Introduction
Electroencephalography (EEG) provides a noninvasive method for directly measuring the brain's electrical activity. Waveform traces are recorded using electrodes placed on the scalp following standardized protocols. These EEG recordings offer valuable insights into the underlying brain function while helping to clarify seizure disorders and assess overall neurological status.
EEG is widely recognized for its role in seizure evaluation and epilepsy diagnosis. In addition, EEG also aids in diagnosing sleep disorders such as rapid eye movement (REM) sleep behavior disorder, narcolepsy, and parasomnias. Additionally, EEG plays a crucial role in evaluating and monitoring complex, multifactorial conditions, including encephalopathy, coma, medication effects, trauma impact, and brain death.
EEG tracings are particularly valuable in identifying and characterizing epileptiform and other pathological patterns associated with various disease states. Neurologists interpreting these tracings must refine their skills in recognizing abnormal patterns and determining whether they are benign (normal variants) or epileptiform, ensuring accurate diagnosis and effective management of neurological conditions.
Overinterpreting normal EEG variants of uncertain significance can create unnecessary concern, leading to additional testing and potential misdiagnoses. EEG interpreters must be equally knowledgeable about benign EEG variants and their significance as they are about epileptiform and other disease-specific patterns.[1]
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
An EEG is a noninvasive test used to measure the neurophysiological activity of the brain. The test involves placing electrodes on the scalp following standardized protocols to capture neuronal activity. Please see StatPearls' companion resource, "Breach Rhythm," for more information. Dynamic extracellular currents from transmembrane ion flow generate the signals captured by EEG for analysis.[1] The EEG machine amplifies these signals and records them as waveforms with varying frequencies and amplitudes, enabling the identification of normal and abnormal electrical activity patterns based on waveform characteristics.
A conventional EEG uses a standard set of 21 recording electrodes and one ground (reference) electrode, with odd-numbered electrodes positioned on the left side of the head and even-numbered ones on the right. Letter designations indicate different cortical regions (eg, F for frontal, C for central, T for temporal, O for occipital, and Z for midline). Please see StatPearls' companion resource, "EEG Neonatal Visual Analysis," for more information. Consistent and reproducible electrode placement ensures accurate interpretation and allows for reliable comparison between studies.
Overinterpreting EEG results can lead to misdiagnosing epilepsy. To ensure high diagnostic value and specificity, interpreters must recognize benign waveforms—including artifactual rhythms, normal rhythms, and normal variants of uncertain significance—that could be mistakenly identified as abnormal patterns. Please see StatPearls' companion resource, "Breach Rhythm," for more information.[2] The term "normal variants of uncertain significance" refers to waveforms that are considered normal but may be misinterpreted as abnormal or even epileptiform. Accurately distinguishing between artifactual, normal, and normal variant waveforms is crucial to avoid overreading and prevent misdiagnosis.[2]
Issues of Concern
Artifacts
Artifacts are electrical potentials observed on the EEG that are not generated by the brain. When artifacts are present, they can obscure important findings or lead to misleading conclusions. Some artifactual tracings, such as those related to muscle or eye movements, can aid in correctly identifying sleep-wake stages or provide other clinically relevant information. Artifacts can originate from physiological or extraphysiological sources. Extraphysiological artifacts are produced outside the body and are usually either environmental (eg, electrodes, equipment, and cellphones) or device-related (eg, pacemakers and neurostimulators).
Physiological artifacts: These artifacts originate within the body but outside the brain, as mentioned below.
- Muscle artifacts: These artifacts result from myogenic activity generated near the EEG electrodes. Known as EMG (electromyography) artifacts, they arise from contracting skeletal muscles. Common triggers include jaw clenching, swallowing, chewing, talking, grimacing, or frowning. These artifacts appear as sharply contoured, "spiky" waveforms with a short duration (<20 ms) and typically dissipate with jaw relaxation or sleep. An experienced EEG technologist can often confirm the presence of an artifact by instructing the patient to perform specific actions, such as saying "la, la, la," followed by relaxing the tongue on the floor of the mouth to eliminate tongue muscle artifacts. When available, video correlation with EEG waveforms provides valuable confirmation.
- Eye artifacts: These artifacts arise from the dipole between the positively charged cornea and the retina. When the eyes close, the globe rotates upward, creating a positively, sharply contoured, symmetrical waveform—most prominently recorded at the frontopolar electrodes with a steep posterior gradient—assuming the patient has not undergone enucleation. Conversely, eye-opening produces the opposite effect.
- Movement artifacts: These artifacts arise from a highly variable array of actions generated by motion-related electrical fields. Depending on which leads are affected, these artifacts can appear focal or widespread. Common sources include hiccups, tremors, shivering, and respiratory movements, all of which can produce artifactual waveforms on the EEG. Movement monitors and specialized electrodes can aid in identifying these artifacts, while video recordings often provide crucial confirmation.
- Cardiovascular artifacts: These artifacts originate from the heart and are more likely to occur when the heart is positioned closer to the brain, such as in infants or adults with short necks.
- Sweat artifacts: These artifacts arise from perspiration and are relatively easy to eliminate by using either special filters or simply by drying the scalp.
Normal rhythms: These rhythms fall within specific ranges of frequency, amplitude, morphology, and distribution. Standard frequency bands include delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (>30 Hz). Some waveforms vary with age, while others differ based on arousal state (eg, sleep versus wakefulness), spatial distribution, or responses to stimuli. Normal rhythms typically exhibit interhemispheric symmetry.
- Wake state rhythms: These rhythms include classical waveforms such as the alpha rhythm (also known as the posterior dominant rhythm) and the mu rhythm, which appears during rest with eyes closed. Posteriorly oriented lambda waves emerge during visual scanning with eyes open. Additionally, typical waveforms can be induced by factors such as hyperventilation, youthfulness, flashing lights, sleep-wake transitions, and skull defects (referred to as the "breach" rhythm).
- Sleep state rhythms: These rhythms include vertex waves, sleep spindles, K complexes, and positive occipital sharp transients of sleep (POSTS), which are typically observed during light sleep.
Normal variants: These variants are often referred to as variants of uncertain clinical significance. They include nearly a dozen waveforms that can be misclassified as abnormal, including epileptiform patterns, yet are considered normal.[2]
The normal variants of uncertain significance consist of 10 subtypes, as mentioned below.
- Benign sporadic sleep spikes (BSSS)
- Wicket spikes
- 14- and 6-Hz positive spikes
- 6-Hz spike and waves
- Rhythmic temporal theta-burst of drowsiness (RTTD)
- Subclinical rhythmic electrographic discharge of adults (SREDA)
- Slow fused transients
- Occipital spikes of blindness
- Midline central theta (also known as a Cigánek) rhythm
- Benign central transients of the older populations [2]
This section will describe the major variants in detail.
Benign Sporadic Sleep Spikes
BSSS, also known as small, sharp spikes or benign epileptiform transients of sleep (BETS), are characterized by low amplitude (<50 mV), brief duration (<50 ms), and sharply contoured monophasic or biphasic electronegative spikes that occur in the theta- or alpha frequency range. BSSS are typically observed in a widespread horizontal dipole distribution, without causing noticeable disruption to the ongoing background activity. Occasionally, BSSS may be accompanied by a slow wave that follows the spikes, which usually has a lower amplitude than the spikes themselves. These spikes are sporadic and do not appear in runs.[2]
BSSS typically occur in the temporal region and are characteristically challenging to localize precisely. They often appear in both hemispheres, either independently or bisynchronously, and may exhibit shifting asymmetries. BSSS occur almost exclusively during drowsiness or light non-REM sleep. The appearance of stereotyped, isolated, small-amplitude spikes that do not disrupt the background activity and occur only during drowsiness or light sleep strongly suggests that the transients may be BSSS. BSSS are among the most common benign EEG variants, appearing in 1.85% to 24% of scalp EEG recordings.[3] BSSS are usually observed in adults and seldom in children.
Until the early 1990s, BSSS were considered epileptogenic. However, a 1991 study by Molaie et al concluded that BSSS are likely benign variants.[4] A more recent study by Issa et al conducted using implanted electrodes in the hippocampi demonstrated that some sporadic sleep spikes were time-locked to hippocampal epileptiform discharges and high-frequency oscillations. In that study, the authors suggested that sporadic sleep spikes might serve as early indicators of a hippocampal pathological condition (see Image. BSSS [aka BETS]).[5]
Wicket Spikes or Wicket Rhythms
Wicket spikes, also known as wicket rhythms, are medium to high-voltage, monophasic wave bursts within the theta- or alpha frequency range (6-11 Hz). These spikes typically manifest in the anterior or middle temporal areas with negative polarity, evolving from the background into arcuate-shaped, brief rhythmic discharges lasting between 0.5 and 1 second. Wicket spikes are most commonly observed in adults aged 30 or older during light sleep or in individuals whose background activity features sharply contoured waveforms. Although wicket spikes were initially classified as a temporal lobe phenomenon—often with a left-lateralizing tendency—similar isolated and sharply contoured waveforms can occur in any region of the scalp exhibiting sharply contoured background activity (see Image. Typical Wicket Waves).[6]
Several extensive studies report a prevalence of wicket spikes of approximately 1%. This pattern's rarity, unilateral distribution, temporal location, and spike-like single-fragment appearance often lead to their misinterpretation as interictal epileptiform discharges. Several features can help differentiate wicket spikes from interictal epileptiform discharges. Wicket spikes do not disrupt background activity or exhibit an after-going slow wave. Instead, they typically appear repetitively in trains of arch-shaped rhythms that resemble a mu rhythm.[7] Notably, wicket spikes are not associated with epilepsy but are observed more frequently in patients with cerebrovascular disease, dizziness or vertigo, and headache.[8][9]
6-Hz Spike and Wave Discharges (Phantom Spike and Wave)
6-Hz spike and wave discharges consist of a repetitive spike-and-wave complex with a mitten-like morphology in the 4 to 7 Hz range. Also referred to as "phantom" spike waves, these discharges have a relatively low amplitude (<40 mV), featuring a fast spike (<30 ms) followed by a wave of equal or greater amplitude at 5 to 7 Hz. Typically, 6-Hz spike and wave discharges occur in young adults, predominantly during relaxed wakefulness, drowsiness, or light sleep, and occasionally in REM sleep.[10] They usually occur bilaterally and synchronously.
The 6-Hz spike and wave discharges have been described in 2 different forms. The classical form, known as "FOLD" (female occipital low amplitude spike, drowsy), is characterized by relatively low amplitude and maximal activity over the posterior head regions, and it is not associated with seizures. The second form, termed "WHAM" (waking, high amplitude spike, anterior, male), exhibits moderate-to-high amplitude and is maximal over the frontal regions.[11] This form of the 6-Hz spike and wave discharges overlaps with abnormal generalized atypical spike and wave patterns with rapid repetition rates. In WHAM, characteristics such as high spike amplitude, a frequency of less than 5 Hz, anterior location, and persistence into slow wave sleep should prompt further investigation, as these factors are associated with seizures (see Image. Phantom Spike and Wave).
14- and 6-Hz Positive Spikes
14- and 6-Hz positive spikes are most effectively observed using long interelectrode distances and ear-referential montages. These spikes consist of brief runs (<1 s) of positive spikes occurring at rates of 14 Hz or 6 to 7 Hz. The amplitude of these spikes is variable but rarely exceeds 75 mV. These bursts typically consist of "negative" arched waveforms located over the posterior temporal regions of the head, interspersed with alternating "positive" spiky components. The pattern can occur bisynchronously or unilaterally, meaning the 2 hemispheres may exhibit independent activity at different times. The 14-Hz pattern often resembles a sleep spindle with a sharp positive phase, although its location is distinct. Bursts of both 6- and 14 Hz can occur independently of each other.
This benign variant pattern occurs in 20% to 60% of the normal population, predominantly among adolescents, especially during drowsiness and light non-REM sleep.[12] The incidence decreases with age. Studies from the 1950s to the 1970s suggested that this pattern was commonly associated with toxic-metabolic encephalopathies, including coma. However, a 1968 study indicated that the presence of this pattern could serve as a favorable prognostic indicator in such cases.[13] The 14- and 6-Hz positive spike pattern is currently regarded as a benign EEG variant without any pathological or epileptogenic significance (see Image. Positive Spiky Waveforms).[2]
Rhythmic Mid-Temporal Theta Burst of Drowsiness
Rhythmic mid-temporal discharges or RMTDs, also referred to as the "psychomotor variant," share similarities with epileptiform discharges seen during psychomotor seizures. RMTD consists of bursts of rhythmic, non-evolving sharp waves in the theta frequency range ( 4-7 Hz), lasting from a few seconds to a few minutes. RMTD typically appears in the mid-temporal regions but may spread parasagittally or to the posterior temporal areas. This can occur unilaterally or bilaterally in an independent manner. Distinguishing RMTD from ictal activity is possible due to its lack of evolution and monorhythmic and monomorphic features.[12]
RMTDs occur in approximately 0.1% to 2.1% of young adults, and their prevalence can reach up to 10% in older adults.[14] RMTDs typically manifest during drowsiness and light sleep.[3] Currently, RMTDs are considered benign variants due to the absence of associated clinical changes, although earlier studies suggested they were among the least epileptogenic temporal discharges.[15] Research indicates that RMTD originates from the inferior temporal fissure, as supported by EEG and magnetoencephalographic studies.[16]
Subclinical Rhythmic Electrographic Discharge of Adults
SREDA is rare, with a prevalence ranging from 0.04% to 0.07%. A recent study reported an incidence of 0.03%.[3][17][18]
SREDA is characterized by monomorphic, diffuse, sharply contoured, or sinusoidal theta frequency waveforms within the range of 5 to 7 Hz, typically seen most prominently in the parietal and posterior temporal regions. SREDA can be generalized either symmetrically or asymmetrically. Atypical SREDA may evolve in frequency from delta to theta and can change abruptly or gradually. In contrast to actual ictal activities, SREDA shows minimal changes in frequency, morphology, and distribution.[19]
Similar to RMTD, SREDA can be misinterpreted as ictal epileptiform activity. However, several distinctive features can help differentiate it, as mentioned below.[20]
- SREDA occurs while the patient is awake, and response testing can demonstrate that consciousness and mentation are preserved.
- SREDA is likely to appear in subsequent EEG recordings, even if several years have passed between recordings.
- SREDA exhibits a relative lack of evolution in frequency, morphology, or distribution compared to most abnormal seizure patterns, and in some cases, background activity may persist.
- SREDA is further distinguished from seizure activity by the absence of postictal slowing.
Although SREDA primarily occurs in adults, particularly older adults, it has also been reported in children and adolescents. SREDA typically manifests in relaxed, awake, and drowsy states, but it can also occur during sleep. SREDA has been associated with vascular and nonvascular neurological conditions, as well as non-neurological diseases, including migraine, syncope, transient global ischemia, and hemolytic uremic syndrome.[21][22]
Clinical Significance
Electroencephalographers risk misclassifying benign variant EEG patterns as epileptiform discharges. This misclassification can lead to diagnostic delays in other paroxysmal disorders, such as psychogenic nonepileptic seizures, anxiety or panic disorders, and near syncope.[8] Additionally, unnecessary treatments may arise from these misclassifications.
Careful interpretation of EEG patterns, along with a meticulous review of clinical history and demographic features, typically aids in distinguishing normal variant patterns from interictal epileptiform discharges or seizures. When nonspecific transients appear on the EEG, it may be challenging to determine whether a transient represents an epileptiform discharge or a benign variant. Notably, it is advisable not to "overcall" a finding as abnormal; instead, it is best to examine the finding in the context of the clinical history.
Enhancing Healthcare Team Outcomes
EEG is crucial for the accurate diagnosis of seizures and psychogenic nonepileptic spells, enabling clinicians to differentiate between pathological activity and normal physiological findings that may appear similar. Identifying benign epileptiform variants in EEG readings is essential to prevent diagnostic confusion and reduce the risk of unnecessary treatment with antiseizure medications, which can have significant side effects. Neurologists, primary care physicians, and nurses will all benefit from precise and accurate EEG interpretation.
Recent studies indicate that benign variants may go unrecognized, potentially leading to misdiagnosis as epileptiform discharges.[23][18] Therefore, it is essential for all members of the interprofessional healthcare team caring for patients with seizures and other paroxysmal events to be familiar with these EEG waveforms. The epileptologist interpreting the EEG benefits from input provided by other team members, who can offer details about the patient’s presentation, intra-EEG physiological and behavioral events, and other distinctive characteristics.
Media
(Click Image to Enlarge)
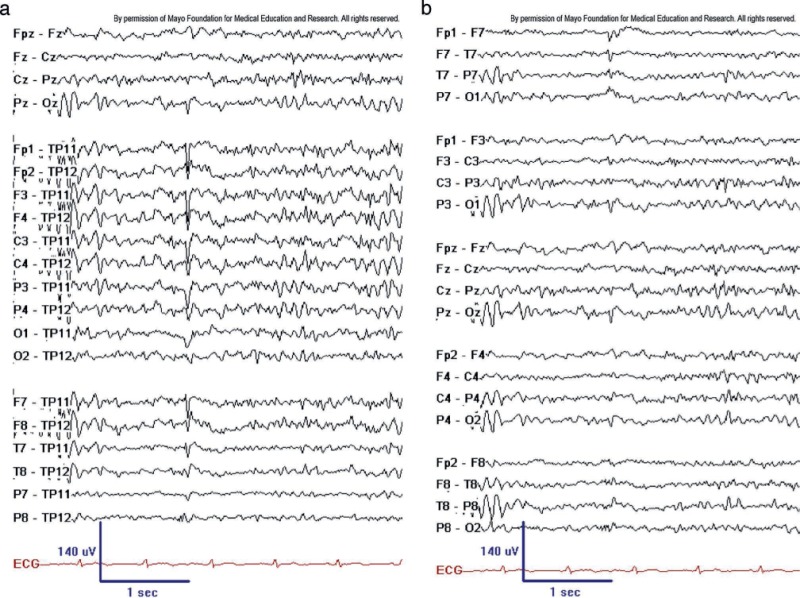
BSSS (aka BETS). BSSS on ipsilateral ear reference (left) and longitudinal bipolar montage (right). Note steep descending slope of low-amplitude spike and small after-coming slow wave, particularly at T7 to TP11. BSSS are a common feature in adult EEGs during drowsiness and sleep. Note the typical steep descending waveform limb and lack of background disturbance. Copyright ©2016 by American Epilepsy Society.
Copyright of all cited figures: Copyright ©2016 by American Epilepsy Society. No permission is required from the editors, authors, or publisher to reuse or repurpose content, provided the original work is properly cited. Figures, tables, and images included in a publication of “Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants” are also published under the CC BY-NC-SA license and should be properly cited when reused or repurposed. Image.
(Click Image to Enlarge)
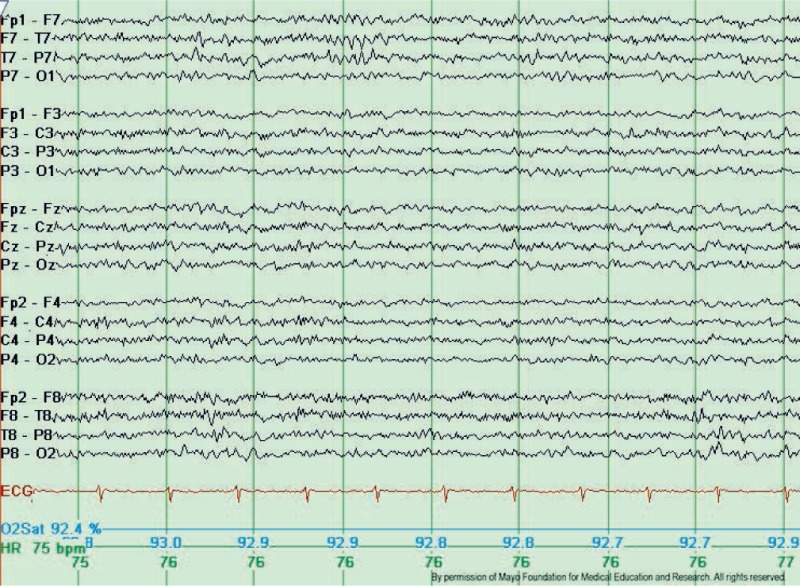
Typical Wicket Waves. Note the arciform appearance, lack of after-going slow wave, and lack of background disruption or disturbance. The wicket waves are seen in the left temporal region with phase-reversal at T7 in seconds 3 and 4 of the tracing, longitudinal bipolar montage. Copyright ©2016 by American Epilepsy Society.
Copyright of all cited figures: Copyright ©2016 by American Epilepsy Society. No permission is required from the editors, authors, or publisher to reuse or repurpose content, provided the original work is properly cited. Figures, tables, and images included in a publication of “Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants” are also published under the CC BY-NC-SA license and should be properly cited when reused or repurposed. Image.
(Click Image to Enlarge)
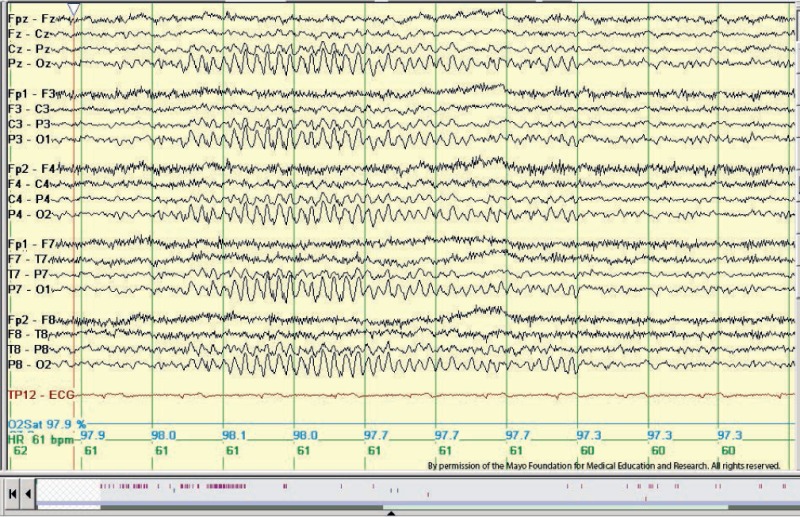
Phantom Spike and Wave. Example of 6-Hz “phantom” spike and wave in an adolescent patient. There are bilaterally symmetrical diffuse tiny spikes with prominent wave components (“mitten-like” morphology) in seconds 3 through 6 below. Longitudinal bipolar montage. This is the posterior variant, sometimes called the FOLD (female occipital low-amplitude drowsiness) subtype. Copyright ©2016 by American Epilepsy Society.
Copyright of all cited figures: Copyright ©2016 by American Epilepsy Society. No permission is required from the editors, authors, or publisher to reuse or repurpose content, provided the original work is properly cited. Figures, tables, and images included in a publication of “Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants” are also published under the CC BY-NC-SA license and should be properly cited when reused or repurposed. Image.
(Click Image to Enlarge)
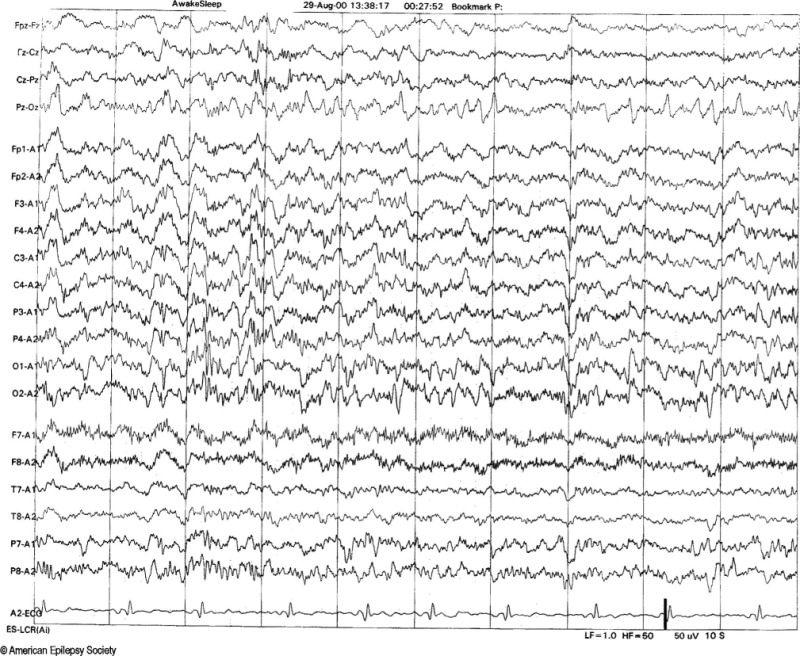
Positive Spiky Waveforms. Example of 14- and 6-Hz positive spikes in a 13-year-old girl. Note that the 14-Hz positive spiky waveforms are best appreciated over the posterior temporal and biposterior head regions, and they are best seen in the third second. Copyright ©2016 by American Epilepsy Society.
Copyright of all cited figures: Copyright ©2016 by American Epilepsy Society. No permission is required from the editors, authors, or publisher to reuse or repurpose content, provided the original work is properly cited. Figures, tables, and images included in a publication of “Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants” are also published under the CC BY-NC-SA license and should be properly cited when reused or repurposed. Image.
References
Tatum WO 4th. EEG Essentials. Continuum (Minneapolis, Minn.). 2022 Apr 1:28(2):261-305. doi: 10.1212/CON.0000000000001129. Epub [PubMed PMID: 35393960]
Amin U, Nascimento FA, Karakis I, Schomer D, Benbadis SR. Normal variants and artifacts: Importance in EEG interpretation. Epileptic disorders : international epilepsy journal with videotape. 2023 Oct:25(5):591-648. doi: 10.1002/epd2.20040. Epub 2023 Jul 27 [PubMed PMID: 36938895]
Santoshkumar B, Chong JJ, Blume WT, McLachlan RS, Young GB, Diosy DC, Burneo JG, Mirsattari SM. Prevalence of benign epileptiform variants. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009 May:120(5):856-61. doi: 10.1016/j.clinph.2009.03.005. Epub 2009 Apr 10 [PubMed PMID: 19362516]
Level 2 (mid-level) evidenceMolaie M, Santana HB, Otero C, Cavanaugh WA. Effect of epilepsy and sleep deprivation on the rate of benign epileptiform transients of sleep. Epilepsia. 1991 Jan-Feb:32(1):44-50 [PubMed PMID: 1985829]
Issa NP, Wu S, Rose S, Towle VL, Warnke PC, Tao JX. Small sharp spikes as EEG markers of mesiotemporal lobe epilepsy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2018 Sep:129(9):1796-1803. doi: 10.1016/j.clinph.2018.06.011. Epub 2018 Jun 28 [PubMed PMID: 30005212]
Azzam RH, Arain AM, Azar NJ. Revisiting the laterality of wicket spikes with continuous EEG. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2015 Apr:32(2):e8-11. doi: 10.1097/WNP.0000000000000124. Epub [PubMed PMID: 25159738]
Reiher J, Lebel M. Wicket spikes: clinical correlates of a previously undescribed EEG pattern. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1977 Feb:4(1):39-47 [PubMed PMID: 837263]
Kang JY, Krauss GL. Normal Variants Are Commonly Overread as Interictal Epileptiform Abnormalities. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2019 Jul:36(4):257-263. doi: 10.1097/WNP.0000000000000613. Epub [PubMed PMID: 31274688]
Vallabhaneni M, Baldassari LE, Scribner JT, Cho YW, Motamedi GK. A case-control study of wicket spikes using video-EEG monitoring. Seizure. 2013 Jan:22(1):14-9. doi: 10.1016/j.seizure.2012.09.009. Epub 2012 Oct 5 [PubMed PMID: 23041030]
Level 2 (mid-level) evidenceGelisse P, Crespel A. Phantom spike-and-wave bursts during REM-sleep. Neurophysiologie clinique = Clinical neurophysiology. 2008 Aug:38(4):249-53. doi: 10.1016/j.neucli.2008.04.002. Epub 2008 May 7 [PubMed PMID: 18662622]
Level 3 (low-level) evidenceHughes JR. Two forms of the 6/sec spike and wave complex. Electroencephalography and clinical neurophysiology. 1980 May:48(5):535-50 [PubMed PMID: 6153962]
St. Louis EK, Frey LC, Britton JW, Frey LC, Hopp JL, Korb P, Koubeissi MZ, Lievens WE, Pestana-Knight EM, St. Louis EK. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. 2016:(): [PubMed PMID: 27748095]
Long MT, Johnson LC. Fourteen- and six-per-second positive spikes in a nonclinical male population. Neurology. 1968 Jul:18(7):714-6 [PubMed PMID: 4386209]
Macorig G, Crespel A, Nilo A, Tang NPL, Valente M, Gigli GL, Gélisse P. Benign EEG variants in the sleep-wake cycle: A prospective observational study using the 10-20 system and additional electrodes. Neurophysiologie clinique = Clinical neurophysiology. 2021 Jun:51(3):233-242. doi: 10.1016/j.neucli.2021.03.006. Epub 2021 Apr 16 [PubMed PMID: 33875321]
Level 2 (mid-level) evidenceHughes JR, Olson SF. An investigation of eight different types of temporal lobe discharges. Epilepsia. 1981 Aug:22(4):421-35 [PubMed PMID: 7262049]
Lin YY, Wu ZA, Hsieh JC, Yu HY, Kwan SY, Yen DJ, Yiu CH, Ho LT. Magnetoencephalographic study of rhythmic mid-temporal discharges in non-epileptic and epileptic patients. Seizure. 2003 Jun:12(4):220-5 [PubMed PMID: 12763469]
Azman Iste F, Tezer Filik FI, Saygi S. SREDA: A Rare but Confusing Benign EEG Variant. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2020 May:37(3):225-230. doi: 10.1097/WNP.0000000000000623. Epub [PubMed PMID: 31393275]
Wüstenhagen S, Terney D, Gardella E, Meritam Larsen P, Rømer C, Aurlien H, Beniczky S. EEG normal variants: A prospective study using the SCORE system. Clinical neurophysiology practice. 2022:7():183-200. doi: 10.1016/j.cnp.2022.06.001. Epub 2022 Jun 30 [PubMed PMID: 35865124]
Gil L, Moosa A, Gupta A. Atypical SREDA During Wakefulness, NREM and REM Sleep in a Young Teenager: A Diagnostic Challenge. Clinical EEG and neuroscience. 2019 Jul:50(4):296-299. doi: 10.1177/1550059418824446. Epub 2019 Jan 23 [PubMed PMID: 30672308]
Dash GK, Radhakrishnan A. What does "subclinical rhythmic electrographic discharge of adults" in EEG signify? Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2013 Jun:30(3):255-60. doi: 10.1097/WNP.0b013e3182933d4d. Epub [PubMed PMID: 23733089]
Burakgazi E, Akbar U, Kelly J. Subclinical rhythmic electrographic discharges of adults in a patient with migraine. Clinical EEG and neuroscience. 2012 Apr:43(2):165-8. doi: 10.1177/1550059412443938. Epub 2012 Apr 16 [PubMed PMID: 22715490]
Level 3 (low-level) evidenceBegum T, Ikeda A, Takahashi J, Tomimoto H, Shimohama S, Satow T, Nagamine T, Fukuyama H, Shibasaki H. Clinical outcome of patients with SREDA (subclinical rhythmic EEG discharge of adults). Internal medicine (Tokyo, Japan). 2006:45(3):141-4 [PubMed PMID: 16508227]
Level 3 (low-level) evidenceRathore C, Prakash S, Rana K, Makwana P. Prevalence of benign epileptiform variants from an EEG laboratory in India and frequency of their misinterpretation. Epilepsy research. 2021 Feb:170():106539. doi: 10.1016/j.eplepsyres.2020.106539. Epub 2021 Jan 5 [PubMed PMID: 33461042]