Introduction
The electron transport chain is a series of four protein complexes that couple redox reactions, creating an electrochemical gradient that leads to the creation of ATP in a complete system named oxidative phosphorylation. It occurs in mitochondria in both cellular respiration and in chloroplasts for photosynthesis. In the former, the electrons come from breaking down organic molecules, and energy is released. In the latter, the electrons enter the chain after being excited by light, and the energy released is used to build carbohydrates.
Fundamentals
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Fundamentals
Aerobic cellular respiration is made up of three parts: glycolysis, the citric acid (Krebs) cycle, and oxidative phosphorylation. In glycolysis, glucose metabolizes into two molecules of pyruvate, with an output of ATP and nicotinamide adenine dinucleotide (NADH). Each pyruvate oxidizes into acetyl CoA and an additional molecule of NADH and carbon dioxide (CO2). The acetyl CoA is then used in the citric acid cycle, which is a chain of chemical reactions that produce CO2, NADH, flavin adenine dinucleotide (FADH2), and ATP. In the final step, the three NADH and one FADH2 amassed from the previous steps are used in oxidative phosphorylation, to make water and ATP.
Oxidative phosphorylation has two parts: the electron transport chain (ETC) and chemiosmosis. The ETC is a collection of proteins bound to the inner mitochondrial membrane and organic molecules, which electrons pass through in a series of redox reactions, and release energy. The energy released forms a proton gradient, which is used in chemiosmosis to make a large amount of ATP by the protein ATP-synthase.
Photosynthesis is a metabolic process that converts light energy into chemical energy to build sugars. In the light-dependent reactions, light energy and water are used to make ATP, NADPH, and oxygen (O2). The proton gradient used to make the ATP forms via an electron transport chain. In the light-independent reactions, sugar is made from the ATP and NADPH from the previous reactions.
Cellular Level
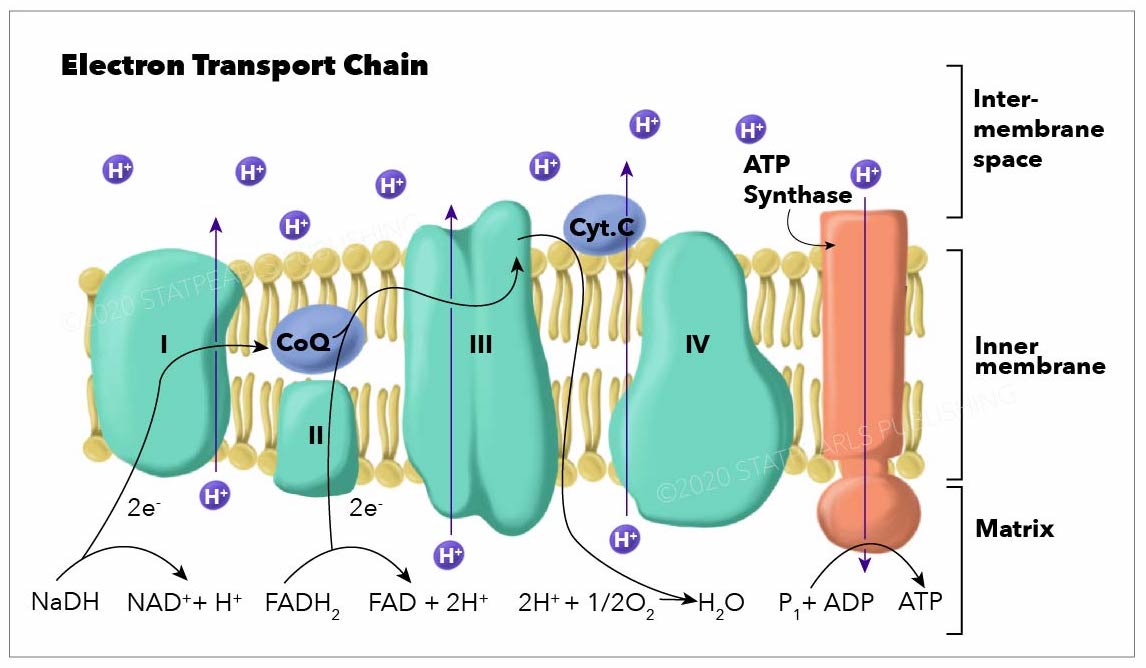
In the electron transport chain (ETC), the electrons go through a chain of proteins that increases its reduction potential and causes a release in energy. Most of this energy is dissipated as heat or utilized to pump hydrogen ions (H+) from the mitochondrial matrix to the intermembrane space and create a proton gradient. This gradient increases the acidity in the intermembrane space and creates an electrical difference with a positive charge outside and a negative charge inside. The ETC proteins in a general order are complex I, complex II, coenzyme Q, complex III, cytochrome C, and complex IV.
- Complex I, also known as ubiquinone oxidoreductase, is made up of NADH dehydrogenase, flavin mononucleotide (FMN), and eight iron-sulfur (Fe-S) clusters. The NADH donated from glycolysis, and the citric acid cycle is oxidized here, transferring 2 electrons from NADH to FMN. Then they are transferred to the Fe-S clusters and finally from Fe-S to coenzyme Q. During this process, 4 hydrogen ions pass from the mitochondrial matrix to the intermembrane space, contributing to the electrochemical gradient. Complex I may also play an important role in causing apoptosis in programmed cell death.[1][2][3][4][1]
- (NADH + H+) + CoQ + 4 H+(matrix) -> NAD+ + CoQH2 + 4 H+(intermembrane)
- Complex II, also known as succinate dehydrogenase, accepts electrons from succinate (an intermediate in the citric acid cycle) and acts as a second entry point to the ETC. When succinate oxidizes to fumarate, 2 electrons are accepted by FAD within complex II. FAD passes them to Fe-S clusters and then to coenzyme Q, similar to complex I. However; no protons are translocated across the membrane by complex II, therefore less ATP is produced with this pathway.[5][6]
- Succinate + FAD -> Fumarate + 2 H+(matrix) + FADH2
- FADH2 + CoQ -> FAD + CoQH2
- Glycerol-3-Phosphate dehydrogenase and Acyl-CoA dehydrogenase also accept electrons from glycerol-3-P and fatty acyl-CoA, respectively. Inclusion of these protein complexes allows for the donation to the ETC by cytosolic NADH (glycerol-3-P acts as a shuttle to regenerate cytosolic NAD from NADH) and fatty acids undergoing beta-oxidation within the mitochondria (acyl-CoA is oxidized to enoyl-CoA in the first step, producing FADH2).[7][8]
- Coenzyme Q, also known as ubiquinone (CoQ), is made up of quinone and a hydrophobic tail. Its purpose is to function as an electron carrier and transfer electrons to complex III. Coenzyme Q undergoes reduction to semiquinone (partially reduced, radical form CoQH-) and ubiquinol (fully reduced CoQH2) through the Q cycle. This process receives further elaboration under Complex III.
- Complex III, also known as cytochrome c reductase, is made up of cytochrome b, Rieske subunits (containing two Fe-S clusters), and cytochrome c proteins. A cytochrome is a protein involved in electron transfer that contains a heme group. The heme groups alternate between ferrous (Fe2+) and ferric (Fe3+) states during the electron transfer. Because cytochrome c can only accept a single electron at a time, this process occurs in two steps (the Q cycle), in contrast to the single-step complex I and II pathways. Complex III also releases 4 protons into the intermembrane space at the end of a full Q cycle, contributing to the gradient. Cytochrome c then transfers the electrons one at a time to complex IV.[9][10][11]
- Q Cycle:
- Step 1 in the Q cycle involves ubiquinol (CoQH2) and ubiquinone (CoQ) binding to two separate sites on complex III. CoQH2 transfers each electron to a different path. One electron goes to Fe-S and then cytochrome c, while the second electron is transferred to cytochrome b and then to CoQ bound at the other site. While this occurs, 2 H+ ions are released into the intermembrane space, contributing to the proton gradient. CoQH2 is now oxidized to ubiquinone and dissociates from the complex. The CoQ bound at the second site enters a transitional CoQH- radical state from accepting one of the electrons.
- The second step of the cycle involves a repeat of the first: a new CoQH2 binds to the first site and transfers two electrons like before (and 2 more H+ ions released). Again, one electron passes to cytochrome c and one to cytochrome b, which this time works to reduce CoQH- to CoQH2 before it dissociates from complex III and can be recycled. In this way, one full cycle appears as follows:[12]
- 2 CoQH2(site 1) + CoQ(site 2) + 2 Cyt c(ox) + 2 H+(matrix) -> 2 CoQ(site 1) + CoQH2(site 2) + 2 Cyt c(red) + 4 H+(intermembrane)
- Q Cycle:
- Complex IV, also known as cytochrome c oxidase, oxidizes cytochrome c and transfers the electrons to oxygen, the final electron carrier in aerobic cellular respiration. The cytochrome proteins a and a3, in addition to heme and copper groups in complex IV transfer the donated electrons to the bound dioxygen species, converting it into molecules of water. The free energy from the electron transfer causes 4 protons to move into the intermembrane space contributing to the proton gradient. Oxygen reduces via the following reaction:[13][14]
- 2 cytochrome c(red) + ½O2 + 4 H+(matrix) -> 2 cytochrome c(ox) + 1 H2O + 2 H+(intermembrane)
ATP synthase, also called complex V, uses the ETC generated proton gradient across the inner mitochondrial membrane to form ATP. ATP-synthase contains up of F0 and F1 subunits, which act as a rotational motor system. F0 is hydrophobic and embedded in the inner mitochondrial membrane. It contains a proton corridor that is protonated and deprotonated repeatedly as H+ ions flow down the gradient from intermembrane space to matrix. The alternating ionization of F0 causes rotation, which alters the orientation of the F1 subunits. F1 is hydrophilic and faces the mitochondrial matrix. Conformational changes in F1 subunits catalyze the formation of ATP from ADP and Pi. For every 4 H+ ions, 1 ATP is produced. ATP-synthase can also be forced to run in reverse, consuming ATP to produce a hydrogen gradient, as is seen in some bacteria.[15][16][17]
Molecular Level
Nicotinamide adenine dinucleotide has two forms: NAD+ (oxidized) and NADH (reduced). It is a dinucleotide connected by phosphate groups. One nucleoside has an adenine base and the other nicotinamide. When involved in metabolic redox reactions, the mechanism is as shown in Reaction 1.
- Reaction 1: RH2 + NAD+ -> R + H+ + NADH
R is the reactant, for example, sugar.
NADH enters the ETC at complex I and produces a total of 10 H+ ions through the ETC (4 from complex I, 4 from complex III, and 2 from complex IV). ATP-synthase synthesizes 1 ATP for 4 H+ ions. Therefore, 1 NADH = 10 H+, and 10/4 H+ per ATP = 2.5 ATP per NADH (**some sources round up**). When NADH is oxidized, it breaks into NAD+, H+, and 2 e- as shown in Reaction 2.
- Reaction 2: NADH -> H+ + NAD+ + 2 e-
Flavin adenine dinucleotide has 4 redox states, 3 of them being FAD (quinone, fully oxidized form), FADH- (semiquinone, partially oxidized), and FADH2 (hydroquinone, fully reduced). FAD is made up of an adenine nucleotide and a flavin mononucleotide (FMN), connected by phosphate groups. FMN is synthesized in part from vitamin B2 (riboflavin). FAD contains a highly stable aromatic ring, and FADH2 does not. When FADH2 oxidizes, it becomes aromatic and releases energy, as seen in Reaction 3. This state makes FAD a potent oxidizing agent, with an even more positive reduction potential than NAD. FADH2 enters the ETC at complex II and creates a total of 1.5 ATP (4 H+ from complex III, and 2 H+ from complex IV; 6/4 H+ per ATP = 1.5 ATP per FADH2 **some sources round up**).[18]
- Reaction 3: FADH2 -> FAD + 2 H+ + 2 e-
FAD also functions in several metabolic pathways outside of the ETC, including DNA repair (MTHF repair of UV damage), fatty acid beta-oxidation (acyl-CoA dehydrogenase), and synthesis of coenzymes (CoA, CoQ, heme).
Clinical Significance
Uncoupling Agents
An uncoupling agent dissociates the electron transport chain from phosphorylation by ATP-synthase, preventing the formation of ATP. Disruption of the phospholipid bilayer of membranes causes a fluid-like and disorganized state, which allows protons to flow through more freely. This proton leak weakens the electrochemical gradient, while also transferring protons without the use of ATP-synthase such that no ATP is produced.
While the cell becomes starved of ATP, the ETC will overwork in an attempt to shuttle more and more electrons to ATP-synthase without success. The ETC regularly produces heat as the electrons transfer from one carrier to the next, and this overactivity will raise the body temperature as a result. Additionally, cells will adapt to utilizing fermentation as if in anaerobic conditions; this may cause a type B lactic acidosis in affected patients.[19]
Aspirin (Salicylic Acid)
- Salicylic acid is an uncoupler. Unique to salicylate poisoning, however, are signs of tinnitus and early respiratory alkalosis, which transitions to a mixed metabolic acidosis and respiratory alkalosis as the process progresses. Early treatment involves activated charcoal if presenting within 1 hour of ingestion, or sodium bicarbonate otherwise.[20]
Thermogenin
- Thermogenin, also known as uncoupling protein 1 (UCP1), is found in brown adipose tissue. Brown adipose tissue has many small lipid droplets and a high concentration of mitochondria (which provide the "brown" color), in contrast to white adipose tissue, which has a single droplet. This difference supports that brown fat is classically abundantly present in hibernating animals or newborns, who have delayed neurologic thermoregulation (ex. shivering) and are therefore at risk for hypothermia. These brown fat mitochondria contain more thermogenin than other cells, allowing for increased inner mitochondrial membrane disruption and proton leakage. [21][22]
Oxidative Phosphorylation Inhibitors
Certain poisons can inhibit cellular oxidative phosphorylation such as rotenone, carboxin, antimycin A, cyanide, carbon monoxide (CO), sodium azide, and oligomycin. Rotenone inhibits complex I, carboxin inhibits complex II, antimycin A inhibits complex III, and cyanide and CO inhibit complex IV. Oligomycin inhibits ATP synthase.[23][24]
Rotenone (and some barbiturates) – inhibits complex I (coenzyme Q binding site)
- Rotenone is a broadly used pesticide, but more often in the US as a piscicide (fish). Rotenone blocks complex I from passing electrons from the Fe-S clusters to ubiquinone. It is poorly absorbed through the skin, but rarely deadly as poisoning can cause vomiting and removal of the substance. However, purposeful ingestion can be fatal. [25][26]
Carboxin – inhibits complex II (coenzyme Q binding site)
- Carboxin is a fungicide that is no longer in use because of newer, more broad-spectrum agents. Similar to rotenone, carboxin interferes with ubiquinone at the binding site.
Doxorubicin – coenzyme Q (theoretical)
- Doxorubicin is used in cancer chemotherapy, typically breast and bladder carcinomas, and lymphoma. A well-known side effect of doxorubicin is dilated cardiomyopathy. One proposed mechanism of causation is the generation of reactive oxygen species within myocardial tissue as the drug interferes with electron transfer by coenzyme Q. [27]
Antimycin A – inhibits complex III (cytochrome c reductase)
- Antimycin A is a piscicide that binds to cytochrome c reductase at the Qi binding site. This activity prevents ubiquinone from binding and accepting an electron, thereby blocking the recycling of ubiquinol (CoQH2) by the Q cycle.
Carbon Monoxide (CO) – inhibits complex IV (cytochrome c oxidase)
- Carbon monoxide binds to and inhibits cytochrome c oxidase (complex IV). In addition to the disruption of the ETC, carbon monoxide also binds to hemoglobin at an oxygen-binding site converting it to carboxyhemoglobin. In this state, oxygen is displaced from hemoglobin, effectively blocking delivery to body tissues. The cardiac and central nervous systems, both organ systems which are highly dependent on oxygen consumption, manifest the common signs of CO poisoning. Symptoms such as tachycardia, hypotension, or arrhythmias may couple with fatigue, headache, nausea, vomiting, and changes in vision. More serious cases may display seizure, coma, retinal hemorrhages, or a characteristic cherry-red blood hue of the skin, though more often useful on autopsy (caution is critical: some patients may appear "normal" rather than pale/dusky because of inadequate tissue oxygenation).[28]
- Sources of CO are paint strippers, house fires, wood-burning stoves, automobile exhaust, and other gasoline- or propane-fueled equipment. A CO saturation monitor can detect CO levels. Ratios of carboxyhemoglobin to hemoglobin greater than 10% are likely to show as symptomatic. Regular pulse oximetry devices read the percent of bound hemoglobin, irrespective of what is bound. Therefore, when CO is bound rather than O2, a patient's pulse Ox may still appear normal and cannot be used reliably. Instead, a co-oximeter should be used. Treatment for CO poisoning is to dissociate the bound CO with O2. Providing 100% supplemental oxygen via non-rebreather or administering hyperbaric oxygen are options.[29][30][31]
Cyanide (CN) – inhibits complex IV (cytochrome c oxidase)
- Cyanide also binds to and inhibits cytochrome c oxidase (complex IV). Similar symptoms as a result of tissue hypoxia can present in affected patients. In contrast, these patients tend to have hypoxia that is not responsive to supplemental O2 and an almond breath odor. Typical sources of cyanide include house fires (furniture or rugs), jewelry cleaning solutions, plastic or rubber manufacturing, iatrogenic from prescribed nitroprusside, or even some fruit seeds (apricots, peaches, apples).
- Treatment can include nitrites to oxidize hemoglobin iron from Fe2+ to Fe3+, also known as methemoglobin, a conformation that binds cyanide, preventing it from contacting the ETC. However, this prevents blood cells from transporting oxygen, therefore requiring further treatment with methylene blue to reduce Fe3+ back to Fe2+. Another option is administering hydroxocobalamin, a form of vitamin B12, or thiosulfate, although thiosulfate is not time efficient and typically requires combination therapy with nitrites.[32]
Oligomycin – inhibits ATP-synthase (complex V)
- Oligomycin is a macrolide antibiotic synthesized by Streptomyces species that inhibits the F0 subunit of ATP-synthase, preventing ATP production. Its predominant use is for research purposes.[33]
Media
(Click Image to Enlarge)
References
Lencina AM, Franza T, Sullivan MJ, Ulett GC, Ipe DS, Gaudu P, Gennis RB, Schurig-Briccio LA. Type 2 NADH Dehydrogenase Is the Only Point of Entry for Electrons into the Streptococcus agalactiae Respiratory Chain and Is a Potential Drug Target. mBio. 2018 Jul 3:9(4):. doi: 10.1128/mBio.01034-18. Epub 2018 Jul 3 [PubMed PMID: 29970468]
Hirst J. Towards the molecular mechanism of respiratory complex I. The Biochemical journal. 2009 Dec 23:425(2):327-39. doi: 10.1042/BJ20091382. Epub 2009 Dec 23 [PubMed PMID: 20025615]
Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science (New York, N.Y.). 2006 Mar 10:311(5766):1430-6 [PubMed PMID: 16469879]
Level 3 (low-level) evidenceHirst J. Energy transduction by respiratory complex I--an evaluation of current knowledge. Biochemical Society transactions. 2005 Jun:33(Pt 3):525-9 [PubMed PMID: 15916556]
Level 3 (low-level) evidenceYankovskaya V, Horsefield R, Törnroth S, Luna-Chavez C, Miyoshi H, Léger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science (New York, N.Y.). 2003 Jan 31:299(5607):700-4 [PubMed PMID: 12560550]
Horsefield R, Iwata S, Byrne B. Complex II from a structural perspective. Current protein & peptide science. 2004 Apr:5(2):107-18 [PubMed PMID: 15078221]
Level 3 (low-level) evidenceGeertman JM, van Maris AJ, van Dijken JP, Pronk JT. Physiological and genetic engineering of cytosolic redox metabolism in Saccharomyces cerevisiae for improved glycerol production. Metabolic engineering. 2006 Nov:8(6):532-42 [PubMed PMID: 16891140]
Thorpe C, Kim JJ. Structure and mechanism of action of the acyl-CoA dehydrogenases. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995 Jun:9(9):718-25 [PubMed PMID: 7601336]
Level 3 (low-level) evidenceSun C, Benlekbir S, Venkatakrishnan P, Wang Y, Hong S, Hosler J, Tajkhorshid E, Rubinstein JL, Gennis RB. Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature. 2018 May:557(7703):123-126. doi: 10.1038/s41586-018-0061-y. Epub 2018 Apr 25 [PubMed PMID: 29695868]
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science (New York, N.Y.). 1998 Jul 3:281(5373):64-71 [PubMed PMID: 9651245]
Level 3 (low-level) evidenceTrumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. The Journal of biological chemistry. 1990 Jul 15:265(20):11409-12 [PubMed PMID: 2164001]
Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS letters. 2003 Jun 12:545(1):39-46 [PubMed PMID: 12788490]
Level 3 (low-level) evidenceCalhoun MW, Thomas JW, Gennis RB. The cytochrome oxidase superfamily of redox-driven proton pumps. Trends in biochemical sciences. 1994 Aug:19(8):325-30 [PubMed PMID: 7940677]
Level 3 (low-level) evidenceSchmidt-Rohr K. Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics. ACS omega. 2020 Feb 11:5(5):2221-2233. doi: 10.1021/acsomega.9b03352. Epub 2020 Jan 28 [PubMed PMID: 32064383]
Lovero D, Giordano L, Marsano RM, Sanchez-Martinez A, Boukhatmi H, Drechsler M, Oliva M, Whitworth AJ, Porcelli D, Caggese C. Characterization of Drosophila ATPsynC mutants as a new model of mitochondrial ATP synthase disorders. PloS one. 2018:13(8):e0201811. doi: 10.1371/journal.pone.0201811. Epub 2018 Aug 10 [PubMed PMID: 30096161]
Okuno D, Iino R, Noji H. Rotation and structure of FoF1-ATP synthase. Journal of biochemistry. 2011 Jun:149(6):655-64. doi: 10.1093/jb/mvr049. Epub 2011 Apr 26 [PubMed PMID: 21524994]
Level 3 (low-level) evidenceJunge W, Nelson N. ATP synthase. Annual review of biochemistry. 2015:84():631-57. doi: 10.1146/annurev-biochem-060614-034124. Epub 2015 Mar 23 [PubMed PMID: 25839341]
Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochimica et biophysica acta. 2005 Jan 7:1706(1-2):1-11 [PubMed PMID: 15620362]
Barrett MA, Zheng S, Roshankar G, Alsop RJ, Belanger RK, Huynh C, Kučerka N, Rheinstädter MC. Interaction of aspirin (acetylsalicylic acid) with lipid membranes. PloS one. 2012:7(4):e34357. doi: 10.1371/journal.pone.0034357. Epub 2012 Apr 17 [PubMed PMID: 22529913]
Warrick BJ, King A, Smolinske S, Thomas R, Aaron C. A 29-year analysis of acute peak salicylate concentrations in fatalities reported to United States poison centers. Clinical toxicology (Philadelphia, Pa.). 2018 Sep:56(9):846-851. doi: 10.1080/15563650.2018.1435887. Epub 2018 Feb 12 [PubMed PMID: 29431532]
Cinti S. The adipose organ. Prostaglandins, leukotrienes, and essential fatty acids. 2005 Jul:73(1):9-15 [PubMed PMID: 15936182]
Level 3 (low-level) evidenceEnerbäck S. The origins of brown adipose tissue. The New England journal of medicine. 2009 May 7:360(19):2021-3. doi: 10.1056/NEJMcibr0809610. Epub [PubMed PMID: 19420373]
Level 3 (low-level) evidenceZhou W, Faraldo-Gómez JD. Membrane plasticity facilitates recognition of the inhibitor oligomycin by the mitochondrial ATP synthase rotor. Biochimica et biophysica acta. Bioenergetics. 2018 Sep:1859(9):789-796. doi: 10.1016/j.bbabio.2018.03.019. Epub 2018 Apr 7 [PubMed PMID: 29630891]
Kamalian L, Douglas O, Jolly CE, Snoeys J, Simic D, Monshouwer M, Williams DP, Kevin Park B, Chadwick AE. The utility of HepaRG cells for bioenergetic investigation and detection of drug-induced mitochondrial toxicity. Toxicology in vitro : an international journal published in association with BIBRA. 2018 Dec:53():136-147. doi: 10.1016/j.tiv.2018.08.001. Epub 2018 Aug 7 [PubMed PMID: 30096366]
Wood DM, Alsahaf H, Streete P, Dargan PI, Jones AL. Fatality after deliberate ingestion of the pesticide rotenone: a case report. Critical care (London, England). 2005 Jun:9(3):R280-4 [PubMed PMID: 15987402]
Level 3 (low-level) evidenceLupescu A, Jilani K, Zbidah M, Lang F. Induction of apoptotic erythrocyte death by rotenone. Toxicology. 2012 Oct 28:300(3):132-7. doi: 10.1016/j.tox.2012.06.007. Epub 2012 Jun 19 [PubMed PMID: 22727881]
Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacology & toxicology. 2003 Sep:93(3):105-15 [PubMed PMID: 12969434]
Weaver LK. Clinical practice. Carbon monoxide poisoning. The New England journal of medicine. 2009 Mar 19:360(12):1217-25. doi: 10.1056/NEJMcp0808891. Epub [PubMed PMID: 19297574]
Sato K, Tamaki K, Hattori H, Moore CM, Tsutsumi H, Okajima H, Katsumata Y. Determination of total hemoglobin in forensic blood samples with special reference to carboxyhemoglobin analysis. Forensic science international. 1990 Nov:48(1):89-96 [PubMed PMID: 2279722]
Barker SJ, Tremper KK. The effect of carbon monoxide inhalation on pulse oximetry and transcutaneous PO2. Anesthesiology. 1987 May:66(5):677-9 [PubMed PMID: 3578881]
Level 3 (low-level) evidenceRaub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide poisoning--a public health perspective. Toxicology. 2000 Apr 7:145(1):1-14 [PubMed PMID: 10771127]
Level 3 (low-level) evidenceJensen P, Wilson MT, Aasa R, Malmström BG. Cyanide inhibition of cytochrome c oxidase. A rapid-freeze e.p.r. investigation. The Biochemical journal. 1984 Dec 15:224(3):829-37 [PubMed PMID: 6098268]
Shchepina LA, Pletjushkina OY, Avetisyan AV, Bakeeva LE, Fetisova EK, Izyumov DS, Saprunova VB, Vyssokikh MY, Chernyak BV, Skulachev VP. Oligomycin, inhibitor of the F0 part of H+-ATP-synthase, suppresses the TNF-induced apoptosis. Oncogene. 2002 Nov 21:21(53):8149-57 [PubMed PMID: 12444550]