Introduction
Epilepsy is characterized by transient disruptions in brain synchronization leading to the occurrence of 2 or more unprovoked seizures separated by at least 24 hours. These seizures are typically linked to abnormal hypersynchronous discharges in the brain, resulting in observable clinical manifestations.[1][2] Detailed descriptions of seizures often play a crucial role in establishing an accurate diagnosis, particularly given the considerable overlap in the clinical presentation of focal epilepsies. Different forms of focal epilepsy produce seizure manifestations that depend on the specific anatomical structures involved. Clinicians can better pinpoint potential seizure localizations by identifying the symptoms typically associated with each brain region.
Magnetic resonance imaging (MRI) and electroencephalogram (EEG) remain the primary tools for diagnosing focal epilepsy. While most forms of epilepsy exhibit distinct EEG changes that aid in accurate localization, there are some inherent challenges. Imaging techniques have proven effective in identifying epilepsy lesions and improving the localization of brain seizures.[3] EEG is a valuable tool for recording electrical activity in the cortex and deeper brain structures, facilitating the diagnosis and classification of various seizure types.[4] Recent research results have emphasized the importance of video-EEG monitoring for confirming seizure types and estimating the epileptogenic zone within the brain. Additionally, scalp EEG-based seizure-detection algorithms utilized in clinical settings should demonstrate high sensitivity and selectivity across a wide range of seizure types while being user-friendly for patients with consistent parameters.[5]
Localization-related epilepsies, also known as focal epilepsies, arise from abnormal neuronal activity localized to a specific focus and involving a limited area of the cortex. Seizures without impairment of consciousness are termed "focal onset aware seizures," previously known as simple partial seizures. Conversely, seizures accompanied by a loss of consciousness are termed "focal impaired awareness seizures," formerly referred to as complex partial seizures.[6]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The neocortex comprises 6 distinct layers, each with unique anatomical and physiological characteristics supporting activity integration across cortical areas. Anatomically, associative connections terminate predominantly in the superficial layers, while slow cortical rhythms facilitate physiological integration.[7] Within the cortex, neurotransmitter release triggers stimulation of the postsynaptic endplate on adjacent dendrites, forming endplate postsynaptic potentials (EPSPs). This process establishes an electrical dipole across the neuron's soma, with positive internal and negative external charges. Subsequently, this dipole rapidly propagates along the neuron's axon as an action potential. The EEG captures the summation of EPSPs, representing a collective outcome of excitatory and inhibitory postsynaptic potentials originating from groups of synchronously firing pyramidal cells.[8][9]
Recent studies utilizing laminar microelectrode arrays in 19 human participants have supported the primary involvement of superficial slow rhythms in generating the EEG and coordinating cortical activity. These investigations demonstrated that most EEG activity, particularly below 10 Hz (delta/theta), originates from the superficial cortical layers during wakefulness and sleep. Through cortical surface grid, grid-laminar, and dual-laminar recordings, it was observed that these slow rhythms exhibit synchronous activity within the upper layers across extensive cortical regions. The progression of this superficial slow activity is influenced by infrequent stimuli and accompanied by variations in the amplitude of faster oscillations and neuronal firing across all cortical layers.[7] The synchronization among various cerebral functions is intricately linked to the dynamic interactions within segregated brain regions.[10] During a seizure, there is a disruption in the normal brain network, leading to an abnormal and excessively synchronized neuronal discharge.[11][12] Evaluating the abnormal waveforms of these discharges and their propagation aids in understanding the transmission pathways involved and identifying the specific seizure focus within the brain.[10]
Indications
When interpreting EEG results in the context of seizure activity, a skilled specialist adeptly identifies the origin of the seizure, even if the recorded waveform appears abnormal and distant from the actual lesion. This proficiency relies on analyzing waveform morphology and frequency coupled with the patient's clinical presentation. Together, these factors aid in categorizing the localization of lesions into various types of epilepsy, including temporal lobe epilepsy, mesial temporal lobe epilepsy (MTLE), lateral temporal lobe epilepsy (LTLE), frontal lobe epilepsy, parietal lobe epilepsy, or occipital lobe epilepsy.
In cases where lesions are confined to the temporal lobe, the distinction between MTLE and LTLE is made based on the involvement of mesial temporal structures. Furthermore, this diagnostic framework also considers individuals exhibiting asymmetrical hippocampal sclerosis with lateralization of a smaller hippocampus.[13] Diagnosing MTLE with hippocampal sclerosis relies on a comprehensive assessment encompassing typical semiological signs and symptoms, interictal and ictal EEG findings, cerebral imaging, and neuropsychological testing.[14] This multifaceted approach ensures a thorough understanding of the patient's condition, facilitating accurate diagnosis and informed treatment decisions.
Contraindications
While EEG has no true contraindications, its use should be judiciously guided by clinical history to mitigate the risk of overdiagnosis. In adults, EEG should primarily support a diagnosis of epilepsy when the clinical presentation strongly suggests an epileptic origin of the seizure. This cautious approach is vital to prevent the misinterpretation of EEG results, which could lead to erroneous diagnoses and unnecessary treatments.
The potential for overdiagnosis of epilepsy presents a significant challenge in seizure management. Thus, a comprehensive clinical history is indispensable to determine the necessity of EEG testing. In cases where syncope is suspected, EEG may yield false-positive results, complicating diagnostic accuracy and treatment decisions. Likewise, EEG should not be solely relied upon to rule out epilepsy in patients whose clinical presentation indicates a nonepileptic event. Typically, EEG is conducted after the occurrence of a second epileptic seizure, though exceptions may be made under specialist evaluation, allowing for testing after the first seizure in certain circumstances.[15]
In addition to clinical history, factors such as ongoing medication, history of cerebrovascular disease, migraine headaches, and sleep deprivation must be carefully considered to avoid misdiagnosis of seizure disorders. Research suggests that EEG following sleep deprivation may enhance the detection of specific epileptiform abnormalities, particularly in the initial diagnosis of idiopathic generalized epilepsy. However, its utility in focal epilepsy diagnosis may vary.[16]
Equipment
EEG synthesizing involves equipment such as electrodes, amplifiers, and plotting devices. Traditionally, electrolytic gel and salts were utilized to enhance conductivity from the scalp through the electrodes. Introducing "dry electrodes" has revolutionized scalp preparation by eliminating the need for gels and salts, resulting in more accurate EEG recordings. Despite their potential benefits, dry electrodes have not yet been widely adopted. EEG electrode caps are currently well-tolerated across all age groups. Recent studies have explored the relationship between EEG source localization and the number of scalp recording channels. While increasing the number of electrodes enhances source localization, the incremental improvement in accuracy diminishes with higher electrode counts.[17]
Historically, amplifiers and plotting equipment consisted of mechanical pen and paper recording devices. However, these have been superseded by modern digital EEG systems. These innovative systems offer faster sampling rates and the ability to record from expanding channels simultaneously. Contemporary EEG systems used in clinical practice typically feature at least 128 channels, each capable of sampling at over 10 kHz and boasting a 24-bit resolution at each amplifier.[8] The advancement of EEG technology continues to enhance the quality and efficiency of neurological assessments, providing clinicians with valuable insights into brain function and pathology.
The presentation of a comparative study between scalp EEG and behind-the-ear EEG for patients with focal epilepsy underscores the potential of wearable EEG devices for continuous monitoring, which can offer valuable insights into epilepsy management. Currently, no EEG setup is sufficiently compact and discreet for daily use. However, recording behind the ear may present a promising solution for wearable EEG setups.
The similarities between behind-the-ear EEG and scalp EEG are notable, particularly regarding temporal waveform and frequency content during seizures and meaningful epileptic discharges. Moreover, automatic seizure detection algorithms based on support vector machines have demonstrated comparable performance between the 2 modalities.[18] These findings suggest that behind-the-ear EEG holds promise as a viable alternative to traditional scalp EEG for continuously monitoring patients with epilepsy due to its potential to provide reliable seizure detection in a wearable format, which could significantly improve the management and treatment of epilepsy—offering patients greater freedom and flexibility in their daily lives.
Interpreting an EEG involves understanding the electrical wave progression over the brain. The basic electrode placement follows the universal 10-20 system and is set to the required montages. Montages are EEG electrode settings that record the EPSP from a specific focus point of interest.[19] These montages fit broadly under 3 headings.
Referential Montages
EEG analysis involves plotting waveforms from a suspected focal point on the head (active electrode) to a reference point elsewhere on the body or scalp. However, this setup does not guarantee achieving neutrality with the reference electrode. Various types of reference montages are utilized in EEG recordings:
- Central reference
- Historically, the potential at the midline electrode (eg, 'Cz') or the average potential across all electrodes was chosen as the reference. This approach aims to maintain a high signal-to-noise ratio for successful EEG recordings.
- Average reference
- This method uses the average potential across all electrodes as the reference and provides a broader representation of brain activity, but outliers may influence it.
- Localized reference
- In this approach, only the surrounding potentials of a specific electrode are averaged to calculate the reference. This method aims to reduce the influence of distant brain regions on the reference signal.
A modern approach proposes a dynamic selection method for the reference electrode, allowing all electrodes to be treated as active. An electrode is statistically chosen based on a specific frequency stimulus's highest estimated signal-to-noise ratio.[20] This dynamic selection optimizes the reference choice for each recording session, potentially improving the accuracy and reliability of EEG analyses.
By employing these various reference montages, researchers and clinicians can tailor EEG recordings to specific research or clinical needs, optimizing signal quality and enhancing the interpretation of brain activity.
Bipolar Montages
- The term 'bipolar' in EEG electrode placement is derived from the recording mechanism utilized in this configuration. In a bipolar montage, 2 electrodes are positioned along an anteroposterior or left-over-right axis. The difference in electrical potential between these 2 electrodes is then plotted, providing information about the activity between the 2 regions of the brain being monitored. This setup is also referred to as a differential montage due to the comparison between 2 specific brain regions.
- Bipolar montages can capture localized activity in EEG recordings and provide insights into the functional connectivity and interactions between different brain regions. This approach allows for a more focused analysis of neural activity and can be particularly useful in detecting localized abnormalities or changes in brain function.
Laplacian Montages
- This montage type involves a second derivative computation, where the combined weighted average of voltages surrounding a specific electrode of interest is calculated. This technique requires relatively complex computation, resulting in a net output that depends on the particular electrode in the montage. The Laplacian montage is particularly effective when focal discharges generate a minimal field, allowing for a more precise assessment of localized brain activity.[21] By focusing on the surrounding voltages and applying weighted averages, this montage can enhance the sensitivity to subtle changes in electrical activity within specific brain regions.
Different montage settings are employed in EEG evaluation to accurately isolate the suspected lobular involvement and localize the epileptic focus.[22] These montages are tailored based on the specific characteristics of the patient's seizure activity and the suspected region of brain involvement.
The digital nature of EEG technology enables ease of reformatting and remontaging to facilitate the localization of abnormalities. Clinicians can adjust the montage settings as needed, allowing for a more precise evaluation of the epileptic focus. This capability enhances the diagnostic accuracy of EEG recordings and aids in developing targeted treatment plans for individuals with epilepsy.
Technique or Treatment
A systemic approach is paramount for interpreting an EEG recording. Before starting the analysis, certain factors, including the patient's age, physical activity level, mental state, level of consciousness, factors, environmental and pharmacological agents, and biological factors that can potentially influence the morphology of the waveforms, need to be considered.
There is a wide variation in the EEG waveforms. A good understanding of the normal or benign variants is necessary to differentiate normal or benign variants from pathologic waveforms.[23] Some of these normal variants include:
- Wicket spikes: These waveforms appear over the temporal (anterior or mid-temporal) region during relaxed wakefulness, drowsiness, or light sleep.
- Benign epileptiform transients of sleep: These are also called small sharp spikes and occur in stage 1 or 2 of sleep.
- 6 Hz "phantom" spike-and-wave complex (PhSW): PhSW can be considered a smaller version of the 3 Hz spike-and-wave pattern. These waveforms have low amplitudes and appear in the frequency range of 5 to 7 Hz.
- Rhythmic midtemporal theta of drowsiness: This psychomotor variant pattern is usually in the midtemporal region and appears in relaxed wakefulness and drowsiness.
- Positive occipital sharp transients of sleep: These waveforms are asymmetrically distributed and appear in the occipital regions during nonrapid eye movement sleep.
- Subclinical rhythmic EEG discharge in adults: This is a very rare benign EEG pattern resembling ictal discharges and is, at times, interpreted as such, leading to a misdiagnosis of epilepsy.
- 14 Hz and 6 Hz positive spikes: These are typically seen in the younger age group.
- Repetitive vertex waves: These are found mainly in children.
- Breach rhythm: These waveforms are seen over the regions with a skull defect. Because of the skull defect, faster frequencies that are otherwise less appreciated on scalp EEG increase visibility.
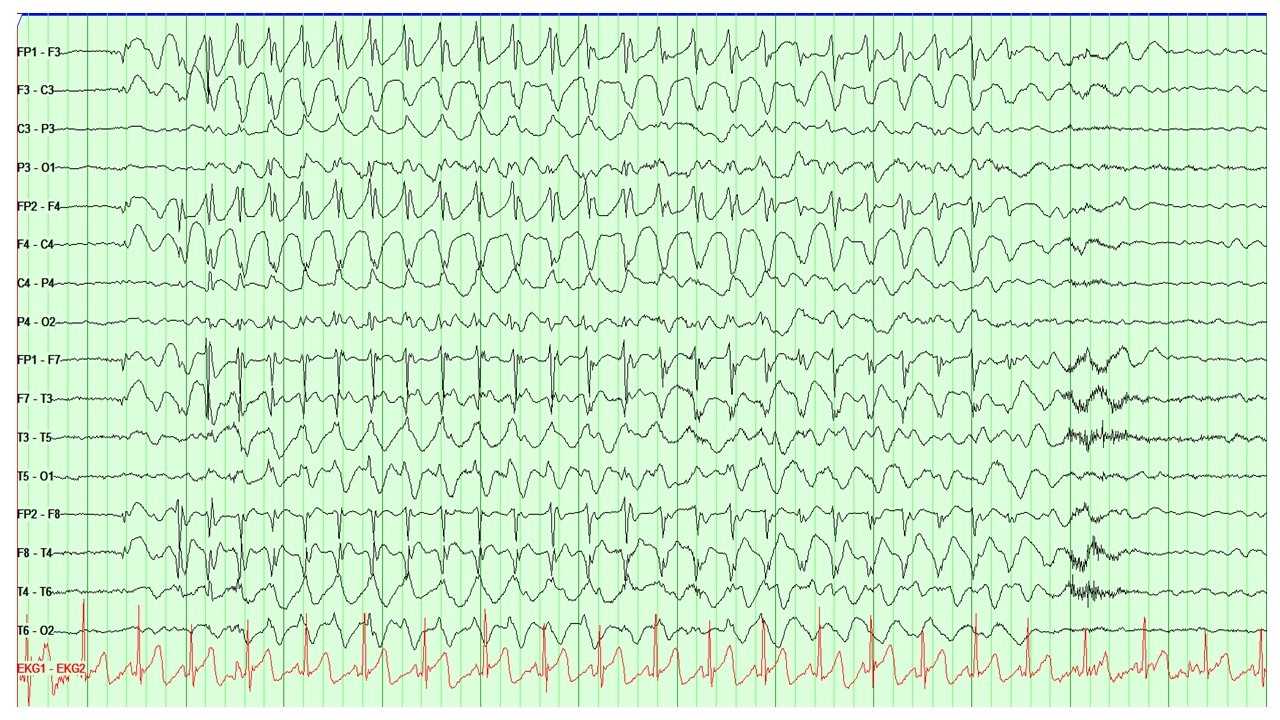
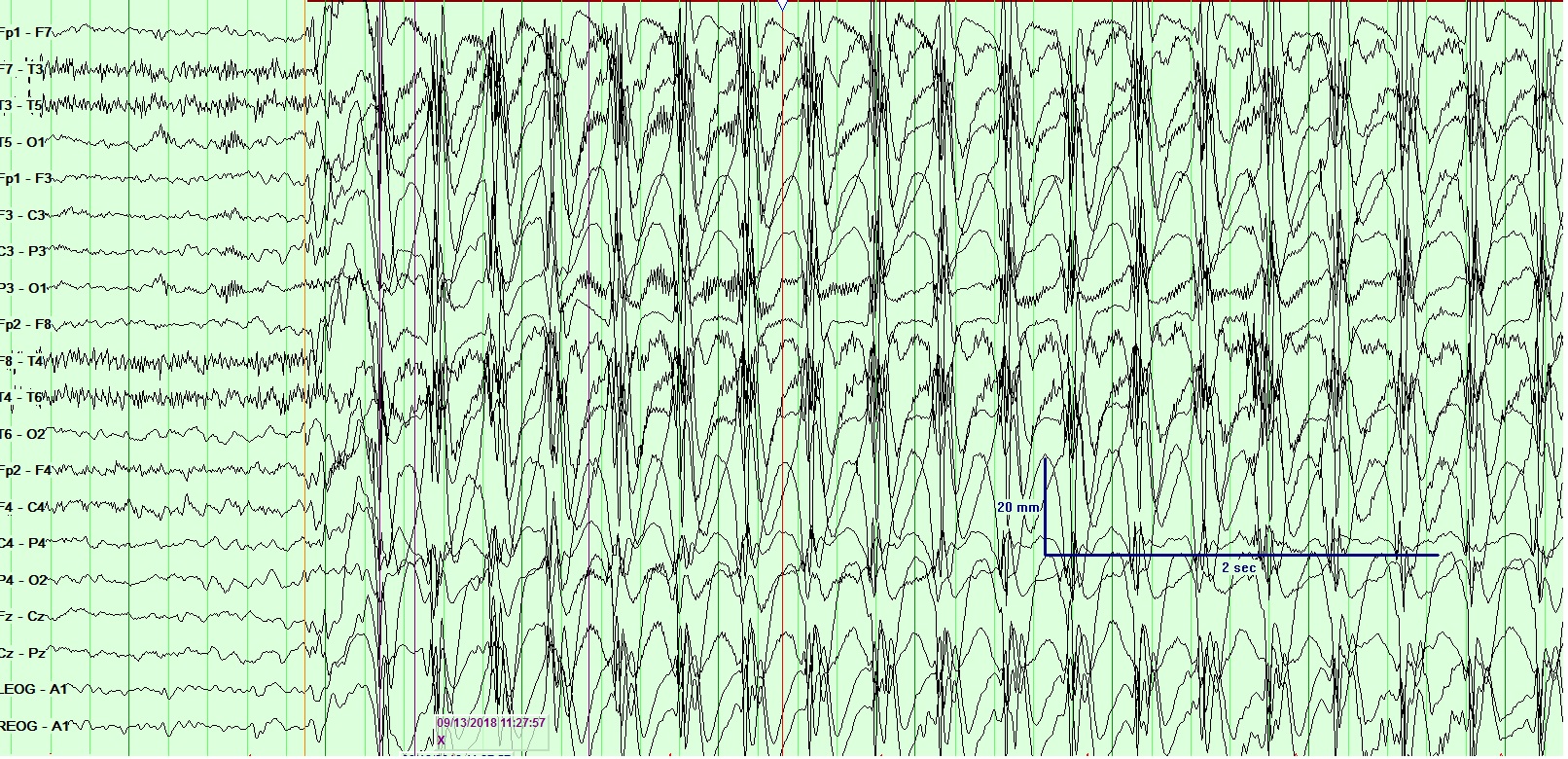
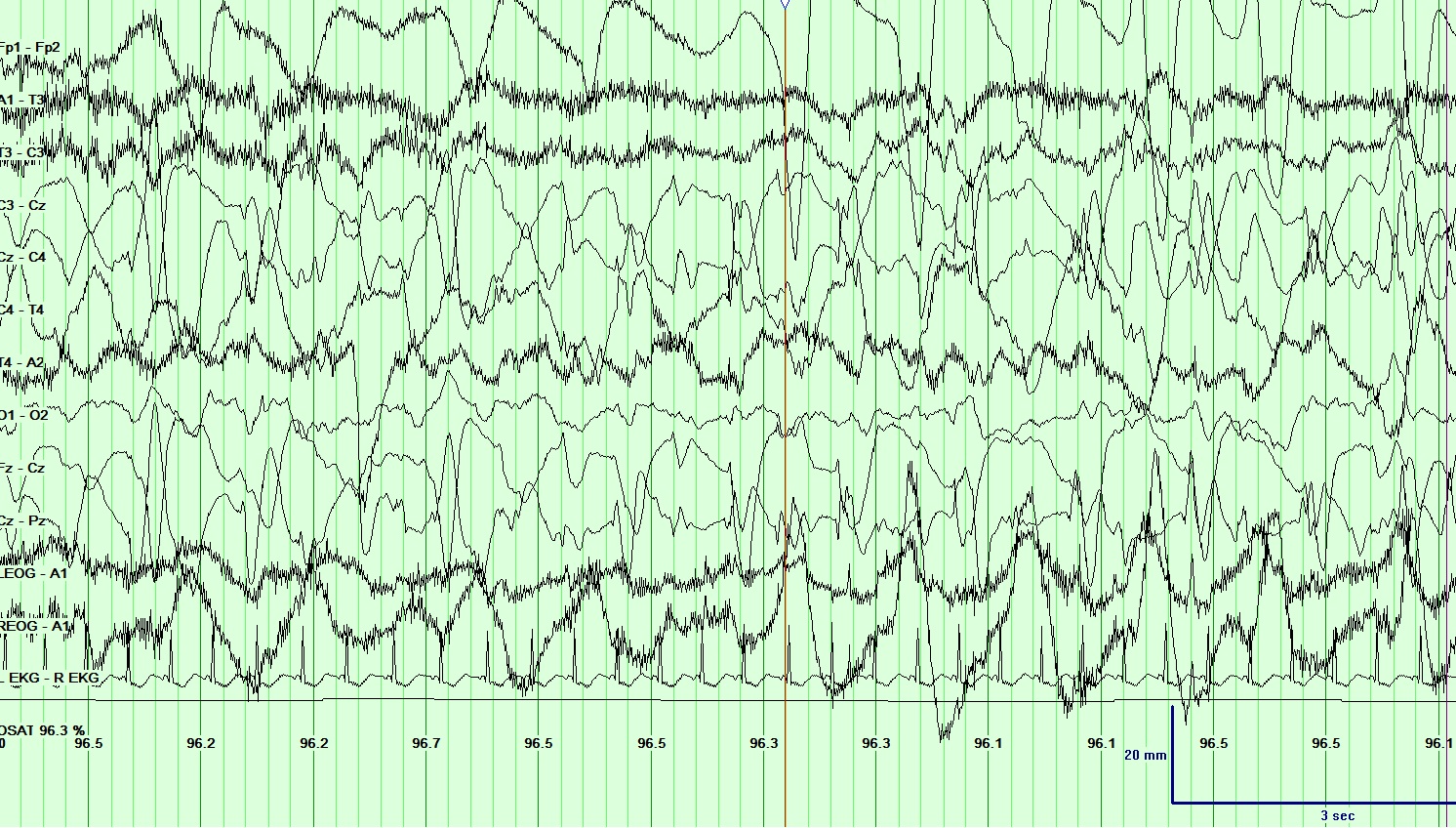
In EEG recordings of seizures, the onset often begins with the emergence of abnormal discharges in bursts, termed ictal epileptiform discharges. These discharges escalate in frequency, evolving into rapid continuous spikes and waves, and ultimately peak with numerous spikes accompanied by buried waves. As the seizure activity subsides, the waves reappear, decrease in frequency, and eventually cease.[24] (See Image. Electroencephalogram (EEG), Absence Epilepsy and Image. Electroencephalogram (EEG), General Epileptiform and Image. Electroencephalogram (EEG), Neonatal Seizure.)
The period during which seizure activity occurs is known as the ictal period, while the intervals between seizures are termed interictal periods. EEG activity during interictal periods also reveals abnormal discharges, referred to as interictal epileptiform discharges (IEDs). Given that most patients present either immediately after or before a seizure, the presence of IEDs serves as a key diagnostic indicator, validating clinical suspicion of seizure activity in epilepsy.
Multiple EEG recordings are often necessary to capture IEDs effectively. For example, approximately every fourth consecutive EEG in a patient with epilepsy exhibits an IED frequency ranging from 60% to 90%. In contrast, the frequency of IEDs in those who are nonepileptic is lower, typically ranging from 0.5% to 2.5% in healthy young men and around 12% in patients without epilepsy of all age groups with progressive cerebral disorders. Notably, the specificity of IED detection may be lower; sensitivity tends to be higher in children than adults.[25] Activation methods such as hyperventilation, sleep deprivation, and photic stimulation are employed to enhance the appearance of IEDs. These methods can be valuable for localization purposes and can help strengthen the diagnosis of epilepsy by increasing the likelihood of detecting abnormal epileptiform activity.
EEG literature has extensively studied IEDs with negative polarity. However, recent research has shed light on the pathophysiological, neuroimaging, and clinical correlates of IEDs with positive polarity, specifically positive sharp waves (PSWs), in scalp EEG recordings. Although PSWs are rare and underreported EEG abnormalities, they, like negative IEDs, indicate focal epileptogenicity.
Analysis of PSWs has revealed them to be an epileptogenic pattern with localizing significance, primarily observed in younger age groups. Moreover, there is a strong association between PSWs and chronic and static central nervous system (CNS) pathology, particularly congenital CNS anomalies, often accompanied by psychomotor developmental delays. Patients exhibiting "multifocal" PSWs typically present with severe intellectual and motor deficits, consistently associated with a variety of congenital CNS insults.[26]
While surface EEG recordings are less sensitive than invasive studies, they remain efficient in approximating the epileptogenic zone in many common epilepsies. In cases where scalp EEG does not yield definitive results or when the focus is adjacent to eloquent cortex regions, invasive studies become particularly useful.[27] The most commonly employed invasive electrodes include stereotactically implanted depth and subdural strip or grid electrodes.
Case reports detailing EEG abnormalities in partial epilepsy with simultaneous EEG with functional MRI (EEG-fMRI) recordings have provided valuable insights into the epileptogenic network. Scalp EEG recordings and the classification of IEDs among patients with epilepsy offer significant information, particularly during the preoperative evaluation of patients with severe refractory epilepsies.[26]
Moreover, while most patients with localization-related epilepsy and genetic generalized epilepsy are categorized based on semiology and video-EEG findings, these characteristics may sometimes fail to provide a definitive diagnosis. Recent studies have investigated the frequency of index finger pointing during generalized motor convulsions as a lateralizing semiology in localization-related epilepsy. The findings revealed that index finger pointing is more prevalent among those with localization-related epilepsy compared to those with nonepileptic attacks, indicating its potential utility as a diagnostic marker in certain cases.[28]
These advancements in understanding EEG abnormalities and seizure semiology contribute to the refinement of diagnostic strategies and treatment approaches for patients, particularly those with refractory seizures who may benefit from surgical intervention.
Complications
EEG signals offer valuable insights into brain activity with high temporal resolution and relatively stable outcomes. However, the complexity involved in generating and analyzing brain functional networks poses significant challenges due to the complexity of the process.[29] One notable challenge lies in the unconventional diagnostic accuracy of EEG devices, which is further complicated by the poor reliability of interrater assessments.[30] Concerns regarding EEG waveforms also merit attention. Firstly, selecting the appropriate reference electrode is crucial to effectively cancel out normal waveforms and amplify pathological waveforms.
Given that EEGs are influenced by both local and remote electrical activity, the reference electrode should be positioned to effectively capture interfering waveforms. Moreover, the reference electrode must maintain a significant potential difference to facilitate charge movement without acceleration. Placing the reference electrode too close to the pathological site may result in a negligible potential difference compared to the active electrode, rendering the recording less interpretable.[8] Addressing these concerns is essential for optimizing the diagnostic utility of EEG recordings and enhancing their reliability in clinical practice.
Clinical Significance
EEG is a valuable tool in clinical evaluation, providing insights into various cerebral pathologies and neural network modifications. The most important waveforms for clinical assessment include delta (0.5-4 Hz), theta (4-7 Hz), alpha (8-12 Hz), sigma (12-16 Hz), and beta (13-30 Hz) waves. Additionally, infraslow and high-frequency oscillations are increasingly recognized as clinically relevant, thanks to advancements in digital signal processing.[31][32]
Recent study results have highlighted the significance of EEG phase synchrony analyses, revealing associations between large-scale phase synchrony of brain activity and clinical symptoms.[33] EEG has proven useful in evaluating epilepsy, altered states of consciousness, parasomnias, dementias, toxic confusional states, cerebral infections, and encephalopathies.
Alteration of consciousness has emerged as a critical clinical manifestation of focal seizures, significantly impacting the quality of life of patients. Research suggests partial seizures disrupt consciousness by disturbing the global workspace theory, which posits that access to consciousness requires coordinated activity from associative cortices, particularly the prefrontal and posterior parietal cortices. Abnormal synchrony in global workspace regions and thalamocortical synchrony changes during seizure spreading are linked to an alteration of consciousness, offering new perspectives for mitigating consciousness alteration in seizures and improving patient outcomes.[34]
Abnormal EEG waveforms reflect a spectrum of pathophysiological processes, including raised intracranial pressure, cerebral anoxia, edema, epileptogenesis, and more, and do not usually specify a particular disease.[35] EEG plays a fundamental role in detecting localized epileptiform activity in the cerebral cortex.
In patients with focal epilepsy, there is a disruption of interregional communication between specialized brain regions involved in information processing.[36] Clinical studies have observed abnormal activity extending beyond the region of pathology.[37][38][39][40]
In a study by Foldvary et al, localized ictal onset was observed in 57% of seizures, including mesial temporal lobe epilepsy (MTLE), left frontal lobe epilepsy (LFLE), and parietal lobe epilepsy (PLE). Lateralized onsets predominated in neocortical temporal lobe epilepsy (TLE), and generalized onsets were seen in medial frontal lobe epilepsy (MFLE) and occipital lobe epilepsy (OLE).[41] These findings underscore the multifaceted role of EEG in clinical neurology. EEG provides valuable insights into brain function and pathology and guides diagnostic and therapeutic interventions. Classical EEG morphologies, based on specific lobular involvement and epileptic foci, are described below:
TLE
- Ictal EEG
- In patients with focal impaired awareness seizures, ictal EEG abnormalities are observed in approximately 95% of cases, with about 66% exhibiting an electrodecremental pattern. A rhythmic theta discharge ranging from 5 to 7 Hz in the temporal regions is specific to TLE. Depth electrodes are particularly useful in capturing this pattern and accurately diagnosing ipsilateral mesial temporal structures. However, scalp electrodes are more effective lateralizing than localizing the seizure focus. Frontal lobe seizures challenge scalp EEG interpretation due to movement artifacts, making it difficult to appreciate ictal EEG changes.
- Recent studies have highlighted that the ictal pattern observed in TLE on scalp EEG may not become evident until the intracranial ictal discharge reaches ≤10 Hz and has propagated from its site of onset, involving structures such as the hippocampus, medial paleocortex, and lateral temporal neocortex.[42]
- In many cases, EEG findings may necessitate further assessment using various neuroimaging modalities such as magnetic resonance imaging (MRI), interictal fluorodeoxyglucose (FDG), positron emission tomography (PET), ictal single-photon emission computed tomography (SPECT), magnetic encephalography (MEG), or functional MRI (fMRI) to provide a comprehensive evaluation of abnormalities.[43]
- IEDs
- Isolated IED-like sharps and spikes occurring over the temporal region, along with temporal intermittent rhythmic delta activity, are strongly associated with a diagnosis of TLE. These EEG abnormalities, in conjunction with clinical history, can aid in diagnosing TLE.[44][45]
- In seizures originating from the medial temporal lobe, the amplitude of mesial temporal spikes is most prominent at anterior temporal scalp electrodes and sphenoidal electrodes if utilized.[46] This spatial distribution of spike amplitude can provide valuable information for localization in TLE cases.
- In cases where IEDs are absent, microstate abnormalities can still be utilized to identify patients with TLE using machine learning techniques. Microstates refer to quasi-stable electrical distributions in EEG recordings that convey significant information about the dynamics of large-scale brain networks.[47] By analyzing microstate patterns, machine learning algorithms can detect abnormalities indicative of TLE even in the absence of overt interictal discharges.
- Seizure semiology
- Auras associated with epilepsy provide valuable insights into the localization and characterization of seizure onset. In MTLE, auras often manifest as visceral sensations and fear. Conversely, neocortical TLE is frequently accompanied by auditory and vertiginous auras.
- In neocortical TLE, patients typically experience a diverse range of hallucinations or illusions at seizure onset, while automatisms and dystonic posturing are uncommon features.[44][48]
- In contrast, MTLE presents distinctive features such as a behavioral arrest characterized by a blank facial expression and loss of awareness. This is followed by oral, facial, or alimentary automatisms such as lip-smacking, chewing, sucking, or swallowing. Additionally, ipsilateral automatisms such as repetitive hand movements, picking, fidgeting behavior, and contralateral abnormal limb posturing may occur. Following the seizure, patients often experience a period of postictal confusion, with rare progression to secondary generalization.[49]
- Intracranial EEG recordings commonly reveal low-voltage, fast, and hypersynchronous patterns as the predominant seizure-onset patterns in MTLE cases.[50]
FLE
FLE, ranking second after TLE, is a prevalent form of localization-related epilepsy in childhood.[51] Due to the complexity and multifunctionality of the frontal lobes, FLE can manifest as complex psychiatric conditions associated with motor, cognitive, and medical changes, thereby including FLE as a differential diagnosis for complex neuropsychiatric reports.[52]Characterizing frontal lobe seizures proves challenging due to their brevity, intricate motor behaviors, and emotional expressions. This challenge extends to interpreting semiologic and EEG patterns, compounded by the extensive connectivity of the frontal lobe with other brain regions, facilitating rapid and generalized seizure propagation.[51]
Localizing seizures in the frontal lobe using the 10-20 system scalp EEG can be challenging due to several factors, including the rapid spread of neocortical seizures, significant muscle artifacts, and limited spatial resolution, especially for seizure generators involving the mesial frontal lobe cortex. Recent studies have suggested that high-density EEG monitoring, such as the 10-10 system, may offer improved EEG source localization capabilities.[53]
Additionally, research results have shown that categorizing frontal seizures based on their semiology and correlating them with the anatomical organization along a rostrocaudal axis aligns with current hypotheses regarding the hierarchical organization of the frontal lobe. This electroclinical categorization provides insights into the probable zones of network organization essential for producing specific semiologic features, thereby aiding in presurgical localization efforts.
Furthermore, analyzing ictal motor behavior in prefrontal seizures, such as stereotypies, can offer valuable information for interpreting the underlying cortical/subcortical networks responsible for generating these behaviors.[54] Overall, integrating advances in EEG monitoring techniques, electroclinical categorization, and motor behavior analysis can enhance our understanding and localization of seizures originating from the frontal lobe.
- Ictal EEG
- Scalp ictal EEG changes are difficult to appreciate in most frontal lobe seizures due to the movement artifacts obscuring the visibility of the underlying ictal waveforms.[55] A recent study described the additional lateralizing and localizing value of the postictal EEG in FLE since ictal EEG in FLE is difficult to localize. This study showed that a close examination of the postictal EEG can provide additional information to identify a potentially resectable epileptogenic zone.[56]
- Interictal EEG
- IEDs are observed in only 60% to 80% of patients with FLE and have a lower localizing value than in TLE since they can be bilateral, involving multiple lobes, or even secondarily generalized.[57] The medial frontal epilepsies only rarely reveal any IEDs, and even if they do, they are bifrontal spikes and wave discharges.[58]
- Seizure semiology
- Since the ictal EEG in FLE is of less value due to frequent muscle and motion artifacts, analyzing the ictal semiology and clinical history is important to differentiate FLE from psychogenic nonepileptic spells. This condition is frequently misdiagnosed as epilepsy.[59]
- In dorsolateral FLE, the clinical presentations of seizures are notably diverse. Seizures originating from the premotor cortex often manifest as forced contralateral head deviation or head-turning, termed versive seizures. Activation of the contralateral frontal eye field can lead to lateral deviation of the eyes. Aphasic seizures may occur due to involvement of the Broca area.[60]
- Seizures involving the prefrontal cortex exhibit even more variable clinical manifestations. These are often referred to as "hypermotor seizures." Typically, they commence with somatosensory auras and progress to include behaviors such as bizarre gestures, laughing, shouting, bicycle peddling motions, and vigorous thrashing of the extremities.[60]
- Mesial FLE: Associated features of this type include loss of consciousness followed by conjugate eye and head deviation, behavioral arrest, and immediate recovery of consciousness.[61]
- Auras serve as valuable indicators of seizure onset zones. In FLE, they occur in descending order of frequency as follows: autonomic aura, emotional aura, somatosensory aura, psychic aura, cephalic aura, abdominal aura, whole-body sensory aura, visual aura, auditory aura, vestibular aura, and unclassified aura. Among these, autonomic auras are reported most frequently, while somatosensory auras are often linked with contralateral motor areas.[61]
- Orbitofrontal epilepsy: This is considered among patients with sleep-related, hyperkinetic seizures without specific aura and frontotemporal interictal discharges.[62]
PLE
- Ictal EEG
- Ictal scalp EEG is not usually helpful in localizing PLE. Even with extensive invasive studies, localization can be inconclusive.[63]
- Interictal EEG
- Seizure semiology
- Seizures originating from the parietal lobe can present with various symptoms. While many patients may not exhibit signs suggestive of parietal lobe involvement, specific symptoms, if present, often include unilateral paresthesias and pain at the onset of the seizure. Other manifestations may arise from diffuse cortical involvement, including hallucinations and spatial distortions.[66]
- Recent studies have uncovered a notable commonality among patients despite clinical and EEG features that may not be easily localized. These investigations revealed that seizures originating from the precuneus in the mesial parietal lobe appear to display a discernible electroclinical phenotype. The precuneus holds significance within the default mode network, playing a crucial role in internal reflective thinking. Interestingly, deactivation of this region is a prominent feature of generalized spike and wave epileptiform activity. The seizure semiology in these patients appears to mirror the activation of the precuneus and the propagation of ictal activity along intrinsically connected default mode network components.[67] These findings shed light on the complex interplay between brain regions and networks in the manifestation of seizure symptoms originating from the parietal lobe.
OLE
- Ictal EEG
- During the ictal period, an EEG recording is more likely to show diffuse bi-occipital activity spreading to the temporal regions than well-localized unifocal discharges in the occipital region.
- Interictal EEG
- IEDs may manifest either spontaneously or in response to photic stimulation. Spontaneous IEDs often present as unilateral posterior EEG slowing rather than spike waves. While infrequently localized over the occipital cortex, the posterior temporal region emerges as the most common site of occurrence.
- In contrast, photosensitive OLE necessitates intermittent photic stimulation to evoke IEDs. These IEDs typically appear as either spikes and polyspikes localized to the occipital region or as generalized spikes and polyspikes diffusely spreading across the posterior cortex.[68][69]
- Seizure semiology
- Seizures in OLE commonly manifest with visual symptoms such as hallucinations, blindness, nystagmus, and rapid blinking of the eye. In rare instances, they may present as a generalized tonic-clonic seizure accompanied by impaired consciousness. This spread to neighboring cortical regions complicates focus localization, adding to the diagnostic challenge.[70]
- Recent study results have revealed that patients with OLE exhibit a widespread organization of the epileptogenic zone, which may involve parietal or temporal regions. Among these structures, the fusiform gyrus emerges as the most epileptogenic, further highlighting the complex nature of OLE and its implications for diagnosis and treatment.[71]
In most cases of focal onset aware seizures, the scalp EEG often does not exhibit significant changes during simple partial seizures. The focal ictal discharge may be distant, deep, or involve too small a neuronal aggregate to detect a synchronized activity on the scalp. However, as the seizure progresses and involves a larger cortical area before potentially leading to secondary generalization, changes in the EEG become more apparent.
Enhancing Healthcare Team Outcomes
An epileptic syndrome is a chronic disorder that heavily depends on an interprofessional team to provide a holistic and integrated approach to provide the best possible long-term seizure control.[72] Effective management of EEG localization-related epilepsy requires a collaborative effort among healthcare professionals, including physicians, advanced practitioners, nurses, pharmacists, and other team members. Each member brings unique skills, from the specialized knowledge of physicians and advanced practitioners in epilepsy diagnosis and treatment to the patient education and monitoring nurses provide. Pharmacists contribute expertise in medication management, ensuring appropriate drug selection and dosing to optimize patient outcomes. By leveraging these diverse skills, the team can develop comprehensive treatment plans tailored to each patient's needs, enhancing patient-centered care.
Interprofessional communication is essential for coordinating care and ensuring patient safety. Clear and open communication channels allow team members to share information about the patient's condition, treatment plan, and any changes in their status. Regular team meetings and case conferences facilitate collaboration and ensure alignment of treatment goals across disciplines. Care coordination further enhances patient-centered care by integrating services across different healthcare settings and disciplines, ensuring continuity of care and streamlining the care process. By working together effectively, healthcare professionals can deliver high-quality care, improve patient outcomes, and enhance team performance in managing EEG localization-related epilepsy. The interprofessional care provided to the patient must use an integrated care pathway combined with an evidence-based approach to planning and evaluating all joint activities.[73]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 Apr:58(4):512-521. doi: 10.1111/epi.13709. Epub 2017 Mar 8 [PubMed PMID: 28276062]
Donos C, Maliia MD, Dümpelmann M, Schulze-Bonhage A. Seizure onset predicts its type. Epilepsia. 2018 Mar:59(3):650-660. doi: 10.1111/epi.13997. Epub 2018 Jan 11 [PubMed PMID: 29322500]
Skidmore CT. Adult Focal Epilepsies. Continuum (Minneapolis, Minn.). 2016 Feb:22(1 Epilepsy):94-115. doi: 10.1212/CON.0000000000000290. Epub [PubMed PMID: 26844732]
Guerreiro CA. Epilepsy: Is there hope? The Indian journal of medical research. 2016 Nov:144(5):657-660. doi: 10.4103/ijmr.IJMR_1051_16. Epub [PubMed PMID: 28361817]
Baumgartner C, Koren JP. Seizure detection using scalp-EEG. Epilepsia. 2018 Jun:59 Suppl 1():14-22. doi: 10.1111/epi.14052. Epub [PubMed PMID: 29873826]
Ighodaro ET, Maini K, Arya K, Sharma S. Focal Onset Seizure. StatPearls. 2024 Jan:(): [PubMed PMID: 29763181]
Halgren M, Fabó D, Ulbert I, Madsen JR, Erőss L, Doyle WK, Devinsky O, Schomer D, Cash SS, Halgren E. Superficial Slow Rhythms Integrate Cortical Processing in Humans. Scientific reports. 2018 Feb 1:8(1):2055. doi: 10.1038/s41598-018-20662-0. Epub 2018 Feb 1 [PubMed PMID: 29391596]
Biasiucci A, Franceschiello B, Murray MM. Electroencephalography. Current biology : CB. 2019 Feb 4:29(3):R80-R85. doi: 10.1016/j.cub.2018.11.052. Epub [PubMed PMID: 30721678]
Kirschstein T, Köhling R. What is the source of the EEG? Clinical EEG and neuroscience. 2009 Jul:40(3):146-9 [PubMed PMID: 19715175]
Mei T, Wei X, Chen Z, Tian X, Dong N, Li D, Zhou Y. Epileptic foci localization based on mapping the synchronization of dynamic brain network. BMC medical informatics and decision making. 2019 Jan 31:19(Suppl 1):19. doi: 10.1186/s12911-019-0737-8. Epub 2019 Jan 31 [PubMed PMID: 30700279]
Stam CJ. Modern network science of neurological disorders. Nature reviews. Neuroscience. 2014 Oct:15(10):683-95. doi: 10.1038/nrn3801. Epub 2014 Sep 4 [PubMed PMID: 25186238]
Level 3 (low-level) evidenceRavindra VM, Sweney MT, Bollo RJ. Recent developments in the surgical management of paediatric epilepsy. Archives of disease in childhood. 2017 Aug:102(8):760-766. doi: 10.1136/archdischild-2016-311183. Epub 2017 Jan 17 [PubMed PMID: 28096104]
Ye BS, Cho YJ, Jang SH, Lee MK, Lee BI, Heo K. The localizing and lateralizing value of auras in lesional partial epilepsy patients. Yonsei medical journal. 2012 May:53(3):477-85. doi: 10.3349/ymj.2012.53.3.477. Epub [PubMed PMID: 22476989]
Level 2 (mid-level) evidenceChatzikonstantinou A. Epilepsy and the hippocampus. Frontiers of neurology and neuroscience. 2014:34():121-42. doi: 10.1159/000356435. Epub 2014 Apr 16 [PubMed PMID: 24777136]
National Clinical Guideline Centre (UK). The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care: Pharmacological Update of Clinical Guideline 20. 2012 Jan:(): [PubMed PMID: 25340221]
Renzel R, Baumann CR, Poryazova R. EEG after sleep deprivation is a sensitive tool in the first diagnosis of idiopathic generalized but not focal epilepsy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016 Jan:127(1):209-213. doi: 10.1016/j.clinph.2015.06.012. Epub 2015 Jun 18 [PubMed PMID: 26118491]
Sohrabpour A, Lu Y, Kankirawatana P, Blount J, Kim H, He B. Effect of EEG electrode number on epileptic source localization in pediatric patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2015 Mar:126(3):472-80. doi: 10.1016/j.clinph.2014.05.038. Epub 2014 Jul 11 [PubMed PMID: 25088733]
Gu Y, Cleeren E, Dan J, Claes K, Van Paesschen W, Van Huffel S, Hunyadi B. Comparison between Scalp EEG and Behind-the-Ear EEG for Development of a Wearable Seizure Detection System for Patients with Focal Epilepsy. Sensors (Basel, Switzerland). 2017 Dec 23:18(1):. doi: 10.3390/s18010029. Epub 2017 Dec 23 [PubMed PMID: 29295522]
Kutluay E, Kalamangalam GP. Montages for Noninvasive EEG Recording. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2019 Sep:36(5):330-336. doi: 10.1097/WNP.0000000000000546. Epub [PubMed PMID: 31490450]
Wu Z, Su S. A dynamic selection method for reference electrode in SSVEP-based BCI. PloS one. 2014:9(8):e104248. doi: 10.1371/journal.pone.0104248. Epub 2014 Aug 6 [PubMed PMID: 25100038]
Gordon R, Rzempoluck EJ. Introduction to Laplacian montages. American journal of electroneurodiagnostic technology. 2004 Jun:44(2):98-102 [PubMed PMID: 15328706]
Foldvary N, Caruso AC, Mascha E, Perry M, Klem G, McCarthy V, Qureshi F, Dinner D. Identifying montages that best detect electrographic seizure activity during polysomnography. Sleep. 2000 Mar 15:23(2):221-9 [PubMed PMID: 10737339]
Kang JY, Krauss GL. Normal Variants Are Commonly Overread as Interictal Epileptiform Abnormalities. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2019 Jul:36(4):257-263. doi: 10.1097/WNP.0000000000000613. Epub [PubMed PMID: 31274688]
Rosenow F, Klein KM, Hamer HM. Non-invasive EEG evaluation in epilepsy diagnosis. Expert review of neurotherapeutics. 2015 Apr:15(4):425-44. doi: 10.1586/14737175.2015.1025382. Epub 2015 Mar 16 [PubMed PMID: 25779862]
Aanestad E, Gilhus NE, Brogger J. Interictal epileptiform discharges vary across age groups. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2020 Jan:131(1):25-33. doi: 10.1016/j.clinph.2019.09.017. Epub 2019 Nov 4 [PubMed PMID: 31751836]
Janati AB, Umair M, Alghasab NS, Al-Shurtan KS. Positive sharp waves in the EEG of children and adults. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2014 May:35(5):707-13. doi: 10.1007/s10072-013-1588-1. Epub 2013 Nov 27 [PubMed PMID: 24281945]
Noachtar S, Rémi J. The role of EEG in epilepsy: a critical review. Epilepsy & behavior : E&B. 2009 May:15(1):22-33. doi: 10.1016/j.yebeh.2009.02.035. Epub 2009 Feb 25 [PubMed PMID: 19248841]
Siegel J, Tatum WO 4th. Index-finger pointing in generalized tonic-clonic seizures. Epilepsy & behavior : E&B. 2016 May:58():18-21. doi: 10.1016/j.yebeh.2016.02.022. Epub 2016 Mar 18 [PubMed PMID: 26994878]
Yao R, Xue J, Yang P, Wang Q, Gao P, Yang X, Deng H, Tan S, Li H. Dynamic Changes of Brain Networks during Working Memory Tasks in Schizophrenia. Neuroscience. 2021 Jan 15:453():187-205. doi: 10.1016/j.neuroscience.2020.11.007. Epub 2020 Nov 27 [PubMed PMID: 33249224]
Grant AC, Abdel-Baki SG, Omurtag A, Sinert R, Chari G, Malhotra S, Weedon J, Fenton AA, Zehtabchi S. Diagnostic accuracy of microEEG: a miniature, wireless EEG device. Epilepsy & behavior : E&B. 2014 May:34():81-5. doi: 10.1016/j.yebeh.2014.03.015. Epub 2014 Apr 12 [PubMed PMID: 24727466]
Gondeck AR, Smith JR. Dynamics of human sleep sigma spindles. Electroencephalography and clinical neurophysiology. 1974 Sep:37(3):293-7 [PubMed PMID: 4136646]
Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomarkers in medicine. 2011 Oct:5(5):557-66. doi: 10.2217/bmm.11.74. Epub [PubMed PMID: 22003904]
Kawano T, Hattori N, Uno Y, Kitajo K, Hatakenaka M, Yagura H, Fujimoto H, Yoshioka T, Nagasako M, Otomune H, Miyai I. Large-Scale Phase Synchrony Reflects Clinical Status After Stroke: An EEG Study. Neurorehabilitation and neural repair. 2017 Jun:31(6):561-570. doi: 10.1177/1545968317697031. Epub 2017 Mar 22 [PubMed PMID: 28506148]
Bartolomei F, McGonigal A, Naccache L. Alteration of consciousness in focal epilepsy: the global workspace alteration theory. Epilepsy & behavior : E&B. 2014 Jan:30():17-23. doi: 10.1016/j.yebeh.2013.09.012. Epub 2013 Oct 6 [PubMed PMID: 24103816]
Binnie CD, Prior PF. Electroencephalography. Journal of neurology, neurosurgery, and psychiatry. 1994 Nov:57(11):1308-19 [PubMed PMID: 7964803]
Dahal P, Ghani N, Flinker A, Dugan P, Friedman D, Doyle W, Devinsky O, Khodagholy D, Gelinas JN. Interictal epileptiform discharges shape large-scale intercortical communication. Brain : a journal of neurology. 2019 Nov 1:142(11):3502-3513. doi: 10.1093/brain/awz269. Epub [PubMed PMID: 31501850]
Bettus G, Ranjeva JP, Wendling F, Bénar CG, Confort-Gouny S, Régis J, Chauvel P, Cozzone PJ, Lemieux L, Bartolomei F, Guye M. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PloS one. 2011:6(5):e20071. doi: 10.1371/journal.pone.0020071. Epub 2011 May 19 [PubMed PMID: 21625517]
Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016 Oct:57(10):1546-1557. doi: 10.1111/epi.13510. Epub 2016 Aug 24 [PubMed PMID: 27554793]
Lagarde S, Roehri N, Lambert I, Trebuchon A, McGonigal A, Carron R, Scavarda D, Milh M, Pizzo F, Colombet B, Giusiano B, Medina Villalon S, Guye M, Bénar CG, Bartolomei F. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain : a journal of neurology. 2018 Oct 1:141(10):2966-2980. doi: 10.1093/brain/awy214. Epub [PubMed PMID: 30107499]
Tong X, An D, Xiao F, Lei D, Niu R, Li W, Ren J, Liu W, Tang Y, Zhang L, Zhou B, Gong Q, Zhou D. Real-time effects of interictal spikes on hippocampus and amygdala functional connectivity in unilateral temporal lobe epilepsy: An EEG-fMRI study. Epilepsia. 2019 Feb:60(2):246-254. doi: 10.1111/epi.14646. Epub 2019 Jan 17 [PubMed PMID: 30653664]
Foldvary N, Klem G, Hammel J, Bingaman W, Najm I, Lüders H. The localizing value of ictal EEG in focal epilepsy. Neurology. 2001 Dec 11:57(11):2022-8 [PubMed PMID: 11739820]
Vossler DG, Kraemer DL, Bell AJ. The Hippocampus and Cortex Together Generate the Scalp EEG Ictal Discharge in Temporal Lobe Epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2017 Sep:34(5):448-455. doi: 10.1097/WNP.0000000000000394. Epub [PubMed PMID: 28574952]
Pourmotabbed H, Wheless JW, Babajani-Feremi A. Lateralization of epilepsy using intra-hemispheric brain networks based on resting-state MEG data. Human brain mapping. 2020 Aug 1:41(11):2964-2979. doi: 10.1002/hbm.24990. Epub 2020 May 13 [PubMed PMID: 32400923]
Bercovici E, Kumar BS, Mirsattari SM. Neocortical temporal lobe epilepsy. Epilepsy research and treatment. 2012:2012():103160. doi: 10.1155/2012/103160. Epub 2012 Jul 16 [PubMed PMID: 22953057]
Tatum WO 4th. Mesial temporal lobe epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2012 Oct:29(5):356-65. doi: 10.1097/WNP.0b013e31826b3ab7. Epub [PubMed PMID: 23027091]
Murro AM, Park YD, King DW, Gallagher BB, Smith JR, Yaghmai F, Toro V, Figueroa RE, Loring DW, Littleton W. Seizure localization in temporal lobe epilepsy: a comparison of scalp-sphenoidal EEG and volumetric MRI. Neurology. 1993 Dec:43(12):2531-3 [PubMed PMID: 8255452]
V KR, Rajagopalan SS, Bhardwaj S, Panda R, Reddam VR, Ganne C, Kenchaiah R, Mundlamuri RC, Kandavel T, Majumdar KK, Parthasarathy S, Sinha S, Bharath RD. Machine learning detects EEG microstate alterations in patients living with temporal lobe epilepsy. Seizure. 2018 Oct:61():8-13. doi: 10.1016/j.seizure.2018.07.007. Epub 2018 Jul 10 [PubMed PMID: 30044996]
Maizuliana H, Usui N, Terada K, Kondo A, Inoue Y. Clinical, semiological, electroencephalographic, and neuropsychological features of "pure" neocortical temporal lobe epilepsy. Epileptic disorders : international epilepsy journal with videotape. 2020 Feb 1:22(1):55-65. doi: 10.1684/epd.2020.1132. Epub [PubMed PMID: 32031536]
Blair RD. Temporal lobe epilepsy semiology. Epilepsy research and treatment. 2012:2012():751510. doi: 10.1155/2012/751510. Epub 2012 Mar 7 [PubMed PMID: 22957241]
Avoli M, de Curtis M, Gnatkovsky V, Gotman J, Köhling R, Lévesque M, Manseau F, Shiri Z, Williams S. Specific imbalance of excitatory/inhibitory signaling establishes seizure onset pattern in temporal lobe epilepsy. Journal of neurophysiology. 2016 Jun 1:115(6):3229-37. doi: 10.1152/jn.01128.2015. Epub 2016 Apr 13 [PubMed PMID: 27075542]
Vaessen MJ, Jansen JF, Braakman HM, Hofman PA, De Louw A, Aldenkamp AP, Backes WH. Functional and structural network impairment in childhood frontal lobe epilepsy. PloS one. 2014:9(3):e90068. doi: 10.1371/journal.pone.0090068. Epub 2014 Mar 4 [PubMed PMID: 24594874]
Level 2 (mid-level) evidenceGold JA, Sher Y, Maldonado JR. Frontal Lobe Epilepsy: A Primer for Psychiatrists and a Systematic Review of Psychiatric Manifestations. Psychosomatics. 2016 Sep-Oct:57(5):445-64. doi: 10.1016/j.psym.2016.05.005. Epub 2016 May 16 [PubMed PMID: 27494984]
Level 1 (high-level) evidenceFeyissa AM, Britton JW, Van Gompel J, Lagerlund TL, So E, Wong-Kisiel LC, Cascino GC, Brinkman BH, Nelson CL, Watson R, Worrell GA. High density scalp EEG in frontal lobe epilepsy. Epilepsy research. 2017 Jan:129():157-161. doi: 10.1016/j.eplepsyres.2016.12.016. Epub 2017 Jan 2 [PubMed PMID: 28073096]
Bonini F, McGonigal A, Trébuchon A, Gavaret M, Bartolomei F, Giusiano B, Chauvel P. Frontal lobe seizures: from clinical semiology to localization. Epilepsia. 2014 Feb:55(2):264-77. doi: 10.1111/epi.12490. Epub 2013 Dec 24 [PubMed PMID: 24372328]
Beleza P, Pinho J. Frontal lobe epilepsy. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2011 May:18(5):593-600. doi: 10.1016/j.jocn.2010.08.018. Epub 2011 Feb 23 [PubMed PMID: 21349720]
Whitehead K, Gollwitzer S, Millward H, Wehner T, Scott C, Diehl B. The additional lateralizing and localizing value of the postictal EEG in frontal lobe epilepsy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016 Mar:127(3):1774-80. doi: 10.1016/j.clinph.2015.11.050. Epub 2015 Dec 11 [PubMed PMID: 26750581]
Laskowitz DT, Sperling MR, French JA, O'Connor MJ. The syndrome of frontal lobe epilepsy: characteristics and surgical management. Neurology. 1995 Apr:45(4):780-7 [PubMed PMID: 7723970]
Unnwongse K, Wehner T, Foldvary-Schaefer N. Mesial frontal lobe epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2012 Oct:29(5):371-8. doi: 10.1097/WNP.0b013e31826b3c60. Epub [PubMed PMID: 23027093]
Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy & behavior : E&B. 2009 May:15(1):15-21. doi: 10.1016/j.yebeh.2009.02.024. Epub 2009 Feb 21 [PubMed PMID: 19236946]
Lee RW, Worrell GA. Dorsolateral frontal lobe epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2012 Oct:29(5):379-84. doi: 10.1097/WNP.0b013e31826b3c7c. Epub [PubMed PMID: 23027094]
Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic disorders : international epilepsy journal with videotape. 2004 Dec:6(4):223-39 [PubMed PMID: 15634619]
Chibane IS, Boucher O, Dubeau F, Tran TPY, Mohamed I, McLachlan R, Sadler RM, Desbiens R, Carmant L, Nguyen DK. Orbitofrontal epilepsy: Case series and review of literature. Epilepsy & behavior : E&B. 2017 Nov:76():32-38. doi: 10.1016/j.yebeh.2017.08.038. Epub 2017 Sep 18 [PubMed PMID: 28928072]
Level 2 (mid-level) evidenceSalanova V. Parietal lobe epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2012 Oct:29(5):392-6. doi: 10.1097/WNP.0b013e31826c9ebc. Epub [PubMed PMID: 23027096]
Ristić AJ, Alexopoulos AV, So N, Wong C, Najm IM. Parietal lobe epilepsy: the great imitator among focal epilepsies. Epileptic disorders : international epilepsy journal with videotape. 2012 Mar:14(1):22-31. doi: 10.1684/epd.2012.0484. Epub [PubMed PMID: 22426412]
Akimura T, Fujii M, Ideguchi M, Yoshikawa K, Suzuki M. Ictal onset and spreading of seizures of parietal lobe origin. Neurologia medico-chirurgica. 2003 Nov:43(11):534-40 [PubMed PMID: 14705319]
Siegel AM, Williamson PD. Parietal lobe epilepsy. Advances in neurology. 2000:84():189-99 [PubMed PMID: 11091867]
Level 3 (low-level) evidenceKhan SA, Carney PW, Archer JS. Brief asymmetric tonic posturing with diffuse low-voltage fast activity in seizures arising from the mesial parietal region. Epilepsy research. 2014 Dec:108(10):1950-4. doi: 10.1016/j.eplepsyres.2014.09.011. Epub 2014 Oct 7 [PubMed PMID: 25445240]
Adcock JE, Panayiotopoulos CP. Occipital lobe seizures and epilepsies. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2012 Oct:29(5):397-407. doi: 10.1097/WNP.0b013e31826c98fe. Epub [PubMed PMID: 23027097]
AIRD RB, GASTAUT Y. Occipital and posterior electroencephalographic rhythms. Electroencephalography and clinical neurophysiology. 1959 Nov:11():637-56 [PubMed PMID: 13792196]
Harward SC, Chen WC, Rolston JD, Haglund MM, Englot DJ. Seizure Outcomes in Occipital Lobe and Posterior Quadrant Epilepsy Surgery: A Systematic Review and Meta-Analysis. Neurosurgery. 2018 Mar 1:82(3):350-358. doi: 10.1093/neuros/nyx158. Epub [PubMed PMID: 28419330]
Level 1 (high-level) evidenceMarchi A, Bonini F, Lagarde S, McGonigal A, Gavaret M, Scavarda D, Carron R, Aubert S, Villeneuve N, Médina Villalon S, Bénar C, Trebuchon A, Bartolomei F. Occipital and occipital "plus" epilepsies: A study of involved epileptogenic networks through SEEG quantification. Epilepsy & behavior : E&B. 2016 Sep:62():104-14. doi: 10.1016/j.yebeh.2016.06.014. Epub 2016 Jul 22 [PubMed PMID: 27454330]
Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surgical neurology international. 2014:5(Suppl 7):S295-303. doi: 10.4103/2152-7806.139612. Epub 2014 Aug 28 [PubMed PMID: 25289149]
Bosch B, Mansell H. Interprofessional collaboration in health care: Lessons to be learned from competitive sports. Canadian pharmacists journal : CPJ = Revue des pharmaciens du Canada : RPC. 2015 Jul:148(4):176-9. doi: 10.1177/1715163515588106. Epub [PubMed PMID: 26448769]