Introduction
Due to the myriad roles the face plays, the impact of facial paralysis can be pervasive, negatively affecting quality of life due to psychosocial and functional effects. While the inability to close the eye, breathe easily through the nose, or maintain oral competence can be very problematic, losing the ability to smile or even to appear symmetric at rest can be even more significant for many patients, especially young women, and may lead to depression in addition to decreased quality of life.[1][2] The differential diagnosis for facial paralysis is extensive, including Bell's palsy, Ramsay Hunt syndrome, Lyme disease, cerebellopontine angle (CPA) tumors, temporal bone fractures, parotid malignancies, autoimmune disorders, iatrogenic and traumatic injuries, and many others (see image).[3][4] This article will focus on the evaluation and management of traumatic facial paralysis, specifically cases that involve transection of the facial nerve.
When the facial nerve is transected, immediate surgical repair with primary neurorrhaphy is generally the preferred treatment option.[5][6] If coaptation without tension is impossible, a cable graft interposition using the greater auricular or sural nerves may be necessary. The success rate of nerve repair, defined as a return to House-Brackmann grade III function or better, varies from 5% to 86% within the literature.[4][7] However, repairing the nerve at the time of the injury is not always feasible; either the injury is not recognized immediately, or the nerve is transected in a location that precludes suture coaptation of the nerve stumps, such as in the CPA. When the proximal facial nerve is unavailable to reconnect to the distal stump, a nerve transfer may be the best method of restoring facial function.[7]
Any technique employed other than direct coaptation of the severed facial nerve stumps at the time of injury will have associated risks. Delayed primary repair often requires a facial nerve exploration, which risks injury to other facial structures, including the parotid gland and duct, as well as other potentially intact facial nerve branches. Interposition grafting and nerve transfer introduce the potential for donor site morbidity, a critical factor when planning for and counseling patients before surgery. Regardless of the technique employed, the return of function after a facial nerve transection injury is a slow process affecting many aspects of facial function and aesthetics. For this reason, an interprofessional team consisting of not only a peripheral nerve surgeon such as a plastic or facial plastic surgeon or otolaryngologist but also an ophthalmologist and physical and speech therapists is the most effective means of providing holistic care for patients with facial paralysis.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Anatomy
The facial nerve, or seventh cranial nerve, originates in the lower pons and crosses the CPA to enter the internal auditory meatus alongside the cochlear nerve and the superior and inferior vestibular nerves.[8] The meatal portion of the facial nerve, within the petrous portion of the temporal bone, is roughly 8 to 10 mm long. The facial nerve is located in the anterosuperior quadrant within the internal auditory canal, while the cochlear nerve lies anteroinferior. The superior and inferior vestibular nerves occupy the posterosuperior and inferior quadrants, respectively.
At the distal aspect of the internal auditory canal, the facial nerve turns anteriorly into the labyrinthine segment. This segment is approximately 4 mm long and ends at the geniculate ganglion. Here the nerve takes a 180-degree turn and gives off the greater superficial petrosal nerve. The canal housing the facial nerve as it travels through the temporal bone is the Fallopian canal, the narrowest portion of which is the labyrinthine segment. At only 0.7 mm in diameter, the labyrinthine segment is where the facial nerve is particularly susceptible to impingement from bony fractures or intraneural edema.[9][10]
Leaving the Fallopian canal, the nerve courses posteriorly for 9 to 10 mm through the middle ear, running superior to the stapes footplate. This "tympanic" or "horizontal" segment of the facial nerve is at risk for injury due to middle ear pathologies, such as infection, cholesteatoma, or barotrauma, particularly if the bone of the Fallopian canal is dehiscent. As the nerve passes into the mastoid cavity, it turns 90º inferiorly; this is the "mastoid" or "vertical" segment, which is 11-12 mm long. Within the mastoid, the approximate 6000 facial nerve axons begin to organize somatotopically as the fascicles prepare to separate within the parotid gland at the pes anserinus. Leaving the pes anserinus, branches of the facial nerve will control the mimetic muscles in the upper face, midface, lower face, and superficial neck.[11] The chorda tympani branch of the facial nerve splits off the main trunk within the mastoid and travels superiorly to exit the temporal bone through the petrotympanic fissure of Huguier; it will travel to the oral cavity to carry gustatory sensation from the anterior two-thirds of the tongue.[12] The intratemporal course of the facial nerve ends when the main trunk passes through the stylomastoid foramen to enter the infratemporal fossa.
Extratemporally, the facial nerve travels for approximately 14 mm before dividing into what is classically described as five main branches: frontal, zygomatic, buccal, marginal mandibular, and cervical. The anatomy is variable, however, with six branching patterns characterized by Davis et al in 1956.[13]
- Type I (13%): The main trunk branches into upper and lower divisions, with the buccal branch leaving the upper division proximally while the frontal and zygomatic branches separate slightly more distally; the marginal mandibular and cervical branches form the lower division.
- Type II (20%): The main trunk branches into upper and lower divisions, with the frontal and zygomatic branches, as well as an upper buccal branch, forming a common upper facial trunk. The upper buccal branch anastomoses distally with the zygomatic branch, but there is also a separate lower buccal branch. The marginal mandibular and cervical branches form the lower division.
- Type III (28%): The main trunk branches into upper and lower divisions, with the frontal and zygomatic branches running together in the upper division until the zygomatic branch separates distally and anastomoses with the buccal branch, which runs with but leaves the lower division proximally. The marginal mandibular and cervical branches form the remainder of the lower division.
- Type IV (24%): The main trunk branches simultaneously into frontal and zygomatic branches and a lower division. The zygomatic branch splits into upper and lower branches proximally, with the frontal and upper zygomatic branches anastomosing proximally. The buccal branch leaves the lower division proximally and anastomoses with the lower zygomatic branch distally. The marginal mandibular and cervical branches form the lower division.
- Type V (9%): The main trunk branches into upper and lower divisions, with the frontal and the upper and lower zygomatic branches constituting the upper division. The upper and lower buccal branches and the marginal mandibular and cervical branches comprise the lower division. The upper buccal branch anastomoses proximally with the lower zygomatic branch and the lower buccal branch anastomoses distally with the upper zygomatic branch and the combined lower-zygomatic-upper-buccal complex.
- Type VI (6%): The main trunk branches into upper and lower divisions, with significant early ramification and anastomosis between frontal and zygomatic branches within the upper division. Within the lower division, an upper buccal branch leaves proximally and anastomoses proximally with the lower aspect of the frontozygomatic complex. The cervical branch separates proximally from a common trunk, giving off a lower buccal branch and the marginal mandibular branch more distally—the lower buccal branch then anastomoses with the zygomatic-upper buccal complex distally.
Despite this substantial anatomic variability among individuals, several surface landmarks facilitate reliable identification of the main extratemporal facial nerve branches. The line of Pitanguy, which runs from a point 5 mm inferior to the tragus to a point 15 mm superior to the lateral brow, approximates the course of the frontal branch (see image).[14] The point of Zuker, halfway between the root of the helix and the oral commissure, consistently overlies the buccal branch that innervates the zygomaticus major muscle, which is particularly useful for masseteric nerve transfer-based smile reanimation (see image).[15] The marginal mandibular branch, which is the most commonly injured individual facial nerve branch, is reliably located superior to the inferior margin of the mandible anterior to the point where it crosses the facial artery, just anterior to the mandibular angle; posterior to that, it lies inferior to the margin of the mandible in only 20% of patients.[16][17][18] The cervical branch can be found 10 mm inferior to the halfway point between the mentum and the tip of the mastoid process.[19]
The extratemporal facial nerve branches course through the parotid gland, separating its superficial and deep lobes. These branches exit the gland at its anterior border and run deep to the superficial musculoaponeurotic system (SMAS) of the face, ultimately innervating the mimetic muscles from their deep surfaces, except for the levator anguli oris, buccinator, and mentalis muscles, which are innervated superficially due to their deep location within the face. The SMAS is a fibrofatty layer superficial to the parotid gland that extends superiorly to the zygomatic arch, beyond which it is contiguous with the temporoparietal fascia (see image).[20] Inferiorly, the SMAS is contiguous with the platysma muscle, and anteriorly, it joins the mimetic muscles.
The frontal branch of the facial nerve innervates the muscles of the forehead and upper eyelid: the frontalis, corrugator supercilii, and procerus muscles, and the superior aspect of the orbicularis oculi. The zygomatic branch of the facial nerve also controls the orbicularis oculi and some of the upper midface muscles, while the buccal branch innervates the remaining midface muscles and the orbicularis oris. The marginal mandibular branch of the facial nerve primarily controls the depressor anguli oris and mentalis muscles, while the cervical branch controls the largest mimetic muscle, the platysma, as well as the mentalis. Innervation of individual muscles may differ among patients, but this general scheme informs the physical examination, which should separately and systematically evaluate the function of each facial nerve branch.
Physical Examination
Assessment of facial nerve function aims to identify abnormalities in any of the main extratemporal branches discussed above. The preferred assessment method begins in the upper face and examines the function of each branch in a descending fashion (see video). The initial examination should be performed with the patient at rest to identify any gross or subtle asymmetries in muscle tone; these are frequently manifested as brow ptosis, lower eyelid laxity, nasolabial fold effacement, deviation of the upper lip philtrum, or inferior malposition of the oral commissure. Older patients may also demonstrate effacement of platysmal banding on the affected side.
A dynamic physical examination should be performed once the static assessment is complete. Raising the eyebrows, closing the eyes gently and again with full effort, smiling, puckering the lips, and depressing the lower lip successively test the functions of the frontal, zygomatic, buccal, marginal mandibular, and cervical branches of the facial nerve. Determining the level of baseline asymmetry and the amount of effort required to close the affected eye, if complete eye closure is even possible, permits grading the severity of the facial paralysis on the House-Brackmann scale, a simple and universally-accepted method of assessing facial nerve function.[21]
- Grade I: Symmetric at rest and with movement, with no abnormalities
- Grade II: Mild weakness, symmetric at rest, minimal asymmetry with movement, complete eye closure with gentle effort, possible mild synkinesis
- Grade III: Moderate weakness, symmetric at rest, moderate asymmetry with movement, complete eye closure only with full effort, possible moderate synkinesis
- Grade IV: Moderate-to-severe weakness, symmetric at rest, moderate asymmetry with movement, incomplete eye closure
- Grade V: Severe weakness, grossly asymmetric at rest, significant asymmetry with movement, incomplete eye closure
- Grade VI: No visible movement, grossly asymmetric at rest ("facial droop")
The House-Brackmann scale offers a convenient means of quantifying overall hemifacial paralysis. Still, it is not designed to provide a detailed description of severe synkinesis or segmental facial paralysis, such as that with distal facial nerve injury. Synkinesis is the involuntary movement of some muscles accompanying the voluntary movement of others, such as eye closure with smiling or cheek and neck twitching with eye closure or brow elevation. Synkinesis is often characterized by an increased resting tone in the affected side of the face, which may manifest as a deepened nasolabial fold or face and neck cramping that progresses throughout the day.
To characterize a broader range of facial paralysis presentations, including not only flaccid hemifacial palsy but also segmental and synkinetic paralysis, several more detailed facial function grading systems have emerged. Systems in regular use are the Sunnybrook scale, developed by Ross et al in Toronto in 1994, and the eFACE scale (see image), developed by Banks et al at Harvard in 2015.[22][23]
Physiology
The variable clinical presentation of facial paralysis is a result of the complex anatomy of the facial nerve and its behavior when injured. The extent of flaccid paralysis in the acute posttraumatic period is dependent upon the severity of the injury and its location. Similarly, the occurrence and degree of synkinesis also depend on the degree and site of injury. The severity of motor nerve injury is commonly described using two similar scales, described by Sunderland and Seddon, that relate the degree of dysfunction to the microanatomy of the injury (see table).[24][25][24] For this reason, a working knowledge of nerve anatomy is critical. Each motor axon is surrounded by myelin produced by Schwann cells, and around the myelin is a connective tissue membrane called the endoneurium. Axons, wrapped in myelin and endoneurium, are grouped into fascicles, each surrounded by perineurium. Multiple fascicles, surrounded by epineurium, constitute the nerve itself.
Sunderland Classification
- Class I: Temporary conduction block due to focal demyelination with axonal integrity. Prognosis: complete recovery.
- Class II: Axonal disruption with intact endoneurium. Prognosis: complete recovery.
- Class III: Axonal disruption with endoneurial violation; the perineurium remains intact. Prognosis: mild to moderate synkinesis.
- Class IV: Axonal disruption with endoneurial and perineurial violation; only the epineurium remains intact. Prognosis: severe synkinesis.
- Class V: Disruption of all nerve components, including the epineurium; a transection injury. Prognosis: persistent flaccid paralysis.
Seddon Classification
- Neurapraxia: Equivalent to Sunderland Class I injury. Prognosis: complete recovery.
- Axonotmesis: Axonal injury without nerve transection, equivalent to Sunderland Classes II through IV injuries. Prognosis: variable degree of synkinesis.
- Neuronotmesis: Nerve transection, equivalent to Sunderland Class V injury. Prognosis: persistent flaccid paralysis.
These classification systems illustrate the relationship between injury severity and the development of synkinesis. While all traumatic facial nerve injuries present with weakness, only the most severe injuries characterized by anatomical discontinuity of the nerve result in permanent flaccid paralysis; this emphasizes the importance of nerve repair for improving outcomes.
An axonal disruption that leaves some amount of internal neural architecture intact may result in synkinesis; the degree of synkinesis is dictated by which membranes are violated. Endoneurial disruption with intact perineurium portends a better prognosis than an injury to both layers. Axonal disruption also results in Wallerian degeneration, the disintegration of the injured axon between the node of Ranvier just proximal to the site of injury and the neuromuscular junction, following which the immune system clears the debris. Wallerian degeneration occurs approximately 72 hours after a neural transection injury but may persist for days or weeks after a neural crush injury, such as a temporal bone fracture.[26][9]
This timeline of Wallerian degeneration is clinically significant; facial nerve exploration and repair conducted within 72 hours of injury can be performed with the aid of a nerve stimulator, and later-attempted repairs may not. Similarly, the electrophysiological consequences of Wallerian degeneration, a decreased compound muscle action potential (CMAP) amplitude, may provide prognostic information to the patient and care team. Electroneuronography (ENoG) is a form of evoked electromyography (EMG) in which a transcutaneous electrical stimulator is placed at the mastoid tip, and surface electrodes are placed over the facial muscles to record the CMAP, allowing for comparison between the injured and unaffected sides.
If there is a significant decrease in CMAP amplitude on the injured side, Wallerian degeneration has occurred; the injury is at least a Sunderland class II, and synkinesis may occur, particularly if the decrease in CMAP amplitude is substantial. If the CMAP on the affected side is entirely absent, the injury is very likely a Sunderland Class V, which may be confirmed with an ENoG. During the ENoG, action potentials are recorded in the facial muscles by needle electrodes during voluntary movements. A lack of voluntary motor unit potentials indicates nerve transection and corroborates the need for nerve repair.[27]
ENoG testing should not be performed before 72 hours post-injury; if Wallerian degeneration has not had time to occur, the test will not accurately indicate the extent of axonal injury. Ultimately, facial nerve repair aims to convert a Sunderland Class V injury to a Class IV injury or a completely transected nerve to one with intact epineurium to improve the prognosis from persistent flaccidity to synkinesis.
Indications
The primary indication for facial nerve repair is a Sunderland Class V injury to the main trunk or an extratemporal branch of the facial nerve. When the injury occurs intraoperatively, the severity may be self-evident and primary repair may be undertaken at the time of injury; this approach is preferred. However, some facial nerve injuries are not apparent until after emergence from general anesthesia, either immediately upon awakening or in a delayed fashion. When paralysis is delayed, the nerve is intact; repair or transfer is unnecessary. If, on the other hand, the paralysis is complete and immediately apparent upon awakening but was not identified intraoperatively, it may be prudent to evaluate the severity of the axonal injury 3 to 5 days later via ENoG and proceed with repair or transfer only if testing reveals a greater than 90% reduction in CMAP amplitude compared to the uninjured side. In cases of facial trauma, particularly lacerations, a similar process may be followed to determine candidacy for surgical intervention. Nerve injuries occurring medial to the lateral canthus do not need to be explored or repaired; the nerve branches in that region are small enough that coaptation is very challenging, and there is enough redundancy of the branches that spontaneous recovery is all but assured.
There must be viable distal and proximal segments of the nerve if either primary coaptation or interposition grafting is to be attempted. Facial paralysis due to a very proximal nerve injury, as at the CPA, or central nervous system pathology, such as a cavernous brainstem hemangioma, is better addressed with nerve transfer techniques. When considering nerve repair or transfer, there must be a target muscle expected to remain viable long enough to accept axonal ingrowth weeks to months after the procedure. While muscle typically undergoes atrophy and fibrosis after 12-18 months of denervation, this is not consistently the case. There are anecdotal reports of successful reinnervation years after a facial nerve injury. Generally, the muscle will be viable if the repair or nerve transfer is performed within six months of the injury; nerve transfer will likely be more successful than repair in such cases.[7]
Axons regenerate at approximately 1 mm/day, although many factors may affect this rate, including age, nutritional status, and general health. Therefore, a rough estimate of the time it will take for axons to travel from the repair site to the target muscles can be obtained by measuring the distance from the point of repair or transfer in millimeters.[28] However, the ideal time for nerve repair is at the time of injury whenever possible; Kannan et al reported improved functional outcomes in patients who underwent immediate facial nerve repair compared to patients whose repair was delayed by 3 weeks to 18 months.[5] When nerve repair or transfer is scheduled six or more months after injury, electromyography (EMG) should be performed to verify muscle viability; insertional activity or fibrillation potentials should be present. If EMG demonstrates polyphasic potentials, reinnervation procedures should be delayed to determine the effectiveness of the ongoing axonal recovery.
An algorithm is provided to guide the selection of evaluation and management techniques for patients with facial nerve trauma (see image). In brief:
- If the proximal and distal nerve stumps can be approximated without tension, direct end-to-end coaptation is indicated.[4]
- If the proximal and distal nerve stumps can be approximated within 6 mm of each other without tension, coaptation using a nerve connector is indicated.[29][30]
- If both stumps are accessible but cannot be approximated without tension, an interposition nerve graft is indicated. Interpositional grafting most commonly utilizes the greater auricular or sural nerve and should ideally only be performed when the duration of paralysis is one year or less.
- If greater than 50% of the diameter of the nerve has been disrupted, as in a drill injury during mastoidectomy, the injured segment should be resected and primary neuorraphy performed when possible; an interposition graft may be used if necessary.
Contraindications
Incomplete and delayed facial paralysis are contraindications to facial nerve repair. The nerve is intact in both situations and should be allowed to recover spontaneously. Additionally, if the facial nerve injury occurs medial to the lateral canthus, not only would repair be technically difficult, the redundancy of innervation close to the target muscles is usually sufficient that spontaneous functional recovery is highly likely.[31]
The absence of electrical activity, specifically the lack of insertion or fibrillation potentials, on preoperative EMG of the target muscles indicates atrophy of the neuromuscular junction with possible fibrosis of the muscles and an inability to accept reinnervation.[32] Patients whose facial muscles are absent due to extensive facial resection, trauma, or congenital facial palsy may not have target muscles for reinnervation. Facial nerve repair is contraindicated in these circumstances.
Patients with advanced-stage malignancies or other chronic diseases likely to shorten life expectancy may not live long enough to justify the surgical risk of facial reinnervation. In cases of malignancy, facial reinnervation should be delayed until tumor resection is completed. Additionally, patients in a persistent vegetative state or those with severe dementia will be unable to participate in facial retraining, reducing the benefits of facial reinnervation procedures.
Preexisting dysphagia, while not a contraindication to facial reinnervation in general, is a contraindication for hypoglossal nerve transfer; the procedure may exacerbate the problem, particularly in older patients.[33]
Equipment
The following equipment is recommended to perform a facial nerve repair:
- Operating microscope (a cross-table arrangement is not necessarily required for neurorrhaphy)
- Microsurgical instruments (jeweler's forceps, straight and curved micro scissors, non-locking micro needle driver)
- 10-0 nylon sutures on cutting spatula-tip needles, such as TG 160-4
- Weck cell spears
- Suction tip, Goodhill is recommended
- Bipolar jeweler's forceps
- Collagen wrap or other nerve sheaths, such as a vein graft
- Straight Iris or tenotomy scissors
- Retractors (Senn, Ragnell, Cummings, or Lone Star)
- Fibrin glue
- Nerve stimulator
Other instruments may be required to perform the approach to the facial nerve, facial nerve exploration, and the approach to any other nerve that will serve as a graft or be transferred. For example, Metzenbaum, Gorney-Freeman, or Reynolds tenotomy scissors may be useful. In addition, petit point Crile clamps, fine right-angle clamps, McCabe dissectors, and DeBakey or Gerald forceps will serve well for nerve dissection.
Personnel
The nerve coaptation is usually performed by a facial plastic surgeon, neurosurgeon, head and neck surgeon, or plastic surgeon. This may be the primary surgeon whose procedure resulted in the facial nerve injury or a consultant whose expertise is required for nerve repair. For cases that require proximal exposure of the facial nerve within the mastoid process of the temporal bone, an otolaryngologist or otologist should perform the mastoidectomy.
In addition to the surgeon, a circulating nurse and a surgical technologist should be present. In some cases, a surgical first assistant may be necessary. An anesthesia provider is also required. The anesthesia provider should be capable of maintaining a general anesthetic plane deep enough to prevent patient movement without requiring long-acting paralytics, as these preclude the use of intraoperative nerve stimulation.
Preparation
Patient education regarding the goals and expectations for the procedure is essential, as facial reinnervation and the return of movement take several months to occur after nerve repair or transfer. Additionally, maximizing surgical results requires postoperative facial rehabilitative physical therapy. Additional minor procedures, such as botulinum toxin injections or static suspension, are often required.
When a donor graft is used, the patient must understand the expected consequences of graft harvest, typically numbness in the distribution of the nerve from which the graft is to be taken. The area where the graft will be harvested must be prepared simultaneously with the face, and the patient must be positioned and draped accordingly. The nerves most commonly used for grafting are the sural and the greater auricular nerves.[34] When the sural nerve is selected, particularly for cross-face nerve grafting, a separate surgical team may harvest the graft while the other team exposes the facial nerve to save time.[7]
Various details unique to facial nerve reinnervation procedures warrant consideration. Preparation of the face requires the protection of the eyes with adhesive film dressings or temporary tarsorrhaphy sutures. The side affected by paralysis must be definitively marked to avoid intraoperative confusion. One marking method is to write a "P" on the cheek of the paralyzed side and an "NP" on the non-paralyzed side while the patient is awake in the holding area.
During anesthesia induction, the endotracheal tube should be placed in the midline and secured to either the lower lip or sutured to the central maxillary incisors to prevent asymmetric distortion of the lower face. If the planned procedure is either a nerve repair performed within 72 hours of the injury or a nerve transfer performed at any time, the anesthesia provider should avoid administering long-acting paralytics so that a nerve stimulator may be used during dissection. Likewise, the preoperative facial injections should consist of plain epinephrine without a local anesthetic to avoid diminishing the nerve stimulation response while providing hemostasis. A simple method of preparing plain epinephrine is to inject the contents of a 1 mL ampule of 1:1,000 epinephrine into a 100 mL bag of normal saline, thus providing 1:101,000 epinephrine; removing 1 mL of saline from the bag before adding the epinephrine will result in an even 1:100,000 concentration.
Technique or Treatment
Direct End-to-End Coaptation
Direct end-to-end coaptation is the treatment of choice for facial nerve injury whenever feasible. Functional outcomes are improved when the nerve is repaired immediately after injury; this situation is only likely to occur during iatrogenic trauma (see image).[5][32] If repair under the same anesthetic as the injury is impossible, facial nerve exploration and repair should ideally be performed within 72 hours of injury before completion of Wallerian degeneration; this permits using an intraoperative stimulator to facilitate identification of the distal nerve stump.
The proximal nerve stump will ideally be encountered near the distal stump, even though it will not be stimulable. If the proximal stump is not apparent, it can be located via dissection of the facial nerve distally from the main trunk. The main trunk of the facial nerve may be identified by tracing the tragal pointer or deep aspect of the anterior surface of the tragal cartilage and the tympanomastoid suture line. The main trunk of the facial nerve is typically located under a small vein, deep to a fat pad, roughly 1 cm deep, and 1 cm inferior to the tragal pointer, within about 3 mm of the medial end of the tympanomastoid suture line.[35] Between where the facial nerve exits the stylomastoid foramen and where it branches at the pes anserinus within the parotid gland, the main trunk is approximately 2.5 mm in diameter and 14 mm long.[36] In some cases, the facial nerve may be approached directly via a facial laceration; the exploration is often performed via a modified Blair or parotidectomy-type incision.
Once the nerve stumps have been identified, they should be trimmed back to healthy tissue, as evidenced by pinpoint bleeding within the nerve. When the injury is fresh, the required debridement is usually minimal. However, if the injury is old, scar tissue may have formed; this may necessitate more aggressive trimming of the nerve with internal neurolysis, removing the section containing scar tissue, or external neurolysis, dissecting scar tissue away from the outside of the nerve to improve exposure. Intraneural scar tissue prevents proper axonal regeneration across the neurorrhaphy and must be removed completely, even if the removal precludes a tension-free neurorrhaphy and a nerve sheath or interposition graft becomes required (see below). Similarly, if ≥50% of the diameter of the nerve has been injured, the injured segment should be resected and the nerve repaired to reduce the amount of intraneural scar tissue that forms.
The neurorrhaphy itself is typically performed under an operating microscope to improve visibility. The sutures should be placed as interrupted stitches in the epineurium, avoiding overtightening of the knots and pushing the nerve ends together with excessive force, likely distorting the fascicles contained therein. If fascicles herniate through the neurorrhaphy suture line, the sutures should be replaced with less tension on the knots, or the fascicles should be trimmed back to allow them to lie within the neurorrhaphy. Individual fascicular repair placing sutures in the perineurium rather than the epineurium is rarely performed; there is no reliable way to determine the correlates between proximal and distal stump fascicles. Outcomes are equivalent between perineurial and epineurial repair; the latter procedure is far less technically challenging.[37][38]
A cutting or spatula-tipped needle is preferable to a tapered needle. Tapered needles, such as those used for microvascular anastomosis, quickly dull with repeated passes through the relatively tough epineurium; cutting and spatula-tipped needles will not. The authors prefer to sew toward themselves whenever possible, placing the first bite of the first stitch through only the epineurium of the distal nerve stump and directing the stitch from outside the nerve to inside the nerve. The second bite is taken after pulling the needle completely through the distal stump epineurium; this bite goes from within the nerve to outside, again taking only epineurium in the bite. To approximate the nerve ends before tying, pull the suture through the nerve back towards the distal stump until the tail is short enough to manage easily. Four flat throws on the knot with the tails cut short will suffice, with a surgeon's knot often placed as the first throw.
The first stitch is generally placed in the center of the superficial aspect of the epineurium, where it is easiest to sew; once the first suture is placed, two or three more interrupted stitches may be added to approximate the epineurium gently in a circumferential fashion (see video). Alternatively, fibrin glue may be used to coapt the nerve stumps, which appears to produce comparable functional outcomes to suture neurorrhaphy. However, using sutures provides a more stable repair more capable of resisting the tension that may be applied by patient movement.[4][7][39] The most common place to employ fibrin glue during facial nerve repair is intracranially in the CPA or internal auditory canal.[7]
If tension-free neurorrhaphy between the proximal and distal nerve stumps is impossible, three other commonly-employed options exist. The first option is to coapt the nerve ends via a nerve sheath and leave a small gap between them. The second option, described below, is to interpose a cable graft from a donor nerve, and the third option is to perform a nerve transfer. Gaps between proximal and distal nerve stumps of ≤6 mm can be bridged by regenerating axons during the healing process, providing a sheath, typically made of collagen, connects the nerve ends and encloses the gap; outcomes are comparable to those achieved with primary neurorrhaphy.[29][30] Gaps greater than 7 mm should be bridged with interposition grafts. However, interposition grafts should be avoided whenever possible; the number of neurorrhaphies required for the repair doubles, and each neurorrhaphy may potentially lose as many as 50% of the axons regenerating across the repair.[40] Despite this, excessive dissection to mobilize the nerve stumps should also be avoided; epineurial dissection and stretching of the nerve stumps compromise the vasa nervorum and adversely affects healing.
Facial Nerve Coaptation with Interposition Grafting
If the gap between the proximal and distal nerve stumps is too long to allow primary neurorrhaphy, an interposition graft, or "cable" graft, may be employed to span the distance. Placing a cable graft necessarily requires two neurorrhaphies instead of the one in a primary repair; this significantly increases the risk of axonal loss. This risk may be mitigated by reversing the anatomical direction of the grafted nerve; the distal end of the graft is sutured to the proximal nerve stump and vice versa. This practice theoretically allows every fascicle present at the distal end of the graft, now placed proximally, to have a continuous tract all the way to the proximal end of the graft, now located distally. Typically, sensory nerves are used for graft material: most commonly, the greater auricular or sural nerves and occasionally the medial or lateral antebrachial cutaneous nerves.[32] In rare cases, motor nerves may be used as grafts, generally when they are already being sacrificed as part of a larger graft harvest. Cadaveric nerve allografts also are available, although reported procedural outcomes are inferior to nerve autografts (see image).[41][42]
The greater auricular nerve arises from the second and third cervical nerve roots, providing sensation to the inferior aspect of the auricle, the lobule, and some skin overlying the mastoid process and parotid gland. It can provide 7 cm or more of length, and its diameter is approximately 1.5 mm, making it a suitable size match for the facial nerve.[43] The greater auricular nerve wraps around the posterior border of the sternocleidomastoid muscle at roughly one-third of the way down the muscle from its insertion on the mastoid tip, a point from which the lesser occipital, transverse cervical, and supraclavicular nerves also emanate. From there, it travels superiorly until it begins to branch 2-3 cm inferior to the lobule, running 1-2 cm posterior to, but in the same plane as, the external jugular vein, just deep to the platysma.[43] Size, length, and location make the greater auricular nerve very convenient for facial nerve repair; it is often accessible during the procedure associated with the initial facial nerve injury.
The sural nerve arises from the fourth and fifth lumbar and first sacral nerve roots, resulting from the confluence of the medial and lateral sural cutaneous nerves.[44] It rises into a subcutaneous plane between the heads of the gastrocnemius muscle and then travels inferiorly towards the lateral malleolus of the ankle, just deep to the lesser saphenous vein.[34] It is easily approached via a 1-2 cm transverse incision placed 1 cm posterior and 1 cm superior to the lateral malleolus; minimal dissection will reveal the lesser saphenous vein, which can be retracted to expose the sural nerve. The nerve may be isolated and harvested via several approaches, including endoscopy, using a vein or tendon stripper, or making multiple "ladder" incisions perpendicular to the course of the nerve to permit dissection under direct visualization. The sural nerve provides up to 30 cm of length and a diameter of roughly 3.5 mm, slightly more than the facial nerve but sufficient to transmit an ample number of axons.[45] The sural nerve is particularly well suited to use in cross-face grafting applications. Donor site morbidity is minimal, typically limited to numbness of the lateral foot and ankle.
Cross-face Nerve Grafting
From a technical standpoint, cross-face nerve grafting is equivalent to placing a long interposition graft; functionally, it is a nerve transfer, as axons from a different nerve are being redirected into the distal stump of the facial nerve (see image). For this reason, the technique may be applied in cases where the proximal stump of the facial nerve is unavailable for coaptation. More frequently, however, the cross-face graft is used to reinnervate a specific muscle group, such as the orbicularis oculi or zygomaticus major and minor muscles, or to control a transferred muscle for facial reanimation, such as the gracilis or sterno-omohyoid muscles.[46][47]
Placement of a cross-face graft requires exposing the recipient facial nerve or branch thereof on the injured side and performing a facial nerve exploration on the contralateral side to identify an appropriate branch to donate axons. The contralateral branch selected should correspond as precisely as possible with the innervated muscle of the target branch; if successful, the cross-face graft will facilitate spontaneous muscle movement on the reinnervated side, and it is important to produce as much facial symmetry as possible. For example, it would be a suboptimal outcome if a smile on the donor side were to cause an eye blink on the injured side.
Cross-face nerve grafts are notoriously unreliable, particularly when performed in older individuals, patients with longstanding facial paralysis, and patients at increased risk for losing facial function on the donor side, such as those with neurofibromatosis type 2, who are at risk of developing bilateral vestibular schwannomas; such patients are not ideal candidates for the procedure. As with any other nerve transfer procedure, donor site morbidity and axon availability should be considered preoperatively. Because a facial nerve exploration is required on the donor side for cross-face nerve grafting, patients should be aware of the potential for a scar. Additionally, sacrificing a facial nerve branch large enough to provide sufficient axons for effective reinnervation runs the risk of causing weakness. In practice, the weakness is seldom clinically apparent and may help to improve symmetry. The axon count in the sural nerve is approximately 1100, enough to permit the transit of the approximately 900 axons required for effective reinnervation.[48][49]
Hypoglossal Nerve Transfer
Nerve transfers are usually performed when the proximal facial nerve stump is unavailable, as when nerve injury occurs at the skull base or intracranially, as many as 12 months have passed since the injury, or the nerve transfer is being performed for reinnervation of a particular facial region as part of a multi-segment reinnervation and reanimation treatment plan. The two most commonly performed procedures in current practice are the hypoglossal and the masseteric nerve transfers.
The hypoglossal nerve is located superficial to the carotid arteries and deep to the central tendon of the digastric muscle and is typically approached via a high, transverse neck incision. The hypoglossal nerve provides motor innervation to the anterior two-thirds of the tongue and contains approximately 9000 axons, 50% more than the 6000 found in the facial nerve.[50] Because of the relatively high resting tone of the tongue, hypoglossal nerve transfer to the main trunk of the facial nerve is often used to restore resting facial tone. The technique may also be employed specifically for smile rehabilitation; this requires the patient to push their tongue into their cheek to smile. While the high axon count of the hypoglossal nerve makes it a very effective donor for facial reinnervation, the significant morbidity associated with denervating half of the oral tongue has led to the development of multiple variations of the procedure that are designed to limit the functional lingual deficit.[33][51]
The classic hypoglossal nerve transfer procedure involves transecting both the hypoglossal and facial nerves and suturing the proximal hypoglossal stump into the distal facial nerve stump; this procedure is so morbid that it is rarely performed in current practice. Another common procedural variation involves transecting the facial nerve in the mastoid, reflecting the distal facial nerve inferiorly, and performing an end-to-side neurorrhaphy into the hypoglossal nerve. Alternatively, the epineurium of the hypoglossal nerve may be incised, and one-third to one-half of the fascicles may be reflected superiorly to be sewn end-to-side or end-to-end into the main trunk of the facial nerve. However, if some return of facial nerve function is expected, but additional muscle tone is required, hypoglossal-facial nerve transfer without division of the main trunk of the facial nerve is preferable. Lastly, epineurial windows may be made, and one-third of the fascicles in the hypoglossal and facial nerves divided so that an interposition or "jump" graft may be sutured end-to-side into both nerves to carry axons from the transected fascicles in the hypoglossal nerve to the transected fascicles in the facial nerve (see image).
Regardless of the technique selected, however, the donor site morbidity and facial movement outcomes are inversely proportional; as more axons are transferred to the face, more axons are taken from the tongue. While younger patients better tolerate donor site morbidity than older patients, the resulting dysphagia and dysarthria may become problematic with time.
Masseteric Nerve Transfer
While the hypoglossal nerve is relatively conveniently located in the upper neck, not far from the facial nerve, the masseteric nerve lies directly deep to the facial nerve and is easily accessed via the same incision used for facial nerve exposure. The nerve to the masseter muscle is a branch of the mandibular division of the trigeminal nerve. The masseteric nerve exits the skull base via the foramen ovale and traverses the infratemporal fossa before entering the deep surface of the masseter muscle via the sigmoid notch of the mandible. The masseteric nerve contains approximately 1500 axons; this may account for the lower resting tone of the masseter muscle compared to that of the tongue.[11] For this reason, masseteric nerve transfer is not as effective in restoring resting facial tone as hypoglossal nerve transfer. However, the anatomical location of the masseteric nerve makes it ideal for smile rehabilitation.
There are multiple methods to locate the masseteric nerve. The authors prefer to mark a point 3 cm anterior to the anterior surface of the tragal cartilage and 1 cm inferior to the inferior border of the zygomatic arch, as described by Borschel et al. At this point, dissection approximately 1.5 cm deep to the SMAS should reveal the masseteric nerve beneath a consistently encountered tendinous layer within the muscle.[52] The masseteric nerve should be dissected distally and transected just before branching, then reflected out of the muscle for coaptation. End-to-end or end-to-side neurorrhaphy into the main trunk of the facial nerve may be performed, or the masseteric nerve may be sutured end-to-end into the buccal branch located at the point of Zuker to rehabilitate a paralytic or synkinetic smile.[53][54][55][56][57][58]
Another advantage of the masseteric nerve transfer over hypoglossal nerve transfer is its comparative lack of donor site morbidity; loss of masseteric function is well tolerated, and not all patients suffer a noticeable loss of facial volume from muscle atrophy. However, as with the hypoglossal nerve transfer, using the masseteric nerve to provide facial movement requires the patient to initiate a conscious movement, such as when biting down or clenching the jaw (see video). Over time, many patients can smile without needing to bite down hard, but achieving a spontaneous smile with either a masseteric or hypoglossal nerve transfer is comparatively uncommon, especially in adult patients.[59]
Complications
The biggest risk of nerve repair or transfer is no return of function is achieved. Risk factors for procedural failure include prolonged paralysis of greater than 6 to 12 months before the intervention, advanced age or poor health status, and performing multiple neurorrhaphies, as are required for interposition grafting. Each neurorrhaphy introduces additional opportunities for axonal loss. Postoperative radiotherapy does not appear to affect functional outcomes, although chemotherapy is known to cause facial paralysis.[60][61]
When the facial nerve is repaired proximal to the pes anserinus or a nerve transfer to the main trunk is performed, the expected outcome is synkinetic movement. Synkinetic movement is not a complication per se but represents a suboptimal outcome. Synkinesis may impair voluntary movements, particularly smiling, resulting in marked facial asymmetry and lower quality of life despite restoration of tone and movement. While numerous theories regarding the etiology of synkinesis have been proposed, it is currently thought that a major contributing factor to synkinesis is the misdirection of axons to different neuromuscular junctions from the ones they innervated before the injury.[62][63]
Additionally, axons that terminate in multiple rather than single neuromuscular junctions will cause discoordinated movement and increase the basal firing rate of the innervated muscle and its resting tone. Botulinum toxin injections and facial physical therapy are the mainstay treatment options for this problem, but selective neurectomy and myomectomy have also been successfully employed.[64][65][66][67][68] When possible, synkinesis may be avoided or reduced by preferentially reinnervating facial nerve branches rather than the main trunk itself. While the site of the injury determines at which point a nerve repair will take place, the surgeon has discretion over where to perform a nerve transfer. For this reason, masseteric nerve to buccal branch transfers are favored over masseteric nerve to main trunk facial nerve transfers; these procedures may also be combined with selective neurectomy or myomectomy to further reduce synkinesis.[54]
One risk of performing nerve transfers, particularly in the case of the masseteric nerve to buccal branch transfer undertaken more than 72 hours after injury, is the possibility of reinnervating the wrong nerve branch. Without the ability to stimulate the recipient nerve, it is possible to select a suboptimal branch that may not produce the desired precise effect. While suboptimal branch selection is unlikely to result in movements completely different from those intended as long as the recipient branch is in roughly the correct location, it may be that the resulting smile vector, for example, is not entirely symmetric with the uninjured side. Most facial reanimation surgeons prefer to reinnervate native facial muscles whenever possible, but in cases of suboptimal outcomes of nerve transfer, intractable synkinesis, or longstanding paralysis of greater than 12-18 months, free muscle transfer may be employed instead of nerve repair or transfer.[69]
When an uninjured nerve is sacrificed in grafting or transfer, there is always a risk of donor site morbidity. Greater auricular and sural nerve harvest site hypesthesia is common, predictable, and usually results in nothing more than auricular lobule numbness and lateral ankle and foot numbness, respectively. While uncommon, the potential for developing painful neuromas at these sites does exist. These neuromas can be treated with excision and burying the cut nerve end into muscle.
Motor nerve sacrifice, on the other hand, may carry a greater risk of morbidity. Masseteric nerve sacrifice is generally well tolerated, with little more than the loss of some facial volume due to muscle atrophy; most patients do not complain of decreased bite force. Cross-face nerve grafting also tends to be well tolerated, despite requiring sural nerve harvest, and there tends to be minimal morbidity other than a scar from the contralateral dissection required to access the uninjured facial nerve. While sacrificing an uninjured facial nerve branch to supply axons to the cross-face graft may risk weakening the normal side smile, this is rarely seen in practice.
The highest risk for donor site morbidity among these procedures occurs with the hypoglossal-facial nerve transfer because of the potential for dysphagia and dysarthria accompanying tongue weakness. When the entire hypoglossal nerve is transferred, facial outcomes are good because of the high axon count in the hypoglossal nerve, but the morbidity is severe.[50] The sacrifice of only part of the hypoglossal nerve via end-to-side coaptation or interfascicular dissection and transfer decreases morbidity dramatically but also compromises results by reducing the number of axons redirected into the facial nerve.[33]
Clinical Significance
Facial paralysis can cause significant decreases in quality of life due to social and functional impacts. Facial nerve repair and transfer provide the potential for restoration of mimetic facial function with a good risk-to-benefit ratio, as only minor complications are typically reported. The best results are usually seen in young, healthy patients with distal facial nerve injuries that are repaired quickly, as in facial lacerations or iatrogenic injuries. Worse outcomes are expected when the injury is proximal, the patient is older or in poor health, and the paralysis is chronic, as in advanced parotid malignancies and recurrent cholesteatomas. Patients with slow-growing benign tumors or trauma fare best, while those with malignant and inflammatory pathologies fare worse. The first clinical signs of facial reinnervation can be expected to appear anywhere from 2 to 12 months postoperatively.[4]
Enhancing Healthcare Team Outcomes
Providing care to patients undergoing facial reinnervation procedures involves a comprehensive clinical team that typically includes a plastic surgeon, facial plastic surgeon, neurotologist or otolaryngologist, and a physical therapist to provide postoperative rehabilitation. In addition, a clinical photographer may be helpful for regular photodocumentation of progress, and if botulinum toxin injections are required to manage synkinesis, a nurse or advanced practice provider may provide them. In many cases, the etiology of the paralysis also necessitates the involvement of other specialists, such as a surgical oncologist, radiation oncologist, medical oncologist, radiologist, audiologist, ophthalmologist, and wound care nurse. Nursing care is particularly vital for these patients who need perioperative care, support, and education.
The outcomes of facial reinnervation depend on the timing of surgery and the rehabilitation process.[70] It may take more than a year for a patient to experience the full benefit of the procedure. To maximize the results of a nerve transfer, facial muscle retraining with physical therapy is essential, and patients must be aware of this before the surgery. Additional procedures, particularly periorbital interventions such as upper eyelid weight implantation, tarsorrhaphy, and tarsal strip-lateral canthopexy, are often performed concurrently with nerve repair or subsequently to it to enhance patient satisfaction.[4] [level 5]
Media
(Click Image to Enlarge)
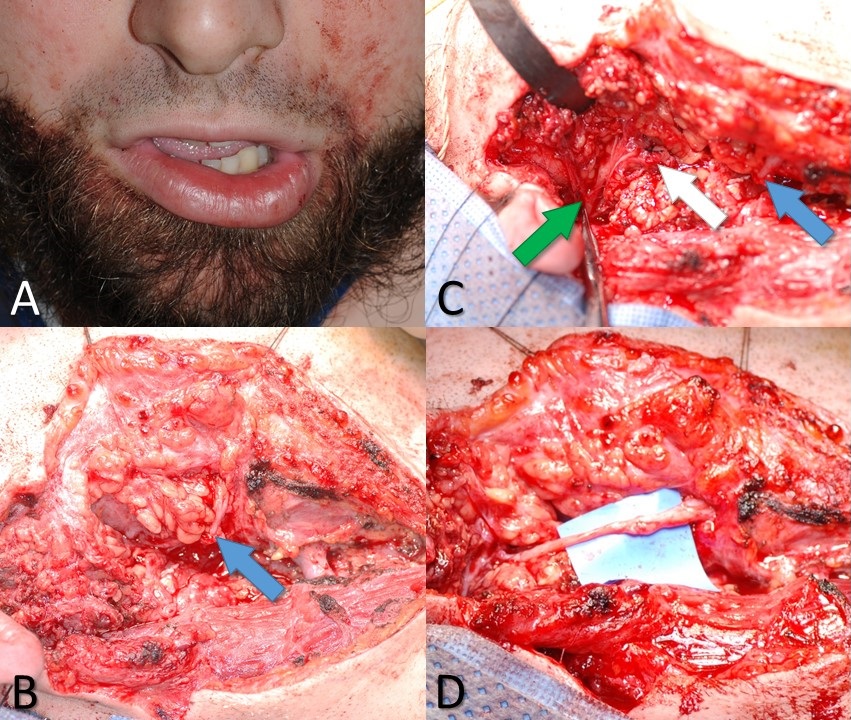
Marginal Mandibular Branch of the Facial Nerve Injury. A 26-year-old male with a knife wound to the right neck. (A) Weakness of the right depressor labii inferioris muscle indicates likely injury to the marginal mandibular branch of the facial nerve. (B) The blue arrow indicates the distal stump of the marginal mandibular nerve, as seen during neck exploration. (C) The white arrow indicates the proximal stump of the marginal mandibular nerve, and the green arrow indicates the pes anserinus of the facial nerve. (D) Proximal and distal stumps of the marginal mandibular nerve coapted microsurgically using 10-0 nylon suture and an interposition graft taken from the ipsilateral greater auricular nerve.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
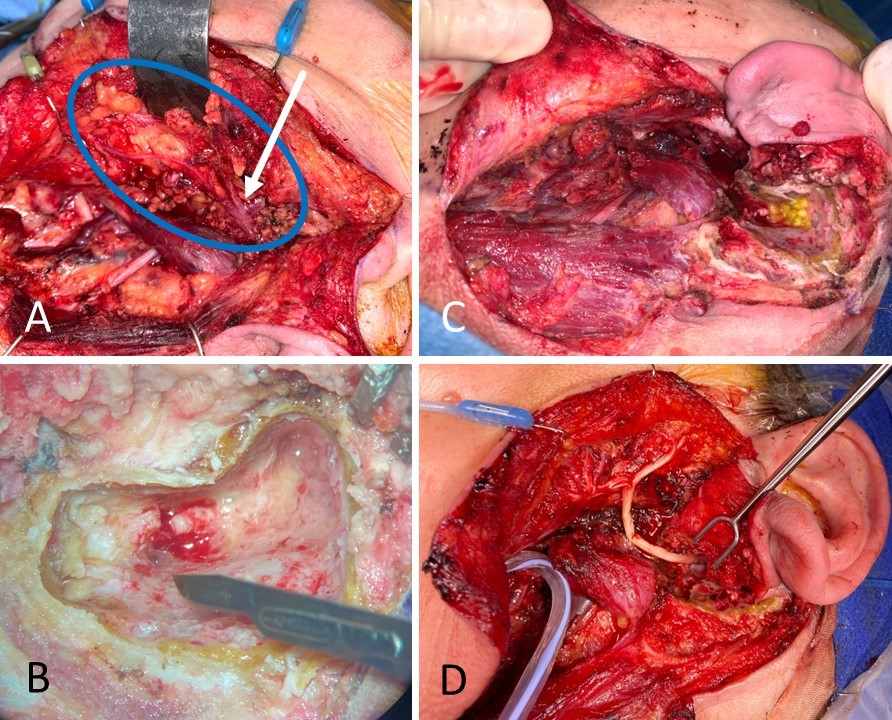
Facial Nerve Reconstruction. A 59-year-old female with carcinoma ex pleomorphic adenoma underwent total parotidectomy with facial nerve sacrifice from halfway down the mastoid segment to the distal branches, past the pes anserinus. (A) The facial nerve is circled in blue, and the pes anserinus is indicated by the white arrow; note the inflamed and dark appearance of the nerve. (B) The mastoid segment of the facial nerve is sectioned to obtain a negative proximal margin. (C) The wound bed is shown with an absent parotid gland, an open mastoid cavity, and a large facial nerve defect. (D) The facial nerve was reconstructed from the mastoid to the distal branches, including the pes anserinus, using an allograft.
Contributed by MH Hohman, MD, FACS, and KG Anderson, MD, FACS
(Click Video to Play)
Facial Nerve Transfer. A 50-year-old female with right-sided House-Brackmann grade VI facial paralysis due to acoustic neuroma resection 6 months status post right masseteric nerve to a buccal branch of facial nerve transfer for smile rehabilitation. Note how she has to bite down to smile.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
House-Brackmann Grade VI Facial Paralysis. A 49-year-old female status post resection of a right-sided acoustic neuroma (vestibular schwannoma) and upper eyelid weight placement for House-Brackmann grade VI facial paralysis.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
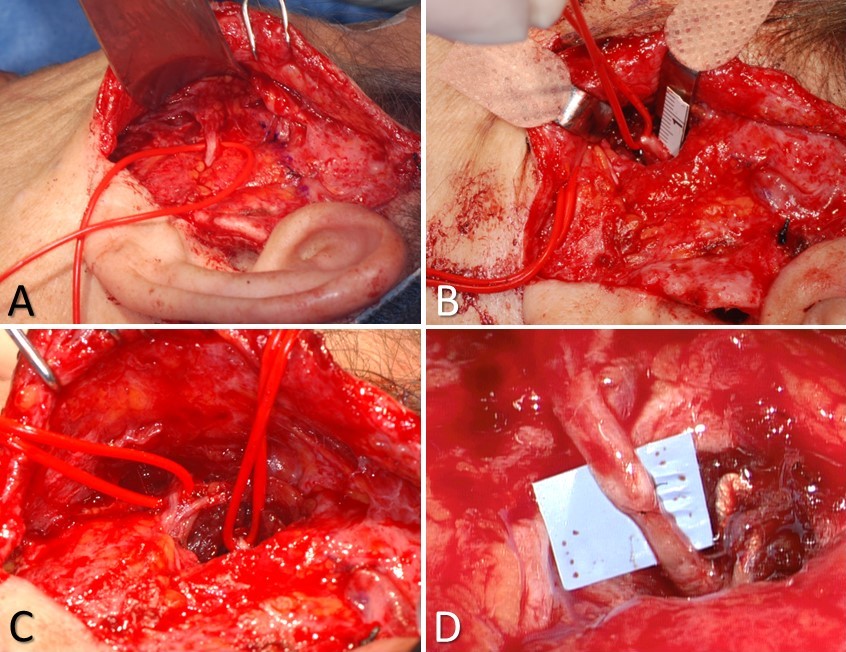
Masseteric Nerve Transfer. A) the buccal branch controlling the zygomaticus major muscle is identified at Zuker's point (halfway between the helical root and the oral commissure), B) the masseteric nerve is identified 3 cm anterior to the tragus, 1 cm inferior to the zygomatic arch, and 1.5 cm deep to the masseteric fascia; C) the 2 nerves are typically located within ~1.5 cm of each other, which obviates the need for an interposition graft; D) the completed nerve transfer, using 10-0 nylon epineurial sutures to coapt the proximal stump of the masseteric nerve to the distal stump of the buccal branch.
Contributed by MH Hohman, MD, FACS
References
Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2015 Dec:40(6):651-6. doi: 10.1111/coa.12434. Epub [PubMed PMID: 25858429]
Level 2 (mid-level) evidenceNellis JC, Ishii M, Byrne PJ, Boahene KDO, Dey JK, Ishii LE. Association Among Facial Paralysis, Depression, and Quality of Life in Facial Plastic Surgery Patients. JAMA facial plastic surgery. 2017 May 1:19(3):190-196. doi: 10.1001/jamafacial.2016.1462. Epub [PubMed PMID: 27930763]
Level 2 (mid-level) evidenceHohman MH, Hadlock TA. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. The Laryngoscope. 2014 Jul:124(7):E283-93. doi: 10.1002/lary.24542. Epub 2014 Jan 15 [PubMed PMID: 24431233]
Level 2 (mid-level) evidenceSánchez-Ocando M, Gavilán J, Penarrocha J, González-Otero T, Moraleda S, Roda JM, Lassaletta L. Facial nerve repair: the impact of technical variations on the final outcome. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019 Dec:276(12):3301-3308. doi: 10.1007/s00405-019-05638-8. Epub 2019 Sep 19 [PubMed PMID: 31538238]
Kannan RY, Hills A, Shelley MJ, Bisase B, Kapoor K, Norris P, Nduka C. Immediate compared with late repair of extracranial branches of the facial nerve: a comparative study. The British journal of oral & maxillofacial surgery. 2020 Feb:58(2):163-169. doi: 10.1016/j.bjoms.2019.11.004. Epub 2019 Nov 24 [PubMed PMID: 31776026]
Level 2 (mid-level) evidenceGardetto A, Kovacs P, Piegger J, Rainer C, Meirer R, Piza-Katzer H. Direct coaptation of extensive facial nerve defects after removal of the superficial part of the parotid gland: an anatomic study. Head & neck. 2002 Dec:24(12):1047-53 [PubMed PMID: 12454942]
Prasad SC, Balasubramanian K, Piccirillo E, Taibah A, Russo A, He J, Sanna M. Surgical technique and results of cable graft interpositioning of the facial nerve in lateral skull base surgeries: experience with 213 consecutive cases. Journal of neurosurgery. 2018 Feb:128(2):631-638. doi: 10.3171/2016.9.JNS16997. Epub 2017 Apr 7 [PubMed PMID: 28387625]
Level 3 (low-level) evidenceSeneviratne SO, Patel BC. Facial Nerve Anatomy and Clinical Applications. StatPearls. 2023 Jan:(): [PubMed PMID: 32119456]
Remenschneider AK, Michalak S, Kozin ED, Barber S, De Venecia RK, Hadlock TA, Jung DH. Is Serial Electroneuronography Indicated Following Temporal Bone Trauma? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Apr:38(4):572-576. doi: 10.1097/MAO.0000000000001337. Epub [PubMed PMID: 28114180]
Darrouzet V, Duclos JY, Liguoro D, Truilhe Y, De Bonfils C, Bebear JP. Management of facial paralysis resulting from temporal bone fractures: Our experience in 115 cases. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001 Jul:125(1):77-84 [PubMed PMID: 11458219]
Level 2 (mid-level) evidenceEngelmann S, Ruewe M, Geis S, Taeger CD, Kehrer M, Tamm ER, Bleys RLAW, Zeman F, Prantl L, Kehrer A. Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves. Scientific reports. 2020 Feb 6:10(1):1935. doi: 10.1038/s41598-020-58917-4. Epub 2020 Feb 6 [PubMed PMID: 32029860]
Kalaiarasi R, Kiran AS, Vijayakumar C, Venkataramanan R, Manusrut M, Prabhu R. Anatomical Features of Intratemporal Course of Facial Nerve and its Variations. Cureus. 2018 Aug 2:10(8):e3085. doi: 10.7759/cureus.3085. Epub 2018 Aug 2 [PubMed PMID: 30324041]
DAVIS RA, ANSON BJ, BUDINGER JM, KURTH LR. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surgery, gynecology & obstetrics. 1956 Apr:102(4):385-412 [PubMed PMID: 13311719]
Pitanguy I, Ramos AS. The frontal branch of the facial nerve: the importance of its variations in face lifting. Plastic and reconstructive surgery. 1966 Oct:38(4):352-6 [PubMed PMID: 5926990]
Dorafshar AH, Borsuk DE, Bojovic B, Brown EN, Manktelow RT, Zuker RM, Rodriguez ED, Redett RJ. Surface anatomy of the middle division of the facial nerve: Zuker's point. Plastic and reconstructive surgery. 2013 Feb:131(2):253-257. doi: 10.1097/PRS.0b013e3182778753. Epub [PubMed PMID: 23357986]
Hohman MH, Bhama PK, Hadlock TA. Epidemiology of iatrogenic facial nerve injury: a decade of experience. The Laryngoscope. 2014 Jan:124(1):260-5. doi: 10.1002/lary.24117. Epub 2013 Apr 18 [PubMed PMID: 23606475]
Level 2 (mid-level) evidenceHazani R, Chowdhry S, Mowlavi A, Wilhelmi BJ. Bony anatomic landmarks to avoid injury to the marginal mandibular nerve. Aesthetic surgery journal. 2011 Mar:31(3):286-9. doi: 10.1177/1090820X11398352. Epub [PubMed PMID: 21385737]
Batra AP, Mahajan A, Gupta K. Marginal mandibular branch of the facial nerve: An anatomical study. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2010 Jan:43(1):60-4. doi: 10.4103/0970-0358.63968. Epub [PubMed PMID: 20924452]
Chowdhry S, Yoder EM, Cooperman RD, Yoder VR, Wilhelmi BJ. Locating the cervical motor branch of the facial nerve: anatomy and clinical application. Plastic and reconstructive surgery. 2010 Sep:126(3):875-879. doi: 10.1097/PRS.0b013e3181e3b374. Epub [PubMed PMID: 20463628]
Mitz V, Peyronie M. The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plastic and reconstructive surgery. 1976 Jul:58(1):80-8 [PubMed PMID: 935283]
House JW, Brackmann DE. Facial nerve grading system. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1985 Apr:93(2):146-7 [PubMed PMID: 3921901]
Ross BR, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 1994 Dec:():S180-1 [PubMed PMID: 10774344]
Banks CA, Jowett N, Azizzadeh B, Beurskens C, Bhama P, Borschel G, Coombs C, Coulson S, Croxon G, Diels J, Fattah A, Frey M, Gavilan J, Henstrom D, Hohman M, Kim J, Marres H, Redett R, Snyder-Warwick A, Hadlock T. Worldwide Testing of the eFACE Facial Nerve Clinician-Graded Scale. Plastic and reconstructive surgery. 2017 Feb:139(2):491e-498e. doi: 10.1097/PRS.0000000000002954. Epub [PubMed PMID: 28121888]
Sunderland S. The anatomy and physiology of nerve injury. Muscle & nerve. 1990 Sep:13(9):771-84 [PubMed PMID: 2233864]
Seddon HJ. A Classification of Nerve Injuries. British medical journal. 1942 Aug 29:2(4260):237-9 [PubMed PMID: 20784403]
Lee DH. Clinical Efficacy of Electroneurography in Acute Facial Paralysis. Journal of audiology & otology. 2016 Apr:20(1):8-12. doi: 10.7874/jao.2016.20.1.8. Epub 2016 Apr 21 [PubMed PMID: 27144227]
Andresen NS, Zhu V, Lee A, Sebetka W, Kimura J, Hansen MR, Gantz BJ, Sun DQ. Electrodiagnostic testing in acute facial palsy: Outcomes and comparison of methods. Laryngoscope investigative otolaryngology. 2020 Oct:5(5):928-935. doi: 10.1002/lio2.458. Epub 2020 Sep 10 [PubMed PMID: 33134541]
Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner journal. 2013 Spring:13(1):100-8 [PubMed PMID: 23531634]
Liu D, Mi D, Zhang T, Zhang Y, Yan J, Wang Y, Tan X, Yuan Y, Yang Y, Gu X, Hu W. Tubulation repair mitigates misdirection of regenerating motor axons across a sciatic nerve gap in rats. Scientific reports. 2018 Feb 21:8(1):3443. doi: 10.1038/s41598-018-21652-y. Epub 2018 Feb 21 [PubMed PMID: 29467542]
Boeckstyns ME, Sørensen AI, Viñeta JF, Rosén B, Navarro X, Archibald SJ, Valss-Solé J, Moldovan M, Krarup C. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. The Journal of hand surgery. 2013 Dec:38(12):2405-11. doi: 10.1016/j.jhsa.2013.09.038. Epub 2013 Nov 5 [PubMed PMID: 24200027]
Level 1 (high-level) evidenceDanner CJ. Facial nerve paralysis. Otolaryngologic clinics of North America. 2008 Jun:41(3):619-32, x. doi: 10.1016/j.otc.2008.01.008. Epub [PubMed PMID: 18436002]
Jandali D, Revenaugh PC. Facial reanimation: an update on nerve transfers in facial paralysis. Current opinion in otolaryngology & head and neck surgery. 2019 Aug:27(4):231-236. doi: 10.1097/MOO.0000000000000543. Epub [PubMed PMID: 31169528]
Level 3 (low-level) evidenceKochhar A, Albathi M, Sharon JD, Ishii LE, Byrne P, Boahene KD. Transposition of the Intratemporal Facial to Hypoglossal Nerve for Reanimation of the Paralyzed Face: The VII to XII TranspositionTechnique. JAMA facial plastic surgery. 2016 Sep 1:18(5):370-8. doi: 10.1001/jamafacial.2016.0514. Epub [PubMed PMID: 27348018]
Piedra Buena IT, Fichman M. Sural Nerve Graft. StatPearls. 2024 Jan:(): [PubMed PMID: 32491647]
Saha S, Pal S, Sengupta M, Chowdhury K, Saha VP, Mondal L. Identification of facial nerve during parotidectomy: a combined anatomical & surgical study. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2014 Jan:66(1):63-8. doi: 10.1007/s12070-013-0669-z. Epub 2013 Jul 24 [PubMed PMID: 24605304]
Khoa TD, Bac ND, Luong HV, Anh TN, Phuong NT, Nga VT, Dinh TC. Anatomical Characteristics of Facial Nerve Trunk in Vietnamese Adult Cadavers. Open access Macedonian journal of medical sciences. 2019 Dec 30:7(24):4230-4238. doi: 10.3889/oamjms.2019.366. Epub 2019 Oct 15 [PubMed PMID: 32215069]
Orgel MG. Epineurial versus perineurial repair of peripheral nerves. Clinics in plastic surgery. 1984 Jan:11(1):101-4 [PubMed PMID: 6368091]
Level 3 (low-level) evidenceCabaud HE, Rodkey WG, McCarroll HR Jr, Mutz SB, Niebauer JJ. Epineurial and perineurial fascicular nerve repairs: a critical comparison. The Journal of hand surgery. 1976 Sep:1(2):131-7 [PubMed PMID: 797698]
Level 3 (low-level) evidenceKnox CJ, Hohman MH, Kleiss IJ, Weinberg JS, Heaton JT, Hadlock TA. Facial nerve repair: fibrin adhesive coaptation versus epineurial suture repair in a rodent model. The Laryngoscope. 2013 Jul:123(7):1618-21. doi: 10.1002/lary.23885. Epub 2012 Nov 27 [PubMed PMID: 23188676]
Level 3 (low-level) evidenceVan Nest DS, Kahan DM, Ilyas AM. Polyethylene Glycol Fusion of Nerve Injuries: Review of the Technique and Clinical Applicability. Journal of hand and microsurgery. 2021 Apr:13(2):49-54. doi: 10.1055/s-0040-1718651. Epub 2020 Dec 10 [PubMed PMID: 33867761]
Peters BR, Wood MD, Hunter DA, Mackinnon SE. Acellular Nerve Allografts in Major Peripheral Nerve Repairs: An Analysis of Cases Presenting With Limited Recovery. Hand (New York, N.Y.). 2023 Mar:18(2):236-243. doi: 10.1177/15589447211003175. Epub 2021 Apr 21 [PubMed PMID: 33880944]
Level 3 (low-level) evidenceJowett N, Pineda Ii R. Acellular nerve allografts in corneal neurotisation: an inappropriate choice. The British journal of ophthalmology. 2020 Feb:104(2):149-150. doi: 10.1136/bjophthalmol-2019-315032. Epub 2019 Nov 12 [PubMed PMID: 31719110]
Altafulla J, Iwanaga J, Lachkar S, Prickett J, Dupont G, Yilmaz E, Ishak B, Litvack Z, Tubbs RS. The Great Auricular Nerve: Anatomical Study with Application to Nerve Grafting Procedures. World neurosurgery. 2019 May:125():e403-e407. doi: 10.1016/j.wneu.2019.01.087. Epub 2019 Jan 28 [PubMed PMID: 30703599]
Rinker B, Fink BF, Barry NG, Fife JA, Milan ME, Stoker AR, Nelson PT. The effect of cigarette smoking on functional recovery following peripheral nerve ischemia/reperfusion injury. Microsurgery. 2011 Jan:31(1):59-65. doi: 10.1002/micr.20820. Epub 2010 Dec 28 [PubMed PMID: 21207501]
Level 3 (low-level) evidenceMahakkanukrauh P, Chomsung R. Anatomical variations of the sural nerve. Clinical anatomy (New York, N.Y.). 2002 Jun:15(4):263-6 [PubMed PMID: 12112352]
Bhama PK, Weinberg JS, Lindsay RW, Hohman MH, Cheney ML, Hadlock TA. Objective outcomes analysis following microvascular gracilis transfer for facial reanimation: a review of 10 years' experience. JAMA facial plastic surgery. 2014 Mar-Apr:16(2):85-92. doi: 10.1001/jamafacial.2013.2463. Epub [PubMed PMID: 24481538]
Level 2 (mid-level) evidenceVincent AG, Bevans SE, Robitschek JM, Groom KL, Herr MW, Hohman MH. Sterno-omohyoid Free Flap for Dual-Vector Dynamic Facial Reanimation. The Annals of otology, rhinology, and laryngology. 2020 Feb:129(2):195-200. doi: 10.1177/0003489419875473. Epub 2019 Oct 3 [PubMed PMID: 31578078]
Frey M, Happak W, Girsch W, Bittner RE, Gruber H. Histomorphometric studies in patients with facial palsy treated by functional muscle transplantation: new aspects for the surgical concept. Annals of plastic surgery. 1991 Apr:26(4):370-9 [PubMed PMID: 1872543]
Terzis JK, Wang W, Zhao Y. Effect of axonal load on the functional and aesthetic outcomes of the cross-facial nerve graft procedure for facial reanimation. Plastic and reconstructive surgery. 2009 Nov:124(5):1499-1512. doi: 10.1097/PRS.0b013e3181babb93. Epub [PubMed PMID: 20009836]
Level 2 (mid-level) evidenceMackinnon SE, Dellon AL. Fascicular patterns of the hypoglossal nerve. Journal of reconstructive microsurgery. 1995 May:11(3):195-8 [PubMed PMID: 7650645]
Elkatatny AAAM, Abdallah HAA, Ghoraba D, Amer TA, Hamdy T. Hypoglossal Facial Nerve Anastomosis for Post-Operative and Post-Traumatic Complete Facial Nerve Paralysis. Open access Macedonian journal of medical sciences. 2019 Dec 15:7(23):3984-3996. doi: 10.3889/oamjms.2019.490. Epub 2019 Jul 29 [PubMed PMID: 32165940]
Borschel GH, Kawamura DH, Kasukurthi R, Hunter DA, Zuker RM, Woo AS. The motor nerve to the masseter muscle: an anatomic and histomorphometric study to facilitate its use in facial reanimation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012 Mar:65(3):363-6. doi: 10.1016/j.bjps.2011.09.026. Epub 2011 Oct 10 [PubMed PMID: 21992936]
Klebuc MJA. Facial reanimation using the masseter-to-facial nerve transfer. Plastic and reconstructive surgery. 2011 May:127(5):1909-1915. doi: 10.1097/PRS.0b013e31820e9138. Epub [PubMed PMID: 21532419]
Level 2 (mid-level) evidenceVincent AG, Bevans SE, Robitschek JM, Wind GG, Hohman MH. Masseteric-to-Facial Nerve Transfer and Selective Neurectomy for Rehabilitation of the Synkinetic Smile. JAMA facial plastic surgery. 2019 Dec 1:21(6):504-510. doi: 10.1001/jamafacial.2019.0689. Epub [PubMed PMID: 31465094]
Salmerón-González E, Simón-Sanz E, García-Vilariño E, Ruiz-Cases A. Masseter-to-facial nerve transfer: Technique and outcomes utilizing a fibrin sealant for coaptation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2018 Aug:71(8):1216-1230. doi: 10.1016/j.bjps.2018.04.004. Epub 2018 Apr 16 [PubMed PMID: 29699849]
Level 3 (low-level) evidenceCassoni A, Catalano C, Di Giorgio D, Raponi I, Di Brino M, Perotti S, Valentini V. Masseter-facial neurorrhaphy for facial palsy reanimation: What happens after masseter denervation? Histomorphometric and stomatognathic functional analysis. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2020 Jul:48(7):680-684. doi: 10.1016/j.jcms.2020.04.009. Epub 2020 Jun 2 [PubMed PMID: 32507669]
Sakthivel P, Singh CA, Thakar A, Thirumeni G, Raveendran S, Sharma SC. Masseteric-Facial Nerve Anastomosis: Surgical Techniques and Outcomes-A Pilot Indian study. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2020 Mar:72(1):92-97. doi: 10.1007/s12070-019-01758-z. Epub 2019 Nov 6 [PubMed PMID: 32158663]
Level 3 (low-level) evidenceMurphey AW, Clinkscales WB, Oyer SL. Masseteric Nerve Transfer for Facial Nerve Paralysis: A Systematic Review and Meta-analysis. JAMA facial plastic surgery. 2018 Mar 1:20(2):104-110. doi: 10.1001/jamafacial.2017.1780. Epub [PubMed PMID: 29222560]
Level 1 (high-level) evidenceHontanilla B, Cabello A. Spontaneity of smile after facial paralysis rehabilitation when using a non-facial donor nerve. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2016 Sep:44(9):1305-9. doi: 10.1016/j.jcms.2016.06.031. Epub 2016 Jul 9 [PubMed PMID: 27460946]
Gidley PW, Herrera SJ, Hanasono MM, Yu P, Skoracki R, Roberts DB, Weber RS. The impact of radiotherapy on facial nerve repair. The Laryngoscope. 2010 Oct:120(10):1985-9. doi: 10.1002/lary.21048. Epub [PubMed PMID: 20824641]
Level 2 (mid-level) evidenceLee RT, Oster MW, Balmaceda C, Hesdorffer CS, Vahdat LT, Papadopoulos KP. Bilateral facial nerve palsy secondary to the administration of high-dose paclitaxel. Annals of oncology : official journal of the European Society for Medical Oncology. 1999 Oct:10(10):1245-7 [PubMed PMID: 10586344]
Level 3 (low-level) evidencePourmomeny AA, Asadi S. Management of synkinesis and asymmetry in facial nerve palsy: a review article. Iranian journal of otorhinolaryngology. 2014 Oct:26(77):251-6 [PubMed PMID: 25320703]
Brown J, Hohman MH, Shermetaro C. Facial Nerve Intratemporal Trauma. StatPearls. 2024 Jan:(): [PubMed PMID: 30137848]
Jacobson J, Rihani J, Lin K, Miller PJ, Roland JT Jr. Outcomes of Direct Facial-to-Hypoglossal Neurorrhaphy with Parotid Release. Skull base : official journal of North American Skull Base Society ... [et al.]. 2011 Jan:21(1):7-12. doi: 10.1055/s-0030-1261263. Epub [PubMed PMID: 22451794]
Moraleda S, Hachoue Z, Abdel-Muti E, Ruiz G, Díez Sebastián J, Lassaletta L. [Satisfaction survey of patients with sequels of peripheral facial palsy treated with botulinum toxin A]. Rehabilitacion. 2020 Oct-Dec:54(4):254-259. doi: 10.1016/j.rh.2020.03.002. Epub 2020 May 4 [PubMed PMID: 32441261]
Level 3 (low-level) evidencevan Veen MM, Quatela O, Tavares-Brito J, Robinson M, Baiungo JH, Werker PMN, Dijkstra PU, Hadlock TA. Patient-perceived severity of synkinesis reduces quality of life in facial palsy: A cross-sectional analysis in 92 patients. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2019 May:44(3):483-486. doi: 10.1111/coa.13322. Epub 2019 Mar 25 [PubMed PMID: 30828959]
Level 2 (mid-level) evidenceHohman MH, Lee LN, Hadlock TA. Two-step highly selective neurectomy for refractory periocular synkinesis. The Laryngoscope. 2013 Jun:123(6):1385-8. doi: 10.1002/lary.23873. Epub 2013 Jan 11 [PubMed PMID: 23315713]
Level 2 (mid-level) evidenceHenstrom DK, Malo JS, Cheney ML, Hadlock TA. Platysmectomy: an effective intervention for facial synkinesis and hypertonicity. Archives of facial plastic surgery. 2011 Jul-Aug:13(4):239-43. doi: 10.1001/archfacial.2011.43. Epub [PubMed PMID: 21768558]
Mistry RK, Hohman MH, Al-Sayed AA. Facial Nerve Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 31971735]
Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Physical therapy. 2010 Mar:90(3):391-7. doi: 10.2522/ptj.20090176. Epub 2010 Jan 21 [PubMed PMID: 20093325]
Level 2 (mid-level) evidence