match for the facial nerve's main trunk and conveniently located so that it can be harvested via the same incision. The greater auricular nerve travels on the superficial surface of the sternocleidomastoid muscle from Erb's point superiorly toward the lobule of the auricle, coursing roughly halfway between the angle of the mandible and mastoid tip, 1 to 2 cm posterior to the external jugular vein.[41]
Other commonly harvested sensory nerve grafts include the sural nerve, which is particularly useful for providing long grafts for cross-face applications, and the medial antebrachial cutaneous nerve, which has a branching pattern similar to the facial nerve. Of note, when using a non-branching graft, it is important to rotate the graft 180 degrees before suturing it to maximize the number of axons that successfully traverse the length of the graft. Because even non-branching grafts, such as the proximal greater auricular nerve, still have microscopic branches, coapting the distal end of the graft to the proximal facial nerve stump and the proximal end of the graft to the distal facial nerve stump helps to prevent axonal loss via unseen branches. Doing everything possible to minimize axonal loss during regeneration is critical because each neurorrhaphy represents the potential to lose up to half of the regenerating axons, which means that up to 75% of axons could be lost traversing the 2 neurorrhaphies required for the use of a cable graft.[42] Some surgeons use cadaveric nerve grafts rather than harvesting sensory nerves from the patient, but there is evidence that these grafts do not perform reliably.[43][44](B3)
From a technical standpoint, the neurorrhaphy should be performed under high magnification, typically using an operating microscope, with a 9-0 or 10-0 nylon suture on a cutting or spatula-tip needle because it can be difficult to pass a tapered needle through epineurium (see Image. Marginal Mandibular Branch of the Facial Nerve Injury). Ideally, the nerve ends are brought together so that they are touching but not pulled firmly together. Sutures are typically placed in an interrupted fashion into the epineurium, and it is rare that more than 3 or 4 are required. If fascicles herniate through the suture line, either the suture line should be replaced with less tension or the fascicles should be amputated to allow them to fit back through the neurorrhaphy. While it may be tempting to place sutures into the perineurium of the fascicles before repairing the endoneurium, it is nearly impossible to determine which fascicles should be coapted to each other with any degree of precision. Therefore, the outcomes of perineurial and epineurial repair are equivalent, despite the greater time and effort required to repair the nerve at a fascicular level.[45][46] Pathophysiologically speaking, the goal of a neurorrhaphy is to take a Sunderland class V injury and convert it to a class IV injury, which in the main trunk of the facial nerve improves the prognosis from long-term flaccidity to synkinetic movement. Patients undergoing repair or grafting of the facial nerve main trunk should be counseled to expect synkinesis and informed that they would require rehabilitation as function returns.(B3)
While primary repair of the facial nerve or cable grafting is preferred, there are situations in which these options are unavailable. The classic example is the case of a skull base tumor in which the proximal facial nerve has been extirpated intracranially, or the facial nucleus itself has been rendered nonfunctional or absent due to central nervous system pathology. A new axonal source is required for these patients to populate the distal facial nerve and reinnervate the mimetic muscles. Historically, the most commonly employed options were the placement of a cross-face nerve graft or using a hypoglossal-facial nerve transfer (see Video. Facial Nerve Transfer). Cross-face nerve grafting typically uses a length of sural nerve to connect the facial nerve on the unaffected side to the paralyzed side, most often sacrificing a single buccal branch on the uninjured side to send axons to a buccal branch on the paralyzed side for smile rehabilitation. The sural nerve contains approximately 1,100 axons, which provides enough room for the ~900 axons of the usual donor buccal branch to regenerate across the face into the recipient nerve.[47][48](B2)
Cross-face nerve grafting is often used to power-free muscle flaps for smile rehabilitation (see below). The decision to use a cross-face nerve graft should only be made after considering the following points: 1) results of cross-face grafting are generally better in younger patients, who presumably have greater neural regeneration potential, and are unreliable in older patients; 2) placement of a cross-face graft requires dissecting the uninjured facial nerve, which places it at risk for injury, even though sacrificing a buccal branch on the uninjured side does not typically result in noticeable weakness, and 3) because the cross-face graft requires normal function on the donor side, patients with bilateral injuries are not candidates, nor are patients at risk for contralateral facial paralysis, such as those with type 2 neurofibromatosis.[49](B2)
However, the primary advantage of a cross-face nerve graft is that it is the only nerve transfer option that reliably provides a spontaneous smile because the signal source for the mimetic muscles after cross-face nerve grafting is still a facial nerve. On the other hand, smiling after hypoglossal-facial transfer requires the patient to move the tongue to activate the facial muscles. The hypoglossal-facial transfer also comes with an important caveat: the outcome of the facial reanimation is proportional to the number of axons transferred, but the functional deficit in the tongue is also proportional to the number of axons transferred. The main trunk of the facial nerve contains about 6,000 axons, while the hypoglossal nerve contains approximately 9,000 axons, making a transfer of the entire hypoglossal nerve a potentially good match for the facial nerve, accounting for a 33% loss of axons due to neurorrhaphy.[12][50][51]
This procedure likely provides good resting facial tone and facial movement with tongue motion, but tongue function is severely compromised, and facial movement is severely synkinetic. Transferring approximately 1/3 of the hypoglossal nerve's diameter end-to-end into the facial nerve provides less tone and movement but also limits the lingual function deficit. An interposition "jump" graft, often using the greater auricular nerve, can also be placed end-to-side between the main trunk of the facial nerve and the hypoglossal nerve if some facial function remains and the surgeon is reluctant to divide the facial nerve. Alternatively, if the main trunk of the facial nerve is already divided, but the surgeon is reluctant to divide the hypoglossal nerve, the facial nerve trunk may be sutured end-to-side into the hypoglossal nerve. Regardless of the chosen transfer technique, end-to-side neurorrhaphy results in fewer transferred axons than an end-to-end coaptation but also causes a less functional loss in the tongue. To perform an end-to-side neurorrhaphy, a window must be cut into the epineurium of the nerve whose side is being used, and dividing some of the fascicles within that nerve also promotes axonal growth. One way to minimize facial synkinesis is to perform the hypoglossal-facial transfer from the hypoglossal nerve to a single branch of the facial nerve, such as the branch to the zygomaticus major muscle, which has the effect of focusing the signal from the hypoglossal nerve on a single facial movement but also prevent the restoration of resting tone. Another solution is to transfer the masseteric nerve to a branch of the facial nerve, which provides control over a single facial movement, such as smiling.[52] The masseteric nerve, which is found 3 cm anterior to the tragus, 1 cm inferior to the zygomatic arch, and 1.5 cm deep to the masseteric fascia, is a reliable reinnervation option for this purpose and contains around 1,500 axons, which should be sufficient to replace the ~900 axons found in the average buccal branch of the facial nerve.[53][12][54](B2)
Unlike the hypoglossal nerve, the masseteric nerve has a low basal firing rate, which makes it a poor choice for restoring resting facial tone.[55] For this reason, a hypoglossal-facial transfer may be employed to restore tone, and a masseteric nerve-facial nerve transfer may be used for smile rehabilitation. As with hypoglossal-facial transfer, masseteric nerve transfer requires a deliberate movement from the patient - biting down, in this case - to activate the facial muscles. Over time, many patients learn to activate their smiles without having to clench their jaws, but it is comparatively uncommon for adults to develop true spontaneity of the smile after a nerve transfer, with women being more likely to do so than men.[56] Unlike with the hypoglossal-facial nerve transfer, using the masseteric nerve results in comparatively little long-term morbidity; the ipsilateral masseter usually atrophys to some extent, which may cause facial hollowing, but the muscle often reinnervate over time and regains some of the lost bulk. Ultimately, the goal of surgical management for facial paralysis is to restore mimetic function with as natural an appearance as possible, which is best accomplished by reinnervating the native musculature.
Temporal Bone Fracture
In cases of temporal bone fracture, the facial nerve generally suffers a blunt injury rather than a transection due to edema occurring within the tight confines of the bony Fallopian canal.[7] The most common area affected is the labyrinthine segment, most often the geniculate ganglion.[18] It can be challenging to differentiate a transection injury, which requires operative repair, from a blunt injury, such as intraneural hematoma, on computed tomography scan; fortunately, blunt injuries account for 86% of facial paralyses in temporal bone fractures, and transection injuries only 14%.[19] As with penetrating or iatrogenic injuries, the severity of the paralysis and the timeline determine the level of suspicion for a transection injury in patients with temporal bone fractures, but a unique aspect of temporal bone fractures is that operative intervention may also be helpful in certain patients who have not suffered nerve transection. Patients with House-Brackmann grade VI paralysis should undergo electroneuronography, waiting at least 3 days from the time of injury for Wallerian degeneration to occur, even if the paralysis is complete. If there is >90% amplitude degeneration of the paralyzed side compared to the unaffected side, facial nerve decompression should be performed.[18] (B2)
The intratemporal facial nerve can be accessed via transmastoid and middle fossa craniotomy approaches if the hearing is intact or via a translabyrinthine craniotomy if the labyrinth was damaged by the fracture and profound hearing loss has occurred.[57] Should a transection injury be encountered during exposure of the facial nerve, an appropriate repair should be performed, either primarily or with an interposition graft, if necessary (see above). Because facial paralysis from temporal bone fractures may evolve slowly, patients should be followed closely until they meet decompression criteria (House-Brackmann grade VI paralysis and >90% amplitude loss on ENoG), clinical recovery becomes apparent, or 2 months from the time of injury elapse, after which decompression is unlikely to provide a benefit to the recovery process.[18] As with patients suffering facial paralysis from other traumatic etiologies, facial paralysis patients with temporal bone fractures should also receive high-dose oral steroids and be monitored for corneal complications, using eyedrops, ocular lubricant, and eyelid taping as necessary. In some cases, periocular surgical procedures may be required to protect the cornea adequately (see above).(B2)
Barotrauma
While barotrauma is an uncommon cause of facial paralysis, it is worth noting that adjunctive treatment along with high-dose oral steroids appears to be helpful. Adding decongestants and antihistamines, as might be recommended for symptomatic relief of an upper respiratory infection, can expedite the return of facial function, as can myringotomy with or without placement of a pressure equalization tube in the ipsilateral ear.[22] Essentially, the goal is to alleviate any acute Eustachian tube dysfunction and permit inflammation in the facial nerve to resolve.[21][23][24][23][21](B3)
Chronic injury: >12 Months Since Injury
Beyond 12 months from paralysis onset, the likelihood of successfully reinnervating the mimetic muscles decreases substantially because they have most likely begun to atrophy and fibrose due to chronic denervation. Attempting to reinnervate them at this late stage runs a higher than usual risk of failure, particularly because after a nerve transfer or repair, axons would still have to grow to the neuromuscular junctions, which occurs at a rate of approximately 1 mm/day; thus, several additional weeks to months typically still pass after the reinnervation procedure has been performed but before the muscles can receive any neural input. If the patient and surgeon feel that reinnervation is the preferred option despite prolonged denervation of the mimetic muscles, for example, if the patient is a poor candidate for a muscle transfer, electromyography may be used to assess the status of the facial muscles. If fibrillation potentials are present, the muscles should be capable of receiving neural input, but electrical silence indicates non-viable muscles. Of course, demonstration of viable muscles on EMG does not guarantee that they remain viable by the time axons from a reinnervation procedure can reach them, although some surgeons anecdotally report successful facial reinnervation performed up to 24 months after the onset of paralysis.
Facial reanimation may be offered for patients not deemed suitable for reinnervation procedures. Reanimation techniques are classically categorized as "static" or "dynamic," with dynamic reanimation procedures further divided into regional and free muscle transfer options. Static reanimation techniques are often applied in the middle and upper thirds of the face, while dynamic techniques are utilized to rehabilitate the smile.
Upper Face
Reanimation of the forehead and periocular region is often performed using "static" techniques that do not provide movement to the face but still improve symmetry and function. Restoring symmetric brow elevation by transferring functional muscle would be challenging because the muscle bulk required would be very noticeable between the skin and soft tissue of the forehead and the frontal bone. For this reason, conventional brow lifting is often employed, commonly using direct, endoscopic, or suture suspension techniques.[58] Endoscopic lifting minimizes the visible scarring but provides a less effective elevation of the brow (approximately 2 mm), particularly when performed unilaterally or asymmetrically, and is therefore only applicable when there is minimal brow ptosis or the patient is reluctant to undergo a procedure that produces a noticeable scar.[59]
Direct brow lifting, on the other hand, provides more elevation and control over brow shape and position, but at the cost of a scar along the superior edge of the eyebrow. For patients who need significant brow elevation but cannot tolerate a scar, suture suspension brow lifting is a compromise between the endoscopic and direct techniques, but it tends to peak the eyebrow and also causes dimpling where the sutures are passed through the dermis of the brow. Regardless of the technique chosen, the goal for the brow lift should be for the paralyzed brow to sit at a height midway between the non-paralyzed brow's resting position and its elevated position, which are typically approximately 10 to 15 mm apart. The average lay bystander notices a difference in brow height of >4 mm; therefore, placing the paralyzed brow at the halfway position between resting and elevation minimizes apparent asymmetry.[60]
Similar to reanimation of the brow, reanimation of the eyelid is generally undertaken using static techniques. Common options include scleral contact lens placement, eyelid weight placement, tarsorrhaphy, tarsoconjunctival flap transfer, and lateral tarsal strip canthopexy. The primary goal of periocular reanimation should be to protect the cornea, and the secondary goal is to produce a synchronous blink. Contact lenses can be very effective for maintaining a healthy corneal environment in patients who tolerate long-term wear, although these lenses do nothing to facilitate eyelid closure per se.[36] (B3)
Weight placement onto or into the upper eyelid via either adhesive or a surgical procedure protects the cornea and improves actual eyelid closure, although the closure produced is slower than that on the unaffected side and often less complete. The other drawback to eyelid weight placement is the compromise between the closure's completeness and the degree of blepharoptosis present at rest. Patients who complain that wind, rain, shampoo, etc, still get into their eyes after weight placement may request larger weights be implanted, but this usually results in noticeable depression of the upper eyelid margin and potential visual field obstruction. Various sizes of weights may be applied with adhesive to determine which is the ideal one for a given patient, and this may be used to inform implantable weight selection if the patient and surgeon feel that an operation is appropriate to improve patient convenience or because a prolonged recovery is anticipated. Most patients do well with a 1.2-gram weight. The weight may be surgically placed in a pretarsal position, roughly 2 mm above and parallel to the lash line, or in a post-septal position, located more superiorly in the upper eyelid. In either case, the weight is centered over the point of maximum lagophthalmos, which is typically between the mid-pupil and the medial limbus. A post-septal location for the weight permits it to be covered by preaponeurotic orbital fat, thereby concealing it and improving cosmesis; the disadvantage to weight placement in this position is that the weight has less of a mechanical advantage than weight in the pretarsal position and therefore a heavier weight is typically required. The post-septal position also means that the weight is likely to pull the eyelid open when the patient is supine, which may necessitate taping the eye closed at night - this is not a problem encountered with pretarsal weight placement.[61]
Pretarsal weight placement, however, does come with its disadvantages: the skin in the pretarsal eyelid is the thinnest in the human body, and the contour of the weight is often visible underneath it. Weight placement in this location is also liable to cause astigmatism due to pressure on the globe, but this can be mitigated using an articulated chain instead of a solid plate.[62] Again, however, a compromise is necessary because using a chain increases the risk of extrusion over solid weight. Lastly, the choice of weight material should be considered: most eyelid weights are made of gold, which has a low tissue reactivity rate (around 5%), but platinum has an even lower rate of tissue reactions and allergies (about 1%) as well as a slightly higher density, making the size of the implant for a given mass even smaller and less visible.[37] Regardless of material or articulation, eyelid weights are easily removed if the patient recovers spontaneous eye closure.
When lower eyelid laxity contributes substantially to a patient's paralytic lagophthalmos, tightening the lower eyelid should be considered. The most common way of accomplishing this is to perform a lateral tarsal strip canthopexy, which removes a portion of the lateral lower eyelid margin and fixates the lateral aspect of the inferior tarsal plate to the lateral orbital wall periosteum posterior and superior to Whitnall's tubercle, thus tightening and elevating the lower eyelid. In some cases, elevation of the medial canthus is also required, particularly in patients with heavy soft tissues and a prior failed lateral canthopexy. Medial canthopexy can be achieved with the placement of a small screw into the maxilla, which is then used to anchor a suture suspension of the medial aspect of the inferior tarsal plate or even a suspension of the entire margin of the lower eyelid using fascia lata or a ribbon of expanded polytetrafluoroethylene (ePTFE).[62]
Avoiding injuring the lacrimal system is critical when operating in the medial canthal area. Lateral tarsal strip canthopexy is often accompanied by a tarsorrhaphy to decrease the resting height of the palpebral fissure and thereby improve the corneal environment, but many patients, particularly younger ones concerned with cosmesis, do not tolerate tarsorrhaphy, whether temporary or permanent. Despite its effectiveness, the end result is often considered unsightly, regardless of whether the tarsorrhaphy is surgical or simply a horizontal mattress suture passed over bolsters. An alternative to tarsorrhaphy is the tarsoconjunctival flap, in which a 3 to 6 mm wide and roughly 4 to 5 mm high inferiorly-based flap of composite tarsal plate and conjunctiva is transferred from the upper eyelid to the lower, thereby coupling the 2 eyelids without suturing the lid margins together in an aesthetically displeasing manner.[63]
These techniques are all capable of providing corneal protection if applied appropriately, but restoration of a synchronous blink is extremely challenging due to the speed of normal eyelid closure because the orbicularis oculi are the fastest muscles in the human body. When the muscle is transferred from a remote site to assist with eyelid closure, the result is not ordinarily worth the effort due to the unnatural appearance of the transferred muscle's bulk and the persistent dyssynchrony of eye closure.[64] Some authors, however, have reported tunneling a portion of the orbicularis oculi of the non-paralyzed side across the nasal root as a pedicled flap, demonstrating improved blinking and corneal health.[65][66]
Mid and Lower Face
The main goals of reanimation in the mid and lower faces are to reduce nasal obstruction, improve nasolabial fold symmetry, and rehabilitate the smile. Static reanimation techniques can be applied to any of these objectives, although smile rehabilitation is most often undertaken with muscle transfer when possible. Alleviation of nasal obstruction in facial paralysis centers on restoring nasal valve patency, compromised due to the nasalis muscle's weakness. While support of the nasal valve may be accomplished using rhinoplasty techniques, such as spreader and alar batten grafting, valve suspension is more often performed using a technique in which a screw is placed into the maxilla. A suspension suture is anchored to it, or a ribbon of fascia lata or ePTFE is used to suspend the lateral aspect of the nasal ala to the temporalis fascia.[67][35] (B2)
Fascia lata or ePFTE is placed via a preauricular incision, which can also be used to place material to suspend the nasolabial fold or the oral commissure. Static oral commissure suspension may be the best smile rehabilitation option for patients who are not healthy enough to tolerate lengthy dynamic reanimation procedures or do not wish to undergo them for other reasons. Suspension of the nasolabial fold, on the other hand, is often performed in conjunction with dynamic smile reanimation to improve the overall symmetry of the face, an oral commissure that moves normally when smiling but that has an effaced or otherwise asymmetric nasolabial fold above it does not provide the overall natural appearance that patients and surgeons are trying to achieve. Nasolabial fold suspension may be performed using an additional incision through the nasolabial fold itself or, more commonly, using a percutaneous approach in which needles are passed through stab incisions just inferior to the fold to anchor a sheet of fascia lata.[68] Similarly, a strip of fascia or ePTFE may be fixated to the modiolus of the oral commissure via an incision in the corner of the mouth or internally under direct visualization through the preauricular incision. However, fascia grafting or alloplastic implantation is not always required for oral commissure repositioning and nasal valve opening. The modiolar rotational cheiloplasty is a simple procedure in which an incision along the nasolabial fold and around the inferior aspect of the nasal ala permits superior advancement of the oral commissure along with the lateral expansion of the nasal vestibule.[69]
While elevating the oral commissure to correct a droop in the corner of the mouth can be readily accomplished with static reanimation techniques, providing movement to the smile on the paralyzed side of the face is substantially more complicated and requires precise control over the vector and amount of oral commissure movement as well as its timing. Dynamic reanimation of the smile can be performed using either regional or free muscle transfer, with the most common muscles used being the temporalis (as a regional flap) and the gracilis (as a free muscle flap). Regional muscle transfer is less time-consuming than free muscle transfer and is technically easier to perform. The primary drawback to regional muscle transfer is that the amount of oral commissure movement produced is not as great as that achieved with free muscle transfer, and there are limitations to the vectors of movement that can be obtained as well. As an example, masseteric muscle transfer is a technique that involves releasing a portion of the inferior aspect of the masseter and suturing it to the oral commissure so that a smile would occur when the patient clenches the jaw because the origin of the masseter muscle is the zygomatic arch, which lies too far posteriorly to the oral commissure, the vector of movement during the smile produced by masseter muscle transfer is much more lateral than that of a natural smile and the technique has been largely abandoned, except some surgeons who reposition the origin of the muscle more anteriorly to achieve the correct vector.[70] (B3)
However, moving the whole masseter muscle redistributes facial volume, leading to additional noticeable asymmetries. For this reason, the temporalis muscle is employed far more frequently for facial reanimation than the masseter muscle, given that its origin is more superior and provides a more natural vector for oral commissure movement than the masseter does. The temporalis may be transferred in 2 different ways: detaching the central portion of the muscle from the skull and reflecting it downward over the zygomatic arch to insert into the oral commissure (temporalis flap transfer) and detaching the insertion of the muscle from the mandible and advancing it to the oral commissure (temporalis tendon transfer). The former method tends to leave a large depression in the side of the head, which can be mitigated somewhat by filling the defect with temporoparietal fascia, but the volume of the muscle lying superficial to the zygomatic arch looks noticeably unnatural regardless.[71] Temporalis tendon transfer has largely supplanted temporalis flap transfer because it can be performed intraorally, thereby avoiding external scars, and it does not transfer facial volume unnaturally.[72](B2)
The drawback of temporalis transfer remains the amount of oral commissure excursion, which is only around 5 mm compared to the 7 to 9 mm obtained with gracilis-free muscle transfer.[73] Other regional muscle transfers that are occasionally performed for smile rehabilitation are the anterior digastric and platysmal transfers, which are used to improve lower lip depression in the case of marginal mandibular nerve injury; the platysmal transfer, of course, is not an available option in the case of a hemifacial paralysis.[74][75]
The gold standard for smile rehabilitation in patients who can tolerate lengthy surgical procedures is free muscle transfer because it has the advantages of precise control of movement vectors, or vectors, as well as greater excursion, albeit at the expense of a more challenging operation. Multiple muscles are available to transfer, including the pectoralis minor, latissimus dorsi, gracilis, serratus anterior, and strap muscles, none of which seem to produce any long-term donor site morbidity. The pectoralis minor and latissimus dorsi muscles are often second-line choices because of unreliable venous outflow and excessive bulk, respectively. Gracilis muscle transfer is widely considered the option of choice because of the consistent anatomy of its vascular pedicle and the reliability of its outcomes.[76][49] (B2)
The gracilis is a large postural muscle of the medial thigh, located between the adductor magnus and the adductor longus, extending from the medial aspect of the pubic and ischial rami to the pes anserinus at the medial tibial epicondyle. It is perfused by the adductor artery, which branches from the deep femoral artery and is drained by the adductor artery's 2 venae comitantes. The motor control to the gracilis comes from the obturator nerve, which arrives at the muscle at the same point and courses in the same plane as the vascular pedicle (between the adductors longus and magnus) but takes a different course because it originates more superiorly, from the lumbar plexus. Typically, a 20-50 gram section of gracilis muscle is harvested, with its neurovascular pedicle, and then inset into the face between the temporalis fascia and the modiolus of the oral commissure, with the adductor artery anastomosed to the facial artery and the dominant vena comitans anastomosed to the facial vein.[77]
In some situations, with awkward flap geometry, the superficial temporal vessels may be used instead of the facial artery and vein. The obturator nerve is then coapted to a donor nerve via primary neurorrhaphy, and the choice of nerve determines the characteristics of the flap's movement. The original description of the microneurovascular gracilis transfer by Harii in 1976 reported the use of the deep temporal nerves, but these are challenging to locate and use; in some cases, the residual facial nerve trunk may be employed, but the basal firing rate in this nerve is so high that it tends to cause excessive contracture of the muscle over time.[76] Currently, the most commonly employed donor nerves are the masseteric nerve and the cross-face nerve graft. Paralleling the foregoing discussion regarding nerve transfer, the use of the masseteric nerve to control a free gracilis muscle transfer provides more reliability (94%) and excursion (8.7 mm) than the use of a cross-face nerve graft (84% and 6.5 mm), although the cross face nerve graft provides a spontaneous smile without the need to clench the jaw as well as better smile symmetry.[49] The other drawback to cross-face grafting is that it requires a 2-stage procedure, with the cross-face graft placement (including sural nerve harvest and facial nerve exploration on the non-paralyzed side) preceding the gracilis transfer by 6 to 9 months.(B2)
Gracilis free muscle transfer, therefore, is a reliable means of restoring a meaningful smile to a paralyzed hemiface, but the technique remains imperfect due to several compromises: 1) the large bulk of gracilis muscle, when transferred to the face, appears unnatural; 2) the single movement vector of a gracilis inset between the temporalis fascia and the oral commissure produces a tight-lipped Mona Lisa smile rather than a genuine Duchenne smile with visible dentition; 3) the surgeon must choose between a reliable donor nerve (the masseteric nerve) and a nerve that can provide spontaneity (a cross-face nerve graft); 4) the gracilis is a postural muscle, which makes it incapable of contracting as rapidly as a native zygomaticus major muscle, thereby introducing dyssynchrony or requiring the patient to smile more slowly to maintain symmetry during motion. The problem of bulk can be solved by thinning the flap, but this introduces a greater risk of devascularization and denervation of the muscle; thinning the muscle also removes the fascia, which in turn increases the risk of the flap tethering to the overlying dermis and creating unnatural dimples during smiling. If the flap is not debulked, it can potentially be split into 2 or more slips of muscle that share the same neurovascular supply to replace not just the zygomaticus major but also the levator labii superioris and other muscles that provide a more natural smile, but doing so then introduces a large amount of excess bulk, and the smile remains somewhat slow.[78]
Some authors report coapting both a cross-face nerve graft and the masseteric nerve into the obturator nerve to combine the advantages of both donor nerves, reliability, and spontaneity, but results remain somewhat variable, perhaps because axons from both nerves must compete for space within the single obturator nerve.[79][49] (B2)
One approach to mitigating these problems is using the serratus anterior muscle, which is harvested as several anatomically separate muscle slips, all perfused by the thoracodorsal artery and controlled by the long thoracic nerve, that permits more precise control of the vector of smile movement.[80] The serratus anterior may provide more rapid contraction than the gracilis, but the volume of the muscle slips remains substantial, and the problem of donor nerve selection persists. A more promising option may be the use of cervical strap muscles, the first description of which was Alam's report of sternohyoid muscle transfer in 2016 that emphasized not only the strap muscle's minimal bulk but also its histological similarity to mimetic muscles, giving it the ability to contract rapidly and maintain a temporally synchronous smile.[81]
One step further is the dual-vector, combined sternohyoid and omohyoid flap described by Vincent et al in 2020, which uses both muscles to provide rapid superolateral excursion to the oral commissure as well as elevation of the upper lip to produce a natural dental show. The strap muscles combined weigh only 10 to 15 grams and introduce minimal additional bulk. Both muscles are perfused by the superior thyroid artery, which is easily coapted to the facial or superficial temporal artery, although the flap's venous outflow, like that of the pectoralis minor, is somewhat variable. The greatest advantage to strap muscle transfer, however, may be that the flap's motor control comes via the ansa cervicalis, which has 2 ends, thereby facilitating dual innervation by the masseteric nerve and a cross-face nerve graft. While the technique remains in its infancy, it appears very promising for ameliorating the compromises inherent to gracilis muscle transfer.[82] Regardless of the muscle transfer procedure ultimately employed, whether regional or free, physical therapy is required to help the patient maximize the use of the new muscle or muscles.
Synkinesis/Non-Flaccid Facial Paralysis
The previous discussion has focused on the management of complete transection injuries and rehabilitation of the flaccidly paralyzed face; however, many, if not the majority, of facial nerve trauma patients ultimately recover function that is dyscoordinated and spastic. While this synkinesis is not as noticeable at rest as complete, flaccid hemifacial paralysis is, it can be nearly as disfiguring with movement and just as disruptive to patients' quality of life. Management of post-paralytic facial dysfunction varies greatly from flaccid paralysis, although some techniques may be applied to both conditions. Patients with synkinesis often complain of involuntary movements accompanying voluntary ones and tightness and soreness that tend to progress throughout the day. Common involuntary movements include eye closure, corner-of-mouth lateralization, chin dimpling, and platysmal contraction. These areas are often sites of chronic pain, particularly the midface and neck. In addition to these involuntary movements, patients frequently note the inability to control their muscles appropriately, persistently complaining of incomplete eye closure and an asymmetric smile. In most cases, the first line of treatment for synkinesis should be physical therapy, which consists of exercises that patients perform before a mirror to improve coordination and symmetry, as well as stretches and massages to reduce discomfort.[83] Once physical therapy has been initiated, additional relaxation of the hyperactive facial muscles can be achieved with chemodenervation, typically with botulinum toxin.[84](B2)
Regular injections of comparatively high doses may be required, which risks developing antibodies and reducing the injections' effectiveness. To mitigate this problem, patients may need to switch isoforms over time, such as shifting from onabotulinumtoxin to abobotulinumtoxin or similar. Most patients are satisfied with improvements made between physical therapy and chemodenervation, but for some, operative intervention may be indicated, typically in patients who do not wish to continue with botulinum toxin injections or for whom regular visits for healthcare are impractical. Surgical options involve division of nerve branches that cause synkinetic movements (selective neurectomy, particularly around the eye, lower face, and neck), removal of muscles causing synkinetic movements (myomectomy, typically the platysma and lower lip depressors), and occasionally reinnervation (such as masseteric nerve transfer) or reanimation of the smile (with free muscle transfer, such as gracilis or sterno-omohyoid), any of which may be combined in the same procedure as necessary.[85][86][87][88][55][89][90] Ultimately, however, non-flaccid facial paralysis is a very challenging problem to solve, as even surgical interventions may provide only temporary relief, given that facial nerve axons tend to regenerate and synkinesis often recur with time.(B2)
Differential Diagnosis
While traumatic facial palsy is typically a straightforward diagnosis to determine from the patient's history, the clinical picture may be clouded in some cases. Viral facial palsy, such as Bell's palsy, zoster sine herpete/Ramsay Hunt syndrome, and COVID-associated facial paralysis may all occur coincidentally with trauma - the first 2 in particular, which may be more likely to occur in times of physiological stress. In some cases, a cerebrovascular accident may also accompany major trauma, causing facial paralysis in the absence of an actual facial nerve injury.
Facial nerve barotrauma may also be challenging to identify, especially when it occurs in conjunction with hearing loss and if a history of diving or high-altitude travel is not elucidated. Lastly, when an infant is born with facial weakness, determining the difference between birth trauma and congenital palsy requires a nuanced physical examination. Typically, birth trauma results in unilateral hemifacial weakness due to either unilateral stretching of the main trunk of the facial nerve or temporal bone trauma. Congenital facial paralysis, in contrast, generally presents with either a unilateral segmental weakness (eg, congenital unilateral lower lip palsy) or bilateral facial weakness with or without other cranial neuropathies, such as in the case of Möbius syndrome.[91] The diagnosis usually appears over several months, however, as paralysis from birth trauma tends to recover somewhat and potentially leads to synkinesis, while congenital palsy remain stable.
Prognosis
The prognosis for patients with traumatic facial paralysis depends entirely on the severity of the injury and, for severe injuries, the effectiveness of the repair. Because of the wide array of traumatic etiologies of facial paralysis, providing objective estimates of functional recovery is challenging due to the small sample sizes reported in the literature. Nevertheless, results of timely nerve repair are encouraging, with a 2013 study indicating that most patients recover at least 90% of their smile symmetry, 60% recover eye closure without requiring eyelid weight placement, and 40% recover brow elevation.[2] In general, patients with mild to moderate injuries, ie, patients whose paralysis is either incomplete or delayed, have a good chance of recovering to normal or near normal function (House-Brackmann grade I-II).
Several authors report that patients with incomplete paralysis from temporal bone fractures have a 100% chance of recovery to normal. Patients with delayed but complete paralysis have nearly as good a prognosis.[16][92] The prognosis is somewhat more guarded for patients with severe injuries characterized by the immediate presentation of complete hemifacial paralysis. If an ENoG is performed and there is less than 90% loss of action potential amplitude, the possibility of complete recovery remains, although it is somewhat less likely to occur than if the paralysis were incomplete or delayed. For patients who require repair of the main trunk of the facial nerve, the best outcome possible is a House-Brackmann grade III with synkinesis. According to Diaz et al, patients who meet indications and undergo facial nerve decompression for complete hemifacial paralysis after temporal bone fracture have a roughly 50% chance of recovery to normal. The rest are very likely to develop synkinesis.[8]
Patients who suffer transection injuries to the main trunk of the facial nerve but do not undergo repair are likely to persist with House-Brackmann grade VI flaccid paralysis in the long term. When the injury in question occurs in a distal branch of the facial nerve rather than in the main trunk, the prognosis is better because the functional deficit is less significant, as is the potential for developing synkinesis. For very distal injuries occurring medial to the lateral canthus of the eye, the likelihood of good recovery is high because of the extensive network of redundant small nerve fibers in that region. Ultimately, regardless of whether operative management is required or not, patients and physicians alike must exercise patience because recovery from nerve injury is slow due to the axonal regrowth rate of approximately 1 mm/day, and the results of a nerve repair or even spontaneous recovery are often not apparent for 6 to 12 months.
Complications
Complications of traumatic facial paralysis include exposure keratopathy and potential blindness, epiphora, xerophthalmia, nasal obstruction, hyperacusis, oral incompetence, and social isolation or decreased self-esteem.[35] In longer-standing cases, synkinesis may also develop, resulting in involuntary movements, dyscoordination, and facial pain. Complications of facial nerve repair include injury to unaffected branches of the facial nerve, failure to recover function after the repair is complete, and donor site morbidity, which may be due to sensory nerve harvest, motor nerve transfer with resulting atrophy of the denervated muscle, or free flap failure.
Consultations
Because of the myriad functions of the facial nerve, its impairment may necessitate the involvement of specialists from many different fields of study during the development of a treatment plan. For any acute facial paralysis, eye care must be a priority to prevent long-term corneal complications; for this reason, early referral to an ophthalmologist for patients with incomplete eye closure is important. In some cases, a plastic surgeon or facial plastic surgeon may be able to address problems with the eye that require surgery, although monitoring of the corneal surface is still best provided by an ophthalmologist. However, a plastic or facial plastic surgeon is still likely best positioned to provide facial nerve exploration, repair, or facial reanimation services, if necessary.
A neurologist or electrophysiologist should be consulted in cases of complete hemifacial paralysis to perform electrodiagnostic testing to determine whether or not surgical repair or decompression is required. For intratemporal injuries, a neurotologist or a neurosurgeon should be a part of the treatment team because their knowledge and technical skills in this area of the body are critical. In the long term, a physical therapist or speech therapist can be useful for helping the patient regain coordinated movement of the face and verbal articulation. Lastly, and perhaps most importantly, for iatrogenic injuries, the operating surgeon should remain a part of the care team because they have the most accurate information about the nature of the facial nerve injury, and including the surgeon helps to provide closure to both the patient and the surgeon.
Deterrence and Patient Education
The simplest prophylactic intervention that can be employed to reduce the rates of traumatic facial paralysis is the routine wear of helmets when performing activities that place the head at risk of receiving a forceful blow, such as driving motorcycles and all-terrain vehicles, operating heavy machinery, and playing contact sports; doing so has been shown to reduce rates of skull fractures.[93][94] However, many mechanisms of injury are challenging to predict or avoid, and their risk is not easily reduced. Nevertheless, iatrogenic injury represents an important target for patient safety initiatives. One of the most common causes of iatrogenic facial nerve injury is temporomandibular joint replacement surgery, an operation in which routine identification of the facial nerve is not necessarily performed; use of a facial nerve monitor, therefore, or even a practice shift towards identification of facial nerve branches within the field of dissection may decrease rates of nerve injury.[2]
Using a facial nerve monitor has long been considered standard practice in otological surgery; however, it limits injuries to the mastoid and tympanic segments of the facial nerve.[95] In head and neck surgical oncology, particularly parotidectomy, the evidence is less clear, although nerve monitoring does appear to reduce the incidence of immediate postoperative facial paralysis and potentially improves long-term facial nerve outcomes (see Image. Facial Nerve Reconstruction).[96][97] For cosmetic surgeons, facial nerve monitoring has not been a common practice, as most surgeons prefer to avoid approaching the nerve and instead rely on anatomical landmarks to avoid it rather than absorb the cost of a nerve monitor.[98]
Understanding the course of the major extratemporal facial nerve branches as they relate to surface anatomy can mean the difference between a weak face and a happy facelift patient, particularly with respect to the frontal and marginal mandibular branches, which are the most commonly injured during rhytidectomy.[2] The frontal branch of the facial nerve has been described as following a course over the zygomatic arch, roughly between its posterior and middle two-thirds, and then running along a line between a point 5 mm inferior to the tragus and a point 15 mm superior to the lateral brow (Pitanguy's line).[99] The marginal mandibular branch of the facial nerve is found closely related to the facial vein and superficial to the facial artery, superior to the inferior border of the mandible when anterior to the gonial notch of the mandible, which is where it crosses over the facial artery. In 80% of patients, the marginal mandibular branch of the facial nerve is superior to the inferior border of the mandible throughout its course, and in 20%, it is inferior to the inferior border of the mandible posterior to the gonial notch.[100]
The midfacial and cervical branches are less commonly injured, although both can cause weakness in the smile. Injuries to the cervical branch may be confused with injuries to the marginal mandibular branch because both provide innervation to depressors of the lower lip, the depressor labii inferioris/depressor anguli oris, and the platysma, respectively. The cervical branch can be found 1 cm inferior to the halfway point of a line between the mentum and the mastoid tip.[101] Midfacial rami of the zygomatic and buccal branches of the facial nerve course throughout the medial cheek, along the surface of the masseter muscle fascia, after exiting the anterior border of the parotid gland.[98] That said, the primary branch that innervates the zygomaticus major and provides oral commissure excursion during smiling is fairly straightforward to locate or avoid, as the case may be. Zuker's point, where this nerve is reliably encountered, lies halfway along a line between the oral commissure and the root of the helix.[102]
Enhancing Healthcare Team Outcomes
Acute traumatic facial palsy is best managed by an interprofessional team, ideally coordinated by a plastic or facial plastic surgeon familiar with emergent, acute, and long-term surgical options and medical management. Because other early interventions may include electrodiagnostic testing and corneal surface evaluation, the inclusion of a neurologist or electrophysiologist and an ophthalmologist or optometrist on the team is essential. In the case of temporal bone fractures, particularly with other sequelae like cerebrospinal fluid leak and hearing loss, a neurosurgeon or a neurotologist may be needed. The interprofessional team may also require a speech therapist or physical therapist in the long term to support the patient's rehabilitation of facial function, and a behavioral health specialist may be necessary to manage the patient's reaction and adjustment to facial paralysis from the immediate post-injury period for months to years afterward.[103]
Media
(Click Image to Enlarge)
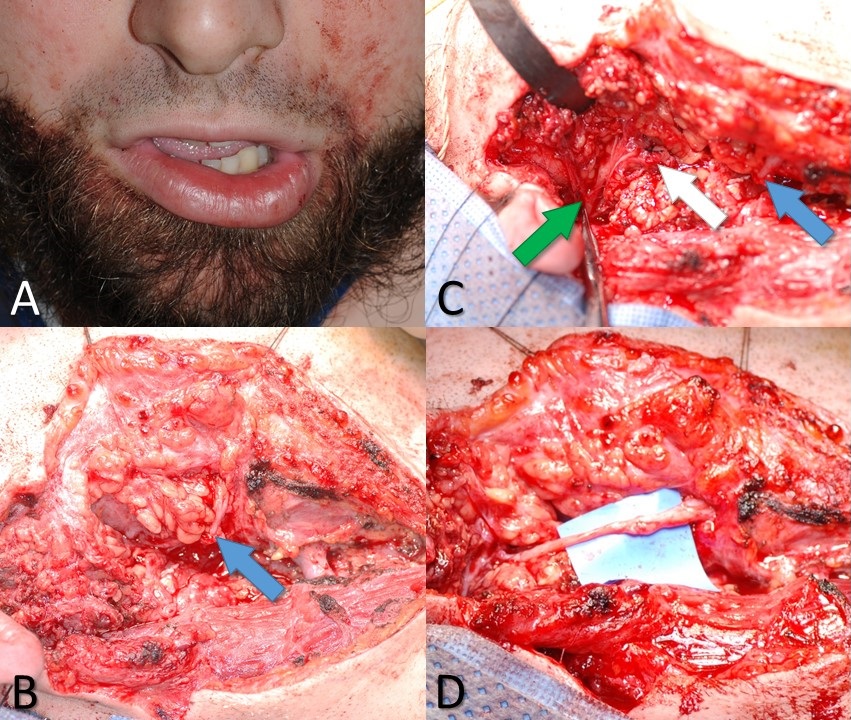
Marginal Mandibular Branch of the Facial Nerve Injury. A 26-year-old male with a knife wound to the right neck. (A) Weakness of the right depressor labii inferioris muscle indicates likely injury to the marginal mandibular branch of the facial nerve. (B) The blue arrow indicates the distal stump of the marginal mandibular nerve, as seen during neck exploration. (C) The white arrow indicates the proximal stump of the marginal mandibular nerve, and the green arrow indicates the pes anserinus of the facial nerve. (D) Proximal and distal stumps of the marginal mandibular nerve coapted microsurgically using 10-0 nylon suture and an interposition graft taken from the ipsilateral greater auricular nerve.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
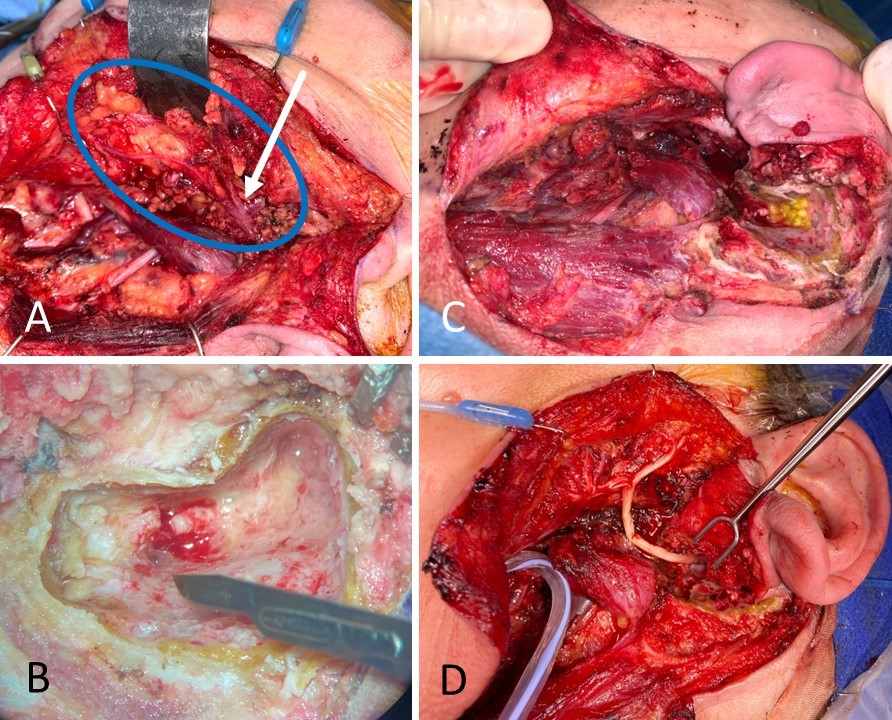
Facial Nerve Reconstruction. A 59-year-old female with carcinoma ex pleomorphic adenoma underwent total parotidectomy with facial nerve sacrifice from halfway down the mastoid segment to the distal branches, past the pes anserinus. (A) The facial nerve is circled in blue, and the pes anserinus is indicated by the white arrow; note the inflamed and dark appearance of the nerve. (B) The mastoid segment of the facial nerve is sectioned to obtain a negative proximal margin. (C) The wound bed is shown with an absent parotid gland, an open mastoid cavity, and a large facial nerve defect. (D) The facial nerve was reconstructed from the mastoid to the distal branches, including the pes anserinus, using an allograft.
Contributed by MH Hohman, MD, FACS, and KG Anderson, MD, FACS
(Click Image to Enlarge)
(Click Video to Play)
Facial Nerve Transfer. A 50-year-old female with right-sided House-Brackmann grade VI facial paralysis due to acoustic neuroma resection 6 months status post right masseteric nerve to a buccal branch of facial nerve transfer for smile rehabilitation. Note how she has to bite down to smile.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
House-Brackmann Grade VI Facial Paralysis. A 49-year-old female status post resection of a right-sided acoustic neuroma (vestibular schwannoma) and upper eyelid weight placement for House-Brackmann grade VI facial paralysis.
Contributed by MH Hohman, MD, FACS
References
Hohman MH, Hadlock TA. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. The Laryngoscope. 2014 Jul:124(7):E283-93. doi: 10.1002/lary.24542. Epub 2014 Jan 15 [PubMed PMID: 24431233]
Level 2 (mid-level) evidenceHohman MH, Bhama PK, Hadlock TA. Epidemiology of iatrogenic facial nerve injury: a decade of experience. The Laryngoscope. 2014 Jan:124(1):260-5. doi: 10.1002/lary.24117. Epub 2013 Apr 18 [PubMed PMID: 23606475]
Level 2 (mid-level) evidenceKleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2015 Dec:40(6):651-6. doi: 10.1111/coa.12434. Epub [PubMed PMID: 25858429]
Level 2 (mid-level) evidenceGordin E,Lee TS,Ducic Y,Arnaoutakis D, Facial nerve trauma: evaluation and considerations in management. Craniomaxillofacial trauma [PubMed PMID: 25709748]
Bleicher JN, Hamiel S, Gengler JS, Antimarino J. A survey of facial paralysis: etiology and incidence. Ear, nose, & throat journal. 1996 Jun:75(6):355-8 [PubMed PMID: 8689964]
Level 3 (low-level) evidenceChávez-Serna E, Telich-Tarriba JE, Altamirano-Arcos C, Nahas-Combina L, Cárdenas-Mejía A. Facial paralysis, etiology and surgical treatment in a tertiary care center in plastic and reconstructive surgery in Mexico. Cirugia y cirujanos. 2021:89(6):718-727. doi: 10.24875/CIRU.20000916. Epub [PubMed PMID: 34851577]
Brown J, Hohman MH, Shermetaro C. Facial Nerve Intratemporal Trauma. StatPearls. 2024 Jan:(): [PubMed PMID: 30137848]
Diaz RC, Cervenka B, Brodie HA. Treatment of Temporal Bone Fractures. Journal of neurological surgery. Part B, Skull base. 2016 Oct:77(5):419-29. doi: 10.1055/s-0036-1584197. Epub 2016 Jun 2 [PubMed PMID: 27648399]
Patel A, Lofgren DH, Varacallo M. Temporal Fracture. StatPearls. 2024 Jan:(): [PubMed PMID: 30571012]
Seddon HJ. A Classification of Nerve Injuries. British medical journal. 1942 Aug 29:2(4260):237-9 [PubMed PMID: 20784403]
Sunderland S. The anatomy and physiology of nerve injury. Muscle & nerve. 1990 Sep:13(9):771-84 [PubMed PMID: 2233864]
Engelmann S, Ruewe M, Geis S, Taeger CD, Kehrer M, Tamm ER, Bleys RLAW, Zeman F, Prantl L, Kehrer A. Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves. Scientific reports. 2020 Feb 6:10(1):1935. doi: 10.1038/s41598-020-58917-4. Epub 2020 Feb 6 [PubMed PMID: 32029860]
Althagafi A, Nadi M. Acute Nerve Injury. StatPearls. 2024 Jan:(): [PubMed PMID: 31751038]
Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner journal. 2013 Spring:13(1):100-8 [PubMed PMID: 23531634]
Gordon T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2016 Apr:13(2):295-310. doi: 10.1007/s13311-015-0415-1. Epub [PubMed PMID: 26754579]
Level 3 (low-level) evidenceBrodie HA, Thompson TC. Management of complications from 820 temporal bone fractures. The American journal of otology. 1997 Mar:18(2):188-97 [PubMed PMID: 9093676]
Level 2 (mid-level) evidenceDulak D, Naqvi IA. Neuroanatomy, Cranial Nerve 7 (Facial). StatPearls. 2024 Jan:(): [PubMed PMID: 30252375]
Remenschneider AK, Michalak S, Kozin ED, Barber S, De Venecia RK, Hadlock TA, Jung DH. Is Serial Electroneuronography Indicated Following Temporal Bone Trauma? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Apr:38(4):572-576. doi: 10.1097/MAO.0000000000001337. Epub [PubMed PMID: 28114180]
Darrouzet V, Duclos JY, Liguoro D, Truilhe Y, De Bonfils C, Bebear JP. Management of facial paralysis resulting from temporal bone fractures: Our experience in 115 cases. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001 Jul:125(1):77-84 [PubMed PMID: 11458219]
Level 2 (mid-level) evidenceKalaiarasi R, Kiran AS, Vijayakumar C, Venkataramanan R, Manusrut M, Prabhu R. Anatomical Features of Intratemporal Course of Facial Nerve and its Variations. Cureus. 2018 Aug 2:10(8):e3085. doi: 10.7759/cureus.3085. Epub 2018 Aug 2 [PubMed PMID: 30324041]
Le Carboulec L, De Mestier J, Boucenna M, Lecanu JB. Recurrent transient facial nerve palsy on commercial air flights. European annals of otorhinolaryngology, head and neck diseases. 2021 May:138(3):187-189. doi: 10.1016/j.anorl.2020.09.009. Epub 2020 Oct 1 [PubMed PMID: 33012666]
Kamide D, Matsunobu T, Shiotani A. Facial baroparesis caused by scuba diving. Case reports in otolaryngology. 2012:2012():329536. doi: 10.1155/2012/329536. Epub 2012 Feb 19 [PubMed PMID: 22953110]
Level 3 (low-level) evidenceWhelan TR. Facial nerve palsy associated with underwater barotrauma. Postgraduate medical journal. 1990 Jun:66(776):465-6 [PubMed PMID: 2216996]
Level 3 (low-level) evidenceWimmer MS, Ali TY. Transient Unilateral Facial Nerve Baroparesis with Vertigo on Ascent in the F-16CM. Aerospace medicine and human performance. 2016 Feb:87(2):141-3. doi: 10.3357/AMHP.4449.2016. Epub [PubMed PMID: 26802380]
House JW, Brackmann DE. Facial nerve grading system. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1985 Apr:93(2):146-7 [PubMed PMID: 3921901]
Banks CA, Jowett N, Azizzadeh B, Beurskens C, Bhama P, Borschel G, Coombs C, Coulson S, Croxon G, Diels J, Fattah A, Frey M, Gavilan J, Henstrom D, Hohman M, Kim J, Marres H, Redett R, Snyder-Warwick A, Hadlock T. Worldwide Testing of the eFACE Facial Nerve Clinician-Graded Scale. Plastic and reconstructive surgery. 2017 Feb:139(2):491e-498e. doi: 10.1097/PRS.0000000000002954. Epub [PubMed PMID: 28121888]
Vrabec JT, Backous DD, Djalilian HR, Gidley PW, Leonetti JP, Marzo SJ, Morrison D, Ng M, Ramsey MJ, Schaitkin BM, Smouha E, Toh EH, Wax MK, Williamson RA, Smith EO, Facial Nerve Disorders Committee. Facial Nerve Grading System 2.0. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009 Apr:140(4):445-50. doi: 10.1016/j.otohns.2008.12.031. Epub [PubMed PMID: 19328328]
Level 1 (high-level) evidenceRoss BR, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 1994 Dec:():S180-1 [PubMed PMID: 10774344]
Hato N, Fujiwara T, Gyo K, Yanagihara N. Yanagihara facial nerve grading system as a prognostic tool in Bell's palsy. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014 Oct:35(9):1669-72. doi: 10.1097/MAO.0000000000000468. Epub [PubMed PMID: 24945585]
Level 2 (mid-level) evidenceSun GH, Shoman NM, Samy RN, Cornelius RS, Koch BL, Pensak ML. Do contemporary temporal bone fracture classification systems reflect concurrent intracranial and cervical spine injuries? The Laryngoscope. 2011 May:121(5):929-32. doi: 10.1002/lary.21718. Epub [PubMed PMID: 21520104]
Level 2 (mid-level) evidenceGantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell's palsy. The Laryngoscope. 1999 Aug:109(8):1177-88 [PubMed PMID: 10443817]
Hohman MH, Warner MJ, Varacallo MA. Bell Palsy. StatPearls. 2025 Jan:(): [PubMed PMID: 29493915]
Crouch AE, Hohman MH, Moody MP, Andaloro C. Ramsay Hunt Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 32491341]
Lin RJ, Klein-Fedyshin M, Rosen CA. Nimodipine improves vocal fold and facial motion recovery after injury: A systematic review and meta-analysis. The Laryngoscope. 2019 Apr:129(4):943-951. doi: 10.1002/lary.27530. Epub 2018 Nov 19 [PubMed PMID: 30450691]
Level 1 (high-level) evidenceHadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. The Laryngoscope. 2006 Aug:116(8):1385-9 [PubMed PMID: 16885741]
Level 2 (mid-level) evidenceGire A, Kwok A, Marx DP. PROSE treatment for lagophthalmos and exposure keratopathy. Ophthalmic plastic and reconstructive surgery. 2013 Mar-Apr:29(2):e38-40. doi: 10.1097/IOP.0b013e3182674069. Epub [PubMed PMID: 23034688]
Level 3 (low-level) evidenceSilver AL, Lindsay RW, Cheney ML, Hadlock TA. Thin-profile platinum eyelid weighting: a superior option in the paralyzed eye. Plastic and reconstructive surgery. 2009 Jun:123(6):1697-1703. doi: 10.1097/PRS.0b013e3181a65a56. Epub [PubMed PMID: 19483568]
Saha S, Pal S, Sengupta M, Chowdhury K, Saha VP, Mondal L. Identification of facial nerve during parotidectomy: a combined anatomical & surgical study. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2014 Jan:66(1):63-8. doi: 10.1007/s12070-013-0669-z. Epub 2013 Jul 24 [PubMed PMID: 24605304]
Liu D, Mi D, Zhang T, Zhang Y, Yan J, Wang Y, Tan X, Yuan Y, Yang Y, Gu X, Hu W. Tubulation repair mitigates misdirection of regenerating motor axons across a sciatic nerve gap in rats. Scientific reports. 2018 Feb 21:8(1):3443. doi: 10.1038/s41598-018-21652-y. Epub 2018 Feb 21 [PubMed PMID: 29467542]
Boeckstyns ME, Sørensen AI, Viñeta JF, Rosén B, Navarro X, Archibald SJ, Valss-Solé J, Moldovan M, Krarup C. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. The Journal of hand surgery. 2013 Dec:38(12):2405-11. doi: 10.1016/j.jhsa.2013.09.038. Epub 2013 Nov 5 [PubMed PMID: 24200027]
Level 1 (high-level) evidenceAltafulla J, Iwanaga J, Lachkar S, Prickett J, Dupont G, Yilmaz E, Ishak B, Litvack Z, Tubbs RS. The Great Auricular Nerve: Anatomical Study with Application to Nerve Grafting Procedures. World neurosurgery. 2019 May:125():e403-e407. doi: 10.1016/j.wneu.2019.01.087. Epub 2019 Jan 28 [PubMed PMID: 30703599]
Van Nest DS, Kahan DM, Ilyas AM. Polyethylene Glycol Fusion of Nerve Injuries: Review of the Technique and Clinical Applicability. Journal of hand and microsurgery. 2021 Apr:13(2):49-54. doi: 10.1055/s-0040-1718651. Epub 2020 Dec 10 [PubMed PMID: 33867761]
Peters BR, Wood MD, Hunter DA, Mackinnon SE. Acellular Nerve Allografts in Major Peripheral Nerve Repairs: An Analysis of Cases Presenting With Limited Recovery. Hand (New York, N.Y.). 2023 Mar:18(2):236-243. doi: 10.1177/15589447211003175. Epub 2021 Apr 21 [PubMed PMID: 33880944]
Level 3 (low-level) evidenceJowett N, Pineda Ii R. Acellular nerve allografts in corneal neurotisation: an inappropriate choice. The British journal of ophthalmology. 2020 Feb:104(2):149-150. doi: 10.1136/bjophthalmol-2019-315032. Epub 2019 Nov 12 [PubMed PMID: 31719110]
Orgel MG. Epineurial versus perineurial repair of peripheral nerves. Clinics in plastic surgery. 1984 Jan:11(1):101-4 [PubMed PMID: 6368091]
Level 3 (low-level) evidenceCabaud HE, Rodkey WG, McCarroll HR Jr, Mutz SB, Niebauer JJ. Epineurial and perineurial fascicular nerve repairs: a critical comparison. The Journal of hand surgery. 1976 Sep:1(2):131-7 [PubMed PMID: 797698]
Level 3 (low-level) evidenceFrey M, Happak W, Girsch W, Bittner RE, Gruber H. Histomorphometric studies in patients with facial palsy treated by functional muscle transplantation: new aspects for the surgical concept. Annals of plastic surgery. 1991 Apr:26(4):370-9 [PubMed PMID: 1872543]
Terzis JK, Wang W, Zhao Y. Effect of axonal load on the functional and aesthetic outcomes of the cross-facial nerve graft procedure for facial reanimation. Plastic and reconstructive surgery. 2009 Nov:124(5):1499-1512. doi: 10.1097/PRS.0b013e3181babb93. Epub [PubMed PMID: 20009836]
Level 2 (mid-level) evidenceBhama PK, Weinberg JS, Lindsay RW, Hohman MH, Cheney ML, Hadlock TA. Objective outcomes analysis following microvascular gracilis transfer for facial reanimation: a review of 10 years' experience. JAMA facial plastic surgery. 2014 Mar-Apr:16(2):85-92. doi: 10.1001/jamafacial.2013.2463. Epub [PubMed PMID: 24481538]
Level 2 (mid-level) evidenceKondo Y, Moriyama H, Hirai S, Qu N, Itoh M. The relationship between Bell's palsy and morphometric aspects of the facial nerve. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2012 Jun:269(6):1691-5. doi: 10.1007/s00405-011-1835-0. Epub 2011 Nov 15 [PubMed PMID: 22083358]
Mackinnon SE, Dellon AL. Fascicular patterns of the hypoglossal nerve. Journal of reconstructive microsurgery. 1995 May:11(3):195-8 [PubMed PMID: 7650645]
Klebuc MJA. Facial reanimation using the masseter-to-facial nerve transfer. Plastic and reconstructive surgery. 2011 May:127(5):1909-1915. doi: 10.1097/PRS.0b013e31820e9138. Epub [PubMed PMID: 21532419]
Level 2 (mid-level) evidenceBorschel GH, Kawamura DH, Kasukurthi R, Hunter DA, Zuker RM, Woo AS. The motor nerve to the masseter muscle: an anatomic and histomorphometric study to facilitate its use in facial reanimation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012 Mar:65(3):363-6. doi: 10.1016/j.bjps.2011.09.026. Epub 2011 Oct 10 [PubMed PMID: 21992936]
Hembd A, Nagarkar PA, Saba S, Wan D, Kutz JW, Isaacson B, Gupta S, White CL 3rd, Rohrich RJ, Rozen SM. Facial Nerve Axonal Analysis and Anatomical Localization in Donor Nerve: Optimizing Axonal Load for Cross-Facial Nerve Grafting in Facial Reanimation. Plastic and reconstructive surgery. 2017 Jan:139(1):177-183. doi: 10.1097/PRS.0000000000002897. Epub [PubMed PMID: 27632395]
Vincent AG, Bevans SE, Robitschek JM, Wind GG, Hohman MH. Masseteric-to-Facial Nerve Transfer and Selective Neurectomy for Rehabilitation of the Synkinetic Smile. JAMA facial plastic surgery. 2019 Dec 1:21(6):504-510. doi: 10.1001/jamafacial.2019.0689. Epub [PubMed PMID: 31465094]
Hontanilla B, Cabello A. Spontaneity of smile after facial paralysis rehabilitation when using a non-facial donor nerve. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2016 Sep:44(9):1305-9. doi: 10.1016/j.jcms.2016.06.031. Epub 2016 Jul 9 [PubMed PMID: 27460946]
Aslan H, Songu M, Eren E, Başoğlu MS, Özkul Y, Ateş D, Katilmiş H, Güvenç G. Results of decompression with middle cranial fossa approach or traumatic intratemporal fascial nerve injury. The Journal of craniofacial surgery. 2014 Jul:25(4):1305-8. doi: 10.1097/SCS.0000000000000772. Epub [PubMed PMID: 25006913]
Level 2 (mid-level) evidenceDe Jong R, Hohman MH. Brow Ptosis. StatPearls. 2024 Jan:(): [PubMed PMID: 32809597]
Perez PB, Gunter AE, Moody MP, Vincent AG, Perez CR, Serra RM, Hohman MH. Investigating Long-Term Brow Stabilization by Endotine-Assisted Endoscopic Brow Lift with Concomitant Upper Lid Blepharoplasty. The Annals of otology, rhinology, and laryngology. 2021 Oct:130(10):1139-1147. doi: 10.1177/0003489421997653. Epub 2021 Feb 25 [PubMed PMID: 33631951]
Hohman MH, Kim SW, Heller ES, Frigerio A, Heaton JT, Hadlock TA. Determining the threshold for asymmetry detection in facial expressions. The Laryngoscope. 2014 Apr:124(4):860-5. doi: 10.1002/lary.24331. Epub 2013 Oct 2 [PubMed PMID: 23900726]
Gendy A,Therattil PJ,Feintisch AM,Lee ES, Postseptal Weight Placement for Paralytic Lagophthalmos. Eplasty. 2016 [PubMed PMID: 27408667]
Bergeron CM, Moe KS. The evaluation and treatment of upper eyelid paralysis. Facial plastic surgery : FPS. 2008 May:24(2):220-30. doi: 10.1055/s-2008-1075838. Epub [PubMed PMID: 18470834]
Dedhia R, Hsieh TY, Chin O, Shipchandler TZ, Tollefson TT. Outcomes From Lateral Eyelid Coupling for Facial Paralysis Using the Modified Tarsoconjunctival Flap. JAMA facial plastic surgery. 2018 Sep 1:20(5):381-386. doi: 10.1001/jamafacial.2018.0070. Epub [PubMed PMID: 29621372]
Frey M, Giovanoli P, Tzou CH, Kropf N, Friedl S. Dynamic reconstruction of eye closure by muscle transposition or functional muscle transplantation in facial palsy. Plastic and reconstructive surgery. 2004 Sep 15:114(4):865-75 [PubMed PMID: 15468391]
Jamshidian-Tehrani M,Kasaee A,Ghadimi H,Nekoozadeh S,Yadegari S,Nowroozzadeh MH, Improved Function of Orbicularis Oculi by Dynamic Transfer of Contralateral Orbicularis Oculi Muscle in Patients with Facial Palsy. Middle East African journal of ophthalmology. 2020 Jul-Sep [PubMed PMID: 33488012]
Sadiq SA, Dharmasena A. Dynamic muscle transfer in facial nerve palsy: the use of contralateral orbicularis oculi muscle. Facial plastic surgery : FPS. 2015 Apr:31(2):145-51. doi: 10.1055/s-0035-1549037. Epub 2015 May 8 [PubMed PMID: 25958901]
Nuara MJ, Mobley SR. Nasal valve suspension revisited. The Laryngoscope. 2007 Dec:117(12):2100-6. doi: 10.1097/MLG.0b013e31814842cd. Epub [PubMed PMID: 18322421]
Level 2 (mid-level) evidenceFaris C, Heiser A, Jowett N, Hadlock T. Minimal Nasolabial Incision Technique for Nasolabial Fold Modification in Patients With Facial Paralysis. JAMA facial plastic surgery. 2018 Mar 1:20(2):148-153. doi: 10.1001/jamafacial.2017.1425. Epub [PubMed PMID: 29049436]
Vincent AG, Hohman MH, Moe KS. Modiolar rotational cheiloplasty: Addressing the central oval in facial paralysis. The Laryngoscope. 2019 Oct:129(10):2262-2268. doi: 10.1002/lary.27700. Epub 2018 Dec 28 [PubMed PMID: 30592307]
Matic DB, Yoo J. The pedicled masseter muscle transfer for smile reconstruction in facial paralysis: repositioning the origin and insertion. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012 Aug:65(8):1002-8. doi: 10.1016/j.bjps.2012.03.021. Epub 2012 Apr 2 [PubMed PMID: 22475686]
Level 3 (low-level) evidenceCheney ML, McKenna MJ, Megerian CA, Ojemann RG. Early temporalis muscle transposition for the management of facial paralysis. The Laryngoscope. 1995 Sep:105(9 Pt 1):993-1000 [PubMed PMID: 7666737]
Level 2 (mid-level) evidenceLabbé D, Huault M. Lengthening temporalis myoplasty and lip reanimation. Plastic and reconstructive surgery. 2000 Apr:105(4):1289-97; discussion 1298 [PubMed PMID: 10744217]
Oyer SL, Nellis J, Ishii LE, Boahene KD, Byrne PJ. Comparison of Objective Outcomes in Dynamic Lower Facial Reanimation With Temporalis Tendon and Gracilis Free Muscle Transfer. JAMA otolaryngology-- head & neck surgery. 2018 Dec 1:144(12):1162-1168. doi: 10.1001/jamaoto.2018.1964. Epub [PubMed PMID: 30325983]
Terzis JK, Kalantarian B. Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation. Plastic and reconstructive surgery. 2000 May:105(6):1917-31; discussion 1932-4 [PubMed PMID: 10839388]
Tzafetta K, Ruston JC, Pinto-Lopes R, Mabvuure NT. Lower Lip Reanimation: Experience Using the Anterior Belly of Digastric Muscle in 2-stage Procedure. Plastic and reconstructive surgery. Global open. 2021 Mar:9(3):e3461. doi: 10.1097/GOX.0000000000003461. Epub 2021 Mar 15 [PubMed PMID: 33747692]
Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis. A preliminary report. Plastic and reconstructive surgery. 1976 Feb:57(2):133-43 [PubMed PMID: 1250883]
Level 3 (low-level) evidenceBraig D, Bannasch H, Stark GB, Eisenhardt SU. Analysis of the ideal muscle weight of gracilis muscle transplants for facial reanimation surgery with regard to the donor nerve and outcome. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2017 Apr:70(4):459-468. doi: 10.1016/j.bjps.2016.12.005. Epub 2017 Jan 9 [PubMed PMID: 28159544]
Boahene KO, Owusu J, Ishii L, Ishii M, Desai S, Kim I, Kim L, Byrne P. The Multivector Gracilis Free Functional Muscle Flap for Facial Reanimation. JAMA facial plastic surgery. 2018 Jul 1:20(4):300-306. doi: 10.1001/jamafacial.2018.0048. Epub [PubMed PMID: 29566121]
Matsumine H, Kamei W, Fujii K, Shimizu M, Osada A, Sakurai H. One-stage reconstruction by dual-innervated double muscle flap transplantation with the neural interconnection between the ipsilateral masseter and contralateral facial nerve for reanimating established facial paralysis: A report of 2 cases. Microsurgery. 2019 Jul:39(5):457-462. doi: 10.1002/micr.30397. Epub 2018 Dec 4 [PubMed PMID: 30512222]
Level 3 (low-level) evidenceSakuma H, Tanaka I, Yazawa M, Shimizu Y. Multivector functioning muscle transfer using superficial subslips of the serratus anterior muscle for longstanding facial paralysis. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2019 Jun:72(6):964-972. doi: 10.1016/j.bjps.2018.12.029. Epub 2018 Dec 23 [PubMed PMID: 30691992]
Alam DS. The Sternohyoid Flap for Facial Reanimation. Facial plastic surgery clinics of North America. 2016 Feb:24(1):61-9. doi: 10.1016/j.fsc.2015.09.005. Epub [PubMed PMID: 26611702]
Vincent AG, Bevans SE, Robitschek JM, Groom KL, Herr MW, Hohman MH. Sterno-omohyoid Free Flap for Dual-Vector Dynamic Facial Reanimation. The Annals of otology, rhinology, and laryngology. 2020 Feb:129(2):195-200. doi: 10.1177/0003489419875473. Epub 2019 Oct 3 [PubMed PMID: 31578078]
Robinson MW, Baiungo J. Facial Rehabilitation: Evaluation and Treatment Strategies for the Patient with Facial Palsy. Otolaryngologic clinics of North America. 2018 Dec:51(6):1151-1167. doi: 10.1016/j.otc.2018.07.011. Epub 2018 Sep 24 [PubMed PMID: 30262166]
Mehta RP, Hadlock TA. Botulinum toxin and quality of life in patients with facial paralysis. Archives of facial plastic surgery. 2008 Mar-Apr:10(2):84-7. doi: 10.1001/archfaci.10.2.84. Epub [PubMed PMID: 18347234]
Level 2 (mid-level) evidenceHohman MH, Lee LN, Hadlock TA. Two-step highly selective neurectomy for refractory periocular synkinesis. The Laryngoscope. 2013 Jun:123(6):1385-8. doi: 10.1002/lary.23873. Epub 2013 Jan 11 [PubMed PMID: 23315713]
Level 2 (mid-level) evidenceAzizzadeh B, Hjelm N. Modified Selective Neurectomy: A New Paradigm in the Management of Facial Palsy with Synkinesis. Facial plastic surgery clinics of North America. 2021 Aug:29(3):453-457. doi: 10.1016/j.fsc.2021.03.005. Epub [PubMed PMID: 34217449]
Henstrom DK, Malo JS, Cheney ML, Hadlock TA. Platysmectomy: an effective intervention for facial synkinesis and hypertonicity. Archives of facial plastic surgery. 2011 Jul-Aug:13(4):239-43. doi: 10.1001/archfacial.2011.43. Epub [PubMed PMID: 21768558]
Derakhshan A, Miller MQ, Malka R, Gadkaree SK, Hadlock TA. Releasing the Smile: Depressor Anguli Oris Excision in the Context of Managing Nonflaccid Facial Palsy. Plastic and reconstructive surgery. 2022 Feb 1:149(2):261e-269e. doi: 10.1097/PRS.0000000000008807. Epub [PubMed PMID: 35077425]
Lindsay RW, Bhama P, Weinberg J, Hadlock TA. The success of free gracilis muscle transfer to restore smile in patients with nonflaccid facial paralysis. Annals of plastic surgery. 2014 Aug:73(2):177-82. doi: 10.1097/SAP.0b013e3182a0df04. Epub [PubMed PMID: 24051452]
Miller MQ, Hadlock TA. Beyond Botox: Contemporary Management of Nonflaccid Facial Palsy. Facial plastic surgery & aesthetic medicine. 2020 Mar/Apr:22(2):65-70. doi: 10.1089/fpsam.2020.0009. Epub [PubMed PMID: 32130060]
Saylam E, Arya K. Congenital Unilateral Lower Lip Palsy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809530]
Dahiya R, Keller JD, Litofsky NS, Bankey PE, Bonassar LJ, Megerian CA. Temporal bone fractures: otic capsule sparing versus otic capsule violating clinical and radiographic considerations. The Journal of trauma. 1999 Dec:47(6):1079-83 [PubMed PMID: 10608536]
Level 2 (mid-level) evidenceAlfrey EJ, Tracy M, Alfrey JR, Carroll M, Aranda-Wikman ED, Arora T, Maa J, Minnis J. Helmet Usage Reduces Serious Head Injury Without Decreasing Concussion After Bicycle Riders Crash. The Journal of surgical research. 2021 Jan:257():593-596. doi: 10.1016/j.jss.2020.08.009. Epub 2020 Sep 12 [PubMed PMID: 32932191]
Rughani AI, Lin CT, Ares WJ, Cushing DA, Horgan MA, Tranmer BI, Jewell RP, Florman JE. Helmet use and reduction in skull fractures in skiers and snowboarders admitted to the hospital. Journal of neurosurgery. Pediatrics. 2011 Mar:7(3):268-71. doi: 10.3171/2010.12.PEDS10415. Epub [PubMed PMID: 21361765]
Level 2 (mid-level) evidenceHarner SG, Daube JR, Ebersold MJ. Electrophysiologic monitoring of facial nerve during temporal bone surgery. The Laryngoscope. 1986 Jan:96(1):65-9 [PubMed PMID: 3079850]
Eisele DW, Wang SJ, Orloff LA. Electrophysiologic facial nerve monitoring during parotidectomy. Head & neck. 2010 Mar:32(3):399-405. doi: 10.1002/hed.21190. Epub [PubMed PMID: 19672866]
Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB. Facial nerve monitoring during parotidectomy: a systematic review and meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015 Apr:152(4):631-7. doi: 10.1177/0194599814568779. Epub 2015 Jan 27 [PubMed PMID: 25628369]
Level 1 (high-level) evidenceBaker DC, Conley J. Avoiding facial nerve injuries in rhytidectomy. Anatomical variations and pitfalls. Plastic and reconstructive surgery. 1979 Dec:64(6):781-95 [PubMed PMID: 515227]
Pitanguy I, Ramos AS. The frontal branch of the facial nerve: the importance of its variations in face lifting. Plastic and reconstructive surgery. 1966 Oct:38(4):352-6 [PubMed PMID: 5926990]
Batra AP, Mahajan A, Gupta K. Marginal mandibular branch of the facial nerve: An anatomical study. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2010 Jan:43(1):60-4. doi: 10.4103/0970-0358.63968. Epub [PubMed PMID: 20924452]
Chowdhry S, Yoder EM, Cooperman RD, Yoder VR, Wilhelmi BJ. Locating the cervical motor branch of the facial nerve: anatomy and clinical application. Plastic and reconstructive surgery. 2010 Sep:126(3):875-879. doi: 10.1097/PRS.0b013e3181e3b374. Epub [PubMed PMID: 20463628]
Dorafshar AH, Borsuk DE, Bojovic B, Brown EN, Manktelow RT, Zuker RM, Rodriguez ED, Redett RJ. Surface anatomy of the middle division of the facial nerve: Zuker's point. Plastic and reconstructive surgery. 2013 Feb:131(2):253-257. doi: 10.1097/PRS.0b013e3182778753. Epub [PubMed PMID: 23357986]
Butler DP, Grobbelaar AO. Facial palsy: what can the multidisciplinary team do? Journal of multidisciplinary healthcare. 2017:10():377-381. doi: 10.2147/JMDH.S125574. Epub 2017 Sep 25 [PubMed PMID: 29026314]