Introduction
Electroencephalography (EEG) was first used in humans by Hans Berger in 1924. The first report was published in 1929. It is a tracing of voltage fluctuations versus time recorded from multiple electrodes placed over the scalp in a specific pattern to sample different cortical regions. It represents fluctuating dendritic potentials from superficial cortical layers, which are recorded in an organized array pattern and require voltage amplification to be captured. Deep electrical activity of the brain is not well sampled in an EEG using extracranial electrode monitoring.
Abnormal waveforms seen in an EEG recording include epileptiform and non-epileptiform abnormalities. In order to identify abnormal waveforms in EEG, the reader should have a basic understanding of the normal EEG pattern in various physiological states in children and adults. The electroencephalographer is expected to have the significant skills to recognize artifacts, and also an understanding of normal, benign variants. This article reviews the abnormal waveforms in EEG recordings.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
EEG has many potential uses:
- To distinguish epileptic seizures from psychogenic non-epileptic seizures, syncope (fainting), sub-cortical movement disorders, and migraine variants
- To differentiate encephalopathy from psychiatric syndromes like catatonia
- To provide ancillary brain death testing
- To determine whether to wean anti-epileptic medications
- To characterize seizures to determine the most appropriate anti-epileptic medication
- To localize the region of the brain from which a seizure originates for workup of possible epilepsy surgery
Issues of Concern
Even normal EEG waveforms can be considered potentially abnormal, depending upon various factors. For example, alpha waves are seen over the posterior head regions in a normal awake person and considered as the posterior background rhythm. However, in certain comatose states, there can be diffuse alpha activity (alpha comma) and may be considered pathognomonic. Delta waves can be seen in drowsiness and also in very young children; however, the appearance of focal delta activity can be abnormal (see below). Beta activity is present in the frontal regions of the brain and can spread posteriorly in early sleep. Focal beta activity sometimes seen in structural lesions and also in various epilepsies (generalized fast activity/GFA). Medications like sedatives (phenobarbital, benzodiazepines) commonly cause diffuse beta activity.
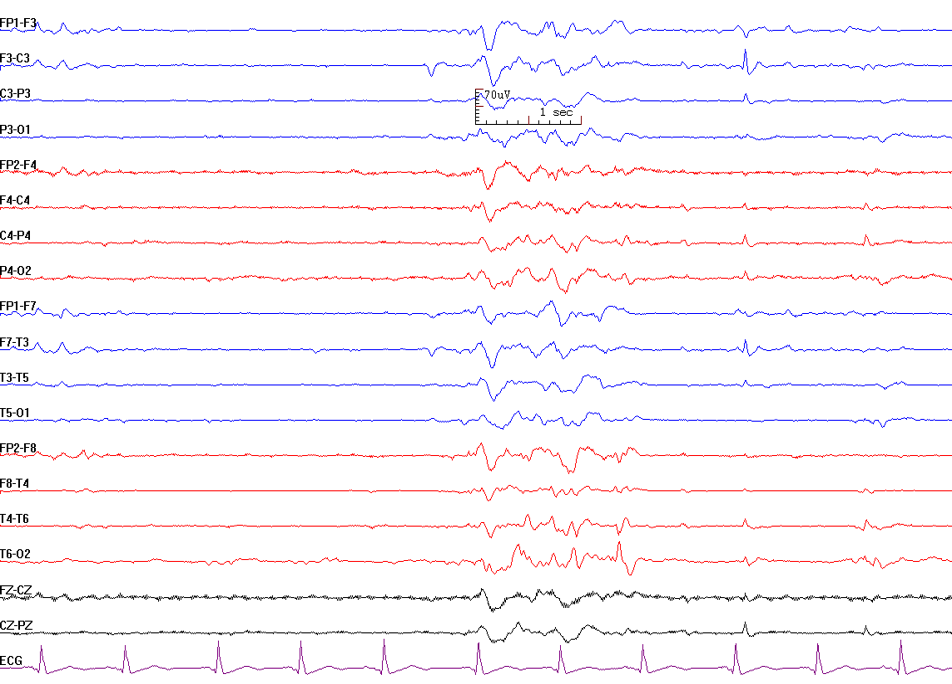
Triphasic waves: Triphasic waves were initially described in 1950 by Foley, and in 1955 Bickford and Butt gave it the name. Triphasic waves were first believed to be pathognomic of hepatic encephalopathy. However, these are nonspecific and can be seen in any metabolic encephalopathy. They are high amplitude sharp waves, with the duration of each phase longer than the next. They are sharply contoured with three phases. The first phase is always negative, hence the name triphasic waves. Triphasic waves are seen diffusely with bifrontal predominance and are synchronous. They are not seen in an awake state. They are seen in patients with altered levels of consciousness. It is hypothesized that they occur due to structural or metabolic abnormalities at the thalamocortical levels due to the changes in the thalamocortical relays.[1][2][3]
Interictal Epileptiform Discharges (IED)
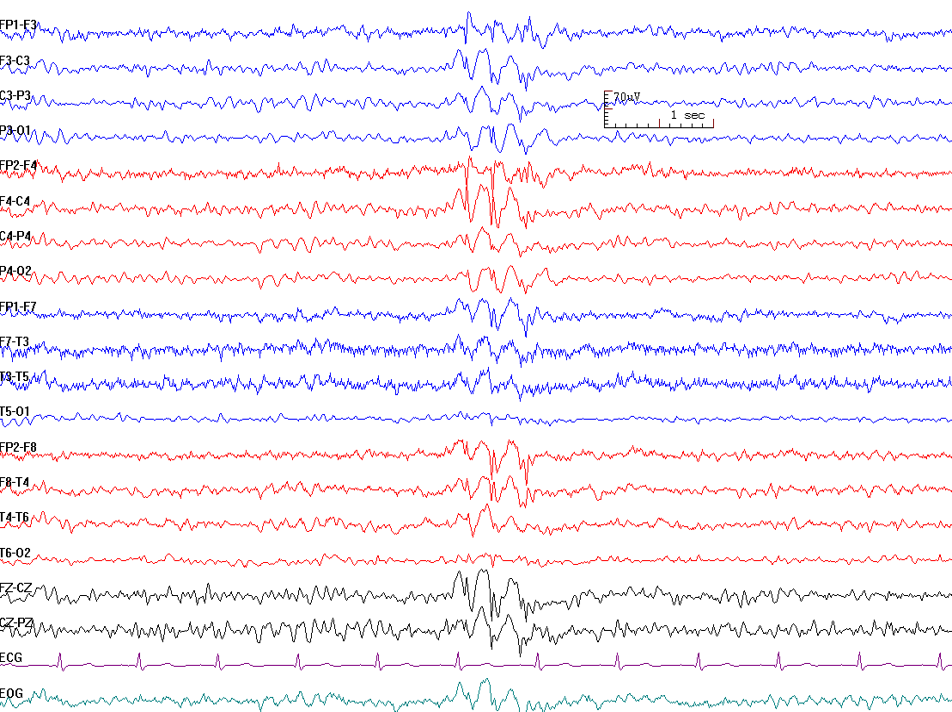
Interictal epileptiform discharge is an abnormal synchronous electrical discharge generated by a group of neurons in the region of the epileptic focus.[4] They represent the epileptic focus in patients with seizures. They have a low sensitivity in routine 30 minute EEG recording, and the yield increases with repeat EEG and prolonged EEG recordings. The presence of IED in a routine EEG in children with a new-onset seizure is 18% to 56%, while in adults, it is 12% to 50%.[5] Though uncommon, they can occur in healthy persons without a history of seizures.[6] IEDs can be subdivided into spikes or sharps.
- Spike and wave: Spikes are very short in duration, with a sharp-pointed peak duration of 20 to 70 milliseconds. A spike is followed by a wave component, and this is generated by GABA-b mediated currents.[7]
- Sharps: Sharps are longer in duration than a spike and last 70 to 200 milliseconds.
The following patterns of interictal epileptiform discharges may be seen:
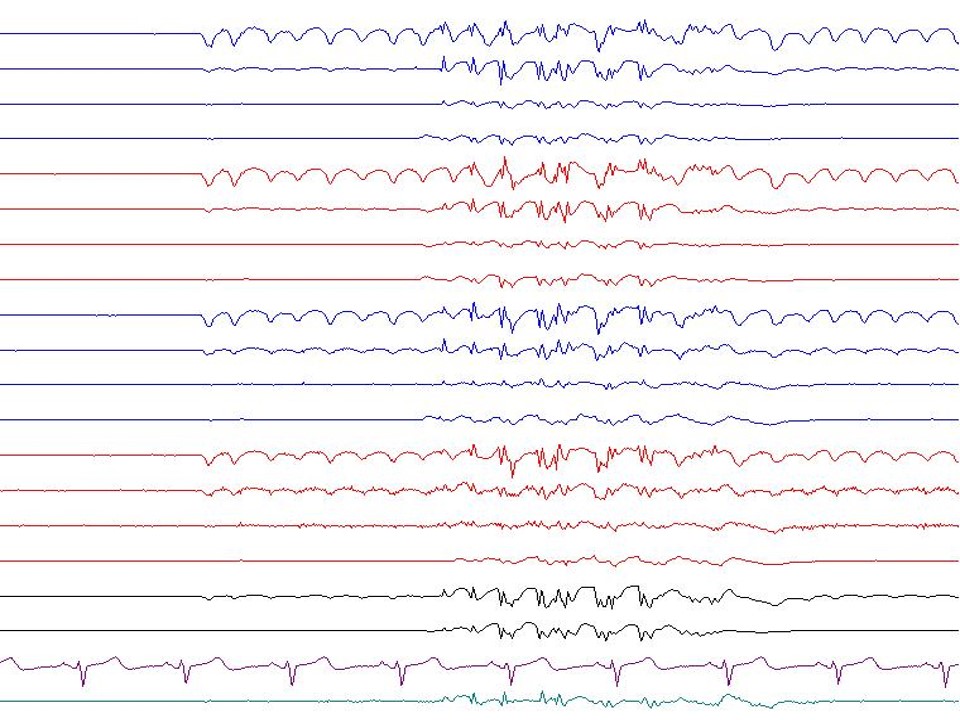
- 3 Hz and spike-wave: These are typical for absence seizures but can also occur in other types of generalized seizures. The waking background EEG activity is normal. The spike-and-wave is a bi-synchronous, symmetric discharge of sudden onset and resolution with a frequency of 3.5 Hz to 4 Hz at the onset, slowing to 2.5 Hz to 3 Hz at resolution. The greatest amplitude is at the superior frontal electrodes. The EEG discharges are reactive and inhibited by eye-opening and alertness. Hyperventilation and hypoglycemia readily activate them. While they are felt to be subclinical, response testing may demonstrate a subtle decline in maximal alertness.[8] These occur secondary to thalamocortical oscillations, which is the same mechanism that results in sleep spindles.[9]
- Centro-temporal spikes/ Rolandic spikes: These are seen in benign focal epilepsy of childhood with centrotemporal spikes (BECTS). Epileptic spikes characterized by horizontal dipoles are common and usually have maximal negativity in the centrotemporal area and positivity in the frontal area. The EEG discharges may be unilateral, bilateral, or have shifting laterality and often asynchronous between the hemispheres. Hyperventilation and photic stimulation do not affect the EEG discharges, drowsiness and sleep activate these spikes.[10] More than 1 seizure focus may be noted, and occasionally, the spike shifts its location toward or away from the centrotemporal area.[11] Seizures are usually brief focal and also secondarily generalized tonic-clonic seizures and seen in sleep, and infrequently during wakefulness.
- Epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS): Continuous spike and wave activity is seen during sleep. This can be seen in many different seizure subtypes and epilepsy syndromes. It can be caused by structural abnormalities of the brain, genetic abnormalities, and metabolic derangements.[12]
- Slow spike and waves: These bilaterally synchronous discharges occur in the symptomatic generalized epilepsies and are the typical EEG feature of children with Lennox–Gastaut syndrome(LGS). The frequency of these discharges is commonly in the range of 1 Hz to 2.5 Hz. Slow spike-and-wave may evolve from a previously normal EEG or patterns of hypsarrhythmia (seen in infantile spasms) or multiple independent sharp-wave foci. The waking background shows generalized slowing. There can be augmentation in sleep to electrical status epilepticus (ESES).[13] The spikes have an amplitude emphasis in the frontal and temporal regions.[14]
- Poly spike and waves: A complex of repetitive spikes is noted, followed by a wave component. These are seen in generalized epilepsy and less commonly in focal epilepsy. Generalized polyspikes and waves are commonly seen in myoclonic epilepsy. Examples of myoclonic epilepsy include Juvenile myoclonic epilepsy and progressive myoclonic epilepsy. Polyspike and wave discharges have a frequency ranging from 3.5 Hz to 5 Hz and termed fast spikes and waves. They show a bifrontal predominance. Myoclonic epilepsy predominantly involves the upper extremities, though it can involve the lower extremities. Photic stimulation often activates these discharges.
- Generalized spike and waves: Single spike is noted, followed by a wave component. These are seen in primary generalized epilepsy. When they occur in idiopathic generalized epilepsy, they occur with a normal background, and other epileptiform abnormalities are not seen.
- Lateralized periodic discharges (LPDs or PLEDs): LPDs are repetitive focal discharges that occur at regular intervals. LPDs can be seen with focal structural lesions (usually acute) and after the resolution of partial-onset status epilepticus.[15] There is no defined morphology for LPDs, and they can be present as spikes, shapes, polyspikes, and waves, etc., Herpes simplex encephalitis is classically described to have temporal LPDs. Other conditions that can cause LPDs are brain infections, tumors, Creutzfeldt-Jacob disease, and other conditions that cause acute brain injuries like subarachnoid hemorrhage, stroke, or traumatic brain injury.[16]
- Bilateral independent periodic discharges (BIPDs/ BiPLEDs): BIPDs are LPDs that occur from 2 different locations, each from different cerebral hemispheres. The 2 LPDs are independent and not synchronous and may occur at different frequencies.
- Generalized periodic discharges (GPDs): GPDs are synchronous, repetitive discharges that occur at regular intervals. The inter-discharge intervals are usually quantifiable. The morphology of each discharge is similar. They can be seen in multiple conditions, including anoxic brain injury, hypothermia, during or after the resolution of status epilepticus, infectious/toxic/metabolic encephalopathy, etc.[17] They occur secondary to disruption of thalamocortical pathways.[18] Prognosis is often guarded, but this is ultimately dependent on the underlying etiology. They can be seen with nonconvulsive status epilepticus, but they do not represent status epilepticus by themselves.
- SREDA (Subclinical EEG discharges of adults): This is a rarely seen pattern some consider as a benign variant but is generally considered epileptiform. This has been reported in children. The appearance can mimic an electrographic seizure as there will be a sudden evolution of high voltage generalized fast (5 Hz to 6 Hz)spike and wave activity and can occur in a recurrent pattern.[19]
- Brief (potentially ictal) rhythmic epileptiform discharges B(i)RDs/ BERDs: This is rare and mostly described in critically ill patients and neonates. The discharges can be sudden runs of sharply contoured theta activity lasting up to 3 seconds. This can be related to epileptogenic foci in refractory epilepsy and also sites of cerebral injury in critically ill patients.[20]
Non-epileptiform Abnormalities
- Slowing: Slowing in the EEG indicates cerebral dysfunction. Slowing can be described as 'polymorphic' based upon the shape of waveforms, and 'rhythmic' based upon the frequency. It is generally accepted that polymorphic slowing is seen in structural dysfunction, and rhythmic slowing may be much more indicative of underlying epileptiform dysfunction. Slowing can be either diffuse or focal, depending on the location or extent of the brain involved.[21]
- Diffuse slowing: Diffuse slowing indicates global cerebral dysfunction. The slowing can be in the theta or delta ranges. The slowing can be high or low amplitude. Several etiologies can cause diffuse slowing, including sedative medications, metabolic encephalopathy, toxic encephalopathy, cerebral infections like meningoencephalitis, or deep midline brainstem structural lesions.
- Focal slowing: Focal slowing indicates focal cerebral dysfunction. This can be continuous or intermittent.
- Continuous focal slowing is often indicative of structural abnormalities and can be seen in conditions like brain tumors, stroke, traumatic brain injury, intracerebral hemorrhage, etc.,
- Intermittent focal slowing can be of the following types based on the location of the slowing:
- Frontal intermittent rhythmic delta activity (FIRDA)
- Occipital intermittent rhythmic delta activity (OIRDA)
- Temporal intermittent rhythmic delta activity (TIRDA)
- These are considered to be patterns seen in patients with epilepsy.
Other Diffuse or Focal Abnormal Patterns in EEG
- Electrocerebral inactivity (ECI): In ECI, no detectable EEG activity is noted at a sensitivity of 2 microvolts. Electrocerebral inactivity can be used as a supportive test in the diagnosis of brain death. It is not specific to brain death and can be seen with deep sedation and severe hypothermia and some metabolic disorders. When performing recording as an ancillary test to determine brain death, certain criteria need to be met, which include 30 minutes of good quality EEG, a complete set of scalp electrodes must be used with the interelectrode impedances between 100 to 10,000 Ohms. Interelectrode distance must be at least 10cms.[22]
- Burst suppression pattern: Burst suppression is characterized by brief bursts of electrographic activity. The bursts may be sharp waves, spikes, or slow waves. The bursts are seen intermittently in a background of isoelectric EEG. It represents a state of cortical hyperexcitability due to compromised inhibition.[23] They can be seen as a medication effect of sedative drugs, hypothermia, metabolic disorders, and anoxic brain injury from cardiac arrest. Further deepening of coma from burst suppression results in severe low amplitude slowing with no reactivity, the EEG appears relatively flat.[24] Burst suppression is often medically induced in the medical management of refractory status epilepticus. The goal is to keep the bursts to 1 per page or less. Myoclonic jerks may be seen accompanying the bursts in anoxic brain injury.[25]
- Breach rhythm: This does not in itself mean any electrical or structural abnormality, but rather a focal abnormal morphology and change in voltage seen over areas of cranial or scalp defects. This is related to decreased impedance in capturing the signal from the cortex, where the overlying bone or tissue is lacking.
Clinical Significance
Understanding abnormal EEG waveforms and differentiating them from normal EEG variations is very important. A normal EEG does not rule out epilepsy, as the sensitivity of an EEG to identify epilepsy is less than 50%. Further, it is also important to understand that even healthy volunteers may have interictal discharges and other EEG abnormalities. Hence unneeded EEG testing can lead to unnecessary and erroneous diagnoses and cause potential harm from treatments if not interpreted properly.
Furthermore, abnormalities like breach rhythm (normal rhythm seen with skull defects) can have focal, sharply contoured morphology.[26] While several EEG characteristics differentiate breach rhythm from epileptiform abnormalities, clinical information of a prior craniotomy or magnetic resonance imaging (MRI) abnormalities showing a skull defect can help with accurate interpretation. Hence, the abnormalities noted on the EEG must always be clinically correlated.
Enhancing Healthcare Team Outcomes
An interprofessional team approach involving EEG technicians, nurses, and physicians will provide the best care for patients with abnormal EEGs. Education of the caregivers and health professionals managing patients who have an abnormal EEG is important. Adequate training in the interpretation of EEG reports and abnormal waveforms will help the clinical team to provide optimal care for the patient. [Level 5]
Media
(Click Image to Enlarge)
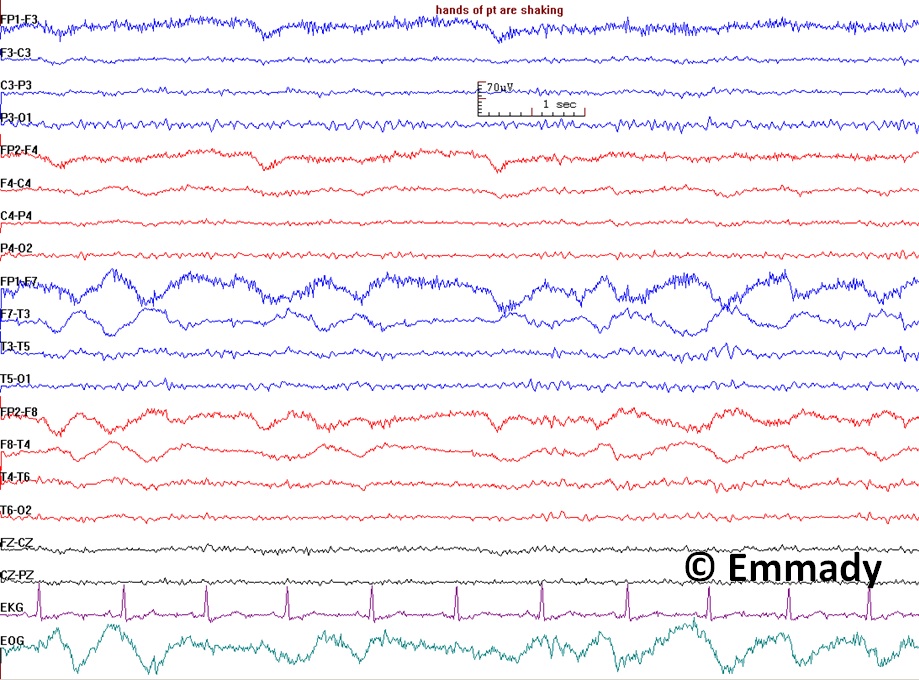
Electroencephalogram Pattern for a Nonepileptic Seizure. This electroencephalogram recording demonstrates features associated with nonepileptic seizures, including handshaking and lateral eye movements. The absence of epileptiform discharges on the EEG, coupled with these physiological movements, suggests a nonepileptic event rather than a seizure originating from epileptic activity. Clinical correlation is essential for correct diagnosis and appropriate management.
Contributed by Prabhu Emmady, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Brigo F, Storti M. Triphasic waves. American journal of electroneurodiagnostic technology. 2011 Mar:51(1):16-25 [PubMed PMID: 21516927]
Van Zandycke M, Orban LC, Vander Eecken HV. [Occurrence of triphasic waves in two cases of thyrotoxic crisis (author's transl)]. Acta neurologica Belgica. 1977 Mar-Apr:77(2):115-20 [PubMed PMID: 868471]
Level 3 (low-level) evidenceBICKFORD RG, BUTT HR. Hepatic coma: the electroencephalographic pattern. The Journal of clinical investigation. 1955 Jun:34(6):790-9 [PubMed PMID: 14381508]
Zacharaki EI, Mporas I, Garganis K, Megalooikonomou V. Spike pattern recognition by supervised classification in low dimensional embedding space. Brain informatics. 2016 Jun:3(2):73-83 [PubMed PMID: 27747608]
Wirrell EC. Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010 Aug:27(4):239-48. doi: 10.1097/WNP.0b013e3181ea4288. Epub [PubMed PMID: 20634717]
So EL. Interictal epileptiform discharges in persons without a history of seizures: what do they mean? Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010 Aug:27(4):229-38. doi: 10.1097/WNP.0b013e3181ea42a4. Epub [PubMed PMID: 20634716]
Destexhe A. Spike-and-wave oscillations based on the properties of GABAB receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998 Nov 1:18(21):9099-111 [PubMed PMID: 9787013]
Albuja AC, Ighodaro ET, Khan GQ. Absence Seizure. StatPearls. 2025 Jan:(): [PubMed PMID: 29763042]
Avoli M. A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia. 2012 May:53(5):779-89. doi: 10.1111/j.1528-1167.2012.03421.x. Epub 2012 Feb 23 [PubMed PMID: 22360294]
Level 3 (low-level) evidenceLee YJ, Hwang SK, Kwon S. The Clinical Spectrum of Benign Epilepsy with Centro-Temporal Spikes: a Challenge in Categorization and Predictability. Journal of epilepsy research. 2017 Jun:7(1):1-6. doi: 10.14581/jer.17001. Epub 2017 Jun 30 [PubMed PMID: 28775948]
Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998:39 Suppl 4():S32-41 [PubMed PMID: 9637591]
Singhal NS, Sullivan JE. Continuous Spike-Wave during Slow Wave Sleep and Related Conditions. ISRN neurology. 2014:2014():619079. doi: 10.1155/2014/619079. Epub 2014 Jan 30 [PubMed PMID: 24634784]
Ikeda A, Yamamoto A, Ichikawa K, Tsuyusaki Y, Tsuji M, Iai M, Enomoto Y, Murakami H, Kurosawa K, Miyatake S, Matsumoto N, Goto T. Epilepsy in Christianson syndrome: Two cases of Lennox-Gastaut syndrome and a review of literature. Epilepsy & behavior reports. 2020:13():100349. doi: 10.1016/j.ebr.2019.100349. Epub 2019 Dec 5 [PubMed PMID: 31879735]
Markand ON. Slow spike-wave activity in EEG and associated clinical features: often called 'Lennox' or "Lennox-Gastaut' syndrome. Neurology. 1977 Aug:27(8):746-57 [PubMed PMID: 407485]
Lin L, Drislane FW. Lateralized Periodic Discharges: A Literature Review. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):189-198. doi: 10.1097/WNP.0000000000000448. Epub [PubMed PMID: 29718828]
García-Morales I, García MT, Galán-Dávila L, Gómez-Escalonilla C, Saiz-Díaz R, Martínez-Salio A, de la Peña P, Tejerina JA. Periodic lateralized epileptiform discharges: etiology, clinical aspects, seizures, and evolution in 130 patients. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2002 Apr:19(2):172-7 [PubMed PMID: 11997729]
Level 2 (mid-level) evidenceSully KE, Husain AM. Generalized Periodic Discharges: A Topical Review. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):199-207. doi: 10.1097/WNP.0000000000000460. Epub [PubMed PMID: 29718829]
Foreman B, Claassen J, Abou Khaled K, Jirsch J, Alschuler DM, Wittman J, Emerson RG, Hirsch LJ. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012 Nov 6:79(19):1951-60. doi: 10.1212/WNL.0b013e3182735cd7. Epub 2012 Oct 3 [PubMed PMID: 23035068]
Level 2 (mid-level) evidenceGoeden M, Bansal LR. Subclinical Rhythmic EEG Discharge of Adult (SREDA) in a Child With Generalized Epilepsy and Literature Review of SREDA in Children. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):270-272. doi: 10.1097/WNP.0000000000000408. Epub [PubMed PMID: 28800038]
Yoo JY, Rampal N, Petroff OA, Hirsch LJ, Gaspard N. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA neurology. 2014 Apr:71(4):454-62. doi: 10.1001/jamaneurol.2013.6238. Epub [PubMed PMID: 24535702]
Level 2 (mid-level) evidenceSt. Louis EK, Frey LC, Britton JW, Frey LC, Hopp JL, Korb P, Koubeissi MZ, Lievens WE, Pestana-Knight EM, St. Louis EK. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. 2016:(): [PubMed PMID: 27748095]
Szurhaj W, Lamblin MD, Kaminska A, Sediri H, Société de Neurophysiologie Clinique de Langue Française. EEG guidelines in the diagnosis of brain death. Neurophysiologie clinique = Clinical neurophysiology. 2015 Mar:45(1):97-104. doi: 10.1016/j.neucli.2014.11.005. Epub 2015 Jan 14 [PubMed PMID: 25687591]
Amzica F. What does burst suppression really mean? Epilepsy & behavior : E&B. 2015 Aug:49():234-7. doi: 10.1016/j.yebeh.2015.06.012. Epub 2015 Jul 17 [PubMed PMID: 26195335]
Niedermeyer E, Sherman DL, Geocadin RJ, Hansen HC, Hanley DF. The burst-suppression electroencephalogram. Clinical EEG (electroencephalography). 1999 Jul:30(3):99-105 [PubMed PMID: 10578472]
Level 3 (low-level) evidenceReeves AL, Westmoreland BF, Klass DW. Clinical accompaniments of the burst-suppression EEG pattern. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1997 Mar:14(2):150-3 [PubMed PMID: 9165410]
Level 3 (low-level) evidenceBrigo F, Cicero R, Fiaschi A, Bongiovanni LG. The breach rhythm. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011 Nov:122(11):2116-20. doi: 10.1016/j.clinph.2011.07.024. Epub 2011 Aug 26 [PubMed PMID: 21872525]
Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2021 Jan 1:38(1):1-29. doi: 10.1097/WNP.0000000000000806. Epub [PubMed PMID: 33475321]